Abstract
Many viruses invade mucosal surfaces to establish infection in the host. Some viruses are restricted to mucosal surfaces, whereas others disseminate to sites of secondary replication. Studies of strain-specific differences in reovirus mucosal infection and systemic dissemination have enhanced an understanding of viral determinants and molecular mechanisms that regulate viral pathogenesis. After peroral inoculation, reovirus strain type 1 Lang replicates to high titers in the intestine and spreads systemically, whereas strain type 3 Dearing (T3D) does not. These differences segregate with the viral S1 gene segment, which encodes attachment protein σ1 and nonstructural protein σ1s. In this study, we define genetic determinants that regulate reovirus-induced pathology following intranasal inoculation and respiratory infection. We report that two laboratory isolates of T3D, T3DC and T3DF, differ in the capacity to replicate in the respiratory tract and spread systemically; the T3DC isolate replicates to higher titers in the lungs and disseminates, while T3DF does not. Two nucleotide polymorphisms in the S1 gene influence these differences, and both S1 gene products are involved. T3DC amino acid polymorphisms in the tail and head domains of σ1 protein influence the sensitivity of virions to protease-mediated loss of infectivity. The T3DC polymorphism at nucleotide 77, which leads to coding changes in both S1 gene products, promotes systemic dissemination from the respiratory tract. A σ1s-null virus produces lower titers in the lung after intranasal inoculation and disseminates less efficiently to sites of secondary replication. These findings provide new insights into mechanisms underlying reovirus replication in the respiratory tract and systemic spread from the lung.
INTRODUCTION
Many viruses enter host organisms by invading mucosal surfaces, including those that line the respiratory tract. Infection by some pneumotropic viruses is restricted to the respiratory tract, whereas others replicate in the lung and disseminate to sites of secondary replication. Mammalian orthoreoviruses (reoviruses) naturally infect both the respiratory and gastrointestinal tracts (1). Reovirus strains differ in the capacity to replicate at mucosal sites and disseminate systemically. Studies of strain-specific differences in reovirus mucosal infection and systemic spread have enhanced an understanding of viral determinants and molecular mechanisms that regulate reovirus pathogenesis. For example, after peroral or intratracheal inoculation, reovirus strain type 1 Lang (T1L) replicates to higher titers than does strain type 3 Dearing (T3D) (2). This difference in replication efficiency at the site of primary replication segregates with the reovirus S1 gene segment (2, 3). Like T1L, reassortant virus 3HA1, with nine gene segments from T3D and an S1 gene from T1L, replicates to high titers in mucosal tissues (2). In contrast, reassortant virus 1HA3, with nine gene segments from T1L and an S1 gene from T3D, fails to replicate to high titers at those sites, like T3D (2). The S1 gene also is associated with the capacity of reovirus to spread systemically from the enteric tract (4, 5). After gastrointestinal infection, 3HA1, like T1L, spreads to sites of secondary replication, whereas 1HA3, like T3D, does not (5). The genetic determinants of viral replication and systemic dissemination from the murine lung are not known, although in a rat model, the S1 gene is linked to reovirus replication efficiency in the respiratory tract (6).
Two distinct viral proteins are encoded by the reovirus S1 gene, viral attachment protein σ1 and nonstructural protein σ1s. The σ1 protein forms filamentous trimers with tail, body, and head domains (7). It is hypothesized that the serotype-specific differences in reovirus gastrointestinal infection are influenced by sensitivity of the σ1 protein (including that of strain T3D) to cleavage by pancreatic serine proteases (8, 9). Differences in sensitivity to protease-mediated cleavage are determined by a single amino acid polymorphism (isoleucine or threonine at position 249) in the body domain of σ1 (8). Proteolysis of sensitive strains by chymotrypsin or trypsin leads to cleavage of σ1 and diminished infectivity in cultured cells. Although high levels of secreted serine proteases are found in the gastrointestinal tract, protease expression in the respiratory tract is limited in the absence of inflammation (10). Following injury or infection of the respiratory tract, there is increased local expression of serine proteases, matrix metalloproteases, and inflammatory proteases (6, 11–17). Some of these proteases are capable of catalyzing reovirus uncoating (18–23).
The nonstructural S1 gene product, σ1s, is encoded by an open reading frame (ORF) that is completely overlapped by the σ1 coding sequence (24–26). Other than a cluster of positively charged amino acids near the amino terminus, little amino acid sequence identity exists in the σ1s proteins of different reovirus serotypes (27). Viruses in which either the T1L (4) or T3D (28) σ1s ORF has been ablated produce yields of viral progeny comparable to those of the corresponding wild-type viruses following replication in cell culture. However, σ1s-null viruses fail to spread hematogenously in mice following either peroral (4) or intramuscular (28) inoculation. It is not known whether σ1s influences viral dissemination following infection in the murine lung.
In this study, we defined genetic determinants that regulate reovirus-induced pathology after respiratory inoculation. We report that two laboratory isolates of T3D, T3D-Cashdollar (T3DC) and T3D-Fields (T3DF), differ in the capacity to replicate in the respiratory tract and spread systemically. Two nucleotide polymorphisms in the S1 gene regulate these differences, and both S1 gene products are involved. Amino acid polymorphisms in both the tail and head domains of σ1 protein influence the sensitivity of virions to protease-mediated loss of infectivity and the capacity of reovirus to disseminate in the host. Viruses that express the T3DC σ1 protein are less sensitive to proteolysis and disseminate systemically from the respiratory tract. Additionally, a recombinant virus that does not express the T3DC σ1s protein produces lower titers in the lung and disseminates less efficiently to sites of secondary replication. These findings provide new insights into mechanisms underlying reovirus replication in the respiratory tract and systemic dissemination.
MATERIALS AND METHODS
Cells and viruses.
Murine L929 cells were maintained as suspension cultures in Joklik's modified minimal essential medium for spinner cultures (S-MEM) (Invitrogen) supplemented to contain 5% heat-inactivated fetal calf serum (HyClone Laboratories, Logan, UT), 2 mM glutamine, 50 U/ml penicillin G, and 50 μg/ml streptomycin sulfate. Baby hamster kidney cells expressing T7 RNA polymerase (BHK-T7 cells) (29) were maintained as monolayer cultures in Glasgow modifed minimal essential medium (G-MEM) (Invitrogen) supplemented to contain 10% heat-inactivated fetal calf serum, 2 mM l-glutamine, 2% minimum essential medium amino acids solution (50×; Invitrogen), 50 U/ml penicillin, and 50 μg/ml streptomycin sulfate. Alternating passages of BHK-T7 cells were made using medium supplemented to contain 1 mg/ml Geneticin (Gibco).
Reovirus strains T1L and T3DF were obtained from Bernard Fields; strain T3DC was obtained from John Parker. Reassortant viruses 3HA1 and 1HA3, isolated from L929 cells coinfected with strains T1L and T3DF (30), were obtained from Bernard Fields. Purified viral stocks were generated as described previously (31). Viral titers were determined by plaque assay using L929 cells (32). The electrophoretic mobility of viral double-stranded RNA (dsRNA) gene segments was verified using 10% polyacrylamide gels and silver staining (33).
Recombinant reoviruses were generated as described previously (34–36). BHK-T7 cells were cotransfected with nine plasmid constructs representing cloned gene segments from the T3DF genome (34, 35): pT7-L1T3D (2 μg), pT7-L2T3D (2 μg), pT7-L3T3D (2 μg), pT7-M1T3D (1.75 μg), pT7-M2T3D (1.75 μg), pT7-M3T3D (1.75 μg), pT7-S2T3D (1.5 μg), pT7-S3T3D (1.5 μg), and pT7-S4T3D (1.5 μg) (35, 36), in combination with 2 μg of pT7-S1T3DF, pT7-S1T3DF/T3DCS1-77, pT7-S1T3DF/T3DCS1-1234, or pT7-S1T3DF/T3DCS1. The S1 gene recombinant plasmids (pT7-S1T3DF/T3DCS1-77, pT7-S1T3DF/T3DCS1-1234, and pT7-S1T3DF/T3DCS1) were generated by altering pT7-S1T3DF using the QuickChange site-directed mutagenesis system (Stratagene). S1T3DF and S1T3DC contain two noncoding nucleotide changes that were not included in the site-directed mutagenesis. The pT7-S1T3DF/T3DCS1 plasmid was altered by QuickChange site-directed mutagenesis to generate pT7-S1T3DF/T3DCS1 σ1s-null. Primer sequences are available upon request. S1 and L1 gene sequences of viruses recovered by reverse genetics were confirmed using viral RNA extracted from purified virions and OneStep reverse transcription-PCR (RT-PCR) (Qiagen) with S1 (GenBank accession numbers NC_004277.1 and EF494441.1)- or L1 (GenBank accession number EF494435)-specific primers (35).
Infections of mice.
Four- to 5-week-old female CBA/J mice were lightly anesthetized with isoflurane and inoculated with 30 μl onto the nares (approximately 15 μl each nare) and depression of the diaphragm (37). Animals were euthanized at various intervals postinoculation, organs were resected, and viral titers in organ homogenates were determined by plaque assay using L929 cells (32). Animal housing and infection and euthanasia procedures were performed in accordance with and approved by the University of Minnesota Institutional Animal Care and Use Committee.
Protease treatment of reovirus virions.
Purified virions (5 × 1010 particles/ml) in virion dialysis buffer (150 mM NaCl, 10 mM MgCl2, 10 mM Tris [pH 7.5]) were incubated with 200 μg/ml chymotrypsin (Sigma), 25 μg/ml neutrophil elastase (Calbiochem), or human airway trypsin-like (HAT) protease (R&D Systems) at 37°C as described previously (9, 21, 38). Chymotrypsin and HAT digestions were terminated by the addition of 2 mM phenylmethylsulfonyl fluoride (PMSF) (Sigma-Aldrich) to the reaction mixture. Neutrophil elastase digests were terminated by the addition of PMSF (2 mM) and 200 μM neutrophil elastase inhibitor (Calbiochem) to the reaction mixture.
Viral replication assays.
Cells were adsorbed with virus at a multiplicity of infection (MOI) of 5 PFU/cell at 4°C for 1.5 h. After adsorption, cells were pelleted by low-speed centrifugation and resuspended in fresh medium. Infected cells were added to dram vials (2 × 105 cells/vial) containing 1 ml of chilled medium. Triplicate samples were prepared for each time point. Time zero samples were frozen immediately at −80°C. The remaining samples were incubated at 37°C for various intervals, and all samples were subjected to three cycles of freezing and thawing. Viral titers were determined by plaque assay using L929 cells (39). Viral yields were calculated according to the following formula: log10 yield at tx = log10(PFU/ml)tx − log10(PFU/ml)t0, where tx is the time postinfection and t0 is the time immediately postadsorption.
Immunoblot analysis of the σ1 protein.
Discontinuous SDS-PAGE was performed as previously described (40). Purified reovirus virions at a concentration of 2 × 1012 particles per ml were mixed with SDS sample buffer, incubated at 98°C for 5 min, and loaded into wells of precast 4 to 20% gradient Tris-tricine polyacrylamide gels (Bio-Rad Laboratories, Hercules, CA). Samples were electrophoresed at a constant voltage of 180 V for 1 h and transferred to nitrocellulose membranes. Immunoblotting was performed as previously described (4) using a rabbit polyclonal antiserum against the T3D σ1 head domain (Cocalico Biologicals). Reovirus proteins were visualized in an SDS-PAGE gel using Novex colloidal blue staining (Invitrogen). Blots were visualized using an Odyssey infrared imaging system (LiCor Biosciences). Band intensities in the scanned images were quantified using Odyssey version 3.0.16 (Li-Cor).
Statistical analysis.
For those experiments in which viral titers were determined in an organ homogenate or blood, the Mann-Whitney test was used to calculate two-tailed P values. This test is appropriate for experimental data that display a non-Gaussian distribution. When all values are less than the limit of detection, a Mann-Whitney test P value cannot be calculated. Grubb's test for outliers was used to compare intensity of bands corresponding to σ2 protein following SDS-PAGE. Statistical analyses were performed using Prism software (GraphPad Software, Inc.).
RESULTS
Strain-specific differences in reovirus infection in the murine respiratory tract.
To determine whether T1 and T3 reovirus strains differ in the capacity to replicate in the murine respiratory tract, we inoculated CBA/J mice intranasally with 107 PFU of T1L, T3DC, or T3DF and quantified viral titers in the lungs at days 1, 3, and 5 postinoculation. Since the T3DC and T3DF isolates of prototype reovirus strain T3D display a variety of distinct in vitro phenotypes (41–43), both isolates were used in our study. We found that inoculation with T1L produced significantly higher titers in the lungs than did T3DF (Fig. 1A), similar to results obtained in previous experiments using a rat model (2). Interestingly, unlike T3DF, the T3DC isolate produced high titers in the lungs, similar to those achieved by T1L (Fig. 1A). At days 3 and 5 postinoculation, titers of T1L and T3DC reached 107 PFU or greater in the lungs of infected mice, 10- to 100-fold higher than those in the lungs of animals inoculated with T3DF. These results reveal that different laboratory isolates of T3D vary in the capacity to replicate in the murine lung.
Fig 1.
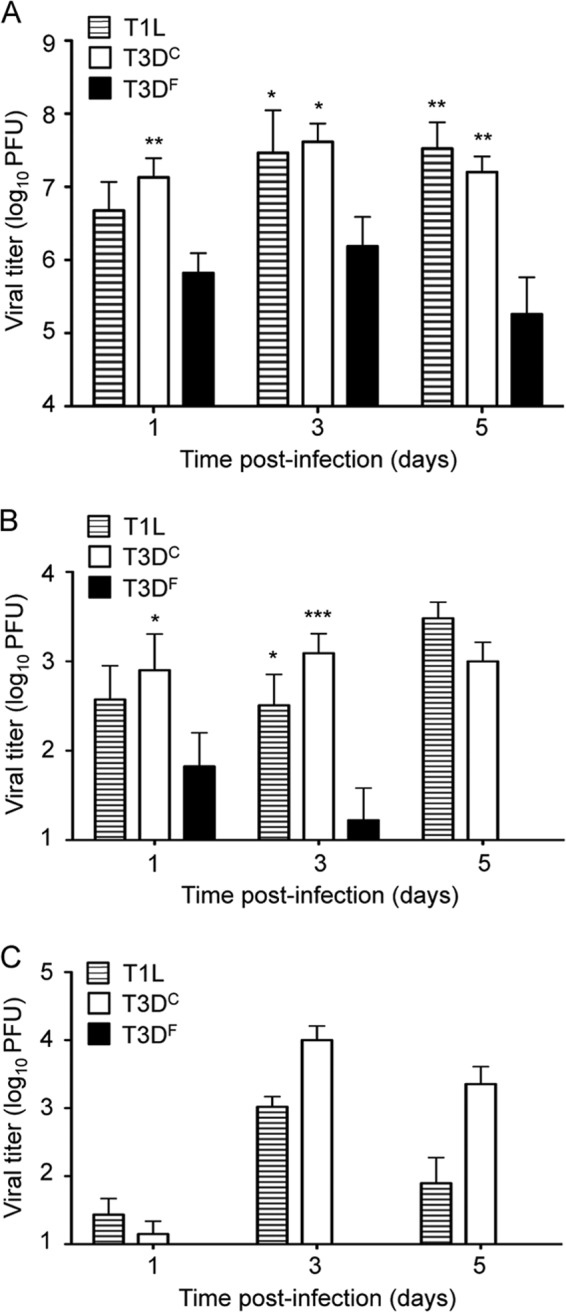
Reovirus strains differ in the capacity to replicate in the respiratory tract and spread systemically. CBA/J mice were inoculated intranasally with 107 PFU of reovirus strain T1L, T3DC, or T3DF. Organs were resected at the indicated times postinoculation, and viral titers in the lungs (A), mediastinal lymph nodes (B), and spleen (C) were determined by plaque assay using L929 cells. Results are expressed as mean viral titers for 6 to 9 mice per time point. Error bars indicate standard errors of the means (SEM). One (P < 0.05), two (P < 0.01), and three (P < 0.001) asterisks reflect significance relative to titers recovered from animals inoculated with T3DF (Mann-Whitney test).
Isolates of T3D differ in the capacity to disseminate from the respiratory tract.
To determine whether the two T3D isolates also differ in the capacity to spread systemically from the respiratory tract, we quantified viral titers at sites of secondary replication following intranasal inoculation. Within a few hours of inoculation, we recovered similar titers of T1L, T3DC, and T3DF in the draining mediastinal lymph nodes (MLN) (data not shown). However, by day 1 postinoculation, titers of T3DC in the MLN were significantly higher than those of T3DF (Fig. 1B). By day 3, both T1L and T3DC reached titers 30- to 100-fold higher than those of T3DF, and by day 5, T3DF was not detected in the MLN but titers of T1L and T3DC remained high. Differences in titers produced by these strains in the spleen were even more striking. T1L and T3DC were recovered from the spleen at days 1, 3, and 5 postinoculation, but T3DF was not (Fig. 1C). Thus, reovirus strains that differ in replication in the murine lung also differ in the capacity to disseminate systemically.
We considered the possibility that T3DF does not spread efficiently to the spleen because it fails to reach a threshold titer in the lungs. To test this hypothesis, we inoculated mice with 30 times more T3DF than that used in the experiments shown in Fig. 1 and quantified viral titers in the lungs and spleen. The higher inoculum of T3DF resulted in titers in the lung approaching 108 PFU at days 1 and 3 postinoculation, slightly higher than titers in the lung of mice inoculated with T3DC (Fig. 2A). However, despite high titers in lung tissue, T3DF did not spread efficiently to the spleen (Fig. 2B). Titers of T3DC in the spleen were in excess of 100-fold higher than those in mice inoculated with the higher dose of T3DF. These results indicate that the T3DC and T3DF laboratory isolates of T3D differ in the capacity to disseminate from the respiratory tract, even when titers in the lung are equivalent.
Fig 2.
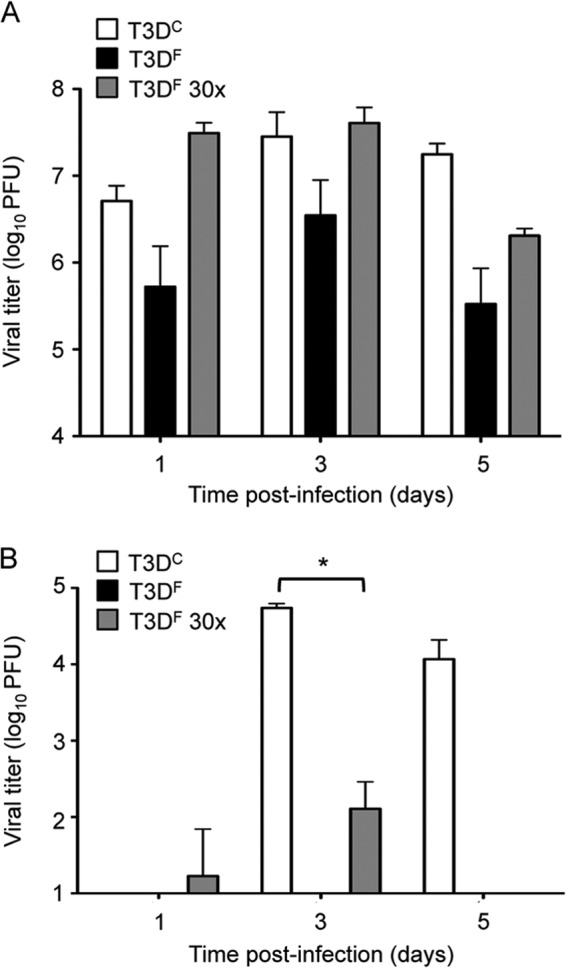
Reovirus systemic dissemination is not dependent on respiratory viral load. CBA/J mice were inoculated intranasally with 107 PFU T3DC, 107 PFU T3DF, or 3 × 108 PFU T3DF. Organs were resected at the indicated times postinoculation, and viral titers in the lungs (A) and spleen (B) were determined by plaque assay using L929 cells. Results are shown from one representative experiment of two performed with 3 mice per time point. Error bars indicate SEM. An asterisk (P < 0.05 by Mann-Whitney test) reflects significance relative to titers recovered from animals inoculated with T3DF.
After peroral inoculation, reovirus is thought to spread from Peyer's patches through lymphatics to regional lymph nodes and the bloodstream (5, 44, 45). From the bloodstream, virus gains access to sites of secondary replication (46). To determine whether reovirus disseminates from the respiratory tract by the hematogenous route, we quantified virus in the blood on days 1, 2, 3, and 5 after intranasal inoculation with 107 PFU of T1L, T3DC, or T3DF. In this experiment, we recovered infectious virus from animals inoculated with either T1L or T3Dc at three of four time points tested (Table 1). At those time points, over 40% of the animals were viremic. In contrast, infectious virus was not detected in the blood of any animal inoculated with T3DF. This finding suggests that access to the bloodstream influences the capacity of strains T1L and T3DC to disseminate systemically from the respiratory tract.
Table 1.
Reovirus strains differ in the capacity to establish viremia after intranasal inoculationa
| Strain and day postinfection | Viremia (no. of positive mice/total no. of mice) |
|---|---|
| T1L | |
| 1 | 0/3 |
| 2 | 2/6 |
| 3 | 1/3 |
| 5 | 2/3 |
| T3DC | |
| 1 | 1/3 |
| 2 | 1/3 |
| 3 | 2/3 |
| 5 | 0/3 |
| T3DF | |
| 1 | 0/3 |
| 2 | 0/3 |
| 3 | 0/3 |
| 5 | 0/3 |
CBA/J mice were inoculated intranasally with 107 PFU of T1L, T3DC, or T3DF. Blood was collected by heart puncture at the indicated times postinoculation, and viral titers were determined by plaque assay using L929 cells. Results are expressed as the number of mice with detectable virus in the blood (limit of detection, 10 PFU/ml).
Construction and characterization of recombinant viruses containing T3DC S1 gene sequences.
The S1 gene is a major determinant of reovirus dissemination from the gastrointestinal tract (28, 34, 44, 45). To determine if sequence polymorphisms in the S1 gene contribute to the differences in dissemination of the T3D laboratory isolates from the lungs, we used reverse genetics (35, 36) to engineer recombinant T3DF viruses that contain T3DC sequences in the S1 gene (Fig. 3A). The T3DC and T3DF S1 genes differ at two nucleotides, 77 and 1234, that result in amino acid changes. Compared to the T3DF S1 gene, the T3DC S1 gene coding change at nucleotide 77 specifies a valine-to-alanine change at position 22 in the σ1 tail and a glutamine-to-histidine change at position 3 in the nonstructural protein σ1s. The T3DC S1 gene coding change at nucleotide 1234 specifies a threonine-to-alanine change at position 408 in the σ1 head. We engineered T3DF viruses that contain each of the individual T3DC coding changes in the S1 gene (rsT3DF/T3DCS1-77 and rsT3DF/T3DCS1-1234) and a virus that contains both T3DC S1 gene coding changes (rsT3DF/T3DCS1).
Fig 3.
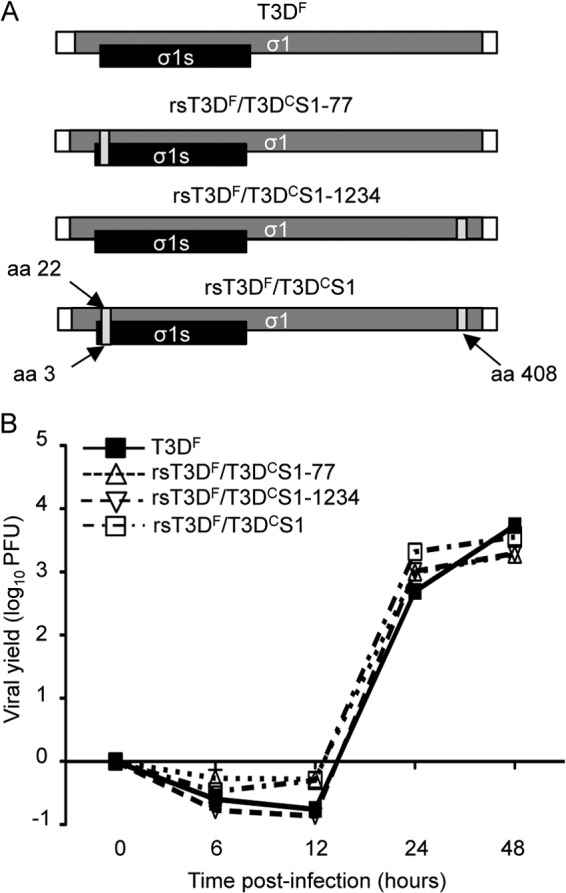
Construction and characterization of T3DF viruses with T3DC sequences in the S1 gene. (A) Schematic of the reovirus S1 gene segment. The σ1 ORF is shown in gray, the σ1s ORF is shown in black, and the 5′ and 3′ UTRs are represented by white rectangles. Each T3DC nucleotide change in the T3DF S1 gene is represented by a dark gray bar. (B) Replication of recombinant viruses. L929 cells were adsorbed with T3DF, rsT3DF/T3DCS1-77, rsT3DF/T3DCS1-1234, or rsT3DF/T3DCS1 at an MOI of 5 PFU/cell. Viral titers were determined at the indicated times postadsorption by plaque assay using L929 cells. The results are expressed as viral yields for triplicate samples. Error bars indicate standard deviations (SD).
To determine whether the T3DC S1 gene sequences influence viral growth in cell culture, we conducted single-cycle replication experiments with the recombinant viruses using mouse L929 fibroblasts. Cells were adsorbed with T3DF, rsT3DF/T3DCS1-77, rsT3DF/T3DCS1-1234, or rsT3DF/T3DCS1 at an MOI of 5 PFU per cell, and viral titers were determined by plaque assay over a 48-h time course. The recombinant viruses with T3DC S1 gene sequences produced yields comparable to those of T3DF and did so with similar kinetics (Fig. 3B). Thus, the T3DC S1 gene sequences do not appear to alter the replication of T3DF in cell culture.
The role of S1 gene sequence polymorphisms in reovirus respiratory infection.
To determine whether the T3DC S1 gene sequences promote reovirus replication in the murine lung, we inoculated mice intranasally with 107 PFU of T3DF or recombinant T3DF with the S1 gene coding sequences of T3DC (rsT3DF/T3DCS1). We recovered similar titers (∼107 PFU) in the lungs of mice infected with these viruses at day 1 postinoculation (Fig. 4A). However, at days 3 and 5, titers of rsT3DF/T3DCS1 in the lungs were ∼10-fold higher than those in animals infected with T3DF. We observed similar trends in viral titers in the MLN, suggesting that viruses in the respiratory tract efficiently gained access to the lymphatic system (Fig. 4B). In contrast, we recovered rsT3DF/T3DCS1 but not T3DF from the spleens of infected mice (Fig. 4C), suggesting that S1 gene sequence polymorphisms in T3DC and T3DF influence systemic dissemination of reovirus from the lung.
Fig 4.
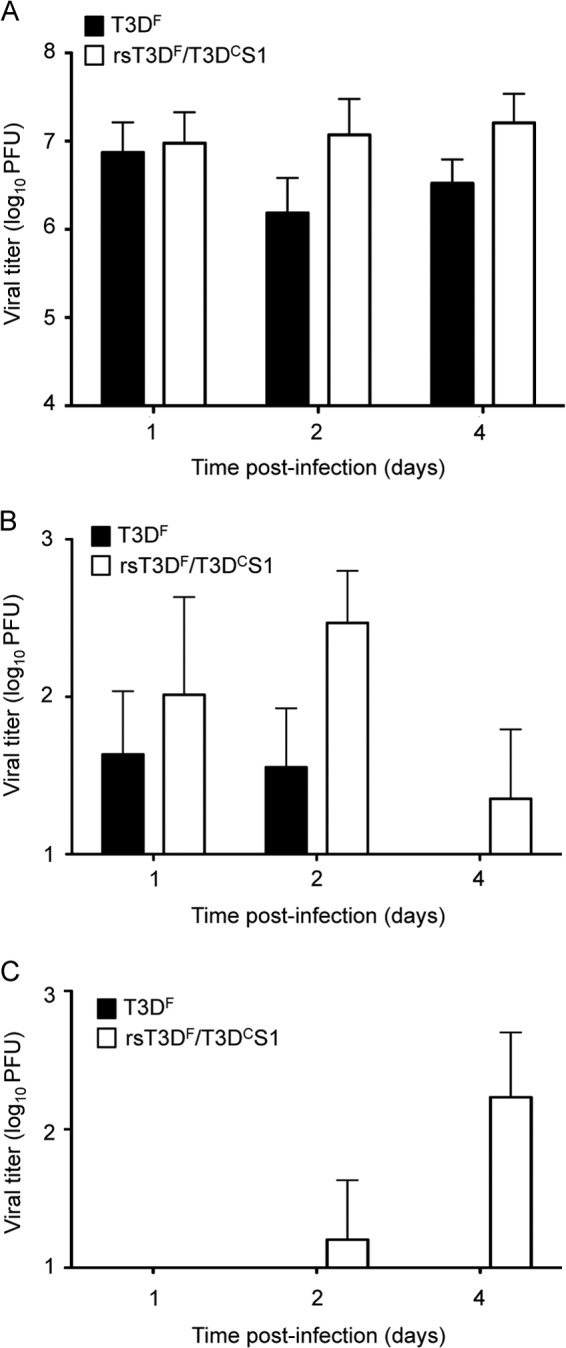
T3DC S1 gene enhances respiratory infection and systemic dissemination. CBA/J mice were inoculated intranasally with 107 PFU of T3DF or rsT3DF/T3DCS1. Organs were harvested at the indicated times postinoculation, and viral titers in the lungs (A), mediastinal lymph nodes (B), and spleen (C) were determined by plaque assay using L929 cells. Results are expressed as mean viral titers for 6 mice per time point. Error bars indicate SEM. No statistically significant differences were observed between groups within panels A and B.
Effect of T3DC S1 gene coding changes on protease-mediated loss of T3D infectivity.
The T3DF reovirus isolate does not replicate to high titers in the small intestine after peroral inoculation and fails to spread systemically (5). These phenotypes are associated with the susceptibility of the T3DF S1 gene-encoded σ1 protein to cleavage by the pancreatic serine proteases chymotrypsin and trypsin (8, 9). While the respiratory tract contains comparably less protease activity than the gastrointestinal tract (47), injury or infection can lead to increases in protease expression in the lung (11, 12, 14–17, 23). Inflammation in the respiratory tract may promote reovirus infection through recruitment of leukocytes and increased expression of inflammatory proteases capable of mediating extracellular reovirus disassembly (6, 23). To explore the possibility that differences in protease sensitivity contribute to differences in the capacity of the two T3D isolates to spread from the respiratory tract, we compared the effects of chymotrypsin on the infectivity of T3DF and the recombinant viruses with T3DC S1 gene polymorphisms (Fig. 5A). We chose chymotrypsin for these experiments, as T3DF virions lose infectivity following treatment with this serine protease, as well as the related protease, trypsin, whereas T1L virions do not (9). We assessed changes in infectivity over time by comparing titers of protease-treated samples to those of mock-treated samples. As anticipated, T3DF virions lost infectivity after 30 min of incubation with chymotrypsin. In contrast, recombinant virions with T3DC S1 gene coding changes retained infectivity after 60 min of chymotrypsin treatment. To test whether the T3DC S1 gene coding changes also affect sensitivity to an inflammatory protease, we performed similar experiments using neutrophil elastase, a serine protease in the same family as chymotrypsin. While T3DF virions lost infectivity within 5 min of treatment with neutrophil elastase (Fig. 5B), recombinant viruses with T3DC S1 gene coding changes retained infectivity through 15 min of protease treatment. Since T3DC and T3DF share sequences in the known protease-sensitive region of σ1 (8), these results indicate that sequences outside this region, in both the head and tail domains of σ1, influence the sensitivity of virions to inactivation by serine proteases.
Fig 5.
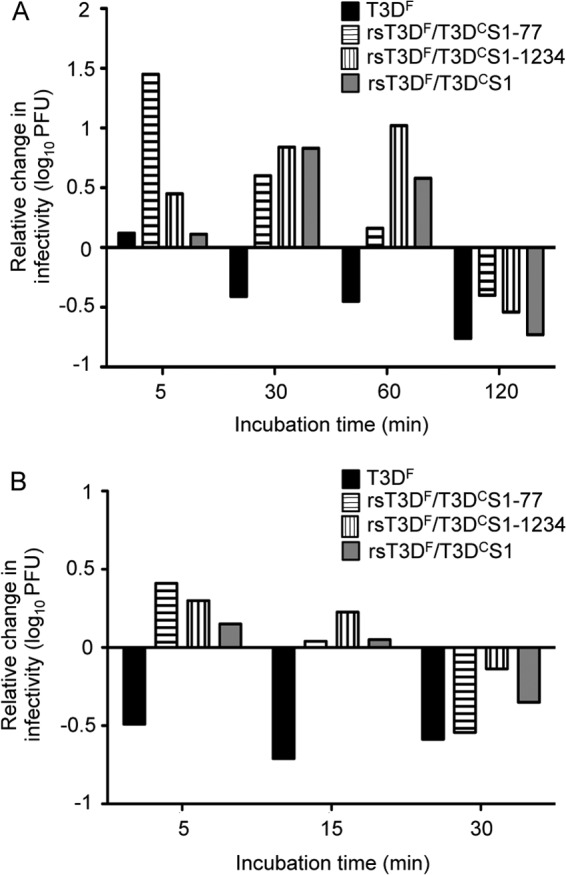
T3DC S1 coding sequences segregate with resistance to protease-mediated loss of infectivity. Purified virions of T3DF, rsT3DF/T3DCS1-77, rsT3DF/T3DCS1-1234, and rsT3DF/T3DCS1 at a concentration of 5 × 1010 particles/ml were treated with either chymotrypsin (A) or neutrophil elastase (B) at 37°C. Viral titers before and after treatment were determined by plaque assay using L929 cells. Changes in viral infectivity are expressed as the ratio of log10 viral titers at each treatment time relative to mock treatment. Results are shown from a representative experiment of three performed.
T3DC and T3DF differ in σ1 virion encapsidation.
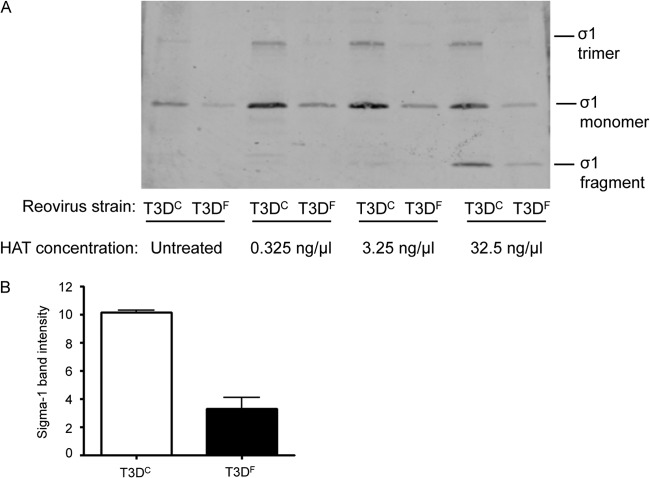
We hypothesized that differences in σ1 protease sensitivity of the T3DC and T3DF isolates affect levels of particle-associated σ1, thereby influencing infectivity in the murine respiratory tract. To test this hypothesis, we treated purified virions of T3DC and T3DF with various concentrations of human airway trypsin-like (HAT) protease, a serine protease present in airway secretions (17). We subjected samples to SDS-PAGE and assessed relative levels of virion-associated σ1 by immunoblotting using a T3D σ1 head-specific polyclonal antiserum. To control for differences in total protein concentration, we prepared a parallel SDS-polyacrylamide gel using aliquots of the analyzed samples and stained it with Coomassie colloidal blue. We compared band intensities of the σ2 protein, a component of the virion core, and found no significant differences between tested samples (P > 0.05 by Grubb's test; data not shown). In all samples assayed by immunoblotting, we observed a band of approximately 50 kDa corresponding to the intact σ1 monomer. In samples treated with higher concentrations of HAT protease, we also observed a faster-migrating band with an apparent molecular mass of ∼26 kDa, which is consistent with the σ1 fragment generated by cleavage with either chymotrypsin or trypsin (Fig. 6A) (8). Surprisingly, we found that the ratio of cleaved σ1 to intact σ1 was approximately equivalent for T3DC and T3DF following treatment with HAT protease. However, we consistently detected more σ1 in samples of T3DC than in samples of T3DF, suggesting that these strains differ in the encapsidation of full-length σ1 protein. To test this hypothesis, we generated fresh preparations of CsCl-purified T3DC and T3DF and quantified the concentration of virion-associated σ1 by immunoblotting. We found that freshly purified virions of T3DC had approximately three times more σ1 (normalized to the band corresponding to core protein σ2 in a parallel Coomassie-stained gel) than detected in virions of T3DF when tested 1 day after purification (Fig. 6B). Moreover, the T3DC σ1 band intensity remained relatively constant following storage of virions at 4°C, while that of T3DF diminished gradually and eventually became undetectable in virion preparations stored at 4°C for several weeks (data not shown). These findings suggest that isolate-specific differences in infectivity are mediated by differences in stability of encapsidated σ1 protein.
Fig 6.
Stoichiometry of virion-associated T3DC and T3DF σ1 protein. (A) Fresh CsCl-banded preparations of T3DC and T3DF virions were treated with HAT protease for 2 h at the concentrations shown. Digestions were terminated, samples were resolved by SDS-PAGE, and intensities of σ1 protein were determined following immunoblotting using a T3D σ1 head-specific polyclonal antiserum. (B) Mean σ1 band intensity relative to σ2 band intensity was determined from Coomassie blue-stained gels using the Odyssey infrared imaging system. Values represent means ± SEM for two independent experiments.
Effect of T3DC S1 gene coding changes on T3D lung replication and systemic dissemination.
To identify the T3DC S1 gene coding polymorphisms that promote viral replication in the lung and dissemination from that site, we infected mice intranasally with T3DF, rsT3DF/T3DCS1-77, or rsT3DF/T3DCS1-1234, resected organs at various intervals postinoculation, and determined viral titers in organ homogenates by plaque assay. We found that animals inoculated with T3DF had titers in the lungs of approximately 106 PFU over the time course, whereas titers in the lungs of animals inoculated with rsT3DF/T3DCS1-1234 tended to be somewhat lower (Fig. 7A). Titers in the lungs of animals inoculated with strain rsT3DF/T3DCS1-77, which contains T3DC amino acid polymorphisms in both the σ1 and σ1s proteins, tended to be somewhat higher at all time points tested (Fig. 7A). However, differences in viral titers in the lungs were not statistically significant.
Fig 7.

Each of the polymorphic coding sequences in the T3D S1 gene influences respiratory infection and dissemination. CBA/J mice were inoculated intranasally with 107 PFU of T3DF, rsT3DF/T3DCS1-77, or rsT3DF/T3DCS1-1234. Organs were harvested at the indicated times postinoculation, and viral titers in the lungs (A), MLN (B), and spleen (C) were determined by plaque assay using L929 cells. Results are expressed as mean viral titers for two independent experiments with 3 to 4 mice per time point. Error bars indicate SEM. There were no statistically significant differences between groups in panels A and B.
To determine whether one or both of the T3DC S1 gene coding polymorphisms contribute to the capacity of T3DC to disseminate systemically from the respiratory tract, we quantified viral titers in the MLN and spleen following intranasal inoculation (Fig. 7B and C). Both recombinant viruses with T3DC S1 gene sequences were capable of disseminating to the MLN. However, only rsT3DF/T3DCS1-77 was detected in the spleen. These results suggest that each of the T3DC coding polymorphisms in the S1 gene influences systemic viral dissemination from the respiratory tract. However, only the polymorphism at nucleotide 77 appears to promote reovirus dissemination.
Role of T3DC S1 gene products in reovirus dissemination from the respiratory tract.
The polymorphism at nucleotide 77 in the T3D S1 gene affects both of its gene products, σ1 and σ1s. Interestingly, both S1 gene products are linked to reovirus pathogenesis in vivo (4, 5, 28, 44, 48). To determine whether the σ1s protein influences viral replication and dissemination from the murine respiratory tract, we used reverse genetics (35, 36) to engineer a recombinant rsT3DF/T3DCS1 σ1s-null virus (Fig. 8A). We assessed the capacity of this σ1s-null virus to replicate in cell culture. As anticipated (4, 49), rsT3DF/T3DCS1 σ1s-null produced yields of viral progeny comparable to those in T3DF- and rsT3DF/T3DCS1-infected L929 cells (Fig. 8B).
Fig 8.
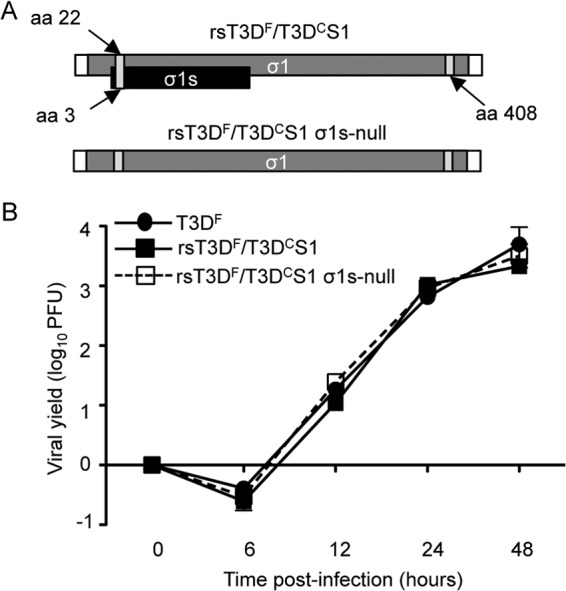
Construction and characterization of rsT3DF/T3DCS1 σ1s-null virus. (A) Schematic of the reovirus rsT3DF/T3DC S1 gene segment. The σ1 ORF is shown in gray, the σ1s ORF is shown in black, and the 5′ and 3′ UTRs are represented by white rectangles. (B) Replication of recombinant viruses. L929 cells were adsorbed with T3DF, rsT3DF/T3DCS1, or rsT3DF/T3DCS1 σ1s-null virus at an MOI of 5 PFU/cell. Viral titers were determined at the indicated times postadsorption by plaque assay using L929 cells. The results are expressed as viral yields for triplicate samples. Error bars indicate SD.
To determine whether the σ1s protein functions in reovirus infection in the lung and dissemination from that site, we inoculated mice intranasally with 107 PFU of T3DF, rsT3DF/T3DCS1, or rsT3DF/T3DCS1 σ1s-null, resected organs at days 1, 2, and 4 postinoculation, and determined viral titers by plaque assay. We found that titers of T3DF, rsT3DF/T3DCS1, and rsT3DF/T3DCS1 σ1s-null were equivalent in the lungs at day 1 postinoculation, and as expected, titers of rsT3DF/T3DCS1 were significantly higher than those of T3DF in the lungs at day 2 postinoculation (Fig. 9A). In contrast, titers of rsT3DF/T3DCS1 σ1s-null were significantly lower in the lung than those produced by either T3DF or rsT3DF/T3DCS1 at days 2 and 4 postinoculation. All viruses gained access to the draining lymph and reached similar titers in the MLN (Fig. 9B). These results suggest that the σ1s protein contributes to reovirus replication in the respiratory tract but is dispensable for access to draining lymph nodes.
Fig 9.

T3DC σ1s protein promotes viral replication in the respiratory tract and enhances systemic dissemination. CBA/J mice were inoculated intranasally with 107 PFU of T3DF, rsT3DF/T3DCS1, or rsT3DF/T3DCS1 σ1s-null. Organs were harvested at the indicated times postinoculation, and viral titers in the lungs (A), MLN (B), and spleen (C) were determined by plaque assay using L929 cells. Results are expressed as mean viral titers for 5 to 6 mice per time point. Error bars indicate SEM. One (P < 0.05) or two (P < 0.01) (Mann-Whitney test) asterisks reflect significance relative to titers recovered from animals inoculated with T3DF.
To determine whether σ1s is required for hematogenous dissemination of reovirus from the respiratory tract, we quantified virus in the blood and spleen after intranasal inoculation with T3DF, rsT3DF/T3DCS1, or rsT3DF/T3DCS1 σ1s-null virus. Consistent with our previous results, we recovered virus in the blood from the majority of mice inoculated with rsT3DF/T3DCS1, but viremia was detected in only a single animal inoculated with T3DF (Table 2). Fewer mice inoculated with the rsT3DF/T3DCS1 σ1s-null virus developed viremia, suggesting that σ1s enhances rsT3DF/T3DCS1 access to the bloodstream. Interestingly, titers of rsT3DF/T3DCS1 σ1s-null in the spleen were only slightly lower than those in animals inoculated with rsT3DF/T3DCS1 (Fig. 9C). These results suggest that the T3DC σ1s protein enhances reovirus access to the bloodstream but is not absolutely required for systemic spread. However, analysis of the S1 gene sequence of five plaque-purified viruses isolated from organs resected from mice inoculated with rsT3DF/T3DCS1 σ1s-null revealed that three of the five had reverted to wild-type σ1s expression. These results emphasize the importance of the T3DC σ1s protein in dissemination from the respiratory tract.
Table 2.
Role of rsT3DF/T3DC S1 gene products in viremiaa
| Strain and day postinfection | Viremia (no. of positive mice/total no. of mice) |
|---|---|
| T3DF | |
| 1 | 1/5 |
| 2 | 0/5 |
| 4 | 0/5 |
| rsT3DF/T3DCS1 | |
| 1 | 7/8 |
| 2 | 6/8 |
| 4 | 4/8 |
| rsT3DF/T3DCS1 σ1s-null | |
| 1 | 3/5 |
| 2 | 3/5 |
| 4 | 1/5 |
CBA/J mice were inoculated intranasally with 107 PFU T3DF, rsT3DF/T3DCS1, or rsT3DF/T3DCS1 σ1s-null. Blood was collected by heart puncture at the indicated times postinoculation, and viral titers were determined by plaque assay using L929 cells. Results are expressed as the number of mice with detectable virus in the blood (limit of detection, 10 PFU/ml).
DISCUSSION
Although much is known about viral and host determinants that regulate reovirus pathogenesis following infection of the gastrointestinal tract, mechanisms of reovirus replication in and systemic dissemination from the respiratory tract are not well understood. In this study, we found that two laboratory isolates of reovirus serotype 3 prototype strain T3D, T3DC and T3DF, differ in the capacity to replicate in the murine lung and spread systemically. These strain-specific differences are influenced by a polymorphism in the S1 gene at nucleotide 77, and both S1 gene products σ1 and σ1s are required for full manifestation of the pulmonary replication and dissemination phenotypes.
We found that reovirus strain T1L replicates to higher titers in the lungs of infected mice than does T3DF, similar to results gathered using a rat model of intratracheal inoculation (2, 50). Interestingly, another laboratory isolate of strain T3D, T3DC, produces titers in the lungs comparable to those of T1L (Fig. 1). Analysis of viral titers over time revealed that increasing lung titers reflected viral replication (progeny) and not recovered input virus (Fig. 2 and data not shown). While the genome sequences of T3DC and T3DF are highly conserved (8), only T3DC disseminates systemically. To probe this key difference in pathogenesis displayed by T3DC and T3DF, we examined whether genes that encode outer-capsid proteins (S1, S4, and M2) segregate with the efficiency of reovirus replication in the lungs and dissemination from the respiratory tract. We found that the T3DC S4 and M2 genes are not associated with either increased titers in the lungs or systemic dissemination from that site (data not shown). In sharp contrast, the coding polymorphisms in the T3DC S1 gene are associated with increased replication in the lungs and enhanced systemic spread (Fig. 4). The failure of T3DF to gain access to the bloodstream may be attributable to failure of this strain to egress from the lungs, as T3DF directly injected into the blood is taken up at levels similar to those of T1L by all organs except the lungs (51). Similar studies have not been performed with the T3DC isolate.
The T3DC S1 gene has two nucleotide polymorphisms relative to the T3DF S1 gene that alter protein coding. The T3DC change at nucleotide 77 confers a valine-to-alanine change at position 22 in the σ1 tail and a glutamine-to-histidine change at position 3 in σ1s, whereas nucleotide 1234 confers a threonine-to-alanine change at position 408 in the σ1 head. These residues are not associated with any known antibody epitope, receptor-binding site, or protease-sensitive region (7, 8, 52–62). Interestingly, our data demonstrate that both T3DC S1 gene nucleotide polymorphisms relative to T3DF result in virions that retain infectivity following protease treatment (Fig. 5). However, only the T3DC polymorphism at nucleotide 77 is associated with increased viral titers in the lungs following intranasal inoculation and systemic viral dissemination (Fig. 7). Based on these observations, we conclude that alanine 22 in σ1, glutamine 3 in σ1s, or a combination of both sequence changes contribute to the T3DC pulmonary replication and spreading phenotypes.
Some serotype 3 reovirus strains, including T3DF, rapidly lose infectivity in the gastrointestinal tract due to a polymorphism in the body domain of the σ1 protein (9). Although T3DC and T3DF share the threonine residue at amino acid 249 in σ1 that confers susceptibility to protease-mediated cleavage (8), our results reveal that virions of T3DC and rsT3DF/T3DCS1, unlike those of T3DF, do not rapidly lose infectivity following treatment in vitro with the serine proteases HAT, chymotrypsin, and neutrophil elastase (Fig. 5 and data not shown). In addition, T3DC but not T3DF is recovered at high titers in the spleen after peroral inoculation (data not shown). These findings suggest that T3DC does not lose infectivity as rapidly as T3DF in vivo, and that sequences outside the known protease-sensitive region in σ1 function to stabilize σ1 in the presence of protease.
How might the coding changes in the T3DC σ1 protein mitigate the infectivity loss that is associated with the protease-sensitive sequence at position 249 in the σ1 body domain? Protease treatment of T3DC and T3DF virions results in nearly equivalent proportions of full-length σ1 and the major σ1 cleavage fragment. However, T3DC has significantly more virion-associated σ1 than does T3DF even prior to protease treatment (Fig. 6). These results suggest that the increased rate at which T3DF loses infectivity in the presence of protease relative to T3DC is not solely attributable to the sensitivity of σ1 to cleavage but also is influenced by the quantity of encapsidated σ1. Thus, the T3DC σ1 polymorphisms may function to enhance assembly of σ1 onto virions or stabilize the molecule once assembled. Mechanisms of σ1 trimerization and encapsidation are not well understood. However, σ1 sequences between amino acids 3 and 107 (63, 64) are required to anchor σ1 into λ2 pentamers at the virion 5-fold symmetry axes (65, 66). Alanine 22 in T3DC σ1 is within the σ1-anchoring region (63, 64), providing a plausible explanation for the enhanced encapsidation of T3DC σ1. How the alanine residue at position 408 in the T3DC σ1 head counterbalances the negative effect of protease on infectivity of T3D virions is not clear, but it may strengthen σ1 interactions with other outer-capsid proteins to promote σ1 assembly or enhance σ1 trimerization. Although it is possible that σ1 encapsidation in vivo differs from what is observed in experiments using L cells, our biochemical results point to an important difference between these isolates that may explain the differences in their pathogenesis.
While attachment protein σ1 is a major determinant of reovirus pathogenesis (5, 30, 44, 45, 67), nonstructural protein σ1s also is required for production of maximal viral titers in the gastrointestinal tract (4) and for hematogenous spread after peroral (4) or intramuscular (28) inoculation. Consistent with a role for σ1s in viral replication at mucosal sites, our results demonstrate that the T3DC σ1s protein enhances viral replication in the respiratory tract and hematogenous dissemination (Fig. 9 and Table 2). Although viral titers in the spleen did not differ significantly after intranasal inoculation of rsT3DF/T3DCS1 or rsT3DF/T3DCS1 σ1s-null virus (Fig. 9), over half of the rsT3DF/T3DCS1 σ1s-null virus isolates from the spleen had reverted to wild-type σ1s expression, and no other reversions were noted in the S1 gene. While we cannot pinpoint the exact timing of the reversion, the σ1s protein is important for reovirus dissemination from the respiratory tract.
Mechanisms by which σ1s promotes reovirus dissemination are unknown. Following intravenous inoculation of humans, oncolytic reovirus has been found associated with circulating blood cells, including granulocytes, monocytes, and platelets (68). Thus, it is possible that σ1s influences systemic dissemination in the mouse model of infection by promoting replication in or efficient release from one or more circulating cell types. Although the means by which reovirus is released from infected cells is not well understood, virus-induced apoptosis contributes to efficient egress in some virus-host cell systems (69). Interestingly, σ1s is required for maximal levels of apoptosis during reovirus infection of the murine heart and central nervous system (70). Failure to induce apoptosis and consequent inefficient escape from infected cells may explain the lower viral yields in the lungs of mice inoculated with rsT3DF/T3DCS1 σ1s-null virus and the recovery of revertant viruses from the spleen.
Collectively, our results reveal that both T3DC S1 gene polymorphisms influence reovirus replication in the murine lung and systemic dissemination from that mucosal site. Furthermore, both S1 gene products σ1 and σ1s are involved in these phenotypes. The capacity of T3DC to replicate in lung tissue and disseminate systemically likely is influenced by increased σ1 encapsidation and resistance to protease-mediated inactivation, as well as functions attributable to nonstructural protein σ1s. These findings provide evidence that systemic viral dissemination depends on coordinated functions of viral attachment and virus-induced cell injury.
ACKNOWLEDGMENTS
We thank Megan Ahl Fischer and Lindsey Foran for excellent technical support and Stephen Rice and Wade Schulz for helpful feedback on the manuscript.
This work was supported by T32 CA09385 (K.W.B.), F32 AI075776 (K.W.B.), K22 AI094079 (K.W.B.), T32 GM008554 (J.D.D), R37 AI038296 (T.S.D.), R01 AI045990 (L.A.S.), and the Elizabeth B. Lamb Center for Pediatric Research. Additional support was provided by Public Health Service awards CA68485 for the Vanderbilt-Ingram Cancer Center and DK20593 for the Vanderbilt Diabetes Research and Training Center.
Footnotes
Published ahead of print 12 June 2013
REFERENCES
- 1. Sabin AB. 1959. A new group of respiratory formerly classified as ECHO type 10 is described. Science 130:1387–1389 [DOI] [PubMed] [Google Scholar]
- 2. Morin MJ, Warner A, Fields BN. 1996. Reovirus infection in rat lungs as a model to study the pathogenesis of viral pneumonia. J. Virol. 70:541–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Farone AL, Frevert CW, Farone MB, Morin MJ, Fields BN, Paulauskis JD, Kobzik L. 1996. Serotype-dependent induction of pulmonary neutrophilia and inflammatory cytokine gene expression by reovirus. J. Virol. 70:7079–7084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boehme KW, Guglielmi KM, Dermody TS. 2009. Reovirus nonstructural protein sigma1s is required for establishment of viremia and systemic dissemination. Proc. Natl. Acad. Sci. U. S. A. 106:19986–19991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kauffman RS, Wolf JL, Finberg R, Trier JS, Fields BN. 1983. The sigma 1 protein determines the extent of spread of reovirus from the gastrointestinal tract of mice. Virology 124:403–410 [DOI] [PubMed] [Google Scholar]
- 6. Ley K. 2004. Weird and weirder: how circulating chemokines coax neutrophils to the lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 286:463–464 [DOI] [PubMed] [Google Scholar]
- 7. Reiter DM, Frierson JM, Halvorson EE, Kobayashi T, Dermody TS, Stehle T. 2011. Crystal structure of reovirus attachment protein sigma1 in complex with sialylated oligosaccharides. PLoS Pathog. 7:e1002166. 10.1371/journal.ppat.1002166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chappell JD, Barton ES, Smith TH, Baer GS, Duong DT, Nibert ML, Dermody TS. 1998. Cleavage susceptibility of reovirus attachment protein sigma1 during proteolytic disassembly of virions is determined by a sequence polymorphism in the sigma1 neck. J. Virol. 72:8205–8213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nibert ML, Chappell JD, Dermody TS. 1995. Infectious subvirion particles of reovirus type 3 Dearing exhibit a loss in infectivity and contain a cleaved sigma 1 protein. J. Virol. 69:5057–5067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shimabukuro DW, Sawa T, Gropper M. 2003. Injury and repair in lung and airways. Crit. Care Med. 31:S524–S531 [DOI] [PubMed] [Google Scholar]
- 11. Chokki M, Yamamura S, Eguchi H, Masegi T, Horiuchi H, Tanabe H, Kamimura T, Yasuoka S. 2004. Human airway trypsin-like protease increases mucin gene expression in airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 30:470–478 [DOI] [PubMed] [Google Scholar]
- 12. Donaldson SH, Hirsh A, Li DC, Holloway G, Chao J, Boucher RC, Gabriel SE. 2002. Regulation of the epithelial sodium channel by serine proteases in human airways. J. Biol. Chem. 277:8338–8345 [DOI] [PubMed] [Google Scholar]
- 13. Matsushima R, Takahashi A, Nakaya Y, Maezawa H, Miki M, Nakamura Y, Ohgushi F, Yasuoka S. 2006. Human airway trypsin-like protease stimulates human bronchial fibroblast proliferation in a protease-activated receptor-2-dependent pathway. Am. J. Physiol. Lung Cell. Mol. Physiol. 290:L385–L395 [DOI] [PubMed] [Google Scholar]
- 14. Moraes TJ, Chow CW, Downey GP. 2003. Proteases and lung injury. Crit. Care Med. 31:S189–S194 [DOI] [PubMed] [Google Scholar]
- 15. Parks WC, Shapiro SD. 2001. Matrix metalloproteinases in lung biology. Respir. Res. 2:10–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reid PT, Sallenave J-M. 2001. Neutrophil-derived elastases and their inhibitors: potential role in the pathogenesis of lung disease. Curr. Opin. Investig. Drugs 2:59–67 [PubMed] [Google Scholar]
- 17. Takahashi M, Sano T, Yamaoka K, Kamimura T, Umemoto N, Nishitani H, Yasuoka S. 2001. Localization of human airway trypsin-like protease in the airway: an immunohistochemical study. Histochem. Cell Biol. 115:181–187 [DOI] [PubMed] [Google Scholar]
- 18. Ebert DH, Deussing J, Peters C, Dermody TS. 2002. Cathepsin L and cathepsin B mediate reovirus disassembly in murine fibroblast cells. J. Biol. Chem. 277:24609–24617 [DOI] [PubMed] [Google Scholar]
- 19. Golden JW, Bahe JA, Lucas WT, Nibert ML, Schiff LA. 2004. Cathepsin S supports acid-independent infection by some reoviruses. J. Biol. Chem. 279:8547–8557 [DOI] [PubMed] [Google Scholar]
- 20. Golden JW, Linke J, Schmechel S, Thoemke K, Schiff LA. 2002. Addition of exogenous protease facilitates reovirus infection in many restrictive cells. J. Virol. 76:7430–7443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Golden JW, Schiff LA. 2005. Neutrophil elastase, an acid-independent serine protease, facilitates reovirus uncoating and infection in U937 promonocyte cells. Virol. J. 2:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Johnson EM, Doyle JD, Wetzel JD, McClung RP, Katunuma N, Chappell JD, Washington MK, Dermody TS. 2009. Genetic and pharmacologic alteration of cathepsin expression influences reovirus pathogenesis. J. Virol. 83:9630–9640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nygaard RM, Golden JW, Schiff LA. 2012. Impact of host proteases on reovirus infection in the respiratory tract. J. Virol. 86:1238–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ernst H, Shatkin AJ. 1985. Reovirus hemagglutinin mRNA codes for two polypeptides in overlapping reading frames. Proc. Natl. Acad. Sci. U. S. A. 82:48–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jacobs BL, Samuel CE. 1985. Biosynthesis of reovirus-specified polypeptides: the reovirus s1 mRNA encodes two primary translation products. Virology 143:63–74 [DOI] [PubMed] [Google Scholar]
- 26. Sarkar G, Pelletier J, Bassel-Duby R, Jayasuriya A, Fields BN, Sonenberg N. 1985. Identification of a new polypeptide coded by reovirus gene S1. J. Virol. 54:720–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cashdollar LW, Chmelo RA, Wiener JR, Joklik WK. 1985. Sequences of the S1 genes of the three serotypes of reovirus. Proc. Natl. Acad. Sci. U. S. A. 82:24–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Boehme KW, Frierson JM, Konopka JL, Kobayashi T, Dermody TS. 2011. The reovirus σ1s protein is a determinant of hematogenous but not neural viral dissemination in mice. J. Virol. 85:11781–11790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Buchholz UJ, Finke S, Conzelmann KK. 1999. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. J. Virol. 73:251–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weiner HL, Powers ML, Fields BN. 1980. Absolute linkage of virulence and central nervous system cell tropism of reoviruses to viral hemagglutinin. J. Infect. Dis. 141:609–616 [DOI] [PubMed] [Google Scholar]
- 31. Smith RE, Zweerink HJ, Joklik WK. 1969. Polypeptide components of virions, top component and cores of reovirus type 3. Virology 39:791–810 [DOI] [PubMed] [Google Scholar]
- 32. Virgin H, Bassel-Duby R, Fields BN, Tyler KL. 1988. Antibody protects against lethal infection with the neurally spreading reovirus type 3 (Dearing). J. Virol. 62:4594–4604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Barton ES, Connolly JL, Forrest JC, Chappell JD, Dermody TS. 2001. Utilization of sialic acid as a coreceptor enhances reovirus attachment by multistep adhesion strengthening. J. Biol. Chem. 276:2200–2211 [DOI] [PubMed] [Google Scholar]
- 34. Boehme KW, Ikizler M, Kobayashi T, Dermody TS. 2011. Reverse genetics for mammalian reovirus. Methods 55:109–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kobayashi T, Antar AAR, Boehme KW, Danthi P, Eby EA, Guglielmi KM, Holm GH, Elizabeth M, Maginnis MS, Naik S, Skelton WB, Denise J, Wilson GJ, Chappell JD, Terence S. 2008. Plasmid-based reverse genetics for animal double-stranded RNA viruses: manipulation of the viral genome and development of a novel gene-transduction system. Cell 1:147–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kobayashi T, Ooms LS, Ikizler M, Chappell JD, Dermody TS. 2010. An improved reverse genetics system for mammalian orthoreoviruses. Virology 398:194–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. London L, Majeski EI, Altman-Hamamdzic S, Enockson C, Paintlia MK, Harley RA, London SD. 2002. Respiratory reovirus 1/L induction of diffuse alveolar damage: pulmonary fibrosis is not modulated by corticosteroids in acute respiratory distress syndrome in mice. Clin. Immunol. 103:284–295 [DOI] [PubMed] [Google Scholar]
- 38. Chandran K, Parker JSL, Ehrlich M, Kirchhausen T, Nibert ML. 2003. The δ region of outer-capsid protein μ1 undergoes conformational change and release from reovirus particles during cell entry. J. Virol. 77:13361–13375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Furlong DB, Nibert ML, Fields BN. 1988. Sigma 1 protein of mammalian reoviruses extends from the surfaces of viral particles. J. Virol. 62:246–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- 41. Coffey CM, Sheh A, Kim IS, Chandran K, Nibert ML, Parker JSL. 2006. Reovirus outer capsid protein micro1 induces apoptosis and associates with lipid droplets, endoplasmic reticulum, and mitochondria. J. Virol. 80:8422–8438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wisniewski ML, Werner BG, Hom LG, Anguish LJ, Coffey CM, Parker JSL. 2011. Reovirus infection or ectopic expression of outer capsid protein micro1 induces apoptosis independently of the cellular proapoptotic proteins Bax and Bak. J. Virol. 85:296–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yin P, Keirstead ND, Broering TJ, Arnold MM, Parker JSL, Nibert ML, Coombs KM. 2004. Comparisons of the M1 genome segments and encoded mu2 proteins of different reovirus isolates. Virol. J. 1:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tyler KL, McPhee DA, Fields BN. 1986. Distinct pathways of viral spread in the host determined by reovirus S1 gene segment. Science 233:770–774 [DOI] [PubMed] [Google Scholar]
- 45. Wilson GA, Morrison LA, Fields BN. 1994. Association of the reovirus S1 gene with serotype 3-induced biliary atresia in mice. J. Virol. 68:6458–6465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Morrison LA, Sidman RL, Fields BN. 1991. Direct spread of reovirus from the intestinal lumen to the central nervous system through vagal autonomic nerve fibers. Proc. Natl. Acad. Sci. U. S. A. 88:3852–3856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Garcia-Verdugo I, Descamps D, Chignard M, Touqui L, Sallenave J-M. 2010. Lung protease/anti-protease network and modulation of mucus production and surfactant activity. Biochimie 92:1608–1617 [DOI] [PubMed] [Google Scholar]
- 48. Tyler KL, Squier MK, Rodgers SE, Schneider BE, Oberhaus SM, Grdina TA, Cohen JJ, Dermody TS. 1995. Differences in the capacity of reovirus strains to induce apoptosis are determined by the viral attachment protein sigma 1. J. Virol. 69:6972–6979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rodgers SE, Connolly JL, Chappell JD, Dermody TS. 1998. Reovirus growth in cell culture does not require the full complement of viral proteins J. Virol. 72:8597–8604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gauvin L, Bennett S, Liu H, Hakimi M, Schlossmacher M, Majithia J, Brown EG. 2013. Respiratory infection of mice with mammalian reoviruses causes systemic infection with age and strain dependent pneumonia and encephalitis. Virol. J. 10:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Verdin EM, Lynn SP, Fields BN, Maratos-Flier E. 1988. Uptake of reovirus serotype 1 by the lungs from the bloodstream is mediated by the viral hemagglutinin. J. Virol. 62:545–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Campbell JA, Schelling P, Wetzel JD, Johnson EM, Forrest JC, Wilson GA, Aurrand-Lions M, Imhof BA, Stehle T, Dermody TS. 2005. Junctional adhesion molecule a serves as a receptor for prototype and field-isolate strains of mammalian reovirus. J. Virol. 79:7967–7978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chappell JD, Duong JL, Wright BW, Dermody TS. 2000. Identification of carbohydrate-binding domains in the attachment proteins of type 1 and type 3 reoviruses. J. Virol. 74:8472–8479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Guglielmi KM, Kirchner E, Holm GH, Stehle T, Dermody TS. 2007. Reovirus binding determinants in junctional adhesion molecule-A. J. Biol. Chem. 282:17930–17940 [DOI] [PubMed] [Google Scholar]
- 55. Helander A, Silvey KJ, Mantis NJ, Hutchings AB, Chandran K, Lucas WT, Nibert ML, Neutra MR. 2003. The viral sigma1 protein and glycoconjugates containing alpha2-3-linked sialic acid are involved in type 1 reovirus adherence to M cell apical surfaces. J. Virol. 77:7964–7977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kirchner E, Guglielmi KM, Strauss HM, Dermody TS, Stehle T. 2008. Structure of reovirus sigma1 in complex with its receptor junctional adhesion molecule-A. PLoS Pathog. 4:e1000235–e1000235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lee PW, Hayes EC, Joklik WK. 1981. Protein sigma 1 is the reovirus cell attachment protein. Virology 108:156–163 [DOI] [PubMed] [Google Scholar]
- 58. Nagata L, Masri S, Pon R, Lee P. 1987. Analysis of functional domains on reovirus cell attachment protein sigma 1 using cloned S1 gene deletion mutants. Virology 160:162–168 [DOI] [PubMed] [Google Scholar]
- 59. Nason EL, Wetzel JD, Mukherjee SK, Barton ES, Prasad BV, Dermody TS. 2001. A monoclonal antibody specific for reovirus outer-capsid protein sigma3 inhibits sigma1-mediated hemagglutination by steric hindrance. J. Virol. 75:6625–6634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nibert ML, Dermody TS, Fields BN. 1990. Structure of the reovirus cell-attachment protein: a model for the domain organization of sigma 1. J. Virol. 64:2976–2989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Vedula SRK, Lim TS, Kirchner E, Guglielmi KM, Dermody TS, Stehle T, Hunziker W, Lim CT. 2008. A comparative molecular force spectroscopy study of homophilic JAM-A interactions and JAM-A interactions with reovirus attachment protein sigma1. J. Mol. Recog. 21:210–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhang B, Lim TS, Vedula SRK, Li A, Lim CT, Tan VBC. 2010. Investigation of the binding preference of reovirus sigma1 for junctional adhesion molecule A by classical and steered molecular dynamics. Biochemistry 49:1776–1786 [DOI] [PubMed] [Google Scholar]
- 63. Leone G, Mah DC, Lee PW. 1991. The incorporation of reovirus cell attachment protein sigma 1 into virions requires the N-terminal hydrophobic tail and the adjacent heptad repeat region. Virology 182:346–350 [DOI] [PubMed] [Google Scholar]
- 64. Mah DCW, Leone G, Jankowski JM, Lee PW. 1990. The amino-terminal quarter of reovirus cell attachment protein sigma-1 possesses intrinsic virion-anchoring function. Virology 179:95–103 [DOI] [PubMed] [Google Scholar]
- 65. Coombs KM, Fields BN, Harrison SC. 1990. Crystallization of the reovirus type 3 Dearing core. Crystal packing is determined by the lambda 2 protein. J. Mol. Biol. 215:1–5 [DOI] [PubMed] [Google Scholar]
- 66. Dryden KA, Wang G, Yeager M, Nibert ML, Coombs KM, Furlong DB, Fields BN, Baker TS. 1993. Early steps in reovirus infection are associated with dramatic changes in supramolecular structure and protein conformation: analysis of virions and subviral particles by cryoelectron microscopy and image reconstruction. J. Cell Biol. 122:1023–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Weiner HL, Drayna DT, Averill DR, Fields BN. 1977. Molecular basis of reovirus virulence. Role of the S1 gene. Proc. Natl. Acad. Sci. U. S. A. 74:5744–5748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Adair RA, Roulstone V, Scott KJ, Morgan R, Nuovo GJ, Fuller M, Beirne D, West EJ, Jennings VA, Rose A, Kyula J, Fraser S, Dave R, Anthoney DA, Merrick A, Prestwich R, Aldouri A, Donnelly O, Pandha H, Coffey M, Selby P, Vile R, Toogood G, Harrington K, Melcher AA. 2012. Cell carriage, delivery, and selective replication of an oncolytic virus in tumor in patients. Sci. Transl. Med. 4:138ra77. 10.1126/scitranslmed.3003578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Marcato P, Shmulevitz M, Pan D, Stoltz D, Lee PW. 2007. Ras transformation mediates reovirus oncolysis by enhancing virus uncoating, particle infectivity, and apoptosis-dependent release. Mol. Ther. 15:1522–1530 [DOI] [PubMed] [Google Scholar]
- 70. Hoyt CC, Richardson-Burns SM, Goody RJ. 2005. Nonstructural protein sigma1s is a determinant of reovirus virulence and influences the kinetics and severity of apoptosis induction in the heart and central nervous system. J. Virol. 79:2743–2753 [DOI] [PMC free article] [PubMed] [Google Scholar]