Abstract
B cells secreting IgG antibodies, but not IgM, are thought to be solely responsible for vaccine-induced protection against rabies virus (RABV) infections in postexposure settings. In this report, we reinvestigated the potential for IgM to mediate protection in a mouse model of RABV vaccination. Immunocompetent mice immunized with an experimental live replication-deficient RABV-based vaccine produced virus neutralizing antibodies (VNAs) within 3 days of vaccination. However, mice unable to produce soluble IgM (sIgM−/−) did not produce VNAs until 7 days postimmunization. Furthermore, sIgM−/− mice were not protected against RABV infection when challenged 3 days postimmunization, while all wild-type mice survived challenge. Consistent with the lack of protection against pathogenic RABV challenge, approximately 50- to 100-fold higher viral loads of challenge virus were detected in the muscle, spinal cord, and brain of immunized sIgM−/− mice compared to control mice. In addition, IgG antibody titers in vaccinated wild-type and sIgM−/− mice were similar at all time points postimmunization, suggesting that protection against RABV challenge is due to the direct effects of IgM and not the influence of IgM on the development of effective IgG antibody titers. In all, early vaccine-induced IgM can limit dissemination of pathogenic RABV to the central nervous system and mediate protection against pathogenic RABV challenge. Considering the importance for the rapid induction of VNAs to protect against RABV infections in postexposure prophylaxis settings, these findings may help guide the development of a single-dose human rabies vaccine.
INTRODUCTION
A hallmark of rabies virus (RABV) infection that makes postexposure prophylaxis (PEP) feasible is the relatively long period of time between exposure at peripheral sites to infection of the central nervous system (CNS). The rapid induction of vaccine-induced VNAs directed against the single transmembrane viral glycoprotein (G) is essential for successful RABV PEP. Historically, RABV-specific VNAs were thought to be produced solely by T cell-dependent B cell responses (1–5). However, using two mouse models of CD4+ T cell deficiency, including mice genetically devoid of T cells (i.e., B6.129P2-Tcrβtm1MomTcrδtm1Mom/J), we recently showed that protection can be afforded via T cell-independent antibody responses in mice immunized with a replication-deficient RABV-based vaccine vector in which the RABV matrix (M) gene is deleted (rRABV-ΔM) (6). In addition, a kinetic analysis of antibody subtype and subclass revealed the potential for early protective IgM antibodies in rRABV-ΔM-immunized mice, although the role for IgM in protection was not directly studied (6). Nonetheless, the role for neutralizing IgM in the protection against RABV infection is thought to be limited in part by previous findings that natural IgM or IgM induced in response to inactivated RABV-based vaccination is not able to protect mice against pathogenic RABV challenge (7). In addition, pathogenic RABV strains are highly neurotropic, and there is no evidence to suggest pathogenic RABV strains are viremic (8). Due to its large pentameric configuration, IgM does not easily traverse vascular endothelial layers into interstitial tissues; therefore, the role for IgM in the clearance of a highly neurotropic virus generally is believed to be limited (9). Finally, the rapid transition in B cells secreting IgM to B cells secreting IgG suggests IgM does not play a significant role in vaccine-induced immunity to RABV in preexposure settings when long-term B cell responses are required for protection. However, in cases of PEP in which rapid short-term B cell responses are required for protection, IgM antibodies might become important.
Natural IgM is present in the blood at high concentrations, while immune IgM is the first antibody isotype induced upon infection or immunization (reviewed in reference 10), suggesting that vaccine-induced IgM may help to improve the efficacy of RABV vaccination in the context of PEP. IgM plays critical roles in limiting virus dissemination for some neurotropic and nonneurotropic viruses, including West Nile virus (WNV) (11), influenza virus (12, 13), and vesicular stomatitis virus (14; reviewed in reference 15). Due to our previous data that show rRABV-ΔM induces early and potent T cell-independent antibody responses in mice (6), we reinvestigated the role of neutralizing IgM in the context of immunization with rRABV-ΔM. In this report, we show that vaccine-induced IgM is able to contribute to the early controlling of pathogenic RABV strains from disseminating to the CNS and causing disease. Understanding these early events in RABV-specific B cell immunity and antibody attributes may help to develop a single-dose vaccine where the rapid induction of VNA is critical (16).
MATERIALS AND METHODS
Viral vaccines.
rRABV-ΔM is a replication-deficient RABV-based vaccine vector in which the RABV M gene is deleted, and it was constructed as described previously (17). rRABV-ΔM was derived from a molecular clone of the SAD-B19 vaccine strain of RABV (18, 19). rRABV-ΔM was propagated on baby hamster kidney cells stably expressing RABV M (BSR-RVM) (17, 20) and purified over a 20% sucrose cushion. The challenge virus used was the pathogenic Challenge Virus Strain-N2c (CVS-N2c), which is a mouse-adapted subclone of CVS-21 RABV (21). CVS-N2c was initially propagated in neonatal mouse brains and then passaged once in vitro on a neuroblastoma cell line (NA cells). The titer of CVS-N2c required to kill unvaccinated mice within 8 days postinfection, which is typical for CVS-N2c (17, 22, 23), was determined experimentally to be 105 focus-forming units (FFU)/mouse.
Immunization and early pathogenic challenge in sIgM−/− and wild-type mice.
Groups of 5 female sIgM−/− (24, 25) (a kind gift from Jianzhu Chen, Massachusetts Institute of Technology, and Kishore R. Alugupalli, Thomas Jefferson University) or 129S6/SvEvTac control mice (Taconic), aged 6 to 9 weeks, were immunized intramuscularly (i.m.) with a single dose of 106 FFU/mouse of rRABV-ΔM or an equal volume of phosphate-buffered saline (PBS). Immunized mice were challenged i.m. with 105 FFU/mouse of CVS-N2c at 3 days postimmunization (the time period during which we previously showed RABV-specific antibody responses are first detected [6]) to evaluate the influence of early vaccine-induced antibody responses (6). Mice were observed daily for approximately 2 weeks, and then weekly for the duration of the study (i.e., day 27 postchallenge), for clinical signs of rabies and were euthanized at the onset of neurological symptoms. Weights were recorded as a measure of overall health. Two independent experiments were completed (total n = 10 mice/group). Kaplan-Meier survival curves were analyzed by the log-rank test. Three (P < 0.001), two (P = 0.001 to 0.01), and one (P = 0.01 to 0.05) asterisk indicates a significant difference between two data points (6, 17, 22, 23). Symbols for control (PBS-immunized) sIgM−/− mice were shifted slightly so they could be visualized. All animal experiments were approved by the Institutional Animal Care and Use Committee of Thomas Jefferson University.
Quantitative reverse transcription-PCR to measure challenge virus spread to the CNS.
Groups of sIgM−/− or wild-type control mice were immunized with rRABV-ΔM and then challenged 3 days later with CVS-N2c as described above. Five days postchallenge, muscle (site of inoculation), spinal cord, and brain were harvested, and tissue samples were immersed into RNAlater RNA stabilization reagent (Qiagen) and stored at 4°C before processing. Total RNA was isolated with the Qiagen RNeasy kit according to the manufacturer's instructions (Qiagen). First-strand cDNA was synthesized by using Omniscript reverse transcriptase according to the manufacturer's protocol (Qiagen). Each 20-μl reaction mixture contained up to 2 μg of total RNA, 10 U RNaseOut RNase inhibitor (Invitrogen), and 0.5 μM random nanomer primer. Quantitative analysis was performed in triplicate on a StepOnePlus real-time PCR system for 45 cycles of two-step PCR amplification (15 s at 95°C and 1 min at 60°C). Each 20-μl reaction mixture contained 1× TaqMan Universal PCR master mix (Applied Biosystems), 500 nM forward primer, 500 nM reverse primer, 100 nM TaqMan probe, and 4 μl cDNA. The forward primer, reverse primer, and probe sequences for CVS-N2C N protein were 5′-CACTTCCGTTCACTAGGCTTGA-3′, 5′-GACCCATGTAGCATCCAACAA-3′, and 5′-6-carboxyfluorescein (FAM)-TGAACACATGACCGACAGCATTCGA-6-carboxytetramethylrhodamine (TAMRA)-3′, respectively (26). A relative standard curve representing eight 10-fold dilutions of a known copy number of DNA was used for analysis of unknown samples as previously described (27), and the copy units were normalized to 2 μg/μl total RNA. Two independent experiments were completed (total n = 8 mice/group). One-way analysis of variance (ANOVA) was used to evaluate statistical significance. Where significant differences were observed, Tukey's multiple comparison test was used to identify significant differences between individual groups (*, P < 0.05).
Evaluation of antibody responses by ELISA and RFFIT.
Groups of sIgM−/− or wild-type mice, aged 6 to 8 weeks, were immunized i.m. with a single dose of 106 FFU/mouse of rRABV-ΔM. On days 3, 5, 7, and 10 postimmunization, blood was collected and sera isolated for analysis. RABV glycoprotein (G)-specific IgG and IgM antibodies were determined by enzyme-linked immunosorbent assay (ELISA) as described previously (6, 17, 22, 23). Data represent two independent experiments (total n = 6 mice/group). For multigroup comparisons, one-way analysis of variance (ANOVA) was used. Where significant differences were observed, Tukey's multiple comparison test was used to identify significant differences between individual groups. VNA titers were determined using the rapid fluorescent focus inhibition test (RFFIT) as described previously (6, 17, 22, 23). Data for the VNA titers represent two independent experiments using the sera also used for RABV G-specific IgG and IgM antibody titers from 3 mice/experiment pooled, and then they were analyzed in duplicate. To compare two groups of data for VNA titers, we used an unpaired, two-tailed t test. Three (P < 0.001), two (P = 0.001 to 0.01), and one (P = 0.01 to 0.05) asterisk indicates a significant difference between two data points (6, 17, 22, 23).
RESULTS
sIgM−/− mice immunized with rRABV-ΔM are not protected against pathogenic RABV challenge.
The effectiveness of RABV PEP relies in part on the speed by which VNAs are induced. We previously showed that rRABV-ΔM induces the production of T cell-independent VNAs within 3 days of vaccination, and that these VNAs could be either IgM or IgG (6). To definitively test for vaccine-induced IgM to aid in protection against pathogenic RABV challenge, we used our previously described mouse model of rabies protection, in which mice are challenged with pathogenic RABV within the first few days postimmunization (the time period during which we previously showed RABV-specific vaccine-induced antibody responses are first detected [6]) as a means to evaluate the potency of early vaccine-induced antibody responses (6). sIgM−/− mice, which are unable to produce secreted IgM but are able to express membrane IgM and all IgG subclasses (24, 25), or wild-type mice, were immunized with 106 FFU/mouse of rRABV-ΔM or an equivalent volume of PBS. Three days later, when initial RABV-specific T cell-independent and early T cell-dependent extrafollicular B cells form (6), mice were challenged with 105 FFU/mouse CVS-N2c, which is a mouse-adapted pathogenic RABV strains that kills nonvaccinated mice by 8 days postinfection. As expected and shown in Fig. 1A (survivorship) and B (weights), all sIgM−/− and wild-type mice mock vaccinated with PBS alone were not protected against challenge with CVS-N2c and succumbed to infection 7 days postchallenge. All wild-type mice immunized with rRABV-ΔM were protected against RABV challenge; however, a significantly reduced level of protection (20% survival) was observed in sIgM−/− mice immunized with rRABV-ΔM compared to protection in similarly immunized and challenged wild-type mice. The reduced protection observed in sIgM−/− mice compared to wild-type mice indicates an important role for IgM in the protection against RABV infection early postvaccination as B cell responses are forming. Furthermore, the finding that nonimmunized control mice succumbed to challenge infection indicates that natural IgM is not sufficient to protect against the dose of pathogenic RABV challenge used in these experiments.
Fig 1.
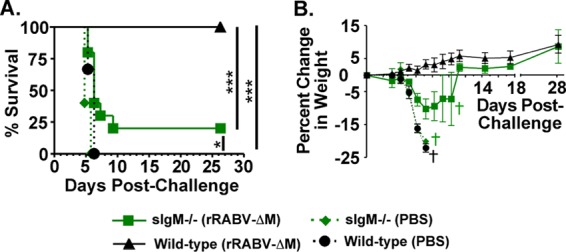
Vaccine-induced IgM helps to protect mice against pathogenic RABV challenge. sIgM−/− or wild-type control mice were immunized i.m. with 106 FFU of rRABV-ΔM or PBS alone and then challenged 3 days later i.m. with 105 FFU of pathogenic RABV CVS-N2c. Survivorship (A) and weights (B) were measured and recorded for 27 days postchallenge. Data shown are a combination of 2 independent experiments consisting of 5 mice per experiment (total n = 10 mice/group). Kaplan-Meier survival curves were analyzed by the log-rank test (***, P < 0.001; **, P = 0.001 to 0.01; *, P = 0.01 to 0.05). Symbols for control (PBS-immunized) sIgM−/− mice were shifted slightly in panel A for clarity. Crosses in panel B indicate the days when the last mice in a specific group were to be euthanized.
Vaccine-induced IgM prevents dissemination of CVS-N2c into the CNS.
The reduced survival afforded vaccinated sIgM−/− mice compared to wild-type mice described in Fig. 1 suggests that vaccine-induced IgM aids in preventing the spread of CVS-N2c into the CNS and causing disease. To investigate the ability of vaccine-induced IgM to limit pathogenic spread into the CNS, sIgM−/− and wild-type mice were immunized i.m. with 106 FFU/mouse of rRABV-ΔM and then challenged 3 days later with 105 FFU/mouse of CVS-N2c, as described above (6). CVS-N2c-specific mRNA (nucleoprotein [N]) was measured from total RNA isolated from the muscle, spinal cord, and brain 5 days postchallenge, which is approximately 2 to 3 days before mice would be expected to show symptoms due to CVS-N2c infection (6, 17, 22, 23). While not significantly different, an approximately 100-fold increase in CVS-N2c-specific mRNA was detected in the muscle (Fig. 2A) of immunized and challenged sIgM−/− mice compared to similarly treated wild-type mice. However, an approximately 50- to 100-fold increase in CVS-N2c-specific mRNA was detected in the spinal cord (Fig. 2B) and brain (Fig. 2C) of immunized and challenged sIgM−/− mice compared to similarly treated wild-type mice, which was significant (P < 0.05). Of note, no CVS-N2c mRNA was detected in the blood or spleen of challenged mice (data not shown), confirming previous suggestions that pathogenic RABV strains are not viremic (8). Together, these data indicate that vaccine-induced IgM is able to prevent dissemination of CVS-N2c from the periphery to the CNS in mice immunized with rRABV-ΔM.
Fig 2.
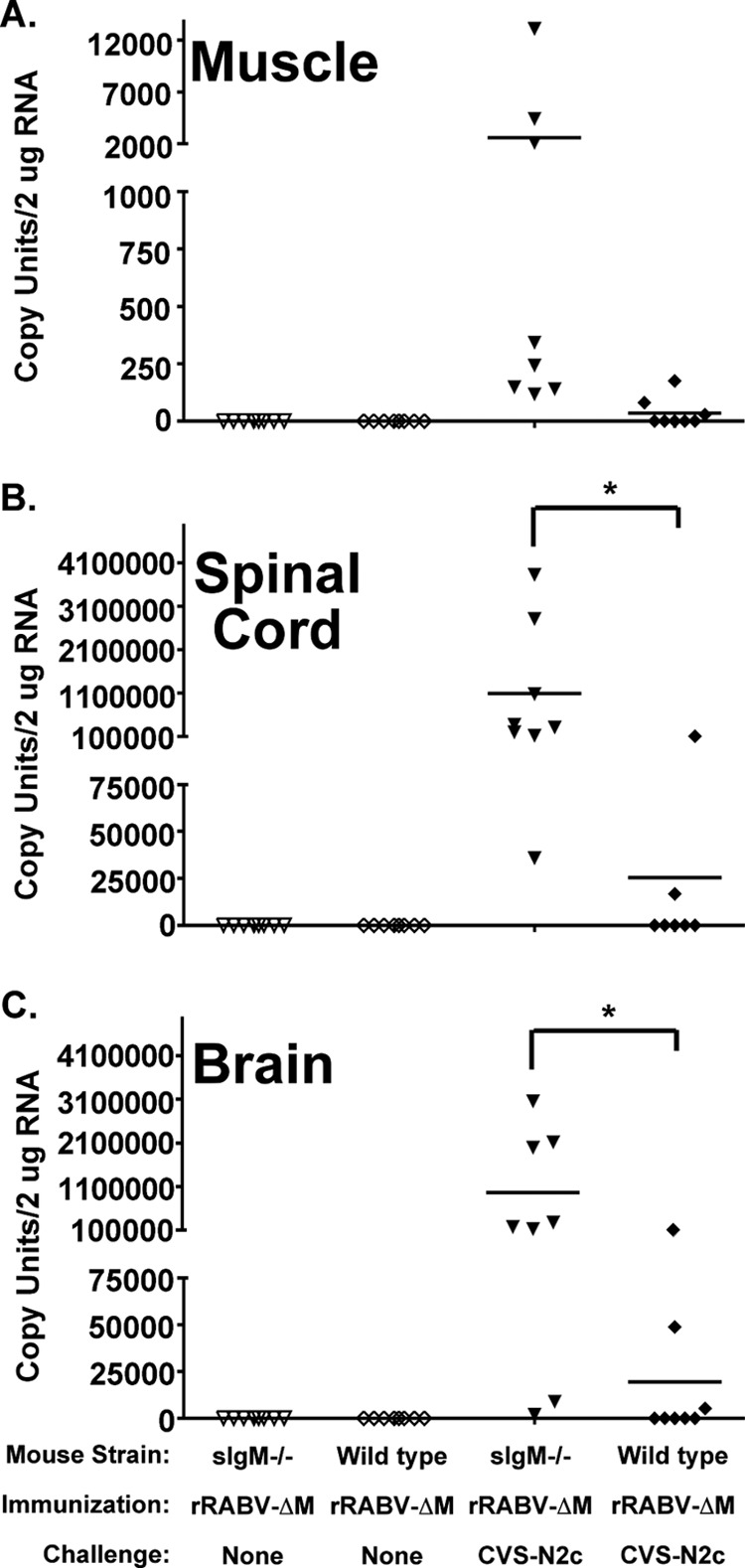
Vaccine-induced IgM helps to prevent the spread of pathogenic RABV into the CNS. Groups of sIgM−/− or wild-type mice were immunized with rRABV-ΔM and then challenged with CVS-N2c as described in the legend to Fig. 1. Five days postchallenge, total RNA was isolated from the muscle (A), the spinal cord (B), and the brain (C) and analyzed by qRT-PCR for the presence of CVS-N2c-specific viral mRNA. Data represent a combination of two independent experiments (total n = 8 mice/group). One-way analysis of variance (ANOVA) was used to evaluate statistical significance. Where significant differences were observed, Tukey's multiple comparison test was used to identify significant differences between individual groups (*, P < 0.05).
VNA responses in sIgM−/− mice.
Protection against RABV infection relies on VNAs directed against the single transmembrane glycoprotein (G) (7, 16, 28). While IgM has been described to be effective against other infectious diseases, such as influenza virus or West Nile virus (reviewed in reference 15), IgM has not been described to play a role in protection against pathogenic RABV infections. To confirm that vaccine-induced IgM has the potential to directly neutralize pathogenic RABV in vitro, sera from sIgM−/− or control mice were collected at various times postimmunization with rRABV-ΔM. As shown in Fig. 3, VNA titers indicative of a satisfactory immunization (0.5 IU/ml) (16, 28–30) were detected in wild-type mice as early as 3 days postimmunization and were significantly higher than titers in similarly immunized sIgM−/− mice. This is consistent with our previous finding that rRABV-ΔM induces early and rapid VNA responses in T cell-dependent and T cell-independent mechanisms (6). VNA titers continued to rise in wild-type mice immunized with rRABV-ΔM through day 10 postimmunization, which was the last time point tested. Conversely, almost 20-fold reduced VNA titers were detected in sIgM−/− mice 5 days postimmunization with rRABV-ΔM compared to wild-type mice, which remained significantly lower during the early phases of B cell development in sIgM−/− mice than titers of wild-type controls. This analysis of serum from immunized sIgM−/− mice, along with our previous finding that nonimmunized sIgM−/− mice are not protected against CVS-N2c challenge (Fig. 1), suggests that induced, and not natural, IgM plays an important role in rRABV-ΔM vaccine-induced protection against pathogenic RABV challenge.
Fig 3.
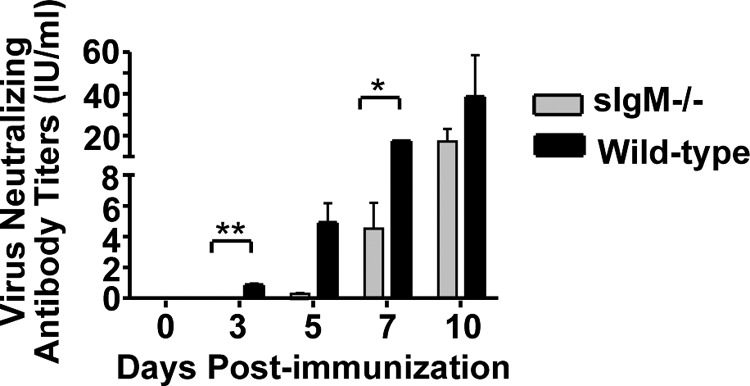
Production of rRABV-ΔM-induced RABV-specific VNAs is delayed in sIgM−/− mice compared to wild-type controls. Groups of sIgM−/− or wild-type mice were immunized i.m. with a single dose of 106 FFU of rRABV-ΔM. Blood was collected on the indicated days postimmunization, and VNA titers from pooled sera were determined using the RFFIT. Neutralization titers, defined as the inverse of the highest serum dilution that neutralizes 50% of the challenge virus (challenge virus strain 11), were normalized to international units/ml (IU/ml) using the WHO anti-RABV antibody reference standard. Data represent 2 independent experiments consisting of sera from 3 mice/experiment pooled and then analyzed in duplicate (total n = 6 mice/group). To compare two groups of data for antibody responses, we used an unpaired, two-tailed t test.
Kinetic analyses of vaccine-induced antibody responses in sIgM−/− mice.
IgM has the ability to influence the development of virus-specific IgG titers (12). Therefore, the potential exists that the lack of protection against pathogenic challenge in sIgM−/− was due to aberrant IgG antibody titers in the sIgM-deficient mice. To investigate whether RABV-specific IgM has the ability to influence the development of RABV-specific IgG responses, sIgM−/− or control mice were immunized with 106 FFU/mouse of rRABV-ΔM, and a kinetic analysis was completed to evaluate RABV G-specific IgM and IgG responses. As expected, only background levels of RABV G-specific IgM antibodies were detected in sIgM−/− mice at all time points tested (Fig. 4A). Significant levels of anti-RABV G IgM antibodies were detected 3 days postimmunization in wild-type mice compared to those in preimmune sera, which peaked around day 7 postimmunization (Fig. 4A). Importantly, significant levels of anti-RABV G IgG were not detected above preimmune levels until day 5 postimmunization in either mouse strain, and equivalent titers of IgG were detected in sIgM−/− and wild-type mice at all time points tested postimmunization (Fig. 4B). Together, the statistically similar anti-RABV G IgG antibody responses detected in immunized sIgM−/− mice and wild-type controls indicate that the lack of protection observed in sIgM−/− mice was not due to the influence of IgM deficiency on the development of anti-RABV IgG antibody responses but due to the direct effects of IgM. This is also supported by the ability of wild-type but not sIgM−/− mice to mount VNA responses within 3 days postimmunization (Fig. 3).
Fig 4.
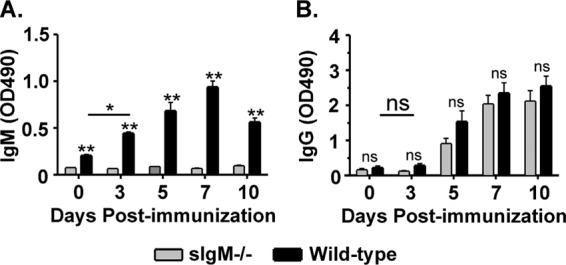
Kinetic analyses of rRABV-ΔM-induced IgM and IgG antibody titers in sIgM−/− mice. Sera collected from individual mice described in the legend to Fig. 3 were analyzed by ELISA to determine anti-RABV G IgM (A) or IgG (B) antibody titers on the indicated days postimmunization. Data represent two independent experiments (total n = 6 mice/group). For multigroup comparisons, ANOVA was used. Where significant differences were observed, Tukey's multiple comparison test was used to identify significant differences between individual groups. Three (P < 0.001), two (P = 0.001 to 0.01), and one (P = 0.01 to 0.05) asterisk indicates a significant difference between two data points (ns, not significant). OD490, optical density at 490 nm.
Together, the data presented in this paper suggest that vaccines that can exploit the induction of early IgM antibodies against RABV infection help in the pursuit of more efficacious single-dose RABV-based postexposure vaccines in which the rapid induction of VNAs is critical for protection.
DISCUSSION
We previously showed that replication-deficient RABV-based vaccines exploit early pathways of B cell activation and development and may hold the key to the development of a single-dose postexposure RABV vaccine wherein the rapid induction of VNA is critical (6, 17, 22, 23). Specifically, a replication-deficient RABV-based vaccine in which the matrix gene is deleted (rRABV-ΔΜ) induced protective antibody responses by T cell-dependent and T cell-independent mechanisms (6). Mice genetically devoid of all T cells were protected against pathogenic RABV challenge, indicating a role for vaccine-induced T cell-independent antibody responses to stunt the virus while high-affinity antibodies are forming in germinal centers (GCs) (6). An evaluation of the antibody isotypes indicated that IgG or IgM antibodies could be responsible for the protection observed in the vaccinated T cell-deficient mice. In this report, we extended these findings by investigating the role for vaccine-induced IgM in the protection against pathogenic RABV infection. We utilized a mutant mouse strain developed by Boes et al. (24), whereby a targeted mutation eliminates IgM secretion but does not affect the overall serum IgG levels. Serum IgG1, IgG2a, IgG2b, and IgG3 levels in adult sIgM−/− mice are similar to those observed in wild-type controls (24, 25), making this mutant mouse strain a valuable model to evaluate induced IgM in the context of RABV vaccination. We show that rRABV-ΔM induces RABV G-specific IgM, which protects mice against pathogenic challenge and reduces the dissemination of pathogenic RABV to the CNS. To the best of our knowledge, this is the first report of vaccine-induced IgM being protective against pathogenic RABV challenge.
The ability of vaccine-induced IgM to enhance survivability upon pathogenic challenge was demonstrated by our finding that only 20% of the sIgM−/− mice immunized with rRABV-ΔM survived challenge with a highly pathogenic RABV strain, while all similarly immunized and challenged wild-type mice survived. Furthermore, our challenge model indicates that induced and not natural IgM is responsible for the protection against pathogenic challenge we observed, since wild-type mice inoculated with PBS alone, which would rely on natural and not induced IgM for protection, succumbed to pathogenic RABV challenge. This is in contrast to the ability of natural IgM to protect against some infectious diseases, such as influenza virus (12), vesicular stomatitis virus (14, 31), lymphocytic choriomeningitis virus, or vaccinia virus (14). One notable difference between pathogenic RABV and these viruses is that there is no evidence that highly neurotropic RABV strains, such as CVS-N2c, enter the circulation (8), where natural IgM most likely serves to prevent hematogenical spread to other organs in the body (32). Nonetheless, our data indicating that vaccine-induced IgM protects mice against pathogenic RABV challenge is in agreement with the ability of induced but not natural IgM to protect against other viruses, such as West Nile virus (WNV) (11). Consistent with the ability of induced IgM to protect against pathogenic WNV infection by limiting viral spread, we showed here that immune IgM is important for vaccine-induced protection against viral spread to the CNS of mice challenged with a highly neurotrophic and pathogenic RABV. Indeed, we detected an approximately 50- to 100-fold increase in challenge virus mRNA in the muscle, spinal cord, and brain of rRABV-ΔM-immunized sIgM−/− mice compared to mice with intact IgM secretory functions.
IgM possesses multiple functions that promote effective antimicrobial properties, including particle agglutination (direct neutralization), complement activation, and enhanced phagocytosis. In addition, IgM has the ability to influence the generation of adaptive immunity (24) and to influence the magnitude of virus-specific IgG antibody responses (12). In the case of West Nile virus infection (11), the lack of IgM resulted in subsequent reduced IgG responses, which may have contributed to the spread of WNV to the CNS in sIgM−/− mice. However, anti-RABV IgG titers of sIgM−/− and wild-type mice were not significantly different at any time points postimmunization (Fig. 4), suggesting the effects of IgM in the context of RABV vaccination are direct and not due to the influence of IgM to prime adaptive IgG antibody titers.
Vaccine strategies that target the induction of immune IgM might have several advantages in the context of RABV PEP treatment. The speed by which immune IgM is induced makes this antibody isotype an attractive target for the development of effective PEP treatment. Since IgM antibodies are the first antibodies produced in response to infection or after immunization, vaccine-induced IgM may help to prevent the spread of pathogenic RABV from peripheral sites to the CNS while higher-affinity IgG antibodies are being formed in GCs. The pentameric structure of IgM results in high valency (i.e., 10 linked antigen-binding sites) (reviewed in references 10 and 33). One IgM antibody is needed to cover 9 or 10 glycoprotein spikes on the surface of RABV particles for neutralization, compared to one or two IgG antibodies to cover only 3 glycoprotein spikes (34). The high valency of IgM is also thought to bind repeating epitopes very efficiently (10), such as the glycoprotein displayed on the surface of RABV particles. Together, the speed by which immune IgM is induced in response to vaccination, the high valency, and lower number of IgM than IgG needed to cover the RABV spike glycoproteins make induced IgM attractive from a PEP vaccination standpoint.
ACKNOWLEDGMENTS
This work was supported by NIH/NIAID grant R01AI079211 to J.P.M.
We thank Jianzhu Chen, Massachusetts Institute of Technology, and Kishore R. Alugupalli, Thomas Jefferson University, for providing us with the sIgM−/− mice.
Footnotes
Published ahead of print 12 June 2013
REFERENCES
- 1. Bunschoten H, Dietzschold B, Claassen I, Klapmuts R, Uytdehaag F, Osterhaus A. 1990. Rabies virus cross-reactive murine T cell clones: analysis of helper and delayed-type hypersensitivity function. Viral Immunol. 3:41–53 [DOI] [PubMed] [Google Scholar]
- 2. Baer GM. 2007. The history of rabies, p 11–19 In Jackson AC, Wunner WH. (ed), Rabies. Elsevier Science, Irvine, CA [Google Scholar]
- 3. Mifune K, Takeuchi E, Napiorkowski PA, Yamada A, Sakamoto K. 1981. Essential role of T cells in the postexposure prophylaxis of rabies in mice. Microbiol. Immunol. 25:895–904 [DOI] [PubMed] [Google Scholar]
- 4. Perry LL, Lodmell DL. 1991. Role of CD4+ and CD8+ T cells in murine resistance to street rabies virus. J. Virol. 65:3429–3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Turner GS. 1976. Thymus dependence of rabies vaccine. J. Gen. Virol. 33:535–538 [DOI] [PubMed] [Google Scholar]
- 6. Dorfmeier CL, Lytle AG, Dunkel AL, Gatt A, McGettigan JP. 2012. Protective vaccine-induced CD4+ T cell-independent B cell responses against rabies infection. J. Virol. 86:11533–11540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Turner GS. 1978. Immunoglobulin (IgG) and (IgM) antibody responses to rabies vaccine. J. Gen. Virol. 40:595–604 [DOI] [PubMed] [Google Scholar]
- 8. Jackson AC. 2011. Update on rabies. Res. Rep. Trop. Med. 2:31–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pone EJ, Zan H, Zhang J, Al-Qahtani A, Xu Z, Casali P. 2010. Toll-like receptors and B-cell receptors synergize to induce immunoglobulin class-switch DNA recombination: relevance to microbial antibody responses. Crit. Rev. Immunol. 30:1–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Racine R, Winslow GM. 2009. IgM in microbial infections: taken for granted? Immunol. Lett. 125:79–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Diamond MS, Sitati EM, Friend LD, Higgs S, Shrestha B, Engle M. 2003. A critical role for induced IgM in the protection against West Nile virus infection. J. Exp. Med. 198:1853–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baumgarth N, Herman OC, Jager GC, Brown LE, Herzenberg LA, Chen J. 2000. B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J. Exp. Med. 192:271–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kopf M, Brombacher F, Bachmann MF. 2002. Role of IgM antibodies versus B cells in influenza virus-specific immunity. Eur. J. Immunol. 32:2229–2236 [DOI] [PubMed] [Google Scholar]
- 14. Ochsenbein AF, Fehr T, Lutz C, Suter M, Brombacher F, Hengartner H, Zinkernagel RM. 1999. Control of early viral and bacterial distribution and disease by natural antibodies. Science 286:2156–2159 [DOI] [PubMed] [Google Scholar]
- 15. Hangartner L, Zinkernagel RM, Hengartner H. 2006. Antiviral antibody responses: the two extremes of a wide spectrum. Nat. Rev. Immunol. 6:231–243 [DOI] [PubMed] [Google Scholar]
- 16. McGettigan JP. 2010. Experimental rabies vaccines for humans. Expert Rev. Vaccines 9:1177–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cenna J, Hunter M, Tan GS, Papaneri AB, Ribka EP, Schnell MJ, Marx PA, McGettigan JP. 2009. Replication-deficient rabies virus-based vaccines are safe and immunogenic in mice and nonhuman primates. J. Infect. Dis. 200:1251–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schnell MJ, Foley HD, Siler CA, McGettigan JP, Dietzschold B, Pomerantz RJ. 2000. Recombinant rabies virus as potential live-viral vaccines for HIV-1. Proc. Natl. Acad. Sci. U. S. A. 97:3544–3549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Conzelmann KK, Cox JH, Schneider LG, Thiel HJ. 1990. Molecular cloning and complete nucleotide sequence of the attenuated rabies virus SAD B19. Virology 175:485–499 [DOI] [PubMed] [Google Scholar]
- 20. Mebatsion T, Weiland F, Conzelmann KK. 1999. Matrix protein of rabies virus is responsible for the assembly and budding of bullet-shaped particles and interacts with the transmembrane spike glycoprotein G. J. Virol. 73:242–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Morimoto K, Hooper DC, Carbaugh H, Fu ZF, Koprowski H, Dietzschold B. 1998. Rabies virus quasispecies: implications for pathogenesis. Proc. Natl. Acad. Sci. U. S. A. 95:3152–3156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cenna J, Tan GS, Papaneri AB, Dietzschold B, Schnell MJ, McGettigan JP. 2008. Immune modulating effect by a phosphoprotein-deleted rabies virus vaccine vector expressing two copies of the rabies virus glycoprotein gene. Vaccine 26:6405–6414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dorfmeier CL, Tzvetkov EP, Gatt A, McGettigan JP. 2013. Investigating the role for IL-21 in rabies virus vaccine-induced immunity. PLoS Negl. Trop. Dis. 3:e2129. 10.1371/journal.pntd.0002129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boes M, Esau C, Fischer MB, Schmidt T, Carroll M, Chen J. 1998. Enhanced B-1 cell development, but impaired IgG antibody responses in mice deficient in secreted IgM. J. Immunol. 160:4776–4787 [PubMed] [Google Scholar]
- 25. Alugupalli KR, Gerstein RM, Chen J, Szomolanyi-Tsuda E, Woodland RT, Leong JM. 2003. The resolution of relapsing fever borreliosis requires IgM and is concurrent with expansion of B1b lymphocytes. J. Immunol. 170:3819–3827 [DOI] [PubMed] [Google Scholar]
- 26. Prosniak M, Zborek A, Scott GS, Roy A, Phares TW, Koprowski H, Hooper DC. 2003. Differential expression of growth factors at the cellular level in virus-infected brain. Proc. Natl. Acad. Sci. U. S. A. 100:6765–6770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tan GS, Preuss MA, Williams JC, Schnell MJ. 2007. The dynein light chain 8 binding motif of rabies virus phosphoprotein promotes efficient viral transcription. Proc. Natl. Acad. Sci. U. S. A. 104:7229–7234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moore SM, Hanlon CA. 2010. Rabies-specific antibodies: measuring surrogates of protection against a fatal disease. PLoS Negl. Trop. Dis. 4:e595. 10.1371/journal.pntd.0000595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Plotkin SA. 2008. Vaccines: correlates of vaccine-induced immunity. Clin. Infect. Dis. 47:401–409 [DOI] [PubMed] [Google Scholar]
- 30. Anonymous 1997. WHO recommendations on rabies post-exposure treatment and the correct technique of intradermal immunization against rabies. World Health Organization, Geneva, Switzerland [Google Scholar]
- 31. Gobet R, Cerny A, Ruedi E, Hengartner H, Zinkernagel RM. 1988. The role of antibodies in natural and acquired resistance of mice to vesicular stomatitis virus. Exp. Cell Biol. 56:175–180 [DOI] [PubMed] [Google Scholar]
- 32. Ochsenbein AF, Zinkernagel RM. 2000. Natural antibodies and complement link innate and acquired immunity. Immunol. Today 21:624–630 [DOI] [PubMed] [Google Scholar]
- 33. Klimovich VB. 2011. IgM and its receptors: structural and functional aspects. Biochemistry 76:534–549 [DOI] [PubMed] [Google Scholar]
- 34. Flamand A, Raux H, Gaudin Y, Ruigrok RW. 1993. Mechanisms of rabies virus neutralization. Virology 194:302–313 [DOI] [PubMed] [Google Scholar]


