Abstract
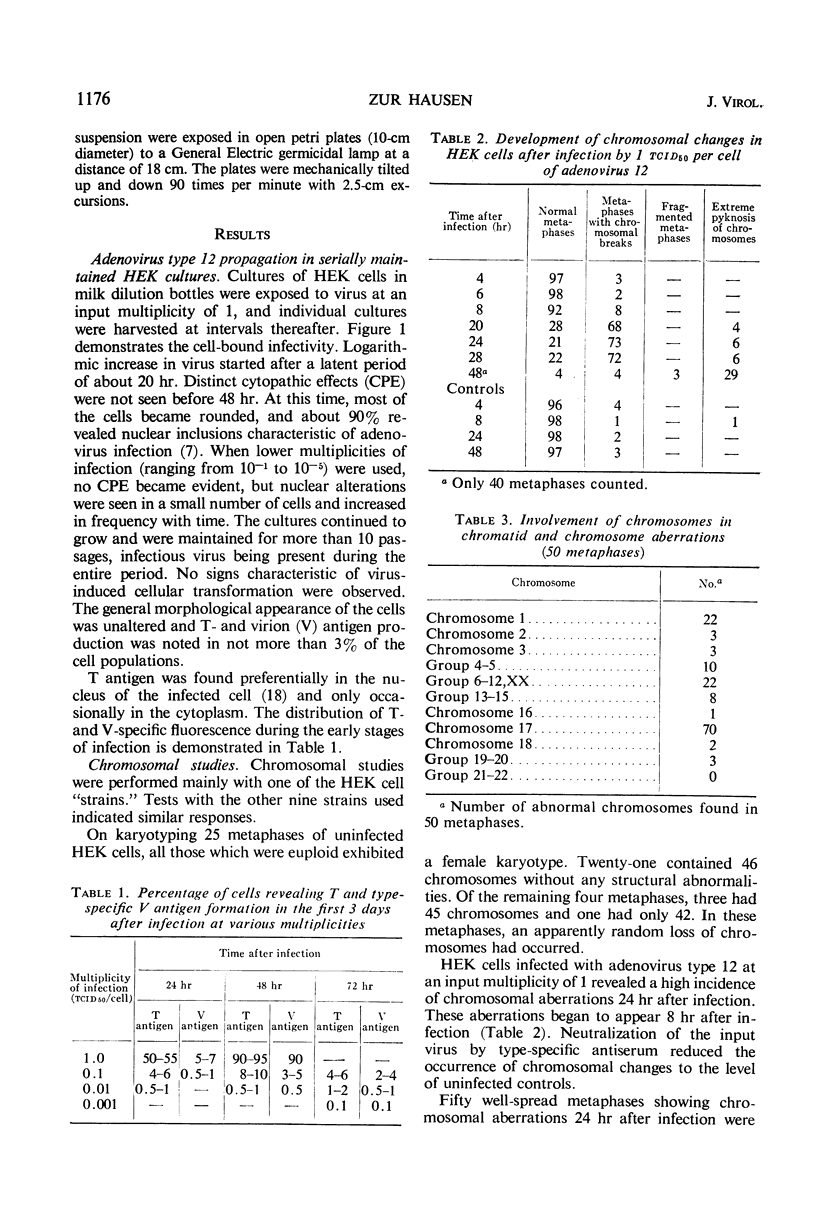
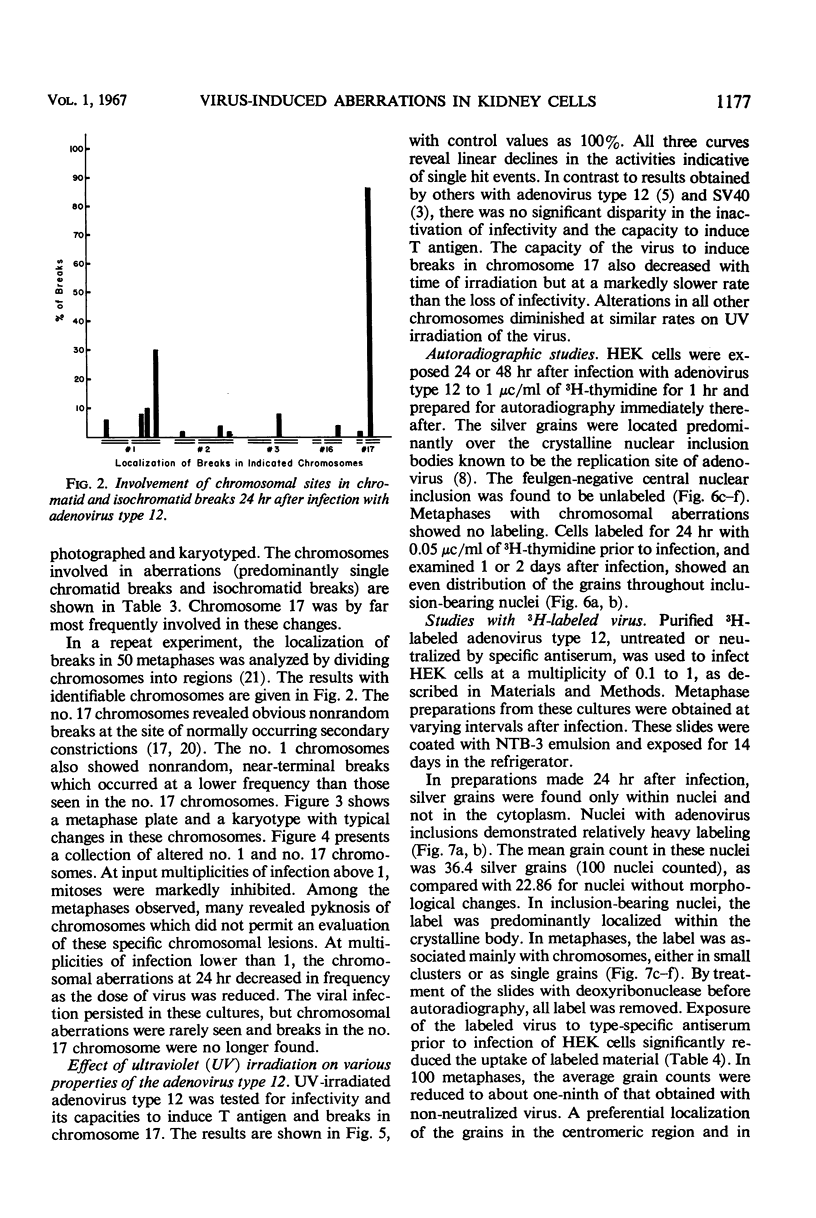
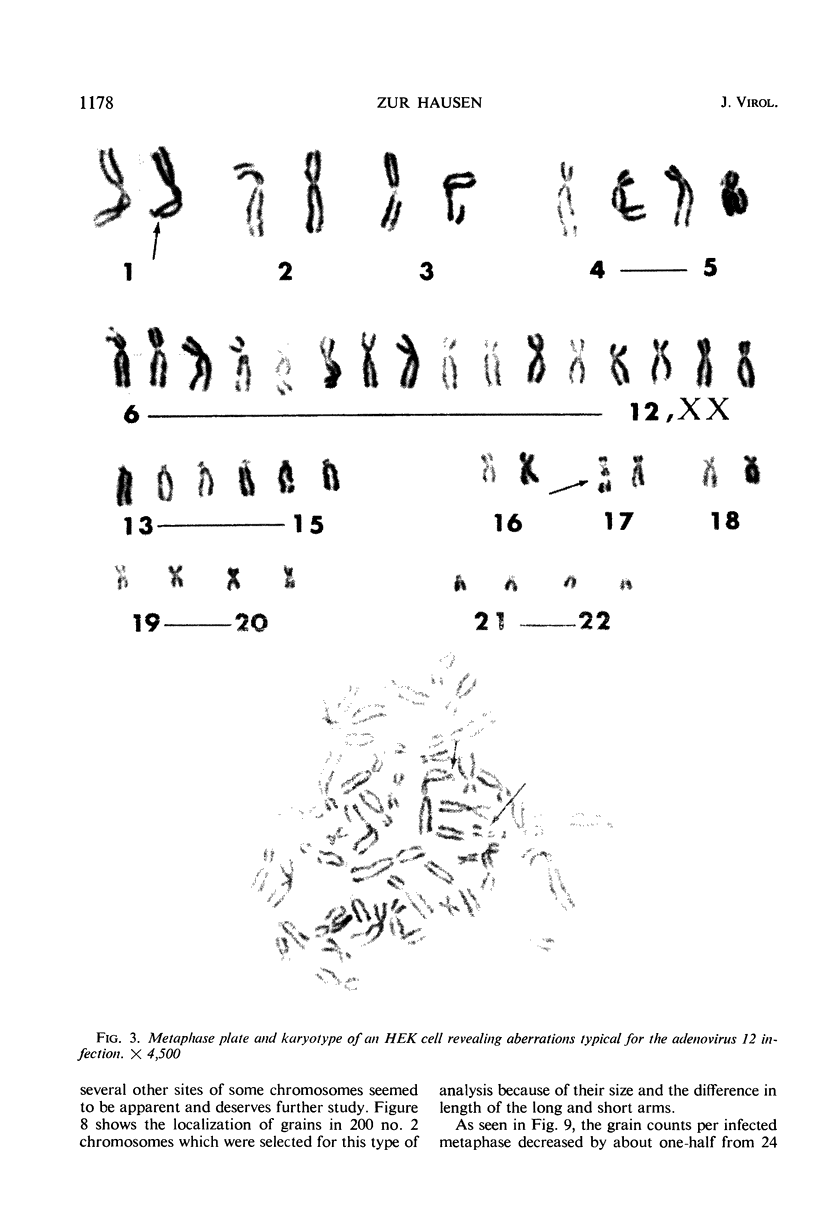
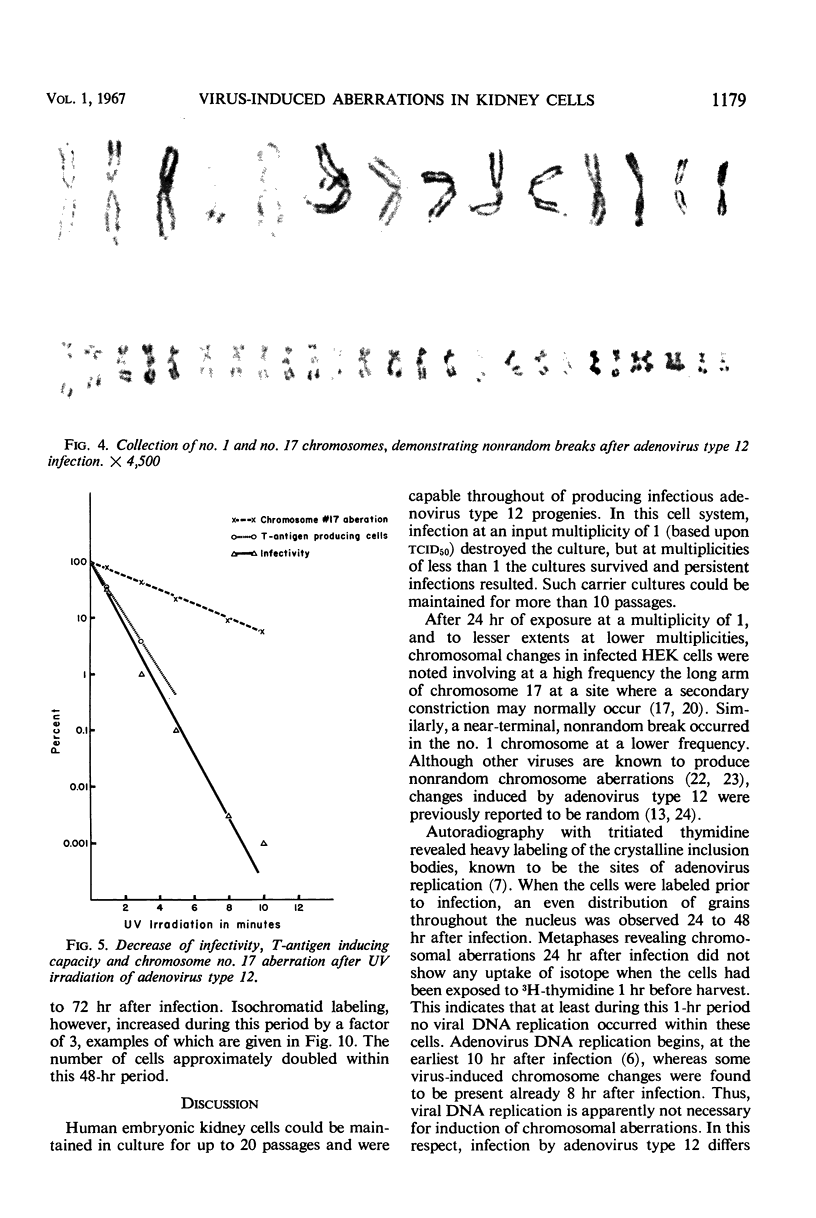
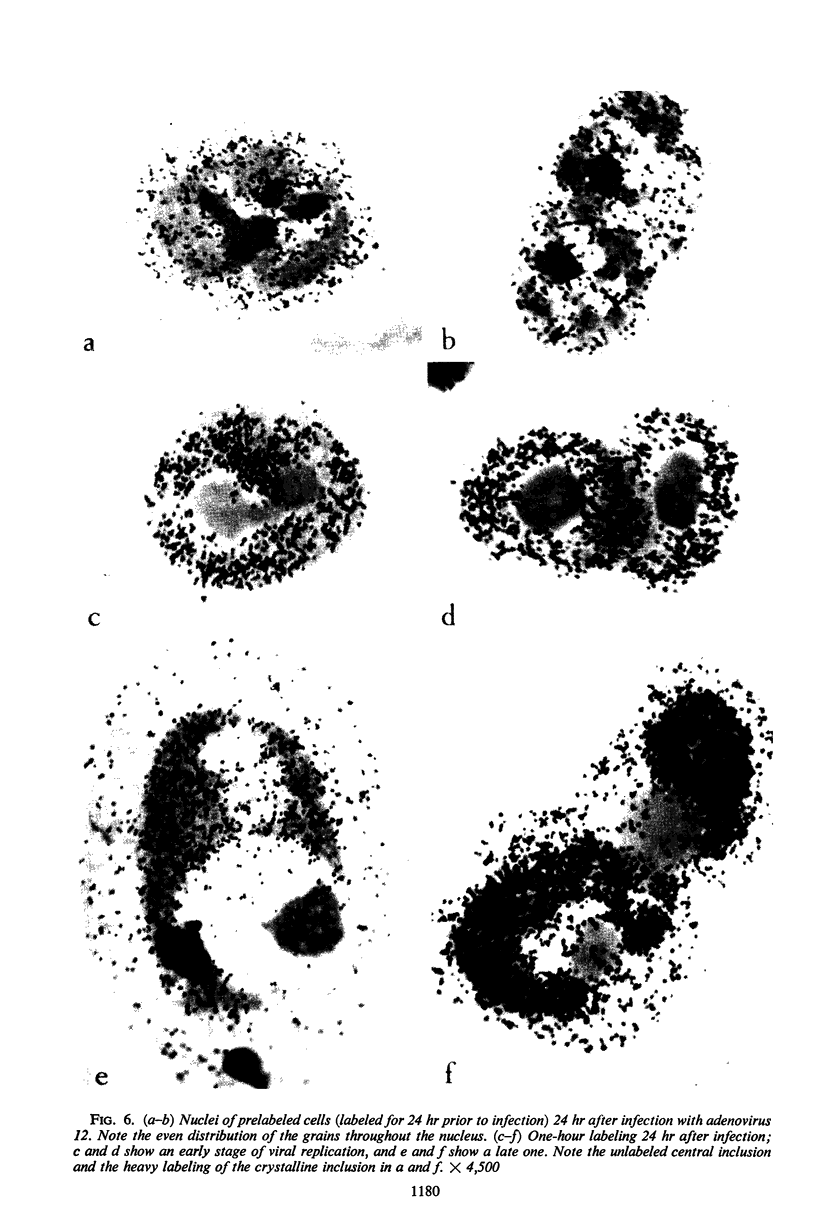
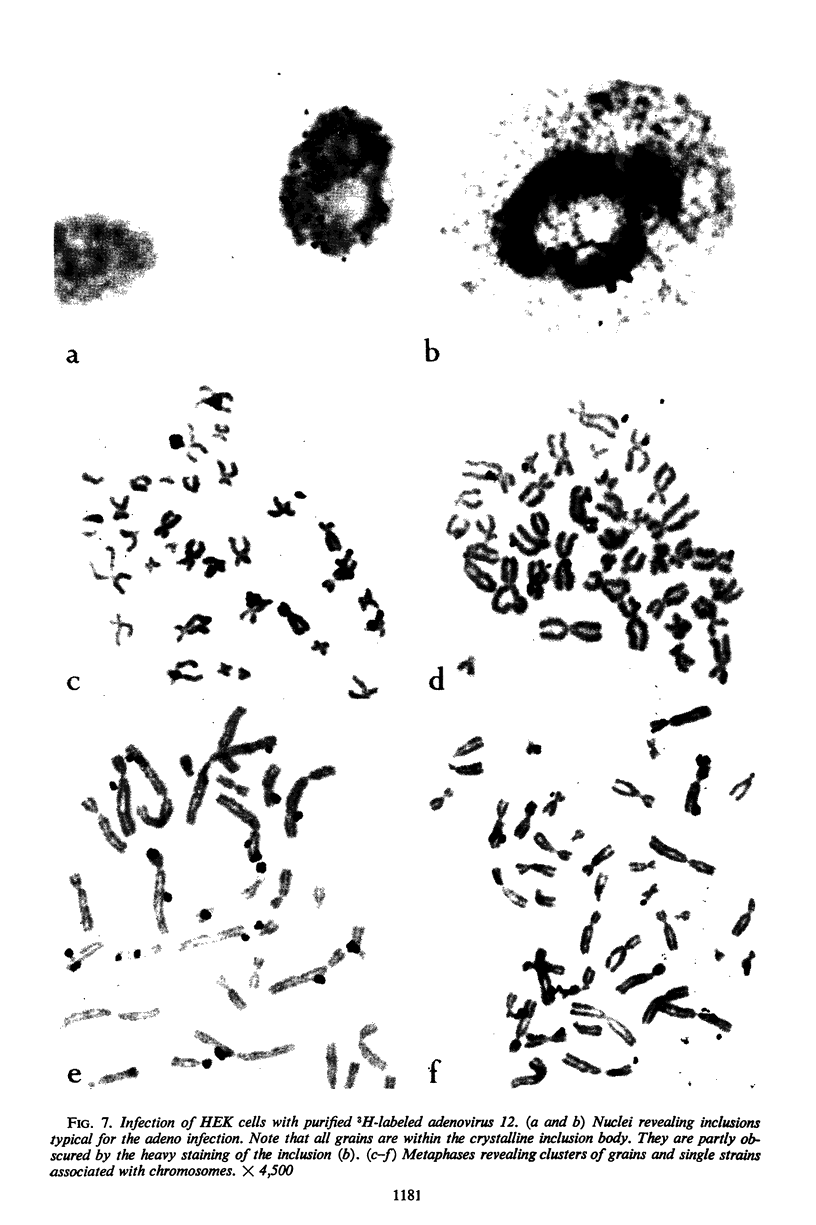
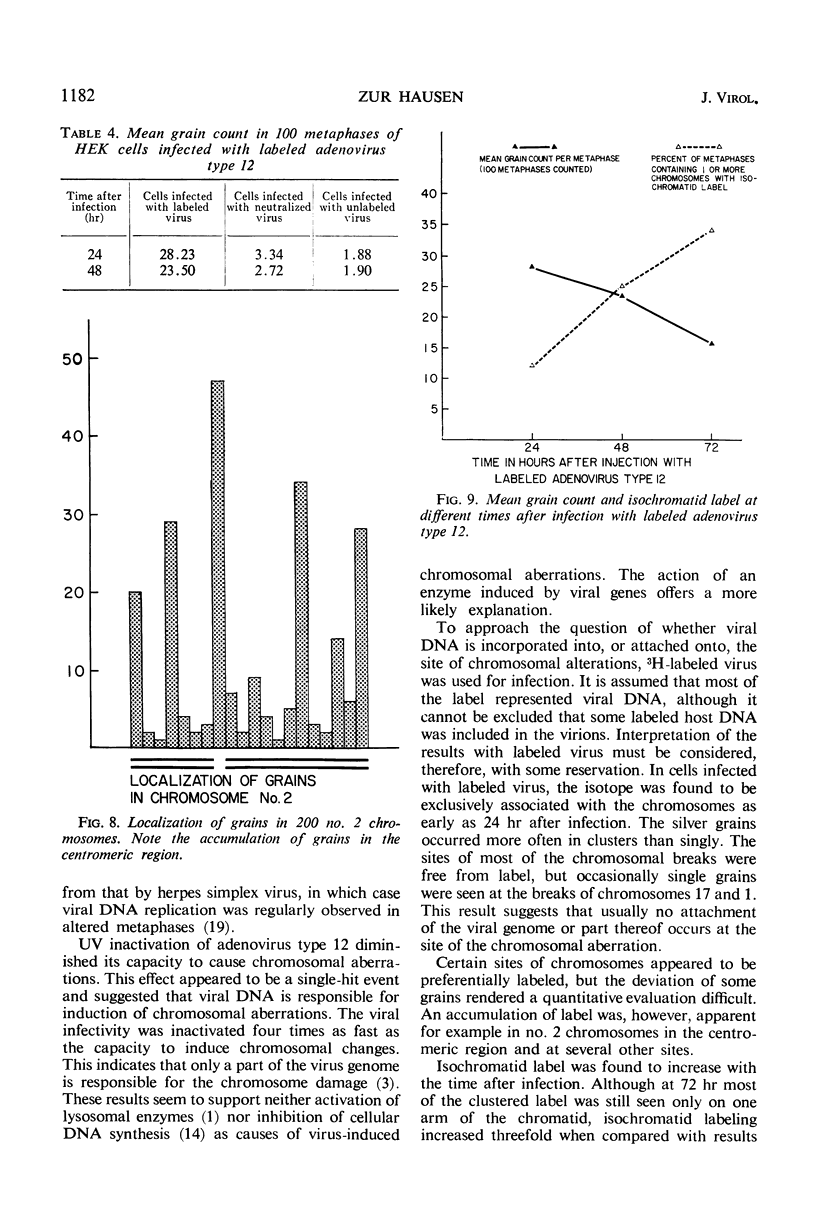
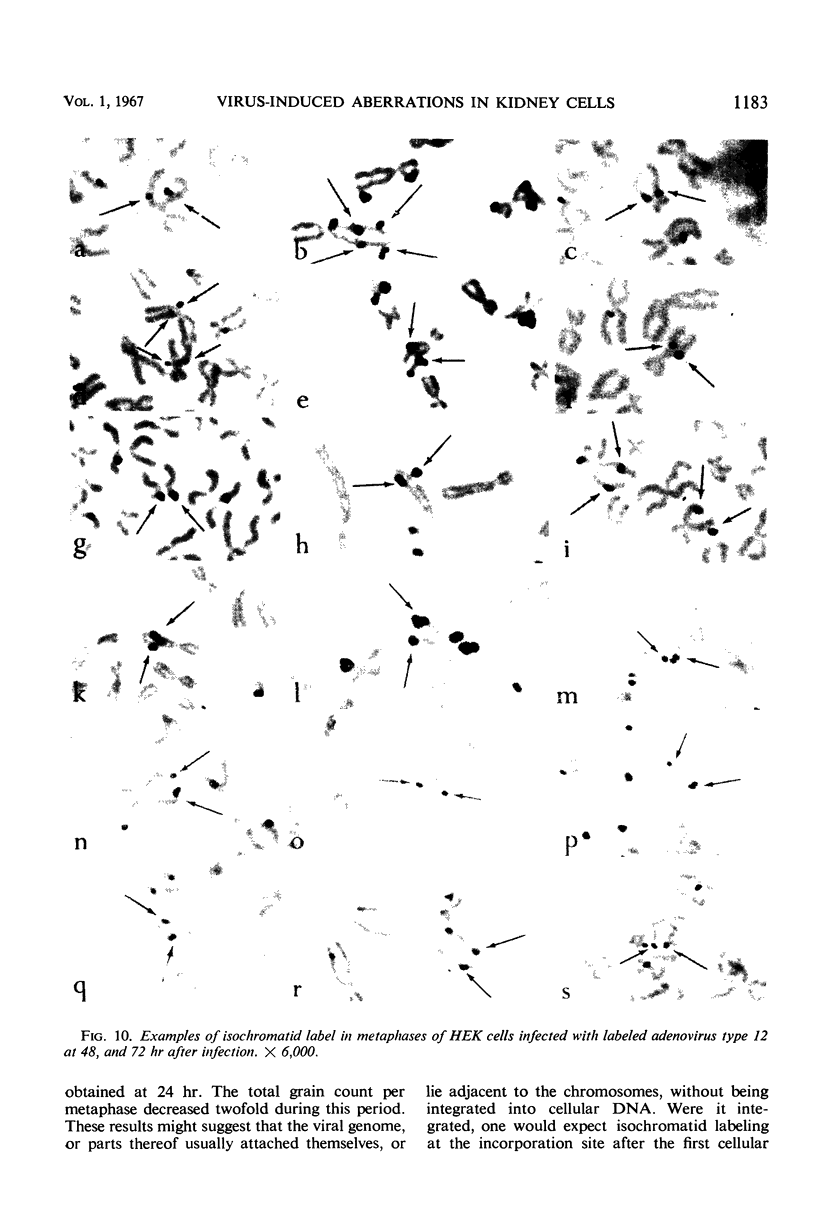
Nonrandom chromosomal breaks in chromosomes 1 and 17 were provoked in human embryonic kidney cells 24 hr after infection with adenovirus type 12. These chromosomal changes disappeared in persistently infected cultures. Neutralization of the virus with type-specific antiviral serum prior to infection prevented the occurrence of chromosomal aberrations. No viral deoxyribonucleic acid (DNA) synthesis, as determined by autoradiography, was seen in metaphases containing adenovirus type 12-induced chromosomal aberrations. Ultraviolet irradiation of the virus reduced chromosomal aberrations linearly. This reduction in aberrations was fourfold slower than the inactivation of viral infectivity. At 24 hr after infection of cells with purified 3H-labeled adenovirus type 12, the isotope was found to be associated with the nuclei. The uptake of isotope was reduced ninefold when the labeled virus was neutralized with type-specific antiviral serum. This difference is considered to account for neutralization of labeled virions. In metaphases infected with labeled viruses, most of the clustered grains were seen only on one arm of the chromatid, even after 72 hr. Isochromatid labeling was found, however, in a small percentage of chromosomes, and increased with time after infection. This increase was threefold between 24 and 72 hr after infection, whereas the mean grain counts decreased twofold during the same period. This has been tentatively interpreted to mean that most of the viral DNA molecules or parts thereof are merely attached to cellular chromatin, but a small fraction of them becomes gradually integrated as time proceeds. Certain chromosomal sites appeared to be preferentially labeled when chromosome 2 was used as a model for evaluation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Paton G. R. Chromosome damage in human diploid cells following activation of lysosomal enzymes. Nature. 1965 Sep 11;207(5002):1170–1173. doi: 10.1038/2071170a0. [DOI] [PubMed] [Google Scholar]
- BLACK P. H., ROWE W. P., TURNER H. C., HUEBNER R. J. A SPECIFIC COMPLEMENT-FIXING ANTIGEN PRESENT IN SV40 TUMOR AND TRANSFORMED CELLS. Proc Natl Acad Sci U S A. 1963 Dec;50:1148–1156. doi: 10.1073/pnas.50.6.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp R. I., Gilden R. V. The inactivation of simian virus 40 infectivity and antigen-inducing capacity by ultraviolet light. Virology. 1965 Dec;27(4):639–641. doi: 10.1016/0042-6822(65)90194-7. [DOI] [PubMed] [Google Scholar]
- GINSBERG H. S., DIXON M. K. Nucleuc acid synthesis in types 4 and 5 adenovirus-infected HeLa cells. J Exp Med. 1961 Feb 1;113:283–299. doi: 10.1084/jem.113.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GODMAN G. C., MORGAN C., BREITENFELD P. M., ROSE H. M. A correlative study by electron and light microscopy of the development of type 5 adenovirus. II. Light microscopy. J Exp Med. 1960 Aug 1;112:383–402. doi: 10.1084/jem.112.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilead Z., Ginsberg H. S. Characterization of a Tumorlike Antigen in Type 12 and Type 18 Adenovirus-Infected Cells. J Bacteriol. 1965 Jul;90(1):120–125. doi: 10.1128/jb.90.1.120-125.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilead Z., Ginsberg H. S. Comparison of the rates of ultraviolet inactivation of the capacity of type 12 adenovirus to infect cells and to induce T antigen formation. J Bacteriol. 1966 Dec;92(6):1853–1854. doi: 10.1128/jb.92.6.1853-1854.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMPAR B., ELLISON S. A. Cellular alterations in the MCH line of Chinese hamster cells following infection with herpes simplex virus. Proc Natl Acad Sci U S A. 1963 Apr;49:474–480. doi: 10.1073/pnas.49.4.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARNDEN D. G. CYTOGENETIC STUDIES ON PATIENTS WITH VIRUS INFECTIONS AND SUBJECTS VACCINATED AGAINST YELLOW FEVER. Am J Hum Genet. 1964 Jun;16:204–213. [PMC free article] [PubMed] [Google Scholar]
- HUEBNER R. J., PEREIRA H. G., ALLISON A. C., HOLLINSHEAD A. C., TURNER H. C. PRODUCTION OF TYPE-SPECIFIC C ANTIGEN IN VIRUS-FREE HAMSTER TUMOR CELLS INDUCED BY ADENOVIRUS TYPE 12. Proc Natl Acad Sci U S A. 1964 Mar;51:432–439. doi: 10.1073/pnas.51.3.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle G., Henle W. Immunofluorescence in cells derived from Burkitt's lymphoma. J Bacteriol. 1966 Mar;91(3):1248–1256. doi: 10.1128/jb.91.3.1248-1256.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon E., Kalnins V. I., Stich H. F., Yohn D. S. Viruses and mammalian chromosomes. VI. Comparative karyologic and immunofluorescent studies on Syrian hamster and human amnion cells infected with human adenovirus type 12. Cancer Res. 1966 Apr;26(4):612–618. [PubMed] [Google Scholar]
- NICHOLS W. W., LEVAN A., CORIELL L. L., GOLDNER H., AHLSTROM C. G. CHROMOSOME ABNORMALITIES IN VITRO IN HUMAN LEUKOCYTES ASSOCIATED WITH SCHMIDT-RUPPIN ROUS SARCOMA VIRUS. Science. 1964 Oct 9;146(3641):248–250. doi: 10.1126/science.146.3641.248. [DOI] [PubMed] [Google Scholar]
- POPE J. H., ROWE W. P. IMMUNOFLUORESCENT STUDIES OF ADENOVIRUS 12 TUMORS AND OF CELLS TRANSFORMED OR INFECTED BY ADENOVIRUSES. J Exp Med. 1964 Oct 1;120:577–588. doi: 10.1084/jem.120.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer C. G., Funderburk S. Secondary constrictions in human chromosomes. Cytogenetics. 1965;4(4):261–276. doi: 10.1159/000129863. [DOI] [PubMed] [Google Scholar]
- RAPP F., HSU T. C. VIRUSES AND MAMMALIAN CHROMOSOMES. IV. REPLICATION OF HERPES SIMPLEX VIRUS IN DIPLOID CHINESE HAMSTER CELLS. Virology. 1965 Mar;25:401–411. doi: 10.1016/0042-6822(65)90061-9. [DOI] [PubMed] [Google Scholar]
- SAKSELA E., MOORHEAD P. S. Enhancement of secondary constrictions and the heterochromatic X in human cells. Cytogenetics. 1962;1:225–244. doi: 10.1159/000129733. [DOI] [PubMed] [Google Scholar]
- SOMERS C. E., HSU T. C. Chromosome damage induced by hydroxylamine in mammalian cells. Proc Natl Acad Sci U S A. 1962 Jun 15;48:937–943. doi: 10.1073/pnas.48.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STICH H. F., HSU T. C., RAPP F. VIRUSES AND MAMMALIAN CHROMOSOMES. I. LOCALIZATION OF CHROMOSOME ABERRATIONS AFTER INFECTION WITH HERPES SIMPLEX VIRUS. Virology. 1964 Apr;22:439–445. doi: 10.1016/0042-6822(64)90064-9. [DOI] [PubMed] [Google Scholar]
- STICH H. F., VANHOOSIER G. L., TRENTIN J. J. VIRUSES AND MAMMALIAN CHROMOSOMES; CHROMOSOME ABERRATIONS BY HUMAN ADENOVIRUS TYPE 12. Exp Cell Res. 1964 Apr;34:400–403. doi: 10.1016/0014-4827(64)90375-1. [DOI] [PubMed] [Google Scholar]
- WOLMAN S. R., HIRSCHHORN K., TODARO G. J. EARLY CHROMOSOMAL CHANGES IN SV40-INFECTED HUMAN FIBROBLAST CULTURES. Cytogenetics. 1964;3:45–61. doi: 10.1159/000129797. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. Chromosomal changes of similar nature in seven established cell lines derived from the peripheral blood of patients with leukemia. J Natl Cancer Inst. 1967 May;38(5):683–696. [PubMed] [Google Scholar]
- zur Hausen H., Henle W., Hummeler K., Diehl V., Henle G. Comparative study of cultured Burkitt tumor cells by immunofluorescence, autoradiography, and electron microscopy. J Virol. 1967 Aug;1(4):830–837. doi: 10.1128/jvi.1.4.830-837.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H., Lanz E. Chromosomale Aberrationen bei L-Zellen nach Vaccinia-Virus-Infektion. Z Med Mikrobiol Immunol. 1966;152(1):60–65. [PubMed] [Google Scholar]