Abstract
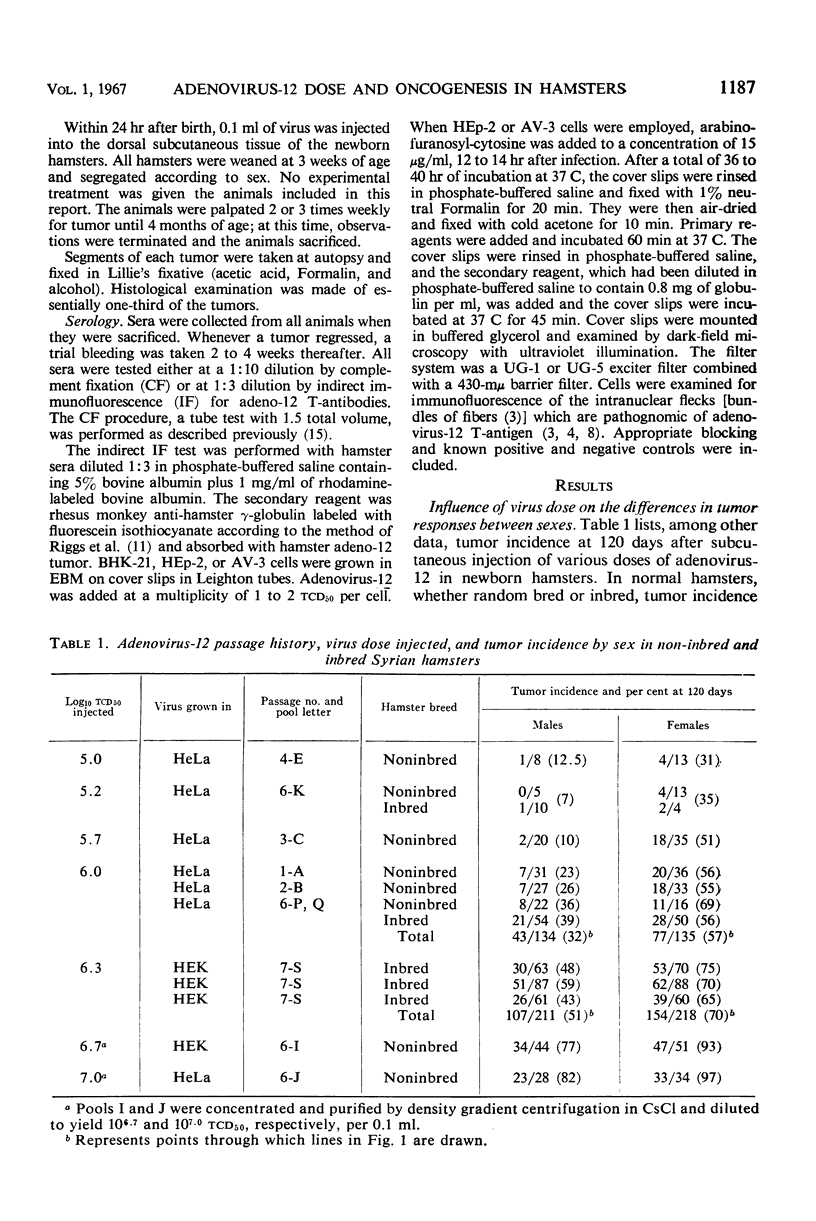
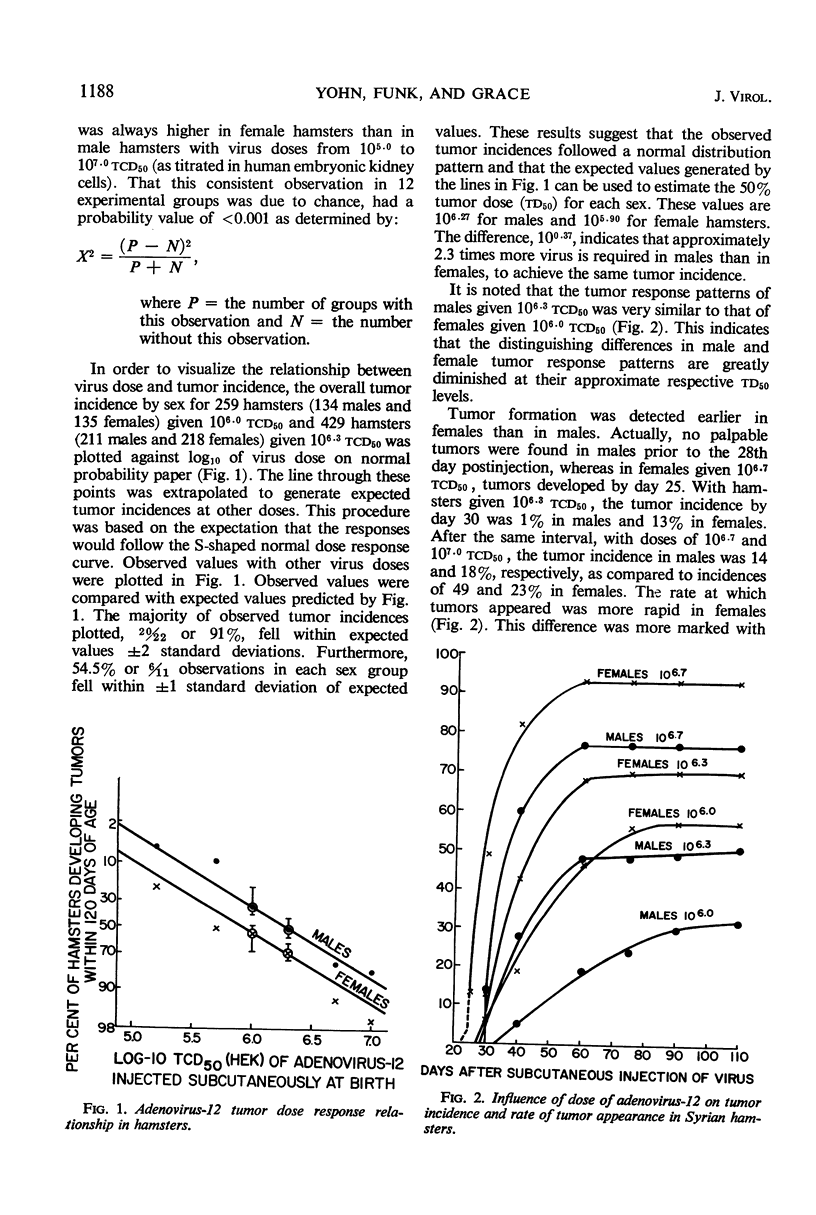
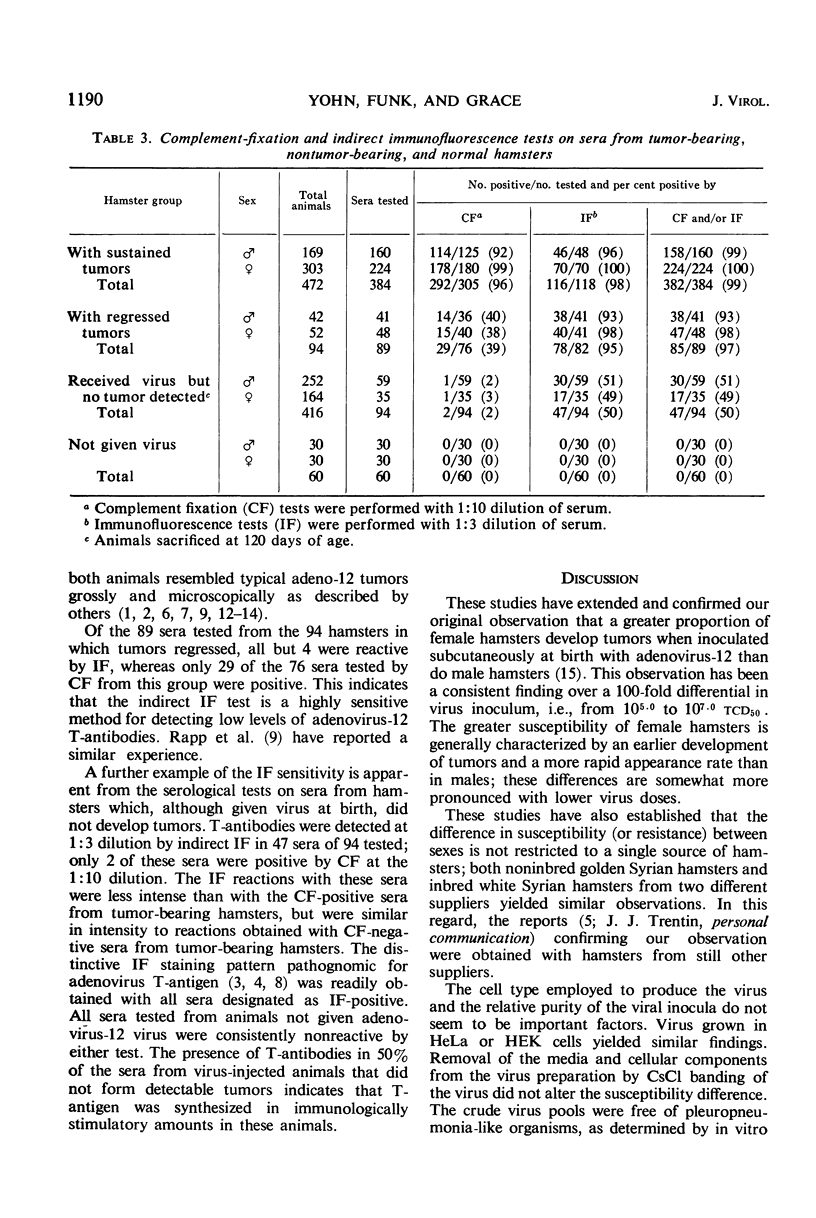
A significantly higher proportion of female hamsters developed tumors than did males given the same dose of adenovirus-12 (Huie) at birth over a dose range from 105.0 to 107.0tcd50 for human embryonic kidney cells. The 50% tumor dose (td50) was calculated to be 105.90tcd50 for females and 106.27 for males. Tumor response patterns induced with approximate td50 inocula, 106.0 for females and 106.3 for males, were quite similar. The greater susceptibility of females was not found to be characteristic of a single strain of hamsters; nor was it attributable to a single lot of virus, to a single type of human cell used to produce the virus, nor to the degree of purification of the virus inoculum. The inoculation route did not appear to be of importance. Inasmuch as the foregoing extrinsic factors were of little influence, it was concluded that the mechanism is host-mediated, presumably hormonally controlled. The possibility that female cells, independent of host control, are more susceptible to adenovirus-12 oncogenesis than male cells has not been explored. Tumor regression occurred in 20% of the 211 tumors in males and in 15% of the 355 tumors in females. Adenovirus-12 T-antibody was detected in all but six of 473 sera tested from tumor-bearing hamsters and in 50% of 94 sera tested from non-tumor-bearing animals given virus at birth. Antibodies in the latter group were detected almost exclusively by indirect immunofluorescence. This technique appears to be extremely sensitive for detection of low levels of adeno-12 T-antibodies. The implications of T-antibody in nontumor-bearing virus-injected hamsters are discussed. Sera from normal hamsters were free of T-antibody.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- HUEBNER R. J., ROWE W. P., LANE W. T. Oncogenic effects in hamsters of human adenovirus types 12 and 18. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2051–2058. doi: 10.1073/pnas.48.12.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUEBNER R. J., ROWE W. P., TURNER H. C., LANE W. T. SPECIFIC ADENOVIRUS COMPLEMENT-FIXING ANTIGENS IN VIRUS-FREE HAMSTER AND RAT TUMORS. Proc Natl Acad Sci U S A. 1963 Aug;50:379–389. doi: 10.1073/pnas.50.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalnins V. I., Stich H. F., Yohn D. S. Electron microscopic localization of virus-associated antigens in human amnion cells (AV-3) infected with human adenovirus, type 12. Virology. 1966 Apr;28(4):751–754. doi: 10.1016/0042-6822(66)90259-5. [DOI] [PubMed] [Google Scholar]
- MacKinnon E., Kalnins V. I., Stich H. F., Yohn D. S. Viruses and mammalian chromosomes. VI. Comparative karyologic and immunofluorescent studies on Syrian hamster and human amnion cells infected with human adenovirus type 12. Cancer Res. 1966 Apr;26(4):612–618. [PubMed] [Google Scholar]
- McFarlane E. S., Embil J. A., Jr Effect of splenectomy and sex in hamsters on adenovirus-12 oncogenesis. Can J Microbiol. 1966 Dec;12(6):1285–1286. doi: 10.1139/m66-171. [DOI] [PubMed] [Google Scholar]
- Ogawa K., Tsutsumi A., Iwata K., Fujii Y., Ohmori M. Histogenesis of malignant neoplasm induced by adenovirus type 12. Gan. 1966 Feb;57(1):43–52. [PubMed] [Google Scholar]
- PEREIRA M. S., MACCALLUM F. O. INFECTION WITH ADENOVIRUS TYPE 12. Lancet. 1964 Jan 25;1(7326):198–199. doi: 10.1016/s0140-6736(64)92290-1. [DOI] [PubMed] [Google Scholar]
- POPE J. H., ROWE W. P. IMMUNOFLUORESCENT STUDIES OF ADENOVIRUS 12 TUMORS AND OF CELLS TRANSFORMED OR INFECTED BY ADENOVIRUSES. J Exp Med. 1964 Oct 1;120:577–588. doi: 10.1084/jem.120.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIGGS J. L., SEIWALD R. J., BURCKHALTER J. H., DOWNS C. M., METCALF T. G. Isothiocyanate compounds as fluorescent labeling agents for immune serum. Am J Pathol. 1958 Nov-Dec;34(6):1081–1097. [PMC free article] [PubMed] [Google Scholar]
- Rapp F., Melnick J. L., Levy B. Correlation of immunology and histopathology of tumors induced by defective SV40-adenovirus hybrids. Am J Pathol. 1967 May;50(5):849–859. [PMC free article] [PubMed] [Google Scholar]
- TRENTIN J. J., YABE Y., TAYLOR G. The quest for human cancer viruses. Science. 1962 Sep 14;137(3533):835–841. doi: 10.1126/science.137.3533.835. [DOI] [PubMed] [Google Scholar]
- YABE Y., SAMPER L., TAYLOR G., TRENTIN J. J. Cancer induction in hamsters by human type 12 adenovirus. Effect of route of injection. Proc Soc Exp Biol Med. 1963 May;113:221–224. doi: 10.3181/00379727-113-28324. [DOI] [PubMed] [Google Scholar]
- Yohn D. S., Funk C. A., Kalnins V. I., Grace J. T., Jr Sex-related resistance in hamsters to adenovirus-12 oncogenesis. Influence of thymectomy at three weeks of age. J Natl Cancer Inst. 1965 Oct;35(4):617–624. [PubMed] [Google Scholar]



