Abstract
Clonal integration of Merkel cell polyomavirus (MCV) DNA into the host genome has been observed in at least 80% of Merkel cell carcinoma (MCC). The integrated viral genome typically carries mutations that truncate the C-terminal DNA binding and helicase domains of the MCV large T antigen (LT), suggesting a selective pressure to remove this MCV LT region during tumor development. In this study, we show that MCV infection leads to the activation of host DNA damage responses (DDR). This activity was mapped to the C-terminal helicase-containing region of the MCV LT. The MCV LT-activated DNA damage kinases, in turn, led to enhanced p53 phosphorylation, upregulation of p53 downstream target genes, and cell cycle arrest. Compared to the N-terminal MCV LT fragment that is usually preserved in mutants isolated from MCC tumors, full-length MCV LT shows a decreased potential to support cellular proliferation, focus formation, and anchorage-independent cell growth. These apparently antitumorigenic effects can be reversed by a dominant-negative p53 inhibitor. Our results demonstrate that MCV LT-induced DDR activates p53 pathway, leading to the inhibition of cellular proliferation. This study reveals a key difference between MCV LT and simian vacuolating virus 40 LT, which activates a DDR but inhibits p53 function. This study also explains, in part, why truncation mutations that remove the MCV LT C-terminal region are necessary for the oncogenic progression of MCV-associated cancers.
INTRODUCTION
Merkel cell polyomavirus (MCV) is the first polyomavirus to be clearly associated with cancer in humans (1). Its genome was recently found integrated into the chromosomes of a highly aggressive skin cancer, Merkel cell carcinoma (MCC) (2). Subsequent analyses of a large number of MCC tumors have revealed that this polyomavirus is associated with at least 80% of all MCC cases (2–4). Integrated MCV genome has also been detected in non-small-cell lung cancer (5). Epidemiological surveys for MCV seropositivity (6, 7) and sequencing analyses of healthy human skin (8) have indicated that MCV represents a common component of the human skin microbial flora.
As with other polyomaviruses, the MCV genome contains an early region that encodes the viral tumor antigens. Differential splicing of the early mRNA produces large tumor antigen (LT), small tumor antigen (sT), and 57kT proteins (9, 10). The highly multifunctional LT protein is involved in a variety of processes, including initiation of viral genome replication, as well as manipulation of the host cell cycle through a number of protein-protein interactions. It has been shown that MCV LT interacts with at least some of the same cellular factors as simian virus 40 (SV40) LT (11). SV40 LT interacts with classic partners including heat shock protein 70 (Hsc70) through the LT DnaJ domain and also interacts with retinoblastoma “pocket protein” (Rb) family members through a classic LxCxE motif in the N-terminal region of LT. SV40 LT binding of Rb abrogates its role as a repressor of E2F transcription factors, thereby promoting transition into S phase. MCV LT is thought to interact with Hsc70 and Rb via similar mechanisms (11–13). SV40 LT is also known to interact with the tumor suppressor protein p53 through two C-terminal LT regions within the helicase domain of LT (14). SV40 LT binding of p53 functionally inactivates its ability to induce cellular senescence or apoptosis in the face of genotoxic stress (see references 1 and 13) for excellent reviews).
The SV40 LT protein has been shown to induce transformation and immortalization in a variety of in vitro (15) and in vivo (16) models. This SV40 LT transforming capability has been attributed, in part, to its ability to inactivate Rb and p53 tumor suppressors (17). SV40 sT's role in cellular transformation is largely supportive in nature, enhancing SV40 LT's ability to induce oncogenesis. In contrast, there is evidence suggesting that MCV sT may have an enhanced transforming ability compared to its SV40 homologue (18). This is consistent with the observation that integrated MCV genomes in MCC tumors nearly always carry mutations resulting in various C-terminal truncations of LT while preserving the full-length sT open reading frame (11). It has also been postulated that the C-terminal helicase domain of LT is selectively truncated in MCC because an intact LT protein would drive over-replication of the integrated viral origin, which would presumably lead to cell growth arrest or death (11). The characteristic truncations of MCV LT C-terminal region found in MCC-associated viral sequences also suggest a selective pressure to remove this MCV LT region during tumor development.
Numerous viruses, including SV40, have been shown to not only elicit but also manipulate the host DDR (19–26). The host DDR is a complex array of signaling pathways that collectively monitor the level of genotoxic stress from DNA replication, cellular metabolism, and exogenous insults such as UV exposure (27). These pathways coordinately recruit the necessary protein complexes required to repair DNA damage, while also signaling to various checkpoints to stall cell cycle progression, allowing for efficient DNA repair or induction of apoptosis (27). The ataxia telangiectasia mutated (ATM) kinase pathway responds primarily to double-stranded breaks (DSBs) and initiates repair through homologous recombination and nonhomologous end-joining repair. DSBs activate the ATM kinase by inducing the autophosphorylation of ATM at serine (Ser) 1981. Activated ATM phosphorylates the threonine (Thr) 68 of the downstream kinase Chk2, which then phosphorylates a variety of target proteins, including p53. In parallel, the ataxia telangiectasia and Rad3-related (ATR) kinase pathway is activated by single-stranded DNA lesions, such as stalled replication forks and other sites of replicative stress. The activated ATR kinase can phosphorylate the downstream kinase Chk1 at Ser 317 and Ser 345 (28). Chk1 can in turn phosphorylate various target proteins, many of which overlap with Chk2's substrates. Phosphorylation of p53 by either Chk1 or Chk2 at particular serines, including Ser 15 and Ser 20, stabilizes p53 and allows it to function as a transcription factor to promote downstream target gene expression that leads to cell cycle arrest, senescence, or apoptosis (29). Both activated ATM and ATR can also phosphorylate a histone variant, H2AX. The phosphorylated H2AX (referred to as γH2AX) has become a classic marker for DNA damage.
SV40 infection activates both ATM and ATR kinase pathways, and certain components of these pathways may be beneficial for viral replication (30–32). However, these pathways do not efficiently signal through p53, as the SV40 LT protein directly inactivates p53's ability to promote cell cycle arrest. A current study by Cheng et al. (33) suggests that, in contrast to SV40 LT, MCV LT does not interact with p53.
In the present study, we show that MCV infection and MCV LT expression also elicit a DDR. Interestingly, we found that MCV LT activates the cellular DDR through its C-terminal region, leading to enhanced p53 phosphorylation and activation of p53 downstream target genes. These signaling events induce cell cycle arrest and inhibit cellular proliferation. These results provide clues for understanding why deletion of the C-terminal region of MCV LT is a critical event during the oncogenic progression of MCV-associated cancers.
MATERIALS AND METHODS
Cell culture, cell lines, and transfection.
U2OS cells were maintained in McCoy's 5A medium (Invitrogen) containing 10% fetal bovine serum (FBS; HyClone). HEK293T cells, HEK293, and NIH 3T3 cells were maintained in Dulbecco modified Eagle medium (Invitrogen) containing 10% FBS. FuGENE6 transfection reagent (Promega), Lipofectamine 2000 (Invitrogen), GeneJammer transfection reagent (Agilent), or calcium phosphate transfection reagent were used for U2OS, HEK293T, and HEK293 transfections according to the manufacturers' instructions. Dharmafect 4 transfection reagent (Thermo Scientific) was used for small interfering RNA (siRNA) transfection.
Recombinant plasmid construction.
Plasmids pIRES-hygromycin and pTIH, which encodes SV40 LT antigen, have been described previously (34). The MCV LT gene used for making the truncation mutants contains the same silent modifications found in pADL* that prevent splicing of the 57kT intron (11, 35, 36). The MCV LT construct is not capable of expressing sT. For the plasmids pcDNA4C-MCV LT 1-211 and pcDNA4C-MCV LT 1-440, DNA fragments encoding the corresponding amino acid sequence of MCV LT were subcloned individually into pcDNA4C using PstI and XhoI sites. For the plasmids pcDNA4C-MCV LT 212-440, pcDNA4C-MCV LT 212-817, and pcDNA4C-full-length MCV LT 1-817, DNA fragments encoding the corresponding amino acid sequence of MCV LT were subcloned individually into pcDNA4C using KpnI and XhoI sites. Each of the pcDNA4C-MCV LT constructs encodes an Xpress tag located at the N terminus of the protein. For pEGFPC1-MCV LT 1-440, pEGFPC1-MCV LT 441-817, and pEGFPC1-full-length MCV LT 1-817, DNA fragments encoding the corresponding amino acid sequence of MCV LT were subcloned individually into pEGFPC1 using XhoI and KpnI sites. A DNA fragment encoding the MCV LT nuclear localization signal (Arg-Lys-Arg-Lys) was inserted at the N terminus of the LT 441-817 fragment. None of the MCV LT constructs used in the present study contain an MCV origin of replication. To prepare religated MCV genome, pMCV-R17a carrying MCV genome was digested with EcoRI to release MCV genome. Linear MCV genome was gel purified and religated with T4 DNA ligase overnight at 16°C. To prepare religated pEGFPC1, pEGFPC1 plasmid was digested with EcoRI, gel purified, and religated with T4 DNA ligase overnight at 16°C. For pLPCX-MCV LT 1-440, pLPCX-MCV LT 223-817, and pLPCX full-length MCV LT 1-817, DNA fragments encoding the corresponding amino acid sequence of MCV LT were subcloned individually into pLPCX using XhoI and NotI sites. For pLPCX-Cherry-LacI cloning, the Cherry-LacI coding DNA fragment was inserted into pLPCX using EcoRI and NotI sites. pLXSN and pLXSN-p53DD were provided by Moshe Oren (The Weizmann Institute). For the MCV LT helicase domain point mutants K599R, E627A, and E640A/D641A, synthetic oligonucleotides containing the desired mutations were annealed to denatured template plasmid pcDNA4C-MCV LT 1-817 and extended by Pfu Turbo polymerase (Agilent) in a PCR. Unmutated plasmid DNA templates were removed by DpnI digestion, and DNA was used to transform DH5α competent cells. For recombinant protein expression and purification, IgG-IgG-TEV (IIT), a DNA sequence encoding two IgG binding domains of Staphylococcus aureus protein A and a tobacco etch virus (TEV) protease cleavage site, was fused to the N terminus of either wild-type or mutant MCV LT in pcDNA4C vector. All constructs were confirmed by restriction enzyme digestion and DNA sequencing.
Antibodies, chemicals, and siRNAs.
The following antibodies were used for immunofluorescence: mouse anti-Xpress (Invitrogen), mouse anti-MCV LT (CM2B4; Santa Cruz), rabbit anti-γH2AX (Cell Signaling), rabbit anti-phosphorylated Chk1S317 (Cell Signaling), rabbit anti-phosphorylated p53S15 (Cell Signaling), rabbit anti-RPA70 (Cell Signaling), Rabbit anti-RPA32S33 (Bethyl Laboratories), Alexa Fluor 594 goat anti-rabbit IgG (Invitrogen), and Alexa Fluor 488 goat anti-mouse IgG (Molecular Probes).
In addition to the antibodies described above, the following antibodies were used for Western blot analyses: mouse anti-SV40 LT (Pab 101; Santa Cruz), rabbit anti-phosphorylated ATMS1981 (Abcam), rabbit anti-ATM (Cell Sigaling), rabbit anti-phosphorylated Chk1S345 (Cell Signaling), mouse anti-Chk1 (Santa Cruz), rabbit anti-phosphorylated Chk2T68 (Cell Signaling), rabbit anti-Chk2 (Cell Signaling), rabbit anti-p53 (Santa Cruz), rabbit anti-p21 (Cell Signaling), rabbit anti-ATR (Abcam), mouse anti-LacI (Millipore), mouse anti-GFP (Santa Cruz), mouse anti-actin (Chemicon), mouse anti-GAPDH (U.S. Biologicals), horseradish peroxidase (HRP)-conjugated horse anti-mouse IgG (Cell Signaling), and HRP-conjugated Goat anti-rabbit IgG (Cell Signaling).
H2O2, hydroxyurea, NU6027, and puromycin were purchased from Sigma. G418 was purchased from American Bioanalytical. AZD7762 was purchased from Selleckchem. Western Lightning Plus-ECL solution was purchased from Perkin-Elmer (NEL). ATR siRNA pools targeting human ATR were purchased from Dharmacon.
Comet assay.
DNA damage in U2OS cells was analyzed using the Comet assay under alkaline conditions as previously described (37). Briefly, the cells were dislodged with cell dissociation solution (Sigma), washed in ice-cold phosphate-buffered saline (PBS), mixed with low gelling temperature agarose, and pipetted onto comet slides. After solidification, the cells were lysed by incubation in alkaline lysis solution (1.2 M NaCl, 100 mM Na2EDTA, 0.1% sodium lauryl sarcosinate, 0.26 M NaOH [pH >13]) for 1 h at 4°C in the dark. For alkaline single-cell electrophoresis that detects both single- and double-stranded DNA breaks, the slides were placed in alkaline electrophoresis buffer (0.03 M NaOH, 2 mM Na2EDTA [pH ∼12.3]). Electrophoresis was carried out at 0.7 V/cm for 25 min. The slides were stained with propidium iodide (Fluka analytical). The slides were then observed at ×200 magnification using an IX81 fluorescence microscope (Olympus) connected to a high-resolution charge-coupled device camera (QImaging, FAST1394). Comet slides were scored using Comet assay III software (Perceptive Instruments). One hundred comets were analyzed for each sample. DNA damage was scored based on the percentage of DNA in the tail.
Immunofluorescence staining (IF) staining.
U2OS cells were fixed with 3% paraformaldehyde in PBS for 20 min. Cells were incubated in blocking/permeabilization buffer (0.5% Triton X-100 and 3% bovine serum albumin [BSA] in PBS) for 10 min at room temperature and stained with specific primary antibodies (as described in the legends) at room temperature for 60 min. After incubation, the cells were washed three times with blocking/permeabilization buffer and incubated with Alexa Fluor 594 goat anti-rabbit IgG and 488 goat anti-mouse IgG (Molecular Probes) for an additional 60 min. After incubation with the secondary antibodies, cells were counterstained with DAPI (4′,6′-diamidino-2-phenylindol) and examined with an Olympus IX81 inverted fluorescence microscope.
Microscopy and image analysis.
All immunofluorescent images were collected using an inverted fluorescence microscope (Olympus, catalog no. IX81) connected to a high-resolution charge-coupled device camera (QImaging, FAST1394). Images were analyzed and presented using SlideBook 5.0 software (Intelligent Imaging Innovations, Inc.). The scale bars were added using ImageJ software.
Western blotting.
Cells were lysed in lysis buffer (10 mM HEPES [pH 7.9], 300 mM NaCl, 3 mM MgCl2, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 3 mM sodium butyrate, 1 mM NaF, and 100 μM Na3VO4, supplemented with protease inhibitor cocktails [Roche] and Ser/Thr protein phosphatase inhibitor cocktails [Sigma]) by passage through a 22-gauge needle 10 times. After a 20-min incubation on ice, the soluble and insoluble fractions were separated by centrifugation at 5,000 rpm for 5 min at 4°C. The supernatants (20 μg) were resolved on an SDS-PAGE gel. Then, 1 mM NaF and 100 μM Na3VO4 were added to the electrophoresis buffer and transfer buffer to inhibit phosphatase activity. Membranes were blocked in 5% TBST-milk for 1 h at room temperature and incubated in TBST-milk containing primary antibodies at 4°C overnight. For anti-phospho protein antibodies, TBST-BSA was used instead of TBST-milk. Membranes were then incubated with HRP-conjugated secondary antibodies in TBST-milk for 1 h at room temperature. Western blots were developed using ECL solution and images were captured using a Fuji imaging system.
Helicase assay.
The helicase assay was performed as previously described with minor modification (38, 39). Wild-type or mutant MCV LT fused to an IIT tag was expressed in 293 cells and purified using IgG Sepharose 6 Fast Flow (GE Healthcare), which were preblocked with 1% BSA in PBS at 4°C for >1 h. IIT-LT purified on beads was split into different parts for SDS-PAGE/Coomassie brilliant blue staining and helicase assays, respectively. To label the substrate, 35 ng of 32-mer primer was annealed to 1 μg of M13mp18 DNA (New England BioLabs). The primer was then elongated using Klenow polymerase (New England BioLabs) in a 50-μl reaction containing 0.1 mM dCTP, dGTP, and [α-32P]dATP. After 20 min of incubation at room temperature, 0.1 mM dATP was added for 20 min. Then, 10 μl of labeled substrate was used in each reaction. LT purified on IgG Sepharose was incubated with the substrate in helicase assay buffer at 37°C for 30 min. The reaction was stopped by adding SDS to a final concentration of 0.2% and EDTA to 50 mM. Total reaction mixtures were resolved by electrophoresis on 11% polyacrylamide gels. The gels were dried and subjected to autoradiography.
Reverse transcription-quantitative PCR (RT-qPCR).
Total RNA was isolated from U2OS cells at 48 h posttransfection using NucleoSpin RNA II kit (Macherey-Nagel) according to the manufacturer's instructions. Reverse transcription (RT) was performed in a 20-μl reaction containing 350 ng of total RNA using oligo(dT) primer (Invitrogen) and SuperScript II reverse transcriptase (Invitrogen) according to the manufacturer's instructions. Real-time PCR using gene-specific primers was performed in triplicate using 1 μl of RT product in a 25-μl reaction containing 12.5 μl of iQ SYBR Green Supermix (Bio-Rad) and 0.5 μM concentrations of each primer. The reaction was carried out on a Bio-Rad iQ 5 multicolor real-time PCR detection system (Bio-Rad). The data were analyzed using Bio-Rad iQ5 software, and mRNA of each gene was normalized to GAPDH mRNA level. The primer sequences were as follows: p21 (sense primer, 5′-CTTGTACCCTTGTGCCTCGCT-3′; antisense primer, 5′-CGGATTAGGGCTTCCTCTTGG-3′, amplicon: 153 bp), GADD45A (sense primer, 5′-TGCGTGCTGGTGACGAATCC-3′; antisense primer, 5′-CAGATGCCATCACCGTTCAGG-3′; amplicon, 138 bp), HDM2 (sense primer, 5′-GTGTATCAGGCAGGGGAGAGTG-3′; antisense primer, 5′-CTTCAGGAAGCCAATTCTCACG-3′; amplicon, 160 bp), and GAPDH (sense primer, 5′-GTGAAGGTCGGAGTCAACGGA-3′; antisense primer, 5′-CCATGGGTGGAATCATATTGGAAC-3′; amplicon, 152 bp).
Flow cytometry.
U2OS cells were transfected with pEGFPC1, pEGFPC1-MCV LT 1-440, pEGFPC1-MCV LT 441-817, or pEGFPC1-MCV LT 1-817. At 48 h posttransfection, the cells were trypsinized and fixed with 0.5% paraformaldehyde in PBS for 20 min prior to permeabilization with 70% ethanol overnight at −20°C. The cells were then washed three times with PBS, resuspended in PBS containing 25 μg of propidium iodide/ml and 100 μg of RNase A/ml, and incubated at room temperature for 30 min. GFP-positive cells were analyzed by flow cytometry using FACSCalibur (Becton Dickinson). The cell cycle profile was analyzed using FlowJo software.
Retrovirus production and stable cell line construction.
To package retrovirus, HEK293T cells were cultured in 10-cm dishes to 95 to 100% confluence. A pLPCX-based plasmid (pLPCX, pLPCX-Cherry-LacI, pLPCX-MCV LT 1-440, or pLPCX-MCV LT 1-817) or a pLXSN-based plasmid (pLXSN or pLXSN-p53DD) was transfected into HEK293T, together with VSVG and pMD-gagpol plasmids, using Lipofectamine 2000 transfection reagent. After 48 h, the packaged retroviruses in the supernatant were harvested and filtered through a 0.45-μm-pore-size filter before infecting U2OS or NIH 3T3 cells. For pLPCX-based U2OS single stable cells, U2OS were infected with pLPCX retroviruses (pLPCX-Cherry-LacI, pLPCX-MCV LT 1-440, or pLPCX-MCV LT 1-817). At 48 h postinfection, the cells were selected using 2 μg of puromycin/ml for 4 days. For pLPCX- and pLXSN-based NIH 3T3 double stable cells, the pLXSN retrovirus (pLXSN or pLXSN-p53DD)-infected NIH 3T3 cells were selected by using 0.4 mg of G418/ml for 10 days, and then the selected stable cells were further infected with pLPCX retroviruses (pLPCX, pLPCX-MCV LT 1-440, or pLPCX-MCV LT 1-817) and selected with 0.4 mg of G418/ml and 2 μg of puromycin/ml for another 4 days. The expression of MCV LT molecules, Cherry-LacI, or p53DD was confirmed by IF and Western blot analyses, and the selected cells were maintained as stable cell lines in medium supplemented with puromycin only or puromycin and G418 together.
Cellular proliferation assay.
U2OS cells stably expressing Cherry-LacI (control), LT 1-440, or LT 1-817 were seeded in triplicate at 5 × 103 cells/well in six-well plates containing growth medium and 2 μg of puromycin/ml. Cells were trypsinized and counted every 24 h for 6 days. NIH 3T3 stable cells were seeded in triplicate at 104 cells/6-cm dish and cultured in medium containing 2 μg of puromycin/ml and 0.4 mg of G418/ml.
Clonogenic assay.
U2OS cells stably expressing Cherry-LacI (control), LT 1-440, or LT 1-817 were plated in triplicate at 5 × 103 cells/dish in 6-cm dishes and cultured in McCoy's 5A medium with 2 μg of puromycin/ml for 10 days. The cells were then fixed with methanol and stained with 0.5% methylene blue.
Soft agar colony formation assay.
NIH 3T3 double stable cells (103 cells) were resuspended in 0.35%, (wt/vol) low-melting-point (LMP) agarose (BD Diagnostic Systems) on a base layer of 0.5%, (wt/vol) LMP agarose in six-well plates. The culture was covered with another layer of 0.5% (wt/vol) LMP agarose 24 h later and incubated at 37°C. After 20 days, colonies were imaged with an Olympus IX81 inverted fluorescence microscope. The diameter of randomly selected colonies was quantified using ImageJ software.
MCV virion preparation and infection.
Native MCV virions and MCV pseudovirions were prepared as previously described (36), with minor modifications. Briefly, an initial seed stock of native virions was produced by transfecting 293-4T cells (which stably express the MCV sT and LT proteins) with the religated recombinant genome of MCV isolate R17b. Five days later, native MCV virions were harvested and purified over an Opti-Prep gradient. This initial seed stock of native MCV virions was used to infect fresh 293-4T cells. The MCV-infected 293-4T cells were harvested and lysed after 5 days of infection, and the amplified native MCV virions were purified over Opti-Prep gradients. For experimental infection, U2OS cells were seeded in six-well plates and incubated with native MCV virions or MCV-GFP pseudovirions at a dose of 5 × 104 MCV genomes (or encapsidated GFP reporter plasmids) per cell for 5 days.
Statistical analysis.
Statistical analysis was performed using the one-way analysis of variance (ANOVA) or Student t test program of GraphPad software (version 5.0). P < 0.05 was considered statistically significant.
RESULTS
MCV infection activates the host DDR pathways.
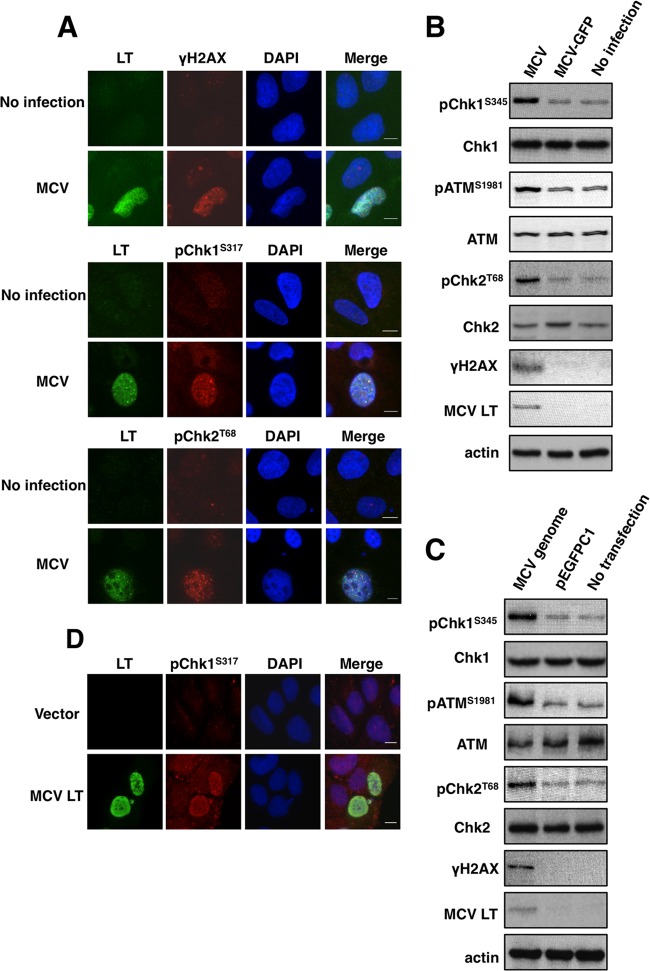
We first examined the host DDR in cells infected with native MCV virions. U2OS cells were transduced using native MCV virions at 5 × 104 viral genomes per cell (Fig. 1A and B). The MCV-infectibility of U2OS cell line was assessed using a MCV-GFP pseudovirus carrying a green fluorescent protein (GFP) reporter construct (36). About 20% of the cells were detectably transduced with the GFP reporter gene (data not shown). It has been shown that the expression of viral genes from the native MCV genome is highly restricted in all of the cell lines thus far tested (9, 36, 40, 41). Consistently, we detected a low level of MCV LT signal in ∼20% of U2OS cells transduced with native MCV virions, and this signal was absent in cells treated with MCV-GFP pseudovirus or uninfected cells (Fig. 1A and B and data not shown). More importantly, a large percentage of the cells expressing MCV LT from the native MCV virions also show increased γH2AX (65% ± 6.3%), pChk1S317 (53.5% ± 7.5%), and pChk2T68 (57.1% ± 8.4%) compared to MCV LT-negative cells (Fig. 1A). In contrast, only 1 to 4% of the uninfected cells showed induction of DDR markers (Fig. 1A). The data were calculated by counting ∼100 cells in each of three independent experiments and averaging the values. We also performed Western blotting to examine MCV LT and DDR markers in MCV-transduced cells. MCV infection stimulated phosphorylation of H2AX, Chk1S345, ATMS1981, and Chk2T68 (Fig. 1B). Stimulation of these DDR makers was detected specifically in MCV-transduced cells but not in cells treated with MCV-GFP pseudovirus or uninfected cells (Fig. 1B). Together, these results demonstrate that natural MCV infection induces activation of both the ATR/Chk1 and the ATM/Chk2 pathways.
Fig 1.
Both MCV infection and MCV genome transfection induce a DNA damage response. (A) U2OS cells transduced with native MCV virions (MCV) and no-infection control cells were harvested at 5 days postinfection and stained with antibodies for MCV LT (green) and DDR markers (red) as indicated. Cells were counterstained with DAPI. Bar, 10 μm. (B) U2OS cells transduced with native MCV virions (MCV) or MCV-GFP pseudovirions (MCV-GFP) and no-infection control cells were harvested at 5 days postinfection and immunoblotted with the indicated antibodies. (C) U2OS cells were transfected with religated MCV genome or pEGFPC1. At 48 h posttransfection, cells were harvested and immunoblotted with the indicated antibodies. (D) U2OS cells were transfected with pcDNA4C (Vector) or pcDNA4C-full-length MCV LT (MCV LT). The cells were fixed at 36 h posttransfection and stained with MCV LT (green) and pChk1S317 (red) antibodies. Cells were counterstained with DAPI. Bar, 10 μm.
Next, we tested whether introducing the MCV genome into cells could also stimulate the host DDR. U2OS cells were transfected with religated MCV genome and examined for MCV LT expression and activation of DDR markers. Nontransfected cells and cells transfected with religated pEGFPC1 were used as negative controls. As shown in Fig. 1C, MCV LT expression was specifically detected in cells transfected with MCV genome. Compared to the negative controls, cells transfected with MCV genome also show increased phosphorylation of H2AX, Chk1S345, ATMS1981, and Chk2T68 (Fig. 1C). The results show that, similar to native MCV infection, introducing MCV genome into cells can also stimulate a host DDR.
In some of the U2OS cells transduced with native MCV virions, we also observed a clear colocalization of MCV LT and phosphorylated Chk1 and Chk2 in large punctate nuclear foci (data not shown). Whether these foci represent the sites of viral or host DNA replication are being investigated. However, these observations indicate that MCV LT may be directly involved in activation of Chk1 and Chk2. To test this possibility, we examined whether expressing MCV LT alone can elicit a host DDR. U2OS cells transfected with either empty vector or full-length MCV LT were stained with MCV LT antibody and either phospho-Chk1S345, phospho-Chk1S317, or phospho-Chk2T68 antibodies. IF results showed that expression of MCV LT could induce phosphorylation of Chk1S345 and Chk1S317 but not Chk2T68 (Fig. 1D, data not shown, and see below). MCV LT-induced phosphorylation of Chk1S345 and Chk1S317 was also observed in C127 cells, 293 and C33A cells (data not shown). In contrast, expression of sT and 57kT proteins did not induce clear activation of DDR makers (data not shown).
MCV LT expression causes DNA damage in host genome.
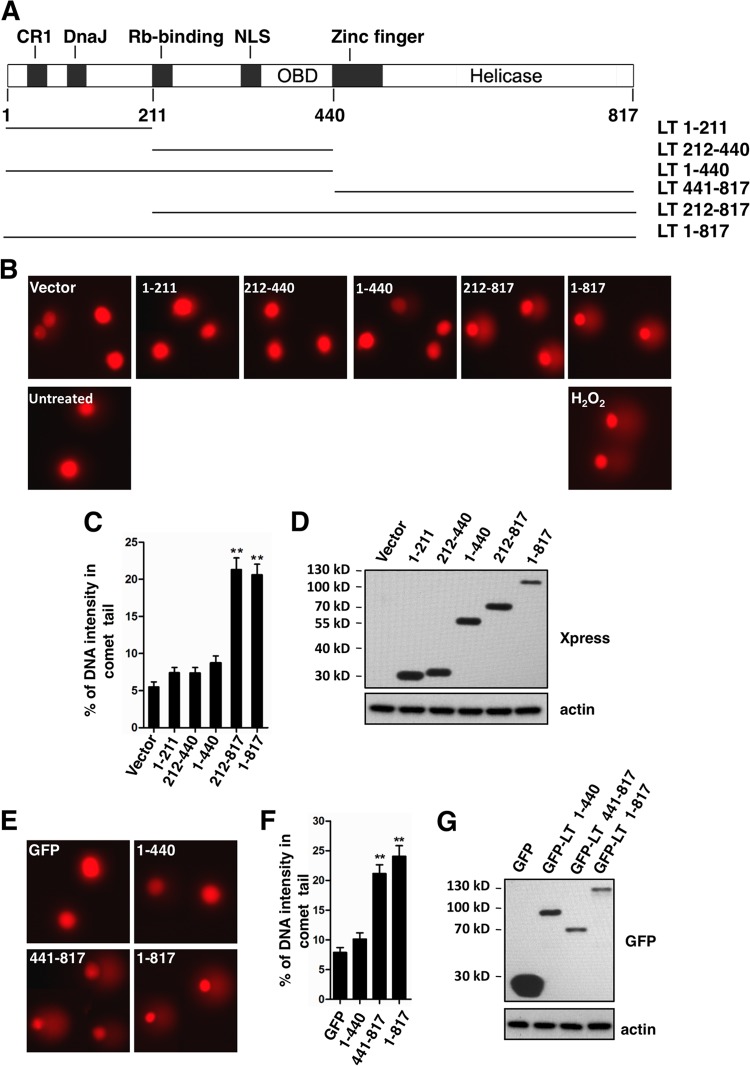
In the next experiment, we investigated whether expression of MCV LT causes DNA damage in the cellular genome. Single cell gel electrophoresis (also known as the comet assay) is a traditional method for detecting DNA damage at the level of individual eukaryotic cells (37). Using this method, DNA breaks can be visualized in structures resembling comets by fluorescence microscopy. U2OS cells were transfected with the empty vector (pcDNA4C) or pcDNA4C encoding Xpress-tagged full-length MCV LT (LT 1-817) or LT truncation mutants as indicated in Fig. 2A. The transfection efficiency was determined by IF to be ca. 50 to 60% for constructs encoding LT molecules. As a positive control, cells were treated with 100 μM hydrogen peroxide (H2O2). The comet assay was performed under alkaline conditions, which can detect both single- and double-stranded DNA breaks (37). Representative comet assay results are shown in Fig. 2B. Comet tails were clearly observed in LT 1-817- or LT 212-817-transfected cells and H2O2-treated cells, whereas PBS-treated cells or cells transfected with empty vector, LT 1-211, LT 212-440, or LT 1-440 produced no significant comet tails. In the comet assay, the extent of DNA breaks can be determined by measuring the percentage of DNA intensity of the comet tail relative to the total DNA (42). Quantification results from a representative comet assay are summarized in Fig. 2C. Expression level of the MCV LT molecules is shown in Fig. 2D. According to the one-way ANOVA statistical analysis, the percentage of DNA in the comet tails of cells transfected with full-length MCV LT and LT 212-817 was significantly higher than cells expressing empty vector (Fig. 2C, P < 0.01, experiment n = 3). There was no significant difference in the percentage of DNA in the comet tail between any of the N-terminal domain mutants (LT 1-211, LT 212-440, and LT 1-440) and empty vector. The MCV LT 441-817 mutant does not express well from the pcDNA4C construct, so it was cloned into the pEGFPC1 vector and tested in a separate comet assay (Fig. 2E and G). Compared to pEGFPC1 empty vector and the GFP-LT 1-440 construct, cells expressing GFP-LT 441-817 and GFP-LT 1-817 showed a clear increase in comet tails (Fig. 2E and F, P < 0.01, experiment n = 3). Together, the comet assay results indicate that MCV LT expression causes DNA damage in the host genome. This function of MCV LT can be assigned to the C-terminal region spanning amino acids 441-817 of MCV LT.
Fig 2.
MCV LT induces DNA damage in host genome. (A) Schematic diagram of the MCV LT protein and truncation mutants used in the present study. CR1, conserved region 1; DnaJ, Hsc70-binding conserved region; NLS, nuclear localization signal; OBD, origin-binding domain. (B) U2OS cells were transfected with pcDNA4C (Vector) or pcDNA4C encoding the indicated LT molecules. Cells were harvested at 36 h posttransfection and analyzed using a comet assay. For positive and negative controls, cells were treated with 100 μM H2O2 and PBS, respectively. Representative images of comets from three independent experiments are shown. (C) The percentage of DNA intensity in the comet tail was quantified from 100 randomly selected comet images for each transfection. Error bars represent mean ± the standard error of the mean (SEM) calculated from each transfection. Asterisks indicate significant difference (P < 0.01) compared to cells transfected with vector. (D) Expression of LT molecules in U2OS cells was detected by Western blotting with the indicated antibodies. (E) U2OS cells were transfected with pEGFPC1 (GFP) or pEGFPC1 encoding the indicated LT molecules. Cells were harvested at 36 h posttransfection and analyzed using the comet assay. Representative images of comets are shown. (F) The percentage of DNA intensity in the comet tail was quantified and presented as in panel C. (G) Expression of GFP-tagged LT molecules was detected by Western blotting with the indicated antibodies.
MCV LT induces RPA accumulation in punctate nuclear foci.
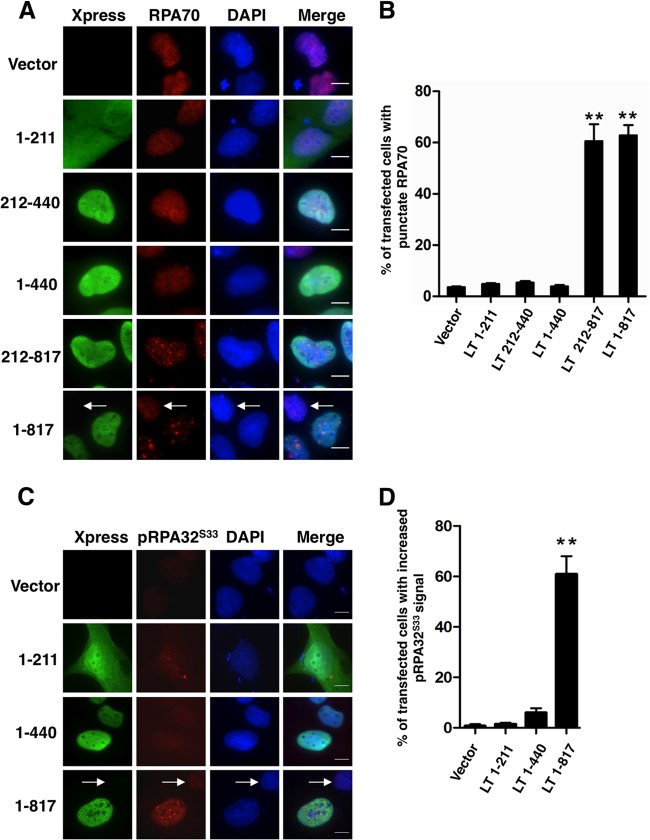
We next sought to detect the MCV LT-induced DNA damage using IF analysis. RPA is the major single-stranded DNA-binding protein that binds to and stabilizes stretches of single-stranded DNA intermediates generated by stalled replication forks or DNA damage (43). It is composed of three subunits, RPA70, RPA32, and RPA14 (43). In the presence of certain forms of DNA damage or active DNA replication, RPA70 is recruited to patches of single-stranded DNA. This results in a punctate nuclear pattern that can be distinguished from the diffuse RPA70 pattern in cells without DNA damage or replication. As shown in Fig. 3A, cells transfected with the empty vector or constructs encoding Xpress-tagged MCV LT N-terminal-domains 1-211, 212-440, or 1-440 showed mostly diffuse RPA70 staining, while RPA70 accumulated in punctate nuclear dots in a large percentage of cells expressing full-length MCV LT or LT 212-817. The percentage of Xpress-positive cells showing RPA70 translocation was calculated from three independent experiments and presented in Fig. 3B. RPA70 foci were found in a small percentage of the cells either carrying empty vector or expressing one of the MCV LT N-terminal domain mutants. Because this low level of RPA70 foci was also observed in untransfected cells, they are likely resulted from RPA70 binding to replicating cellular DNA or single-stranded cellular DNA generated by background level of DNA damage occurring in cells. In contrast, >60% of cells expressing full-length MCV LT or LT 212-817 showed RPA70 foci (Fig. 3B, P < 0.01, experiment n = 3). Immunostaining-fluorescence in situ hybridization (Immuno-FISH) analysis showed that these RPA70 dots do not colocalize with the FISH signal of the transfected plasmids (data not shown), suggesting that RPA70 dots are present on host genome.
Fig 3.
RPA70 and pRPA32S33 accumulate in punctate nuclear foci in LT- expressing cells. (A) U2OS cells were transfected with pcDNA4C (Vector) or pcDNA4C encoding the indicated LT molecules fused to an Xpress tag. At 36 h posttransfection, cells were stained with Xpress (green) and RPA70 (red) antibodies. The cells were counterstained with DAPI. An arrow marks a nontransfected cell. Bar, 10 μm. (B) The percentage of cells showing RPA70 accumulation was quantified from ∼100 positively transfected cells. For cells transfected with the vector, the percentage of cells showing RPA70 accumulation was quantified from ∼100 total cells. The mean ± the standard deviation (SD) was calculated from three independent experiments. Asterisks indicate significant difference (P < 0.01) compared to vector-transfected cells. (C) U2OS cells were transfected and stained as in panel A using Xpress (green) and pRPA32S33 (red) antibodies. (D) The percentage of cells showing increased pRPA32S33 signal was quantified as in panel B.
In response to DNA damage, RPA32 is phosphorylated by ATM, ATR, and DNA-PK to initiate repair DNA synthesis (44). We also detected the signal of RPA32S33 phosphorylation in cells expressing different LT molecules. Interestingly, pRPA32S33 signal was also enriched in punctate nuclear foci in cells expressing full-length MCV LT (Fig. 3C). Cells expressing the LT mutants lacking the C-terminal domain showed only background level of pRPA32S33 signal (Fig. 3C, 3D and data not shown). These results, in combination with the comet assay data from Fig. 2, suggest that expression of full-length MCV LT or LT 212-817 carrying the C-terminal domain causes DNA damage in cells.
MCV LT activates the ATR pathway.
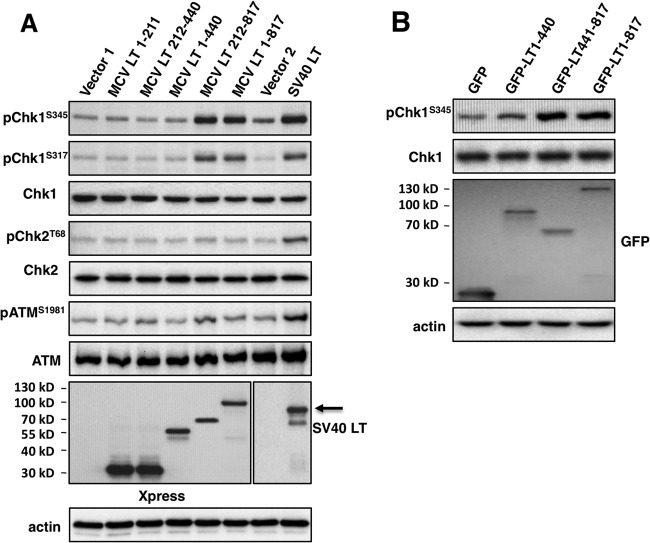
Since it appears that the LT induced DNA damage is mainly caused by the C-terminal region, we tested different LT truncation mutants for the ability to induce DDR. U2OS cells were transfected with pcDNA4C encoding full-length MCV LT or a truncation mutant. SV40 LT, which has been shown to activate both ATM and ATR kinases (32), was used as a positive control. As expected, SV40 LT expression leads to phosphorylation of ATM, Chk2, and Chk1 (Fig. 4A). Full-length MCV LT expression caused clear induction of Chk1S345 and Chk1S317 phosphorylation (Fig. 4A). Among all of the MCV LT truncation mutants tested, only MCV LT 212-817 stimulated Chk1S345 and Chk1S317 phosphorylation. In contrast, none of the MCV LT molecules triggered discernible amounts of ATM or Chk2 phosphorylation (Fig. 4A). These experiments were repeated more than three times with similar results. IF analysis confirmed that full-length MCV LT and the 212-817 truncation mutant (but not other truncation mutants) induce Chk1 phosphorylation (data not shown).
Fig 4.
The MCV LT C-terminal domain induces a DNA damage response. (A) U2OS cells were transfected with pcDNA4C (Vector 1), pcDNA4C encoding Xpress-tagged MCV LT molecules as indicated, pIRES-Hygromycin (Vector 2, control for pTIH) or pTIH encoding SV40 LT. At 36 h posttransfection, cells were lysed and immunoblotted with the indicated antibodies. (B) U2OS cells were transfected with pEGFPC1 or pEGFPC1 encoding MCV LT molecules as indicated. At 36 h posttransfection, cells were immunoblotted with the indicated antibodies. All experiments were repeated more than three times with consistent results.
To further map the domain of MCV LT that induces the ATR-mediated DDR, pChk1S345 was examined in U2OS cells transfected with pEGFPC1 expressing full-length MCV LT, MCV LT 1-440, or MCV LT 441-817. As shown in Fig. 4B, cells transfected with pEGFPC1 vector or expressing GFP-LT 1-440 did not show significantly increased pChk1S345 signal. However, cells expressing GFP-LT 441-817 or GFP-full-length MCV LT displayed an increased pChk1S345 signal (Fig. 4B). These results are consistent with the data from the comet assay and RPA staining, and demonstrate that the MCV LT C-terminal 441-817 region may contain the domain(s) responsible for inducing DNA damage and ATR/Chk1 activation in cells. Notably, in contrast to the MCV infection induced ATM and ATR activation, MCV LT expression only activates the ATR/Chk1 pathway but not the ATM/Chk2 pathway, suggesting that additional components in viral infection may contribute to the difference (see Discussion).
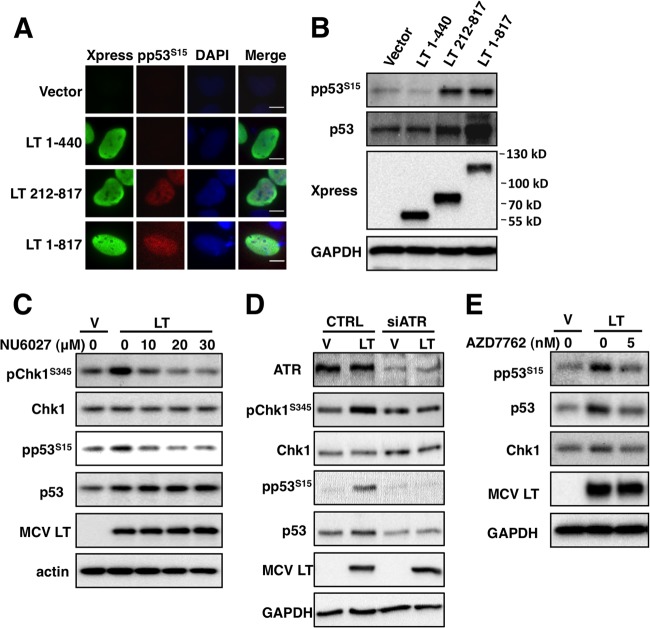
MCV LT-induced DDR activates p53S15 phosphorylation.
In DNA-damaged cells, both ATR and ATM have been shown to stimulate the phosphorylation of p53 at Ser15, which stabilizes p53 and potentiates its downstream activities (45–48). Upon activation, p53 participates in a signal transduction cascade, which can result in cell cycle arrest, DNA damage repair, and/or apoptosis (49). To determine whether the MCV LT-induced ATR activation leads to p53 phosphorylation, we analyzed phospho-p53S15 signal in cells expressing full-length MCV LT or one of the truncation mutants. Representative IF images are shown in Fig. 5A. For cells transfected with the empty vector or a construct encoding MCV LT 1-440, there was no detectable increase of phospho-p53S15 signal. However, expression of full-length MCV LT or LT 212-817 resulted in significantly increased phospho-p53S15 signal (Fig. 5A). More than 100 cells were quantified from each sample of three independent experiments. In contrast to cells transfected with vector or the MCV LT 1-440 construct, which showed little or no stimulation of p53S15 phosphorylation, ca. 65 to 80% of the cells expressing full-length MCV LT or LT 212-817 showed increased phospho-p53S15 signal. Western blot analysis confirmed that expression of full-length MCV LT and MCV LT 212-817 significantly increased p53S15 phosphorylation (Fig. 5B). In contrast, there was no dramatic increase of phospho-p53S15 signal upon expression of MCV LT 1-440 compared to the vector control. Noticeably, the overall p53 protein level was also increased upon full-length MCV LT and LT 212-817 expression. This is consistent with previous studies showing that phosphorylation of p53 at Ser15 stabilizes this protein by inhibiting its interaction with MDM2, preventing p53 degradation (50).
Fig 5.
MCV LT induces p53S15 phosphorylation through ATR/Chk1 pathway. (A) U2OS cells transfected with pcDNA4C (Vector) or pcDNA4C encoding the indicated Xpress-tagged MCV LT molecules were stained with Xpress (green) and pp53S15 (red) antibodies at 36 h posttransfection. The cells were counterstained with DAPI. Bar, 10 μm. (B) U2OS cells were transfected as in panel A. At 36 h posttransfection, cells were lysed and immunoblotted with the indicated antibodies. (C) U2OS cells transfected with pcDNA4C (Vec) or pcDNA4C-LT1-817 were treated with dimethyl sulfoxide (DMSO) or 10, 20, or 30 μM NU6027 for 24 h. Cells were lysed and immunoblotted with the indicated antibodies. (D) U2OS cells were transfected with control (CTRL) or ATR siRNA. At 36 h posttransfection, cells were transfected with pcDNA4C (V) or pcDNA4C-LT1-817 (LT). Cells were lysed and immunoblotted at 72 h after siRNA transfection with the indicated antibodies. (E) U2OS cells transfected with pcDNA4C (Vec) or pcDNA4C-LT1-817 (MCV LT) were treated with DMSO or 5 nM AZD7762 for 24 h. Cells were lysed and immunoblotted with the indicated antibodies. All experiments were repeated three times with consistent results.
Because MCV LT predominantly activates the ATR/Chk1 pathway, we tested whether inhibition of the ATR/Chk1 pathway could abrogate MCV LT-induced p53S15 phosphorylation. U2OS cells transfected with the full-length MCV LT construct were treated with increasing concentrations of an ATR inhibitor, NU6027, for 24 h prior to Western blot analysis. As shown in Fig. 5C, NU6027 could efficiently inhibit ATR activity because the MCV LT-induced Chk1S345 phosphorylation was abolished in cells treated with NU6027. More importantly, the MCV LT-induced p53S15 phosphorylation was also reduced to the background level when the cells were treated with NU6027 (Fig. 5C). MCV LT-induced p53 stabilization was not affected by NU6027, probably because this stabilization occurred before ATR was inactivated by NU6027. We also used ATR siRNA silencing to more specifically test the role of ATR in the MCV LT-induced p53S15 phosphorylation. LT expression-induced Chk1S345 and p53S15 phosphorylation in control siRNA-treated cells, but such stimulation was not detected in ATR knockdown cells (Fig. 5D). In addition to ATR inhibition, treating MCV LT-expressing cells with the Chk1/Chk2 inhibitor AZD7762 also inhibited the MCV LT-induced p53S15 phosphorylation (Fig. 5E). These results provide evidences to support that MCV LT induces p53S15 phosphorylation through ATR/Chk1 pathway.
Examination of the MCV LT helicase activity in DDR activation.
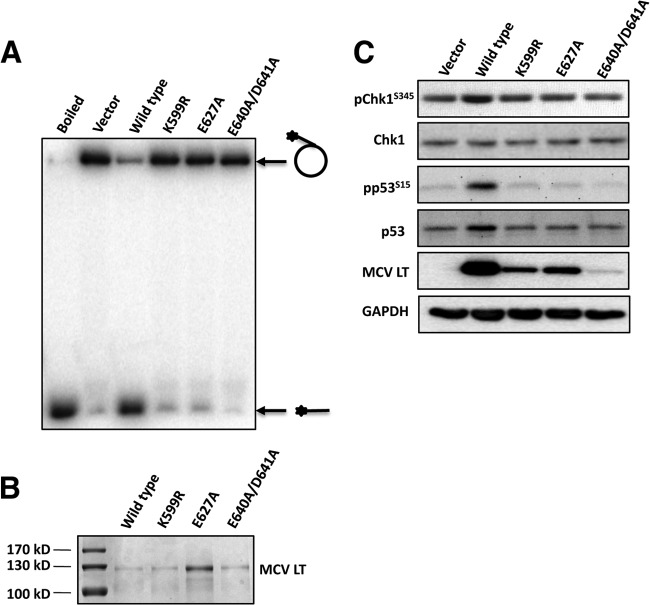
The MCV LT C-terminal 441-817 region is predicted to encode the core helicase. To test whether the MCV LT helicase activity is causing the DNA damage and DDR activation, we generated three MCV LT helicase mutants by mutating the residues that are conserved among all polyomavirus LT helicases (Fig. 6). All three mutants showed abrogated helicase activity in vitro (Fig. 6A and B). These helicase mutants displayed reduced ability to activate Chk1S345 and p53S15 phosphorylation compared to wild-type MCV LT (Fig. 6C). However, they were also expressed at a lower level than wild-type MCV LT protein (Fig. 6C). Therefore, it is uncertain whether the reduced DDR activation observed with these helicase mutants is due to a loss of helicase activity or reduced protein levels. Whether the helicase activity of MCV LT contributes to DDR activation and growth inhibition will be further evaluated once additional MCV LT helicase mutants or anti-helicase drugs become available. Since the MCV LT C-terminal 441-817 fragment is a complex region likely harboring multiple activities, it is also possible that other helicase-independent functions of the region may induce DDR and growth inhibition.
Fig 6.
Examination of the MCV LT helicase activity in DDR activation. (A) Helicase assay of wild-type and mutant MCV LT. Wild-type MCV LT or LT point mutants were expressed in 293 cells. Immunopurified recombinant proteins were used in the helicase assay. The circle with an asterisk indicates radiolabeled single-stranded DNA (ssDNA) annealed to nonradiolabeled M13 ssDNA. The horizontal line with an asterisk indicates unwound radiolabeled ssDNA. The substrate was also boiled to show the unwound ssDNA. (B) SDS-PAGE and Coomassie brilliant blue staining of purified MCV LT proteins. (C) U2OS cells were transfected with pcDNA4C (Vector), wild-type MCV LT (wild type), or one of the MCV LT mutants. At 36 h posttransfection, the cells were lysed and immunoblotted with the indicated antibodies.
MCV LT induces p53 downstream target gene expression.
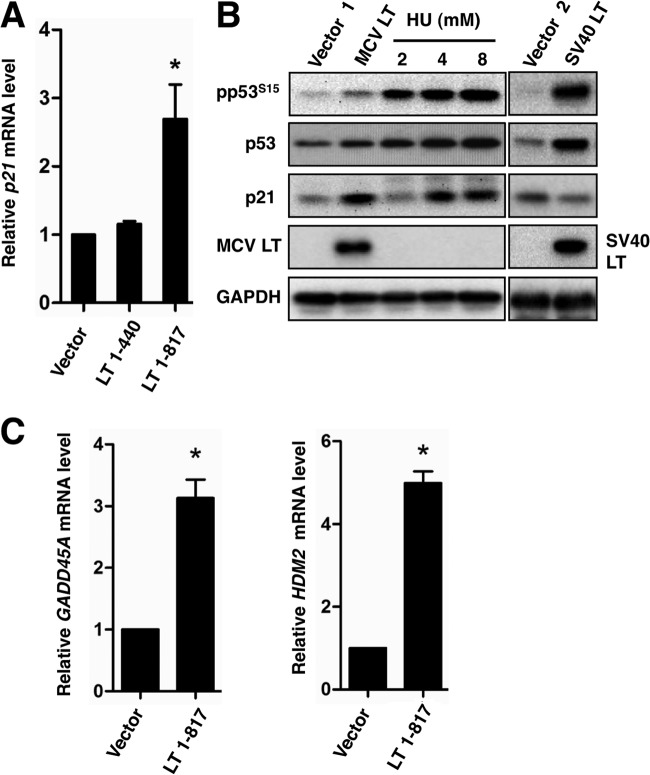
p53 plays a major role in mediating multiple cellular responses to DNA damage (49). It functions through transcriptional activation of a set of downstream target genes. One of the major targets of p53 is p21, a cyclin-dependent kinase inhibitor whose function is to arrest cells in G1 phase (51, 52). To determine whether MCV LT-induced p53 phosphorylation triggers the upregulation of its downstream target genes, we first tested whether p21 mRNA transcription is upregulated in U2OS cells expressing full-length MCV LT. Because the MCV LT C-terminal domain responsible for activating p53 is typically truncated in MCV LT found in MCC tumors, we decided to compare MCV LT 1-440, which lacks the C-terminal domain, with full-length MCV LT in the present study.
Quantitative reverse transcription-PCR (RT-PCR) showed that, compared to the empty vector and MCV LT 1-440, full-length MCV LT increased the p21 mRNA level by ∼2.7-fold (Fig. 7A, P < 0.05, experiment n = 3). Western blot analysis showed that, compared to the empty vector, full-length MCV LT also efficiently increased p21 protein to a level comparable to those observed in hydroxyurea treated cells (Fig. 7B), whereas expression of MCV LT 1-440 did not show significant effect (data not shown). In contrast to MCV LT, expression of SV40 LT, which has been shown to bind to p53 and inhibit its transcriptional activity (53), led to a decrease in p21 protein level compared to the empty vector (Fig. 7B), confirming its ability to functionally inactivate p53. Additional quantitative RT-PCR showed that, besides p21, MCV LT expression also stimulated the expression of other p53 downstream target genes, including GADD45 and HDM2 (Fig. 7C). Taken together, these results demonstrated that, unlike SV40 LT, MCV LT-induced p53 phosphorylation promotes p53 transcriptional activity.
Fig 7.
MCV LT induces expression of p53 downstream targets. (A) U2OS cells were transfected with pcDNA4C (Vector) or pcDNA4C encoding the indicated LT molecules. Total RNA was extracted at 48 h posttransfection and analyzed for p21 expression using RT-qPCR. The p21 mRNA levels were normalized to GAPDH mRNA levels and presented as the ratio of transcript in MCV LT-expressing samples relative to the vector control. Values represent the average of three independent experiments with error bars indicating the standard deviation. Asterisk indicates significant difference (P < 0.05) from vector transfected cells. (B) U2OS cells were transfected with pcDNA4C (Vector 1), pcDNA4C encoding Xpress-tagged MCV LT, pIRES-Hygromycin (Vector 2, control for pTIH), or pTIH encoding SV40 LT. Untransfected cells treated with 2, 4, or 8 mM hydroxyurea (HU) for 12 h serve as positive controls. Cells were harvested and immunoblotted at 48 h posttransfection using the antibodies indicated. This experiment was repeated three times with consistent results. (C) U2OS cells were transfected with pcDNA4C (Vector) or pcDNA4C-MCVLT1-817. Total RNA was extracted at 48 h posttransfection to detect p53 downstream target GADD45A and HDM2 transcription levels using RT-qPCR. Values represent the average of three independent experiments with error bars indicating the standard deviation. Asterisk indicates significant difference (P < 0.05) from the vector control.
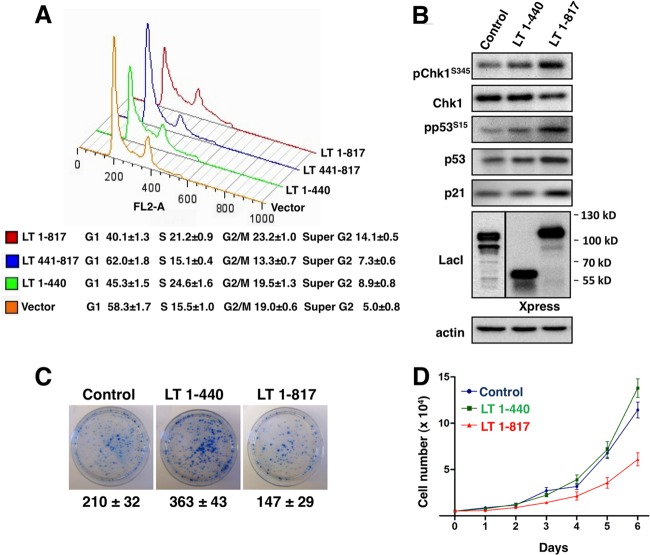
MCV LT expression arrests the host cell cycle.
Our data thus far suggest that MCV LT can activate the ATR kinase pathway, leading to p53 phosphorylation and downstream target gene expression. This activity of MCV LT can be attributed to the C-terminal region, which is frequently deleted in MCV LT mutants typically found in MCC tumors. These observations suggest that the C-terminal domain of MCV LT may present as an obstacle to tumorigenesis, possibly through p53-mediated modulation of the cell cycle. To test this possibility, we investigated how different MCV LT molecules affect the cell cycle. U2OS cells were transfected with pEGFPC1, or a construct encoding GFP tagged LT 1-440, LT 441-817, or full-length MCV LT. The DNA content of the GFP-positive cells was analyzed by using flow cytometry (Fig. 8A). Compared to the vector control, which showed 58% of the cells in G1, LT 1-440 expression reduced the G1 population to 45%, with a corresponding increase of cells found in S phase. In contrast to LT 1-440, the expression of LT 441-817, which has the ability to activate p53, accumulated 62% of the cells in G1 phase (Fig. 8A). In cells transfected with full-length MCV LT, the G1 population was decreased to 40%, with 21% of cells accumulated in S phase and 23% in G2/M phase (Fig. 8A). This experiment was repeated more than three times with similar results. In full-length MCV LT-expressing cells, we consistently detected a significant population (14.1% ± 0.5%) of cells with greater than 4N DNA content (Fig. 8A). The ability of full-length MCV LT to induce S phase arrest is supported by IF and Western blot analyses showing that cyclin A, an S-phase marker, is induced in full-length MCV LT expressing cells (data not shown).
Fig 8.
MCV LT arrests the cell cycle and inhibits cellular proliferation. (A) U2OS cells were transfected with pEGFPC1 or pEGFPC1 encoding the indicated LT molecules. At 48 h posttransfection, cells were fixed and stained with propidium iodide (PI). The DNA content in GFP-positive cells was analyzed by flow cytometry using a BD FACSCalibur. The data were analyzed using FlowJo. (B) U2OS cells stably expressing MCV LT 1-440, MCV LT 1-817, or Cherry-LacI (Control) were analyzed by Western blotting with the indicated antibodies. (C) U2OS stable cells were seeded at 5 × 103 cells/dish in 6-cm dishes and cultured in medium containing puromycin for 10 days. Colonies were stained with methylene blue. Colony numbers and means ± the standard deviations were calculated from three independent experiments. (D) U2OS stable cells were seeded at 5 × 103 cells/well in six-well plates and cultured in medium containing 2 μg of puromycin/ml. Cell numbers were counted on days 1 to 6 after seeding. This experiment was repeated three times with consistent results.
MCV LT inhibits cellular proliferation.
Since the full-length MCV LT and the LT 1-440 mutant have different effects on the cell cycle, we examined how these molecules affect cellular proliferation. Using a pLPCX-based retrovirus construct, we generated U2OS cell lines stably expressing either LT 1-440, full-length MCV LT, or Cherry-LacI, which is an irrelevant molecule that serves as a negative control. Western blot analysis confirmed that LT 1-440 and full-length MCV LT are expressed in U2OS stable cells, and only full-length MCV LT could stimulate the ATR pathway and subsequent p53 phosphorylation and p21 upregulation (Fig. 8B). IF was also performed to confirm that MCV LT, and control proteins were expressed in nearly 100% of the stable cells (data not shown). We then performed a clonogenic assay by seeding an equal number of the stable cells in each dish to test the effects of MCV LT molecules on cellular proliferation. Representative results from three independent experiments are shown in Fig. 8C. The results showed that the LT 1-440 stable cell line generated more colonies than the negative control, whereas fewer colonies were formed in the full-length MCV LT transduced cells compared to the control or LT 1-440 samples (Fig. 8C, experiment n = 3). Compared to the negative control, the U2OS/LT 1-440 cells also appeared to grow into larger colonies (Fig. 8C), suggesting that LT 1-440 can promote cellular proliferation. In accordance with this observation, a proliferation assay showed that U2OS/LT 1-440 cells grew faster than U2OS/full-length MCV LT stable cells (Fig. 8D, experiment n = 3). Compared to MCV LT 1-440, stable expression of full-length MCV LT in mouse C127 cells also led to inhibition of cellular proliferation (data not shown). Taken together, these studies suggest that MCV LT C-terminal domain elicits a DDR and cell cycle arrest, leading to inhibition of cellular proliferation.
Inhibition of cellular proliferation by the MCV LT C-terminal domain can be rescued by a dominant-negative p53 inhibitor.
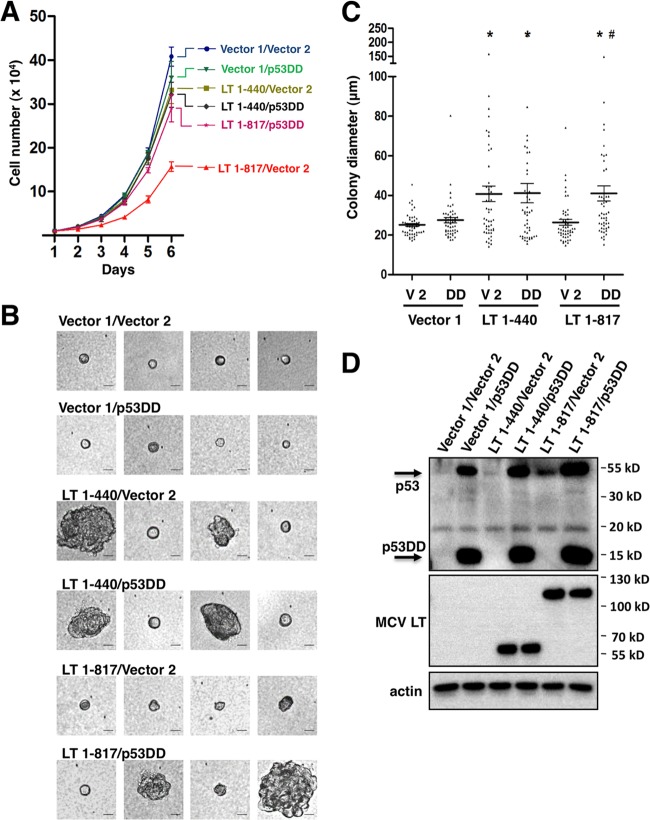
To investigate how the MCV LT- induced DDR and downstream events might affect cellular transformation, we analyzed MCV LT and the N-terminal fragment in a soft agar colony formation assay, which monitors anchorage-independent growth and transformation potential of transduced cells. NIH 3T3 cells were transduced with retroviruses to establish stable expression of either MCV LT 1-440 or MCV LT 1-817. In the soft agar assay, MCV LT 1-440 appeared to stimulate anchorage-independent cell growth compared to the vector control, but MCV LT 1-817 did not show this effect (data not shown and see below). To determine whether MCV LT C terminus-induced p53 activation plays a role in this process, we also generated NIH 3T3 double stable cells expressing either one of the MCV LT molecules together with p53DD, which is a dominant-negative inhibitor of p53 transcription activity (54, 55). Expression of p53DD alleviated the inhibition of cellular proliferation by MCV LT 1-817 without affecting growth rate of the cells stably expressing MCV LT 1-440 or the vector control cells (Fig. 9A, experiment n = 3). The double-stable cells and the respective vector control cells were analyzed in soft agar assay (Fig. 9B and C). Representative images of cells/colonies from each stable cell line are shown in Fig. 9B. The colony sizes were quantified from 50 randomly selected colonies and the data are presented in Fig. 9C. The expression level of the MCV LT molecules and p53DD in NIH 3T3 stable cells are shown by Western blotting (Fig. 9D). Similar to the observation made in previous studies (56), p53DD expression in NIH 3T3 stable cells induces significant stabilization of endogenous p53 (Fig. 9D). This high level of p53 prevents detection of endogenous p53 in the vector control cells at the same exposure level. However, the full-length MCV LT-induced p53 stabilization can be readily detected in the LT 1-817/Vector 2 cells (Fig. 9D).
Fig 9.
Inhibition of cellular proliferation by the MCV LT C-terminal domain can be rescued by a dominant-negative p53 inhibitor. (A) NIH 3T3 cells stably expressing either one of the MCV LT molecules or vector pLPCX (Vector 1), together with p53DD or vector pLXSN (Vector 2), were seeded at 104 cells/6-cm dish and cultured in medium containing 2 μg of puromycin/ml and 0.4 mg of G418/ml. Cell numbers were counted at day 1 to 6 after seeding. This experiment was repeated three times with consistent results. (B) NIH 3T3 double-stable cells were analyzed in the soft agar colony formation assay. Representative images of colonies are shown. Bar, 25 μm. (C) The colony diameter was quantified from 50 randomly selected colonies for each cell line. Error bars represent means ± the standard errors of the mean calculated from three independent experiments. An asterisk (*) indicates significant a difference (P < 0.05) compared to vector 1/vector 2 control. #, Significant difference (P < 0.05) compared to LT-817/Vector 2. (D) Expression of MCV LT molecules and p53DD in NIH 3T3 double-stable cells was detected using Western blotting.
The study showed that the vector control cells and cells carrying only p53DD molecules do not support anchorage-independent growth. However, LT 1-440 expression increased the number of cells that are able to grow into larger colonies in soft agar (Fig. 9B and C). These colonies can be clearly seen under a microscope to be multicellular aggregates (Fig. 9B). p53DD expression in the MCV LT 1-440 stable background did not significantly change the cells' ability to grow in soft agar, and similar numbers of large colonies can be seen as in the LT 1-440/Vector 2 control (Fig. 9B and C). The LT 1-817/Vector 2 stable cells showed similar small colonies as in the vector control cells. Interestingly, expression of p53DD together with MCV LT 1-817 in the LT 1-817/p53DD cell line resulted in significant rescue of anchorage-independent growth compared to LT 1-817/Vector 2 control (Fig. 9B and C, P < 0.05). This experiment was repeated three times, and similar results were obtained. This study showed that, compared to the N-terminal fragment, full-length MCV LT has a decreased potential to support anchorage-independent cell growth and that this defect can be rescued by a p53 dominant-negative inhibitor.
DISCUSSION
The discovery of the role of MCV in human cancer by Chang and coworkers (2) has opened new avenues for investigating the mechanism by which polyomaviruses transform human cells. The characteristic MCV LT truncation mutations found in MCC-associated viral sequences point toward an unknown activity located in the LT C-terminal region that antagonizes tumor formation. This model would make MCV quite different from better-studied polyomaviruses, such as SV40, in which the LT actively antagonizes the cellular tumor suppressor protein p53 (16).
In the present study, we show that MCV infection leads to activation of both ATM and ATR pathways, whereas MCV LT expression alone predominantly activates the ATR pathway. Interestingly, the activity to cause DNA damage and to activate host DDR was mapped to the MCV LT C-terminal region. Our study demonstrates that MCV LT C terminus-induced DDR led to activation of the p53 pathway and inhibition of cellular proliferation. Suppression of cellular proliferation by a C-terminal region of MCV LT was also reported in a recent study by Cheng et al. (33). We show that, compared to the N-terminal mutant 1-440, wild-type MCV LT inhibits cellular proliferation, focus formation, and anchorage-independent cell growth (Fig. 8 and 9). This antiproliferative effect of the MCV LT C-terminal region can be reversed by a p53 dominant-negative inhibitor in clonogenic assays (data not shown), as well as proliferation assays and soft agar transformation assays (Fig. 9). These results suggest that suppression of cellular proliferation by MCV LT is, at least in part, mediated by activation of the p53 pathway.
In cells infected with native MCV virions, low levels of LT expressed from the viral genome were detected. In addition, modest stimulation of both ATR and ATM pathways was also observed (Fig. 1). MCV genomes delivered via native virions do not undergo detectable amounts of viral genome replication in any lines thus far tested (41), suggesting a block against highly functional levels of T antigen expression. This may contribute to the modest induction of DDR markers observed in Fig. 1. DNA damage responses were also activated in cells transfected with a religated MCV genome (Fig. 1). These results demonstrate that, similar to MCV LT expression, introducing the MCV genome into cells either by infection or transfection also stimulates the host DDR, suggesting that the DDR activation observed with MCV LT is not likely to be an overexpression artifact. Interestingly, in cells infected with MCV or transfected with the MCV genome, activation of both ATM and ATR pathway was observed, whereas only the ATR pathway was activated in MCV LT-expressing cells. The activation of ATM by MCV infection may be a result of cross talk between DDR kinases. In addition, other viral activities such as the viral DNA replication process may contribute to this difference.
Analysis of p53S15 phosphorylation, a classic activation marker elicited by both ATR and ATM kinases, showed that the LT-induced DDR might stimulate p53 activity. This activation was mostly through ATR/Chk1 signaling, as treatment with either ATR/Chk1 inhibitors or ATR siRNA abrogated the activation (Fig. 5). Both MCV and SV40 LT could stimulate p53 phosphorylation and stabilize p53 protein levels (Fig. 5 and 7). SV40 LT triggers pp53S15 phosphorylation and stabilizes p53, but a direct interaction between SV40 LT and p53 inhibits its function as a transcription factor (53). Interestingly, analysis of p21, a critical p53 downstream transcriptional target, showed specific upregulation upon MCV LT but not SV40 LT expression (Fig. 7). Additional study showed that MCV LT also stimulates transcription of other p53 downstream target genes (Fig. 7). This seems to indicate that MCV LT, in contrast to SV40 LT, activates p53 and allows it to upregulate downstream genes. This study therefore uncovered important functional differences between MCV LT and SV40 LT.
Cell cycle analysis revealed a complex interaction between the N-terminal and C-terminal regions of MCV LT. Its N-terminal domain promoted progression through the G1/S transition (presumably through inactivation of Rb and/or other cell cycle regulators), while C-terminal domain-induced ATR/p53 activation arrested cells in S-phase and at G2/M checkpoints (57, 58). Cells expressing full-length MCV LT protein are therefore disproportionately found in S and G2 phases. We hypothesize that the intricate balance in the interaction between the N- and C-terminal LT domains coordinately force the host cells into a state that is conducive for efficient viral genome replication. How this phenomenon benefits viral replication during natural infection and how concomitant expression of sT and 57kT might modulate this effect remain to be explored.
Studies of other polyomaviruses implicate the DDR machinery in replication and monomeric resolution of viral genomes, as well as repair of cellular DNA damage caused by the viruses (59, 60). However, the unique feature of MCV discovered in this study is that the MCV LT C terminus functions as an antiproliferation brake by activating the ATR/Chk1/p53 pathway. It is possible that MCV-infected cells can become tumorigenic only after the LT gene develops a truncating mutation, deleting the C-terminal p53-activating region while retaining the N-terminal Rb-inhibiting domain. The transformation induced by the tumor-specific MCV LT truncation mutants may be facilitated by additional host factors and MCV sT, which functions as an oncoprotein through targeting 4E-BP1 (18). Thus, we provide here additional clues for understanding why LT is almost invariably C truncated in MCC tumors.
As the first human polyomavirus clearly shown to cause human cancer (2), MCV represents an exciting opportunity to better understand polyomavirus-mediated tumorigenesis in the context of its natural host. Our studies provide additional examples to support the concept of DDR as an anticancer barrier (61). Future study will investigate how sunlight exposure and other risk factors for MCC (62) can dampen this antitumor barrier to lead to cancer development. Together, these studies may provide molecular insights for understanding the oncogenic mechanism of MCV-associated cancers.
ACKNOWLEDGMENTS
We thank Matthew D. Weitzman (The Children's Hospital of Philadelphia) for critical review and insightful critiques of the manuscript. We thank Eric J. Brown (University of Pennsylvania) for helpful discussion, Juan Muniz (University of Pennsylvania School of Nursing) for advice on the comet assay, Moshe Oren (The Weizmann Institute) for the pLXSN-p53DD construct, Rachel M. Schowalter for providing valuable reagents, Thomas G. Magaldi for providing MCV native virions and reporter pseudovirions, and the members of our laboratories for helpful discussion.
This study was supported by the HIV-Associated Malignancies Pilot Project Award (National Cancer Institute), National Institutes of Health (NIH) grants R01CA148768 and R01CA142723, and the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Published ahead of print 12 June 2013
REFERENCES
- 1. Gjoerup O, Chang Y. 2010. Update on human polyomaviruses and cancer. Adv. Cancer Res. 106:1–51 [DOI] [PubMed] [Google Scholar]
- 2. Feng H, Shuda M, Chang Y, Moore PS. 2008. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 319:1096–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kassem A, Schöpflin A, Diaz C, Weyers W, Stickeler E, Werner M, Zur Hausen A. 2008. Frequent detection of Merkel cell polyomavirus in human Merkel cell carcinomas and identification of a unique deletion in the VP1 gene. Cancer Res. 68:5009–5013 [DOI] [PubMed] [Google Scholar]
- 4. Rodig SJ, Cheng J, Wardzala J, DoRosario A, Scanlon JJ, Laga AC, Martinez-Fernandez A, Barletta JA, Bellizzi AM, Sadasivam S, Holloway DT, Cooper DJ, Kupper TS, Wang LC, DeCaprio JA. 2012. Improved detection suggests all Merkel cell carcinomas harbor Merkel polyomavirus. J. Clin. Invest. 122:4645–4653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hashida Y, Imajoh M, Nemoto Y, Kamioka M, Taniguchi A, Taguchi T, Kume M, Orihashi K, Daibata M. 2013. Detection of Merkel cell polyomavirus with a tumour-specific signature in non-small cell lung cancer. Br. J. Cancer 108:629–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tolstov YL, Pastrana DV, Feng H, Becker JC, Jenkins FJ, Moschos S, Chang Y, Buck CB, Moore PS. 2009. Human Merkel cell polyomavirus infection II. MCV is a common human infection that can be detected by conformational capsid epitope immunoassays. Int. J. Cancer 125:1250–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kean JM, Rao S, Wang M, Garcea RL. 2009. Seroepidemiology of human polyomaviruses. PLoS Pathog. 5:e1000363. 10.1371/journal.ppat.1000363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Foulongne V, Sauvage V, Hebert C, Dereure O, Cheval J, Gouilh MA, Pariente K, Segondy M, Burguière A, Manuguerra Caro J-CV, Eloit M. 2012. Human skin microbiota: high diversity of DNA viruses identified on the human skin by high throughput sequencing. PLoS One 7:e38499. 10.1371/journal.pone.0038499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Feng H, Kwun HJ, Liu X, Gjoerup O, Stolz DB, Chang Y, Moore PS. 2011. Cellular and viral factors regulating Merkel cell polyomavirus replication. PLoS One 6:e22468. 10.1371/journal.pone.0022468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shuda M, Arora R, Kwun HJ, Feng H, Sarid R, Fernandez-Figueras MT, Tolstov Y, Gjoerup O, Mansukhani MM, Swerdlow SH, Chaudhary PM, Kirkwood JM, Nalesnik MA, Kant JA, Weiss LM, Moore PS, Chang Y. 2009. Human Merkel cell polyomavirus infection I. MCV T antigen expression in Merkel cell carcinoma, lymphoid tissues, and lymphoid tumors. Int. J. Cancer 125:1243–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shuda M, Feng H, Kwun HJ, Rosen ST, Gjoerup O, Moore PS, Chang Y. 2008. T antigen mutations are a human tumor-specific signature for Merkel cell polyomavirus. Proc. Natl. Acad. Sci. U. S. A. 105:16272–16277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Houben R, Adam C, Baeurle A, Hesbacher S, Grimm J, Angermeyer S, Henzel K, Hauser S, Elling R, Brocker EB, Gaubatz S, Becker JC, Schrama D. 2012. An intact retinoblastoma protein-binding site in Merkel cell polyomavirus large T antigen is required for promoting growth of Merkel cell carcinoma cells. Int. J. Cancer 130:847–856 [DOI] [PubMed] [Google Scholar]
- 13. An P, Saenz Robles MT, Pipas JM. 2012. Large T antigens of polyomaviruses: amazing molecular machines. Annu. Rev. Microbiol. 66:213–236 [DOI] [PubMed] [Google Scholar]
- 14. Kierstead TD, Tevethia MJ. 1993. Association of p53 binding and immortalization of primary C57BL/6 mouse embryo fibroblasts by using simian virus 40 T-antigen mutants bearing internal overlapping deletion mutations. J. Virol. 67:1817–1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Manfredi J, Prives C. 1994. The transforming activity of simian virus 40 large tumor antigen. Biochim. Biophys. Acta Rev. Cancer 1198:65–83 [DOI] [PubMed] [Google Scholar]
- 16. Ahuja D, Sáenz-Robles MT, Pipas JM. 2005. SV40 large T antigen targets multiple cellular pathways to elicit cellular transformation. Oncogene 24:7729–7745 [DOI] [PubMed] [Google Scholar]
- 17. Pipas JM. 1992. Common and unique features of T antigens encoded by the polyomavirus group. J. Virol. 66:3979–3985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shuda M, Kwun HJ, Feng H, Chang Y, Moore PS. 2011. Human Merkel cell polyomavirus small T antigen is an oncoprotein targeting the 4E-BP1 translation regulator. J. Clin. Invest. 121:3623–3634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lilley CE, Schwartz Ra Weitzman MD. 2007. Using or abusing: viruses and the cellular DNA damage response. Trends Microbiol. 15:119–126 [DOI] [PubMed] [Google Scholar]
- 20. Chaurushiya MS, Weitzman MD. 2009. Viral manipulation of DNA repair and cell cycle checkpoints. DNA Repair 8:1166–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weitzman MD, Lilley CE, Chaurushiya MS. 2010. Genomes in conflict: maintaining genome integrity during virus infection. Annu. Rev. Microbiol. 64:61–81 [DOI] [PubMed] [Google Scholar]
- 22. Fradet-Turcotte A, Bergeron-Labrecque F, Moody CA, Lehoux M, Laimins LA, Archambault J. 2011. Nuclear accumulation of the papillomavirus E1 helicase blocks S-phase progression and triggers an ATM-dependent DNA damage response. J. Virol. 85:8996–9012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gillespie KA, Mehta KP, Laimins LA, Moody CA. 2012. Human papillomaviruses recruit cellular DNA repair and homologous recombination factors to viral replication centers. J. Virol. 86:9520–9526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moody CA, Laimins LA. 2009. Human papillomaviruses activate the ATM DNA damage pathway for viral genome amplification upon differentiation. PLoS Pathog. 5:e1000605. 10.1371/journal.ppat.1000605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sakakibara N, Mitra R, McBride AA. 2011. The papillomavirus E1 helicase activates a cellular DNA damage response in viral replication foci. J. Virol. 85:8981–8995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sowd GA, Li NY, Fanning E. 2013. ATM and ATR activities maintain replication fork integrity during SV40 chromatin replication. PLoS Pathog. 9:e1003283. 10.1371/journal.ppat.1003283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ciccia A, Elledge SJ. 2010. The DNA damage response: making it safe to play with knives. Mol. Cell 40:179–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bakkenist CJ, Kastan MB. 2003. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421:499–506 [DOI] [PubMed] [Google Scholar]
- 29. Zilfou JT, Lowe SW. 2009. Tumor suppressive functions of p53. Cold Spring Harbor Perspect. Biol. 1:a001883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhao X, Madden-Fuentes RJ, Lou BX, Pipas JM, Gerhardt J, Rigell CJ, Fanning E. 2008. Ataxia telangiectasia-mutated damage-signaling kinase- and proteasome-dependent destruction of Mre11-Rad50-Nbs1 subunits in simian virus 40-infected primate cells. J. Virol. 82:5316–5328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shi Y, Dodson GE, Shaikh S, Rundell K, Tibbetts RS. 2005. Ataxia-telangiectasia-mutated (ATM) is a T-antigen kinase that controls SV40 viral replication in vivo. J. Biol. Chem. 280:40195–40200 [DOI] [PubMed] [Google Scholar]
- 32. Hein J, Boichuk S, Wu J, Cheng Y, Freire R, Jat PS, Roberts TM, Gjoerup OV. 2009. Simian virus 40 large T antigen disrupts genome integrity and activates a DNA damage response via Bub1 binding. J. Virol. 83:117–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cheng J, Rozenblatt-Rosen O, Paulson KG, Nghiem P, Decaprio JA. 2013. Merkel cell polyomavirus large T antigen has growth-promoting and inhibitory activities. J. Virol. 87:6118–6126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Buck CB, Pastrana DV, Lowy DR, Schiller JT. 2004. Efficient intracellular assembly of papillomaviral vectors. J. Virol. 78:751–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang X, Li J, Schowalter RM, Jiao J, Buck CB, You J. 2012. Bromodomain protein Brd4 plays a key role in merkel cell polyomavirus DNA replication. PLoS Pathog. 8:e1003021. 10.1371/journal.ppat.1003021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schowalter RM, Pastrana DV, Buck CB. 2011. Glycosaminoglycans and sialylated glycans sequentially facilitate Merkel cell polyomavirus infectious entry. PLoS Pathog. 7:e1002161. 10.1371/journal.ppat.1002161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Olive PL, Banath JP. 2006. The comet assay: a method to measure DNA damage in individual cells. Nat. Protoc. 1:23–29 [DOI] [PubMed] [Google Scholar]
- 38. Stahl H, Droge P, Knippers R. 1986. DNA helicase activity of SV40 large tumor antigen. EMBO J. 5:1939–1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wu C, Roy R, Simmons DT. 2001. Role of single-stranded DNA binding activity of T antigen in simian virus 40 DNA replication. J. Virol. 75:2839–2847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Neumann F, Borchert S, Schmidt C, Reimer R, Hohenberg H, Fischer N, Grundhoff A. 2011. Replication, gene expression and particle production by a consensus Merkel cell polyomavirus (MCPyV) genome. PLoS One 6:e29112. 10.1371/journal.pone.0029112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schowalter RM, Reinhold WC, Buck CB. 2012. Entry tropism of BK and Merkel cell polyomaviruses in cell culture. PLoS One 7:e42181. 10.1371/journal.pone.0042181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, Miyamae Y, Rojas E, Ryu JC, Sasaki YF. 2000. Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen 35:206–221 [DOI] [PubMed] [Google Scholar]
- 43. Binz SK, Sheehan AM, Wold MS. 2004. Replication protein A phosphorylation and the cellular response to DNA damage. DNA Repair (Amst.) 3:1015–1024 [DOI] [PubMed] [Google Scholar]
- 44. Zernik-Kobak M, Vasunia K, Connelly M, Anderson CW, Dixon K. 1997. Sites of UV-induced phosphorylation of the p34 subunit of replication protein A from HeLa cells. J. Biol. Chem. 272:23896–23904 [DOI] [PubMed] [Google Scholar]
- 45. Tibbetts RS, Brumbaugh KM, Williams JM, Sarkaria JN, Cliby WA, Shieh SY, Taya Y, Prives C, Abraham RT. 1999. A role for ATR in the DNA damage-induced phosphorylation of p53. Genes Dev. 13:152–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hall-Jackson CA, Cross DA, Morrice N, Smythe C. 1999. ATR is a caffeine-sensitive, DNA-activated protein kinase with a substrate specificity distinct from DNA-PK. Oncogene 18:6707–6713 [DOI] [PubMed] [Google Scholar]
- 47. Lakin ND, Hann BC, Jackson SP. 1999. The ataxia-telangiectasia related protein ATR mediates DNA-dependent phosphorylation of p53. Oncogene 18:3989–3995 [DOI] [PubMed] [Google Scholar]
- 48. Saito S, Goodarzi AA, Higashimoto Y, Noda Y, Lees-Miller SP, Appella E, Anderson CW. 2002. ATM mediates phosphorylation at multiple p53 sites, including Ser(46), in response to ionizing radiation. J. Biol. Chem. 277:12491–12494 [DOI] [PubMed] [Google Scholar]
- 49. Levine AJ. 1997. p53, the cellular gatekeeper for growth and division. Cell 88:323–331 [DOI] [PubMed] [Google Scholar]
- 50. Prives C. 1998. Signaling to p53: breaking the MDM2-p53 circuit. Cell 95:5–8 [DOI] [PubMed] [Google Scholar]
- 51. Waldman T, Kinzler KW, Vogelstein B. 1995. p21 is necessary for the p53-mediated G1 arrest in human cancer cells. Cancer Res. 55:5187–5190 [PubMed] [Google Scholar]
- 52. Abbas T, Dutta A. 2009. p21 in cancer: intricate networks and multiple activities. Nat. Rev. Cancer 9:400–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Farmer G, Bargonetti J, Zhu H, Friedman P, Prywes R, Prives C. 1992. Wild-type p53 activates transcription in vitro. Nature 358:83–86 [DOI] [PubMed] [Google Scholar]
- 54. Shaulian E, Haviv I, Shaul Y, Oren M. 1995. Transcriptional repression by the C-terminal domain of p53. Oncogene 10:671–680 [PubMed] [Google Scholar]
- 55. Andreassen PR, Lohez OD, Lacroix FB, Margolis RL. 2001. Tetraploid state induces p53-dependent arrest of nontransformed mammalian cells in G1. Mol. Biol. Cell 12:1315–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Heyne K, Schmitt K, Mueller D, Armbruester V, Mestres P, Roemer K. 2008. Resistance of mitochondrial p53 to dominant inhibition. Mol. Cancer 7:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Taylor WR, Stark GR. 2001. Regulation of the G2/M transition by p53. Oncogene 20:1803–1815 [DOI] [PubMed] [Google Scholar]
- 58. Feijoo C, Hall-Jackson C, Wu R, Jenkins D, Leitch J, Gilbert DM, Smythe C. 2001. Activation of mammalian Chk1 during DNA replication arrest: a role for Chk1 in the intra-S phase checkpoint monitoring replication origin firing. J. Cell Biol. 154:913–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Orba Y, Suzuki T, Makino Y, Kubota K, Tanaka S, Kimura T, Sawa H. 2010. Large T antigen promotes JC virus replication in G2-arrested cells by inducing ATM- and ATR-mediated G2 checkpoint signaling. J. Biol. Chem. 285:1544–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jiang M, Zhao L, Gamez M, Imperiale MJ. 2012. Roles of ATM and ATR-mediated DNA damage responses during lytic BK polyomavirus infection. PLoS Pathog. 8:e1002898. 10.1371/journal.ppat.1002898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lilley CE, Weitzman MD. 2010. Keeping viruses in Chk: DNA damage signaling puts the brakes on transformation. Cell Host Microbe 8:464–466 [DOI] [PubMed] [Google Scholar]
- 62. Chang Y, Moore PS. 2012. Merkel cell carcinoma: a virus-induced human cancer. Annu. Rev. Pathol. 7:123–144 [DOI] [PMC free article] [PubMed] [Google Scholar]