Abstract
Lentivirus infection activates CD4+ CD25+ T regulatory (Treg) cells. Activation of Treg cells may be due to direct virus infection or chronic antigenic stimulation. Herein we demonstrate that in vitro feline immunodeficiency virus (FIV) infection, but not UV-inactivated virus, activates Treg cells as measured by immunosuppressive function and upregulation of GARP, FoxP3, and membrane-bound transforming growth factor β (TGF-β). These data demonstrate for the first time that AIDS lentiviruses infect and activate Treg cells, potentially contributing to immune dysfunction.
TEXT
CD4+ regulatory T cells (Treg cells), currently defined by constitutive expression of the high-affinity interleukin-2 (IL-2) receptor CD25 and the transcription factor Foxp3, play an important role in controlling autoimmune disease and shaping the pathogenesis of viral infections by regulating expansion of T and B effector subsets (1–7). Although Treg cells play an important role in preventing excessive inflammation associated with immune responses to infection, they may in the process prematurely abort protective T and B cell immune responses and allow chronic viremia and more severe pathogenesis. This negative effect of Treg cell activation has been demonstrated in herpesvirus, B and C hepatitis virus, and AIDS lentivirus infections (1, 2, 8–10), supporting the speculation that Treg cells may in part be responsible for the chronic nature of these infections. In the case of the lentiviruses human immunodeficiency virus (HIV) and feline immunodeficiency virus (FIV), immunosuppressive Treg cells are first activated during acute infection and remain phenotypically and functionally activated throughout the chronic stage (4, 11), suggesting that they may contribute to virus persistence and subsequent immunodeficiency.
While it is well established that CD4+ and CD8+ T cell immune dysfunction following FIV or HIV infection is associated with early and long-term activation of Treg cells, the precise mechanism(s) of activation has yet to be resolved. While Treg cell activation is associated with virus infection of the host, it is not known whether virus antigens or direct virus infection activates these cells. In support of the latter, we have demonstrated that feline CD4+ CD25+ cells support a productive, noncytopathic FIV infection in vitro that correlates with overexpression of the FIV coreceptor CXCR4 and constitutive activation of transcription factors such as AP1 and ATF that bind to and activate the FIV promoter (12, 13). We have also reported that as early as 1 week post-FIV infection of cats, CD4+ CD25+ T cells support a productive virus infection and are phenotypically and functionally activated (5). Oswald-Richter et al. (14) also reported that Treg cells from HIV-infected individuals express the HIV coreceptor CCR5 and are highly susceptible to HIV infection and replication. Thus, understanding the activation dynamics of these cells during the early stages of lentivirus infection is important to understanding disease progression and treatment approaches.
We (15) and others (16, 17) have presented evidence that membrane-bound transforming growth factor β (mTGF-β) is upregulated on activated Treg cells and is an important mediator of their suppressor function. More recently, we have demonstrated that activated feline Treg cells also display a surface marker, GARP (glycoprotein A repetitions predominant), that anchors mTGF-β on the surface of Treg suppressor cells (18). As Treg cells from FIV-infected cats display increased expression of mTGF-β, GARP, and FoxP3, and express suppressor function (18), we asked if these characteristics could be replicated in Treg cells infected in vitro and thus establish a role for direct Treg cell activation by FIV infection.
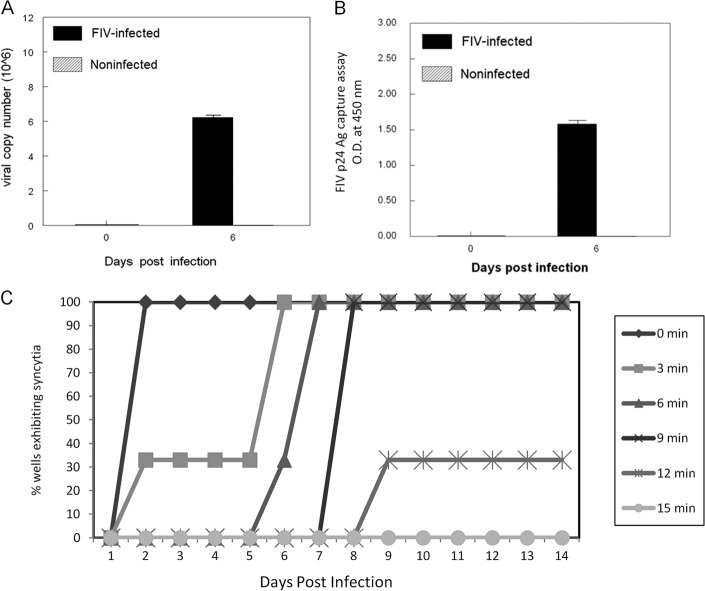
CD4+ CD25+ T cells were fluorescence-activated cell sorter (FACS) purified from peripheral lymph nodes of specific-pathogen-free (SPF) cats as previously described (18), infected with the NCSU1 isolate of FIV at a multiplicity of infection (MOI) of 2.5, and analyzed for virus replication. Six days postinfection (p.i.), a high virus copy number was detected in CD4+ CD25+ cells by real-time PCR (Fig. 1A), and the presence of p24 in the culture supernatant was detected by FIV p24-specific enzyme-linked immunosorbent assay (ELISA) (Fig. 1B), demonstrating a productive infection. As a control, FIV was UV irradiated and shown to be noninfectious for the highly susceptible feline CD4+ cell line CD4E (Fig. 1C). All subsequent experiments were performed using cells infected with FIV at a multiplicity of infection (MOI) of 2.5 and, as negative controls, the equivalent MOI of 30-min UV-inactivated FIV (UV-FIV) or untreated cells.
Fig 1.
Validation of CD4+ CD25+ in vitro infection methods. Purified feline CD4+ CD25+ cells were infected with the NCSU1 isolate of FIV at an MOI of 2.5. Cells and culture supernatant were harvested at 6 days postinfection and analyzed for virus copy by real-time RT-PCR (A) and p24 antigen (Ag) by ELISA (B). Each bar represents the mean ± standard error of the mean (SEM) of 3 replicates. O.D., optical density. (C) UV inactivation of FIV was confirmed using the FIV-susceptible feline T cell line FCD4-E. Virus was exposed to 1 μJ of UV light for various times and then added to FCD4-E cells at an MOI of 2. Five cultures per UV inactivation time were observed daily up to 14 days for the formation of syncytia.
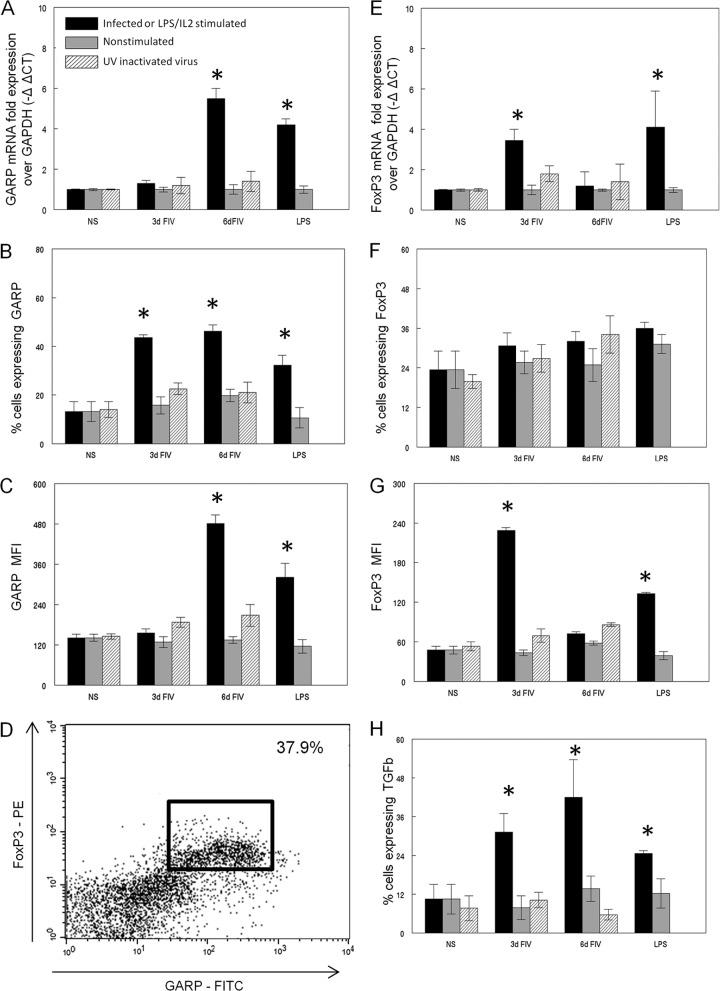
To determine if virus infection alone was capable of inducing a Treg activation phenotype, FACS-purified CD4+ CD25+ cells from SPF cats were untreated, infected with FIV, or treated with UV-FIV and analyzed at 3 and 6 days postinfection for surface expression of GARP, TGF-β, and FoxP3 protein by flow cytometry or for mRNA by real-time reverse transcription (RT)-PCR as described previously (18). As a positive control for activation, purified CD4+ CD25+ cells were stimulated with lipopolysaccharide (LPS) (10 μg/ml) and recombinant human IL-2 (rhIL2) (100 U/ml) as described previously (11). Figure 2A shows that FIV infection of sorted CD4+ CD25+ Treg cells resulted in upregulation of GARP mRNA at 6 days postinfection compared to uninfected controls, and the levels were similar to those in LPS- and rhIL2-activated cells. Both the percentage of cells expressing GARP surface protein (Fig. 2B) and the level of protein expression (mean fluorescence intensity [MFI]) (Fig. 2C) were significantly elevated in FIV-infected CD4+ CD25+ cells. GARP levels were increased at 3 days postinfection, prior to the observed increase in mRNA, suggesting sequestration of intracellular GARP in the absence of stimulation, as described for GARP in human Treg cells (19). The GARP+ CD4+ CD25+ population also coexpressed FoxP3 protein in the FIV-infected cells at 6 days postinfection (Fig. 2D), supporting the classification of this population as Treg cells. No changes in GARP mRNA expression (Fig. 2A) or protein (Fig. 2B) occurred in the UV-FIV-treated cells, suggesting that direct infection of the cells, rather than exposure to viral antigens, is necessary for activation.
Fig 2.
In vitro FIV infection of purified CD4+ CD25+ cells produces an activation phenotype. FACS-purified CD4+ CD25+ cells were either untreated, in vitro infected with FIV at an MOI of 2.5, treated with UV-inactivated virus, or in vitro activated by 4-day LPS and IL-2 stimulation. (A to C) Expression of GARP mRNA (A), cell surface protein (B), and mean fluorescence intensity (MFI) (C) was analyzed. CT, threshold cycle. (D) A representative flow cytometric dot plot showing GARP and FoxP3 expression in FIV-infected cells at 6 days p.i. with the percentage coexpressing GARP and FoxP3 in the upper right corner. (E to G) Expression of FoxP3 mRNA (E), intracellular FoxP3 protein (F), and MFI (G) was analyzed. GARP (C) or FoxP3 (G) detection by flow cytometry was also analyzed by MFI. (H) mTGF-β expression was assessed by flow cytometry; the percentage of cells expressing TGF-β is shown. n = 4 for each treatment group. *, P < 0.05 by Mann-Whitney test for significance. NS, not significant.
FoxP3 message was increased in in vitro FIV-infected CD4+ CD25+ cells by 3 days p.i. but returned to normal levels by 6 days (Fig. 2E). This rapid increase is similar to that seen in vivo, as Mexas et al. demonstrated increased FoxP3 mRNA as early as 1 week post-FIV infection. As described for in vivo studies of FIV and HIV infections (5, 8, 11, 20), the percentage of cells expressing FoxP3 protein remained constant regardless of treatment (Fig. 2F). However, the intracellular protein level as measured by MFI increased at 3 days post-FIV infection (Fig. 2G), consistent with the measured increase in FoxP3 transcription. No change in FoxP3 message (Fig. 2E) or protein (Fig. 2F) was observed following UV-inactivated virus treatment.
Studies have reported that TGF-β is expressed on the surface of activated Treg cells and plays a role in contact-dependent Treg-mediated suppression (3, 15, 17, 18). Furthermore, we have demonstrated that GARP forms a complex with membrane-bound TGF-β (mTGF-β) on activated Treg cells (18). As FIV infection induced GARP expression on CD4+ CD25+ cells, we also analyzed these cells by flow cytometry for surface expression of TGF-β. mTGF-β expression was upregulated following FIV infection of CD4+ CD25+ lymphocytes (Fig. 2H), consistent with GARP upregulation. Taken together, these data suggest that FIV infection is sufficient to induce an activated Treg cell phenotype as measured by the upregulation of GARP, FoxP3, and mTGF-β, whereas exposure to UV-irradiated, noninfectious FIV antigens alone is not.
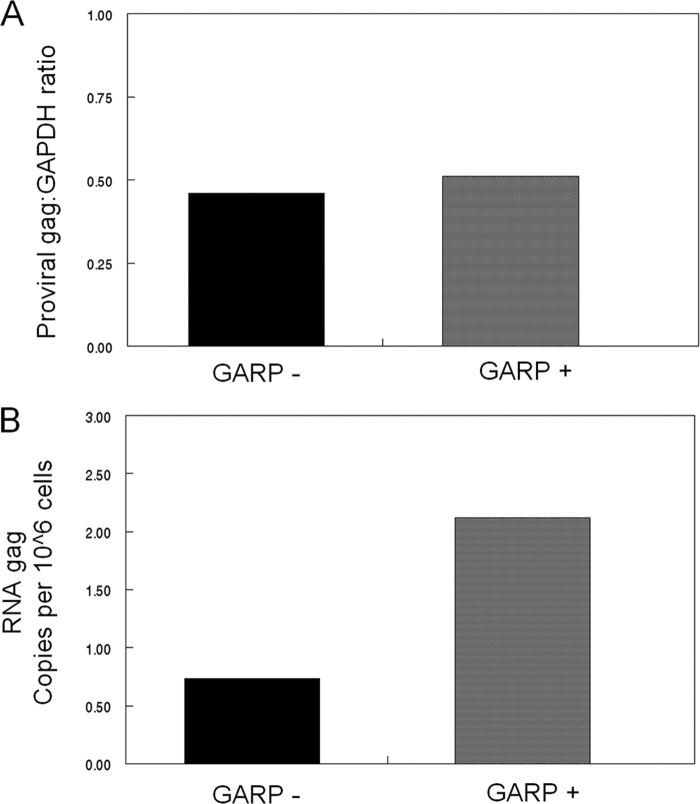
As ∼50% of the CD4+ CD25+ Treg cells become GARP+ by 3 days post-FIV infection, we asked whether there was a difference in virus loads between the GARP+ and GARP− cells following in vitro FIV infection. Purified CD4+ CD25+ cells were infected with FIV for 3 days and FACS sorted into GARP+ and GARP− populations. RNA was collected and analyzed for FIV gag mRNA load by real-time RT-PCR (18), or DNA was collected and analyzed for FIV provirus load using the same gag primers and protocol (18), except that the provirus load was normalized to the housekeeping gene coding for glyceraldehyde-3-phosphate dehydrogenase (GAPDH). While the FIV provirus loads were similar in GARP+ and GARP− cells (Fig. 3A), the FIV mRNA level in the GARP+ cells was 2.5 times that of the GARP− cells (Fig. 3B). These data suggest that although FIV can infect resting Treg cells, conversion to an activation phenotype correlates with and may be dependent on virus replication.
Fig 3.
CD4+ CD25+ GARP+ cells have a higher virus burden than CD4+ CD25+ GARP− cells following in vitro FIV infection. Purified CD4+ CD25+ cells were in vitro infected with FIV at an MOI of 2.5 for 3 days and then FACS sorted into CD4+ CD25+ GARP+ or CD4+ CD25+ GARP− populations. (A) FIV provirus load was analyzed by PCR using gag-specific primers and normalized to GAPDH. (B) Cell-associated FIV gag mRNA was analyzed by real-time RT-PCR.
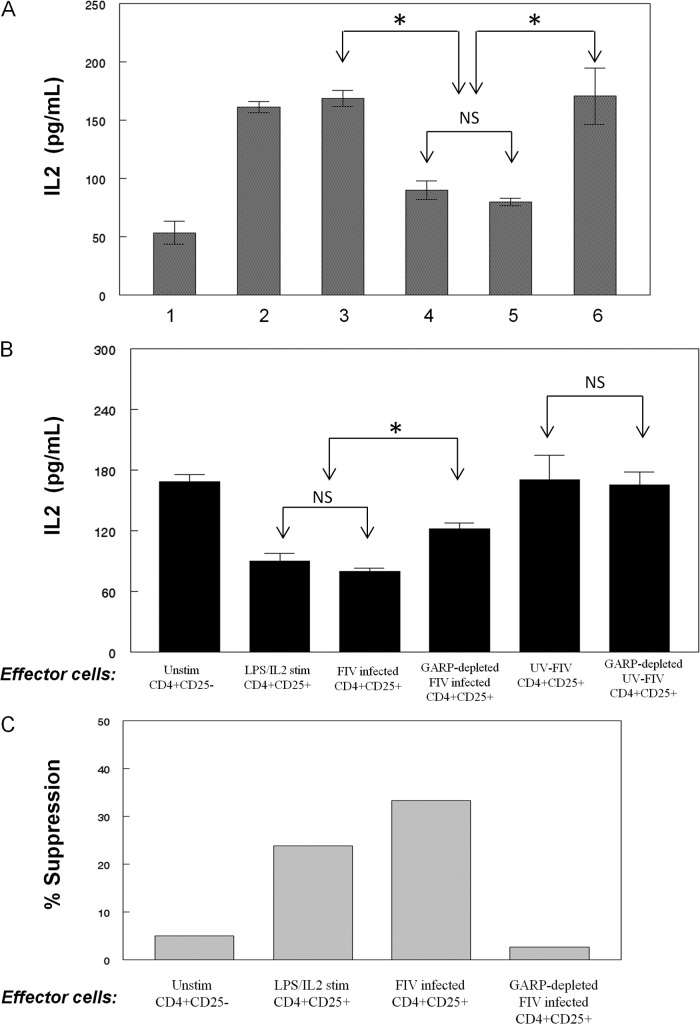
Having demonstrated that FIV infection of purified CD4+ CD25+ Treg cells induces an activation phenotype, we asked whether these cells are functionally activated using an IL-2 suppressor assay described by Miller et al. (18). CD4+ CD25+ cells were purified from FIV-negative cats and infected with FIV or treated with UV-FIV for 3 days prior to coculture with concanavalin A (ConA) (5 μg/ml)-stimulated CD4+ CD25− target cells. As controls, ConA-stimulated target cells were also cocultured with LPS- and rhIL2-stimulated CD4+ CD25+ cells as a positive control for suppressor activity, and unstimulated CD4+ CD25− cells were used in place of Treg cells as a negative control. After 24 h, culture supernatant was collected and assayed for IL-2 by ELISA. As shown in Fig. 4A, CD4+ CD25+ Treg cells infected in vitro with FIV suppressed IL-2 production by activated T helper cells (Fig. 4A, bar 5) to the same degree as LPS- and rhIL2-activated CD4+ CD25+ Treg cells (Fig. 4A, bar 4). Importantly, UV-FIV-treated CD4+ CD25+ cells did not suppress IL-2 production by ConA-stimulated Th cells (Fig. 4A, bar 6). Similarly, stimulated Th cells cocultured with unstimulated Th cells failed to suppress IL-2 production (Fig. 4A, bar 3). To determine the role of GARP in the Treg-mediated suppression, CD4+ CD25+ cells were either infected with FIV or treated with UV-FIV for 3 days, and then GARP+ cells were removed by FACS. The GARP− cells were then cocultured, and the culture supernatant was analyzed for IL-2 as described above. As shown in Fig. 4B, depletion of GARP-expressing cells from the FIV-infected Treg pool reduced the suppressor capability of these effector cells, consistent with our previous studies demonstrating that GARP+ Treg cells are highly efficient suppressors (18). To confirm that the decreased IL-2 production by GARP+ CD4+ CD25+ cells was associated with suppression of target cell proliferation, carboxyfluorescein succinimidyl ester (CFSE)-labeled CD4+ CD25− target cells were cocultured with effector cells for 72 h as described and then analyzed for proliferation by flow cytometry. Percent suppression was calculated as described by Miller et al. (18). In parallel with decreased IL-2 production, FIV-activated CD4+ CD25+ cells suppressed proliferation of ConA-activated CD4+ CD25− T helper cells, and depletion of the GARP+ Treg cells abrogated suppression (Fig. 4C).
Fig 4.
FIV-infected Treg cells suppress CD4+ CD25− T helper cell IL-2 production and proliferation. CD4+ CD25− cells were FACS sorted from FIV-negative cat peripheral blood mononuclear cells (PBMCs) and used as target cells. Antigen-presenting cells (APCs) were prepared by FACS depletion of CD4- and CD8-expressing lymphocytes and added at a 1:1 ratio to target cells. Cells were stimulated for 1 h with 5 μg/ml of ConA unless indicated otherwise (in panels A and C), washed, CFSE labeled to measure proliferation, and then plated. Effector (suppressor) cells were added at an effector/target cell ratio of 1:2. (A) Supernatant was collected after 24 h of coculture, and IL-2 was analyzed by ELISA in triplicate. Bars represent the following conditions: bar 1, unstimulated CD4+ CD25− cells; bars 2 to 6, ConA-stimulated Th cells cocultured with no effector cells (bar 2), unstimulated CD4+ CD25− cells (bar 3), LPS-activated Treg cells (bar 4), FIV-infected Treg cells (bar 5), or UV-FIV-treated Treg cells (bar 6). (B) In the same assay, FIV-infected or UV-FIV-treated Treg cells were depleted of GARP+ cells by FACS and cocultured with ConA-stimulated Th cells. The effector cell controls are the same as those shown in Fig. 3A. (C) Proliferation was measured by flow cytometric analysis of CFSE-labeled cells after 72 h in coculture. The proliferation index (PI) was analyzed using ModFit LT, and the percentage of suppression was calculated as follows: {[(PI of stimulated CD4+ CD25− cells alone) − (PI of stimulated CD4+ CD25− cells with effector cells)]/(PI of stimulated CD4+ CD25− cells alone)} × 100. Two separate experiments were performed, and representative data from one experiment are shown. Error bars in panels A and B represent standard errors of the mean (*, P < 0.05 by Mann-Whitney test).
These data suggest that FIV infection directly activates suppressive CD4+ CD25+ GARP+ Treg cells, rendering them capable of suppressing CD4+ CD25− T helper cells. This virus-induced activation of a suppressor population suggests a novel method for chronic activation of Treg cell-mediated suppression as well as establishing a reservoir for chronic FIV infection.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants R01-A1080288 and K08-AI074445 from the National Institute of Allergy and Infectious Diseases.
We thank Deb Anderson and Janet Dow for excellent technical assistance.
Footnotes
Published ahead of print 12 June 2013
REFERENCES
- 1. Bellou AL, Sarantopoulos A, Gigi E, Sykia A, Beltsis A, Boura P, Raptopoulou-Gigi M. 2011. CD4+CD25+FoxP3+ Tregs: do they inhibit HCV clearance in chronic HCV genotype 1 infection? J. Hepatol. 54(Suppl 1):S111. 10.1016/S0168-8278(11)60274-2 [DOI] [Google Scholar]
- 2. Kinter AL, Horak R, Sion M, Riggin L, McNally J, Lin Y, Jackson R, O'Shea A, Roby G, Kovacs C, Connors M, Migueles SA, Fauci AS. 2007. CD25+ regulatory T cells isolated from HIV-infected individuals suppress the cytolytic and nonlytic antiviral activity of HIV-specific CD8+ T cells in vitro. AIDS Res. Hum. Retroviruses 23:438–450 [DOI] [PubMed] [Google Scholar]
- 3. Fogle JE, Mexas AM, Tompkins WA, Tompkins MB. 2010. CD4(+)CD25(+) T regulatory cells inhibit CD8(+) IFN-gamma production during acute and chronic FIV infection utilizing a membrane TGF-beta-dependent mechanism. AIDS Res. Hum. Retroviruses 26:201–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Holmes D, Jiang Q, Zhang L, Su L. 2008. Foxp3 and Treg cells in HIV-1 infection and immuno-pathogenesis. Immunol. Res. 41:248–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mexas AM, Fogle JE, Tompkins WA, Tompkins MB. 2008. CD4+CD25+ regulatory T cells are infected and activated during acute FIV infection. Vet. Immunol. Immunopathol. 126:263–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Adeegbe D, Bayer AL, Levy RB, Malek TR. 2006. Cutting edge: allogeneic CD4+CD25+Foxp3+ T regulatory cells suppress autoimmunity while establishing transplantation tolerance. J. Immunol. 176:7149–7153 [DOI] [PubMed] [Google Scholar]
- 7. Bettini M, Vignali DA. 2009. Regulatory T cells and inhibitory cytokines in autoimmunity. Curr. Opin. Immunol. 21:612–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Andersson J, Boasso A, Nilsson J, Zhang R, Shire NJ, Lindback S, Shearer GM, Chougnet CA. 2005. Cutting edge: the prevalence of regulatory T cells in lymphoid tissue is correlated with viral load in HIV-infected patients. J. Immunol. 174:3143–3147 [DOI] [PubMed] [Google Scholar]
- 9. Suvas S, Kumaraguru U, Pack CD, Lee S, Rouse BT. 2003. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J. Exp. Med. 198:889–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang G, Liu A, Xie Q, Guo TB, Wan B, Zhou B, Zhang JZ. 2007. Association of CD4+CD25+Foxp3+ regulatory T cells with chronic activity and viral clearance in patients with hepatitis B. Int. Immunol. 19:133–140 [DOI] [PubMed] [Google Scholar]
- 11. Vahlenkamp TW, Tompkins MB, Tompkins WAF. 2004. Feline immunodeficiency virus infection phenotypically and functionally activates immunosuppressive CD4+CD25+ T regulatory cells. J. Immunol. 172:4752–4761 [DOI] [PubMed] [Google Scholar]
- 12. Joshi A, Garg H, Tompkins MB, Tompkins WA. 2005. Preferential feline immunodeficiency virus (FIV) infection of CD4+ CD25+ T-regulatory cells correlates both with surface expression of CXCR4 and activation of FIV long terminal repeat binding cellular transcriptional factors. J. Virol. 79:4965–4976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Joshi A, Vahlenkamp TW, Garg H, Tompkins WAF, Tompkins MB. 2004. Preferential replication of FIV in activated CD4+CD25+T cells independent of cellular proliferation. Virology 321:307–322 [DOI] [PubMed] [Google Scholar]
- 14. Oswald-Richter K, Grill SM, Shariat N, Leelawong M, Sundrud MS, Haas DW, Unutmaz D. 2004. HIV infection of naturally occurring and genetically reprogrammed human regulatory T-cells. PLoS Biol. 2:E198. 10.1371/journal.pbio.0020198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Petty CS, Tompkins MB, Tompkins WA. 2008. Transforming growth factor-beta/transforming growth factor-betaRII signaling may regulate CD4+CD25+ T-regulatory cell homeostasis and suppressor function in feline AIDS lentivirus infection. J. Acquir. Immune Defic. Syndr. 47:148–160 [DOI] [PubMed] [Google Scholar]
- 16. Nakamura K, Kitani A, Fuss I, Pedersen A, Harada N, Nawata H, Strober W. 2004. TGF-beta 1 plays an important role in the mechanism of CD4+CD25+ regulatory T cell activity in both humans and mice. J. Immunol. 172:834–842 [DOI] [PubMed] [Google Scholar]
- 17. Nakamura K, Kitani A, Strober W. 2001. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J. Exp. Med. 194:629–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miller MM, Ross P, Fogle JE, Tompkins MB. 2013. Feline Glycoprotein A Repetitions Predominant (GARP) anchors TGFb on the surface of activated CD4+CD25+ regulatory T cells and mediates AIDS lentivirus-induced T cell immunodeficiency. AIDS Res. Hum. Retroviruses 29:641–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chan DV, Somani AK, Young AB, Massari JV, Ohtola J, Sugiyama H, Garaczi E, Babineau D, Cooper KD, McCormick TS. 2011. Signal peptide cleavage is essential for surface expression of a regulatory T cell surface protein, leucine rich repeat containing 32 (LRRC32). BMC Biochem. 12:27. 10.1186/1471-2091-12-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Estes JD, Wietgrefe S, Schacker T, Southern P, Beilman G, Reilly C, Milush JM, Lifson JD, Sodora DL, Carlis JV, Haase AT. 2007. Simian immunodeficiency virus-induced lymphatic tissue fibrosis is mediated by transforming growth factor beta 1-positive regulatory T cells and begins in early infection. J. Infect. Dis. 195:551–561 [DOI] [PubMed] [Google Scholar]