Abstract
In June of 2012, an H7N3 highly pathogenic avian influenza (HPAI) virus was identified as the cause of a severe disease outbreak in commercial laying chicken farms in Mexico. The purpose of this study was to characterize the Mexican 2012 H7N3 HPAI virus (A/chicken/Jalisco/CPA1/2012) and determine the protection against the virus conferred by different H7 inactivated vaccines in chickens. Both adult and young chickens intranasally inoculated with the virus became infected and died at between 2 and 4 days postinoculation (p.i.). High virus titers and viral replication in many tissues were demonstrated at 2 days p.i. in infected birds. The virus from Jalisco, Mexico, had high sequence similarity of greater than 97% to the sequences of wild bird viruses from North America in all eight gene segments. The hemagglutinin gene of the virus contained a 24-nucleotide insert at the hemagglutinin cleavage site which had 100% sequence identity to chicken 28S rRNA, suggesting that the insert was the result of nonhomologous recombination with the host genome. For vaccine protection studies, both U.S. H7 low-pathogenic avian influenza (LPAI) viruses and a 2006 Mexican H7 LPAI virus were tested as antigens in experimental oil emulsion vaccines and injected into chickens 3 weeks prior to challenge. All H7 vaccines tested provided ≥90% protection against clinical disease after challenge and decreased the number of birds shedding virus and the titers of virus shed. This study demonstrates the pathological consequences of the infection of chickens with the 2012 Mexican lineage H7N3 HPAI virus and provides support for effective programs of vaccination against this virus in poultry.
INTRODUCTION
Avian influenza (AI) is a viral disease of poultry that can occur in many different bird species, and highly pathogenic (HP) forms of the virus result in rapid mortality in susceptible poultry. AI virus (AIV) is classified in the family Orthomyxoviridae, genus Influenza A virus (type A), and contains a negative-sense, segmented RNA genome (1). Antigenically, 16 hemagglutinin (HA) subtypes (H1 to H16) and nine neuraminidase subtypes (N1 to N9) have been detected in birds (2–4). Wild aquatic birds, including ducks, are the natural reservoir for low-pathogenic (LP) AIV, which typically does not cause significant disease or mortality (5). AIV is shed through the intestinal tract of these birds and is primarily spread by fecal contamination of the water or directly to other birds. Although wild birds do not normally get sick from AIV, they have on occasion transmitted the virus to domesticated birds, including chickens, ducks, and turkeys, which are all susceptible to AIV (6). The introduction of H5 or H7 LPAI virus into poultry may result in the emergence of HPAI viruses through various genetic changes of the HA gene (7–11).
H7N3 AIV has been identified in wild birds throughout the world, implicating migration and/or contact (direct or indirect) with other susceptible avian species as the most likely mode of transmission to commercial poultry. Multiple outbreaks of H7N3 HPAI in commercial poultry have been reported in the Americas over the last decade. In May 2002, an outbreak at a broiler breeder farm in Chile was identified and later controlled by depopulation and strict biosecurity (12). Outbreaks of H7N3 HPAI in commercial poultry operations in British Columbia and Saskatchewan, Canada, in 2004 and 2007, respectively, were reported to be caused by LPAI virus precursors from migratory waterfowl (13–15).
In June of 2012, the isolation of an H7N3 HPAI virus was reported in commercial egg layer chickens in the state of Jalisco, Mexico, a poultry-dense region responsible for approximately 55% of Mexican table egg production (16). Initial reports in three layer farms from Acatic and Tepatitlan resulted in the establishment of a 40-km quarantine zone, although the virus would later be reported in poultry farms outside the zone. Initial phylogenetic characterization of the HA gene from H7 viruses has demonstrated three genetically distinct clusters based on the geography of the isolate (17). The Mexican isolate was determined to be closely related to wild bird isolates from North America. Although the origin of the Mexican virus is unknown, it is suspected that an LP AIV from wild birds infected chickens and mutated into an HP form. To date, this outbreak has resulted in the death of over 22 million birds through either disease or culling, at an estimated cost of over $720 million (16).
The current studies were undertaken to characterize the pathobiology of an H7N3 HPAI virus (A/chicken/Jalisco/CPA1/2012 [Ck/J/12]) in poultry and determine the protection against this virus gained through vaccination of chickens with U.S. and Mexican H7 LPAI inactivated viruses. For vaccine studies, U.S. isolates chosen on the basis of available H7 strains previously used for vaccination and a Mexican H7N3 LPAI virus isolate that was obtained from a Cinnamon Teal duck in 2006 were used (18).
MATERIALS AND METHODS
H7 viruses.
A total of six different H7 AIVs were used in these studies, including four isolates from the United States and two isolates from Mexico. The low-pathogenic H7 U.S. vaccine isolates included A/chicken/New York/12273-11/1999 (Ck/NY/99) H7N3, A/quail/Pennsylvania/20304/1998 (Q/PA/98) H7N2, A/turkey/Oregon/1971 (Tk/OR/71) H7N3, and A/turkey/Utah/24721-10/1995 (Tk/UT/95) H7N3. Both of the turkey isolates, Tk/OR/71 and Tk/UT/95, are USDA-approved master seed strains for H7 vaccines for poultry. The Mexican isolates included the LPAI virus A/Cinnamon Teal/Mexico/2817/2006 (CT/MX/06) H7N3 and HPAI virus A/chicken/Jalisco/CPA1/2012 (Ck/J/12) H7N3, which was used as the challenge virus. All viruses were propagated in specific-pathogen-free (SPF) embryonated chicken eggs according to standard procedures (19). Allantoic fluid containing virus was harvested as antigen for vaccine formulation or titration of HPAI challenge virus. All experiments using H7N3 HPAI viruses, including work with animals, were reviewed by the institutional biosecurity committee and were performed in biosecurity level 3 enhanced facilities at SEPRL, with the additional precautions that all personnel were vaccinated against seasonal influenza and were required to wear a powered air protection respirator with a high-efficiency particulate air (HEPA)-filtered air supply (3M, St. Paul, MN).
Vaccine preparation.
Individual H7 vaccines were produced with the five LPAI virus isolates described above following growth in SPF eggs and harvesting of allantoic fluid. Following β-propiolactone inactivation of allantoic fluid, each vaccine virus was diluted to provide an HA concentration of 512 per 0.5 ml when mixed (30/70) with Montanide ISA VG70 oil emulsion (Seppic Inc., Fairfield, NJ) according to the manufacturer's recommendations (20).
Chickens.
For all experiments, SPF chickens (White Rock [meat type] and White Leghorn [egg layer type]) of both sexes were obtained from and housed at the Southeast Poultry Research Laboratory in a biosafety level 2 facility and were transferred to a biosafety level 3E facility for vaccination and challenge. Birds were maintained in HEPA-filtered isolation cabinets with feed and water ad libitum. All bird experimental procedures were approved and performed under the guidelines of the Southeast Poultry Research Laboratory Institutional Animal Care and Use Committee.
Experiment I.
Fifty 7-week-old SPF White Rock chickens were divided into five groups of 10 and wing banded for identification. Birds in groups 1 to 4 received 0.5 ml of Ck/NY/99, Q/PA/98, Tk/OR/71, or Tk/UT/95 vaccine delivered via the subcutaneous route, respectively. Birds in group 5 received a sham vaccination with allantoic fluid from uninoculated SPF embryos as described above. At 3 weeks after vaccination, all birds were bled to determine prechallenge antibody titers, and each bird was immediately challenged with 106 50% egg infective doses (EID50s) of the HPAI Ck/J/12 virus isolate via the intranasal route. The challenge dose was confirmed by backtitration in embryonated eggs. Oropharyngeal and cloacal swab samples were collected on days 2 and 4 postchallenge (p.c.) to determine virus shedding. Following challenge, the groups were monitored twice daily for 14 days for clinical signs, and those with severe clinical signs of disease (e.g., an inability to reach feed or water) were humanely euthanized by the approved protocol and counted as mortalities for that day. Serum was collected from the surviving birds on day 14 p.c., and the birds were then humanly euthanized.
Experiment II.
Twenty 2-week-old SPF White Leghorn chickens were divided into two groups of 10 and wing banded for identification. Birds in group 1 received 0.5 ml of CT/MX/06 vaccine, and birds in group 2 received 0.5 ml of sham vaccine delivered via the subcutaneous route. At 3 weeks after vaccination, all birds were bled to determine prechallenge antibody titers, and each bird was immediately challenged with 106 EID50s of the Ck/J/12 HPAI virus isolate via the intranasal route. Swab samples were collected and processed as described above on days 2 and 4 p.c. Following challenge, groups were monitored twice daily for 14 days for clinical signs, and those with severe clinical signs of disease were humanely euthanized by the approved protocol and counted as mortalities for that day. Serum was collected from the surviving birds on day 14 p.c., and the birds were then humanely euthanized.
Serology.
Serum was obtained from all birds pre- and postchallenge and tested by hemagglutination inhibition (HI) assay using chicken red blood cells. The HI assay was performed using inactivated Ck/J/12 HPAI virus as antigen according to standard procedures (21). Titers were calculated as the highest reciprocal serum dilution providing complete hemagglutination inhibition. Serum titers of 1:8 (3 log2 units) or lower were considered negative for antibodies against avian influenza virus.
Virus titrations.
Virus detection from oropharyngeal and cloacal swabs on days 2 and 4 postchallenge was performed as described previously (22). Briefly, swab specimens were collected from each bird and placed into 2 ml of brain heart infusion (BHI) broth with antibiotics (1,000 units/ml of penicillin G, 200 μg/ml of gentamicin sulfate, and 4 μg/ml of amphotericin B; Sigma Chemical Company, St. Louis, MO), and 0.2 ml was injected into 9- to 11-day-old embryonated SPF chicken eggs. The inoculated eggs were incubated at 37°C for 72 to 96 h, and allantoic fluid was harvested and screened for the presence of AIV by the HA test following standard procedures (23). Virus titers are reported as the log10 numbers of EID50s/ml, and the threshold of detection was 100.9 EID50/ml.
Portions of the brain, lung, muscle, heart, and spleen as well as oral and cloacal swab specimens were also collected in BHI medium with antibiotics and stored at −70°C until use. Titers of infectious virus were determined by weighing and homogenizing the tissues and diluting them 10-fold in BHI to a 10% (wt/vol) concentration. Tissue homogenate supernatants (100 μl) were inoculated into the allantoic sac of chicken embryos, and virus titers were determined as described above. The threshold of detection for virus titers in tissues was 101.9 EID50s/g of tissue.
Sequence analysis.
Viral RNA was extracted from infected chicken allantoic fluid using a MagMax AI/ND viral RNA isolation kit (Life Technologies Corp., Foster City, CA). The extracted RNA was amplified by reverse transcription-PCR using gene-specific primers and a One-Step amplification kit (Qiagen, Corp., Valencia, CA). The primers are available upon request. Cycle sequencing was performed directly on amplicons with a BigDye Terminator kit (Applied Biosystems, Foster City, CA), and the sequences were run on an ABI 3730 DNA analyzer (Applied Biosystems, Foster City, CA) and assembled with the SeqMan (version 10.0) program (DNAStar, Madison, WI). Sequences were aligned with the Clustal V program (Lasergene, version 10.0; DNAStar, Madison, WI), and phylogenetic analysis for the H7 gene was performed with the PAUP* program, version 4.0b10 (Sinauer Associates, Inc., Sunderland, MA), using the maximum parsimony tree-building method with heuristic search and 500 bootstrap replicates.
Pathology.
Clinical signs from sham-vaccinated control chickens challenged with the H7N3 HPAI virus were recorded daily. Two birds from the first study (age, 10 weeks) and two from the second study (age, 5 weeks) were euthanized at 2 days p.c., and gross lesions were recorded. The following tissues were collected in 10% neutral buffered formalin solution to evaluate microscopic lesions and the extent of virus replication in tissues: nasal cavity, trachea, lung, air sac, comb, eyelid, heart, brain, esophagus, proventriculus, ventriculus, duodenum, jejunum, cecal tonsils, pancreas, liver, spleen, bursa, thymus, Harderian gland, kidney, gonads, adrenal gland, and muscle from the left thigh.
Histopathology and immunohistochemistry (IHC).
Samples were prepared as previously described (24). Briefly, collected tissues were fixed by submersion in 10% neutral buffered formalin and embedded in paraffin. In addition, the nasal cavity was decalcified for 2 days. Sections were made at 5 μm and were stained with hematoxylin-eosin (HE). A serial section was immunohistochemically stained by first microwaving the sections in antigen retrieval Citra solution (Biogenex, San Ramon, CA) for antigen exposure. A 1:2,000 dilution of a mouse-derived monoclonal antibody (P13C11) (25) specific for type A influenza virus nucleoprotein (developed at the Southeast Poultry Research Laboratory, USDA) was applied, and the mixture was allowed to incubate overnight at 4°C (26). The primary antibody was then detected by the application of biotinylated goat anti-mouse IgG secondary antibody using a biotin-streptavidin detection system (supersensitive multilink immunodetection system; Biogenex). Fast Red TR (Biogenex) served as the substrate chromogen, and hematoxylin-eosin was used as a counterstain. All tissues were systematically screened for microscopic lesions. Lesions were scored as follows: −, no lesions; +, mild lesions; ++, moderate lesions; +++, severe lesions. The intensity of viral antigen staining in each section was scored as follows: −, no antigen staining; +, infrequent staining; ++, common staining; +++, widespread staining.
Statistical analysis.
Kaplan-Meier survival curves were generated with Prism, version 5, software (GraphPad Co., San Diego, CA). The Mantel-Cox log-rank test was used to compare survival curves between two experimental groups (Prism 5). Statistical differences in the mean HI titers and standard errors were analyzed using the Tukey one-way analysis of variance (ANOVA) (Prism 5). The Fisher exact test was used for pairwise comparison of the group mean HI titer or the frequency of virus isolation between groups (SigmaStat, version 2.0.3; SPSS Inc., Chicago, IL). All statistical tests were performed by consideration of a P value of ≤0.05 to be significant.
RESULTS
Clinical signs of disease.
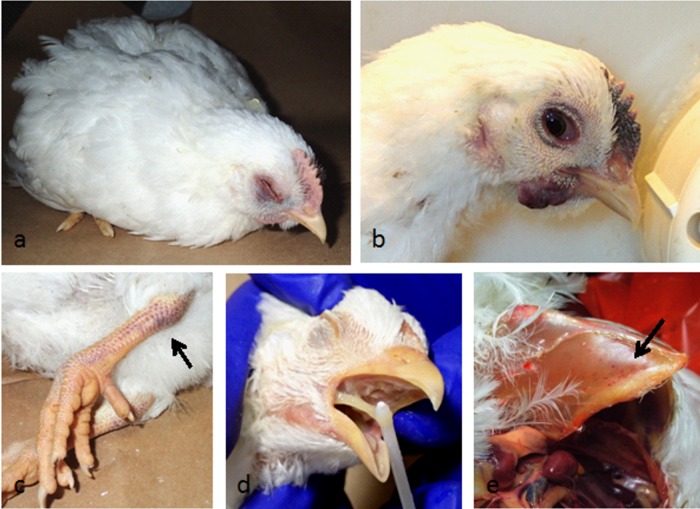
Nonvaccinated (sham-vaccinated) chickens inoculated with the Ck/J/12 HPAI H7N3 virus in both experiments had similar clinical signs and 100% mortality. Some birds showed nonspecific clinical signs, including ruffled feathers, lethargy, anorexia, and prostration, and some birds presented with severe listlessness, respiratory distress, swollen head, and cyanotic or hemorrhagic comb, wattles, and legs (Fig. 1a to c). Some birds died without showing clinical signs (peracute disease). Birds that were found dead had swollen heads and focal hemorrhages in the comb, wattle, and footpads. In some of the birds there were hemorrhages on the surface of the cloaca. Birds that were still alive were lethargic, recumbent, and in some cases trembling. Clinical signs of respiratory distress were prominent in some dying birds and included severe dyspnea and gasping, in association with facial edema; swelling of the infraorbital sinuses; and conjunctivitis. Copious nasal discharge was also observed. Commonly present were swollen and cyanotic combs and wattles and diarrhea.
Fig 1.
Lesions in chickens following experimental infection with the Mexican H7N3 HPAI virus detected at 2 days postinoculation. (a) Prostration and edema of periorbital tissues; (b) subcutaneous hemorrhage of wattles and comb and conjunctivitis and swelling of periorbital area; (c) subcutaneous hemorrhages of leg shanks; (d) mucous in larynx; (e) petechial hemorrhages on breast muscle.
Gross lesions.
Two sham-vaccinated chickens per experiment were necropsied and examined at 2 days p.c. Similar lesions were observed in all 4 chickens examined, and these lesions were consistent with HPAI. A female and a male were examined in each experiment. Grossly, there was congestion and edema of the head and swollen and cyanotic combs, wattles, and legs. The sinuses, nasal cavity, larynx, and trachea were congested and had abundant mucus (Fig. 1d). The lungs were edematous and congested, and the air sacs were thickened. Petechial hemorrhages were observed on the breast and thigh muscles and in the epithelial fat of the heart (Fig. 1e). The proventricular glands were swollen, and the thymus was enlarged and often had petechia. In some chickens, the kidneys were enlarged with parenchymal pallor and accentuated lobular surface architecture. Mild to moderate splenomegaly with parenchymal mottling or petechial hemorrhages was observed in all the birds. Pancreatic lesions characterized by multifocal necrotic areas were also present, as were hemorrhages on the duodenal loop and the cecal tonsils. Hemorrhages were also present on the brain surface of one of the younger birds.
Microscopic lesions and virus antigen staining in tissues.
The results for histologic lesions and viral antigen staining in tissues are shown in Table 1 and Fig. 2. Viral antigen staining was present in multiple tissues of chickens infected with the virus, indicating systemic infection. Microscopic lesions were similar in the four birds that were examined, with minor variations in severity.
Table 1.
Microscopic lesions and viral antigen distribution in tissues from chickens intranasally inoculated with the Mexican H7N3 HPAI virus detected at 2 days p.c.a
| Tissue | Histopathology scoreb | Lesions | IHC scorec | Cell types expressing virus antigen |
|---|---|---|---|---|
| Nasal cavity | +++ | Epithelial cell necrosis and desquamation, rhinitis, sinusitis, lymphocytic infiltrate | ++ | Vascular endothelial cells, epithelial cells, mononuclear cells, necrotic debris |
| Trachea | ++ | Tracheitis, lymphocytic infiltrate | +++ | Vascular endothelial cells, epithelial cells, cellular debris, cells of the tracheolateralis muscle |
| Lung | ++ to +++ | Interstitial pneumonia, bronchitis, edema, congestion, hemorrhage, necrosis, monocytic infiltrate | +++ | Epithelium of air capillaries, mononuclear cells, necrotic debris |
| Air sac | ++ | Airsacculitis, monocytic infiltrate | +++ | Epithelial and mononuclear cells |
| Comb | +++ | Edema, hemorrhages, necrosis | +++ | Vascular endothelial cells, mononuclear cells, necrotic debris |
| Eyelid | +++ | Subcutaneous edema, epidermal and feather follicle necrosis | +++ | Vascular endothelial cells, mononuclear cells, feather follicle epithelium |
| Heart | − | Myocyte necrosis | + | Myocytes |
| Brain | + to ++ | Neuronal necrosis, gliosis, encephalomalacia, chromatolysis of the Purkinge cell layer, lymphoplasmacytic infiltrate | + to ++ | Neurons, glial cells |
| Esophagus | − | − | + | Mononuclear cells in submucosa |
| Proventriculus | ++ | Edema and inflammatory infiltration in submucosa | +++ | Mononuclear cells in submucosa |
| Ventriculus | − | − | + | Mononuclear cells in submucosa |
| Intestine | + to ++ | Lymphohistiocytic infiltration in submucosa, hemorrhage, epithelial necrosis | + | Mononuclear cells in lymphoid-associated tissue, vascular endothelial cells |
| Cecal tonsils | ++ | Hemorrhage, lymphocyte depletion, necrosis | ++ | Macrophages |
| Pancreas | + | Degeneration of individual pancreatic acinar cells | − | − |
| Liver | ++ | Lymphohistiocytic hepatitis | ++ | Kupffer cells, hepatocytes, necrotic debris, vascular endothelial cells |
| Spleen | +++ | Lymphoid depletion, necrosis, splenitis, hyperplasia of macrophage-phagocytic cells | ++ to +++ | Mononuclear cells, necrotic debris, vascular endothelial cells |
| Thymus | + to +++ | Lymphocyte necrosis and apoptosis, lymphocyte depletion, hemorrhage | + to +++ | Histiocytes |
| Cloacal bursa | + to ++ | Lymphocyte necrosis and apoptosis, lymphocyte depletion, phagocytic hyperplasia, necrosis, hemorrhage | − to ++ | Mononuclear cells |
| Harderian gland | + | ++ | Mononuclear cells | |
| Kidney | − to + | Tubular epithelial necrosis | − to ++ | Tubular epithelial cells |
| Gonads | + | Necrosis, lymphoplasmacytic infiltrate | + | Mononuclear and interstitial cells |
| Adrenal gland | − to +++ | Necrosis, mononuclear infiltrate, hemorrhages | − | Corticotrophic and corticotropic cells |
| Muscle | + | Congestion | − | − |
Tissues were collected from 2 birds per age group (n = 4).
Histologic lesion scores by HE staining: −, no lesions; +, mild lesions; ++, moderate lesions; +++, severe lesions.
IHC staining scores: −, no antigen staining; +, infrequent staining; ++, common staining; +++, widespread staining.
Fig 2.
Histopathology and immunohistochemical staining for avian influenza virus antigen in tissues of chickens infected with the Mexican H7N3 HPAI virus performed at 2 days postinoculation. Virus is stained red. (A) Nasal epithelium, showing severe necrotizing rhinitis with submucosal congestion and edema and glandular hyperplasia. (Inset) Demonstration of viral antigen in the epithelial cells and debris, vascular endothelial cells, and infiltrating mononuclear cells. (B) Trachea, showing congestion and lymphocyte, macrophage, and heterophil infiltration in the submucosa. (Inset) viral antigen staining present in the vascular endothelial cells (arrow) and infiltrating macrophages (inset). (C) Lung, showing severe lymphoplasmacytic interstitial pneumonia with necrosis (arrow). (Inset) Viral antigen staining in epithelium of air capillaries, mononuclear cells, and necrotic debris. (D) Cerebrum, showing mild neuronal necrosis and focal gliosis (arrow). (Inset) Viral antigen staining in neurons and necrotic debris. (E) Comb, showing severe congestion. (Inset) Viral antigen staining in vascular endothelial cells (arrow) and infiltrating mononuclear cells. (F) Eyelid, showing severe congestion. (Inset) Viral antigen staining in vascular endothelial cells (arrow) and infiltrating mononuclear cells. (G) Spleen, showing splenitis, moderate lymphocyte depletion, and cell death with heterophilic inflammation. (Inset) Viral antigen staining in mononuclear cells and necrotic debris. (H) Cecal tonsil, showing moderate lymphocyte depletion and cell death with heterophilic inflammation. (Inset) Viral antigen staining in mononuclear cells and necrotic debris. Magnifications, ×400.
In the respiratory system, severe, diffuse catarrhal rhinitis and mild to moderate tracheitis were present in all birds examined. Submucosal vasculitis with mild necrosis and infiltration of heterophils, macrophages, plasma cells, and lymphocytes was present in all birds examined. In the lungs, there was moderate to severe multifocal interstitial pneumonia with infiltrating heterophils, lymphocytes, and macrophages. Multifocal areas of necrosis and mild interstitial edema were also present with moderate airsacculitis, characterized by multifocal thickening with edema and infiltration of moderate numbers of lymphocytes, macrophages, plasma cells, and heterophils.
Severe, diffuse, heterophilic, and histiocytic inflammation of the eyelid and comb was common in all birds. There were moderate to severe edema and congestion in the dermis and multifocal moderate heterophilic dermatitis, characterized by infiltration of moderate numbers of heterophils and macrophages; multifocally, endothelial cells were necrotic or hypertrophic.
In the heart, mild, multifocal myocyte necrosis was present. Lesions in the brains consisted of scattered, occasional neuronal necrosis with gliosis. Lesions in the digestive tract were confined to the lymphoid-associated tissues, including the esophageal-proventricular junction, cecal tonsils, mucosa of the cecum and jejunum, and Peyer's patches of the small intestine. In the lamina propria of the proventriculus there were moderate numbers of infiltrating lymphocytes, plasma cells, macrophages, and heterophils with multifocal small foci of necrosis. Mild degeneration of individual pancreatic acinar cells was also observed.
In the liver, mild to moderate multifocal fibrinoid necrosis, infiltration of heterophils and macrophages, and multifocal periportal moderate lymphohistiocytic hepatitis were common.
In the spleen, there was moderate to severe depletion of the white pulp with multifocal lymphocytic necrosis and diffuse moderate histiocytic splenitis. In the thymus and cloacal bursa, mild to moderate, diffuse lymphocyte depletion and necrosis were present in all birds. The kidneys presented mild multifocal tubular necrosis.
By immunohistochemistry, viral antigen was detected in most tissues, with comparable patterns being found in all four chickens (Table 1; Fig. 2). Virus antigen was often associated with histologic lesions, although virus antigen was also present in areas without detectable lesions. An interesting finding was that viral antigen was detected in most endothelial cells in the nasal cavity, trachea, eyelid, and comb, while in all other tissues viral antigen was detected in only a few, individual endothelial cells. Staining for virus antigen was also present in areas of necrosis and infiltrating mononuclear cells in many tissues, including lymphoid tissues, lung, brain, liver, and spleen. Virus antigen was also observed in the parenchymal cells of some organs, including cardiac myocytes, Kupffer cells, hepatocytes, microglial cells and neurons, lung cells, kidney tubular epithelial and glomerular cells, cells in the feather follicle epithelium, and infiltrating inflammatory cells.
Virus replication in tissues.
Virus replication in brain, heart, lung, spleen, and muscle tissue from sham-vaccinated chickens following intranasal infection with the Ck/J/12 H7N3 HPAI virus was examined at 2 days p.c. As shown in Table 2, high virus titers were detected in all organs examined, indicating extensive systemic viral replication, with the highest viral titers found in lung (>106.8 EID50s per gram of tissue) and the lowest found in muscle (>104.1 EID50s per gram of tissue).
Table 2.
Virus titers in tissues from chickens infected with the Mexican H7N3 HPAI virusa
| Chicken no., expt no. | Virus titer (log10 EID50/g of tissue) |
||||
|---|---|---|---|---|---|
| Lung | Spleen | Heart | Brain | Muscle | |
| 1, 1 | 7.1 | 5.1 | 6.1 | 5.1 | 6.1 |
| 2, 2 | 6.8 | 5.9 | 6.5 | 5.8 | 5.5 |
| 1, 2 | 7.1 | 5.7 | 7.1 | 6.5 | 4.7 |
| 2, 2 | 6.3 | 5.1 | 5.3 | 5.5 | 4.1 |
Tissues collected at 2 days postchallenge were homogenized to a 10% (wt/vol) final concentration. Tenfold dilutions of the 10% homogenates (100 μl) were inoculated into 10-day-old embryonated chicken eggs (ECE), and virus titers were calculated. The threshold of detection was 101.9 EID50/g of tissue.
Phylogenetic analysis.
The complete coding sequences for all eight gene segments of both Ck/J/12 and CT/MX/06 viruses were determined and compared. The nucleotide sequence of Ck/J/12 virus had a high similarity of greater than 97% to the sequences of wild bird viruses from North America in all eight gene segments by BLAST analysis. However, in analyzing the individual genes, the highest sequence similarity was not to the genes of a single virus, but each gene was closest to the gene of different influenza viruses of various hemagglutinin and neuraminidase subtypes. The CT/MX/06 virus, the only H7 wild bird virus isolated in Mexico, also had high similarity to other North American influenza viruses. Although some relatedness exists between the CT/MX/06 virus and the Ck/J/12 virus, the duck isolate was clearly not the precursor virus. The nucleotide sequence relatedness between the CT/MX/06 virus and the HPAI virus isolate varied from 90.5% to 98.1% for all gene segments examined (data not shown).
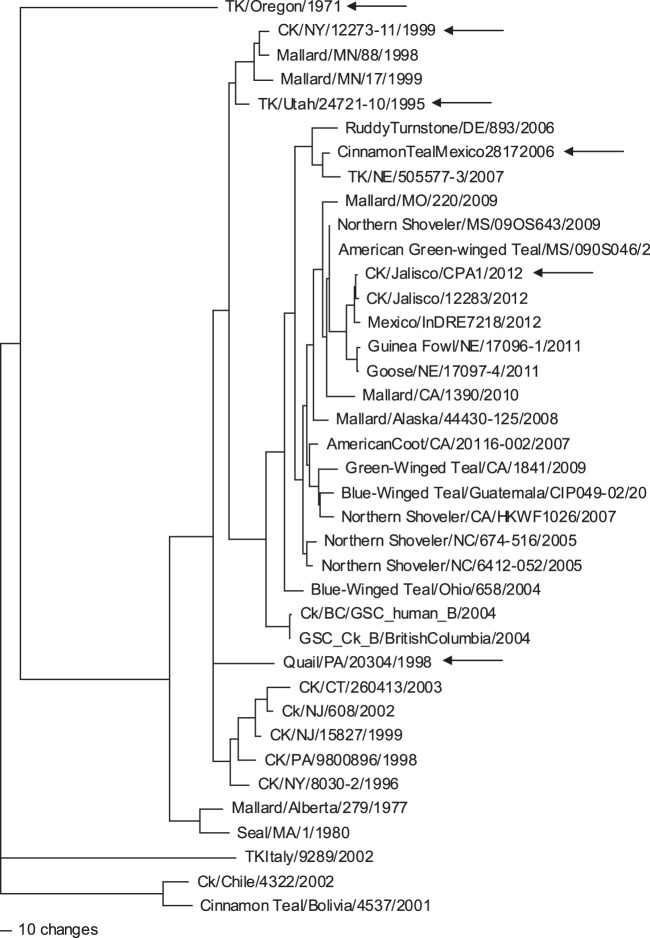
The hemagglutinin gene of Ck/J/12 virus had a 24-nucleotide insert at the hemagglutinin cleavage site. On BLAST analysis, the 24 nucleotides had 100% sequence identity to chicken 28S rRNA (GenBank accession numbers EF552813.1, AC147447.3, and AC186855.3), indicating that the most likely source of the insert sequence was the host. The nucleotide sequence of the H7 gene was most closely related to that of A/Northern Shoveler/Mississippi/09OS643/2009 (H7N7) virus, with 97% sequence similarity. This wild bird virus was collected as part of routine surveillance, and it had a typical low-pathogenic cleavage site with no insert. A nucleotide phylogenetic analysis (Fig. 3) again showed that the most closely related virus sequences were primarily from wild birds, with the most closely related poultry isolate being from a guinea fowl (H7N9) recovered in Nebraska in 2011.
Fig 3.
Phylogenetic tree illustrating the similarity between the nucleotide sequences of the H7 HA gene from the isolates used in these studies (arrows). Sequences were aligned with the Clustal V program (Lasergene, version 10.0; DNAStar, Madison, WI). Phylogenetic analysis for the H7 gene was performed with PAUP*, version 4.0b10 (Sinauer Associates, Inc., Sunderland, MA), using the maximum parsimony tree-building method with heuristic search and 500 bootstrap replicates.
Protection conferred by vaccination using U.S. H7 vaccines.
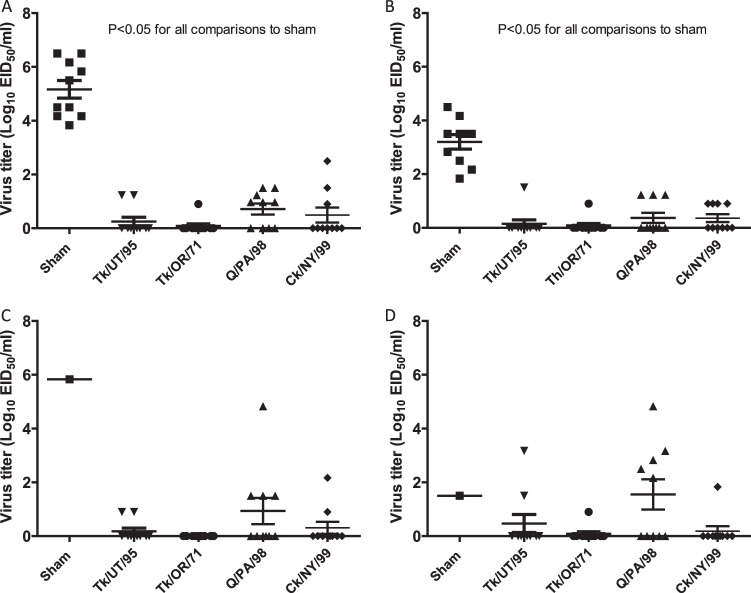
Compared to the 100% mortality by day 4 p.c. observed in sham-vaccinated birds challenged with the Ck/J/12 H7N3 HPAI virus in the first experiment, chickens vaccinated with the Ck/NY/99, Tk/UT/95, or Tk/OR/71vaccine experienced no mortality during the 14-day p.c. period (Fig. 4A). Infection of sham-vaccinated chickens resulted in seven deaths on day 2 p.c., two deaths on day 3 p.c., and one death at day 4 p.c., resulting in a mean death time (MDT) of 2.4 days p.c. The group of birds vaccinated with Q/PA/98 demonstrated 90% protection, with one bird dying on day 6 p.c. All vaccinated groups were significantly protected from mortality, whereas sham-vaccinated birds were not (P < 0.05). Three weeks after vaccination, almost all birds receiving AIV antigen demonstrated an antibody response, with mean HI titers being approximately 8 log2 units (Fig. 5A) when the challenge Ck/J/12 H7N3 HPAI virus was used as the HI antigen. The exception was the birds receiving the Q/PA/98 vaccine, which had a mean HI titer of approximately 4.5 log2 units. However, all groups of vaccinated birds demonstrated a statistically significant increase of titers following challenge (P < 0.01). On day 2 following challenge, all sham-vaccinated birds shed significantly more virus by both the oropharyngeal and cloacal routes (Fig. 6A and B). Only a few birds vaccinated with either Ck/NY/99, Tk/UT/95, Tk/OR/71, or Q/PA/98 shed virus at days 2 and 4 p.c., but the Q/PA/98-vaccinated group consistently had more birds shedding more virus than the other groups (Fig. 6A to D).
Fig 4.
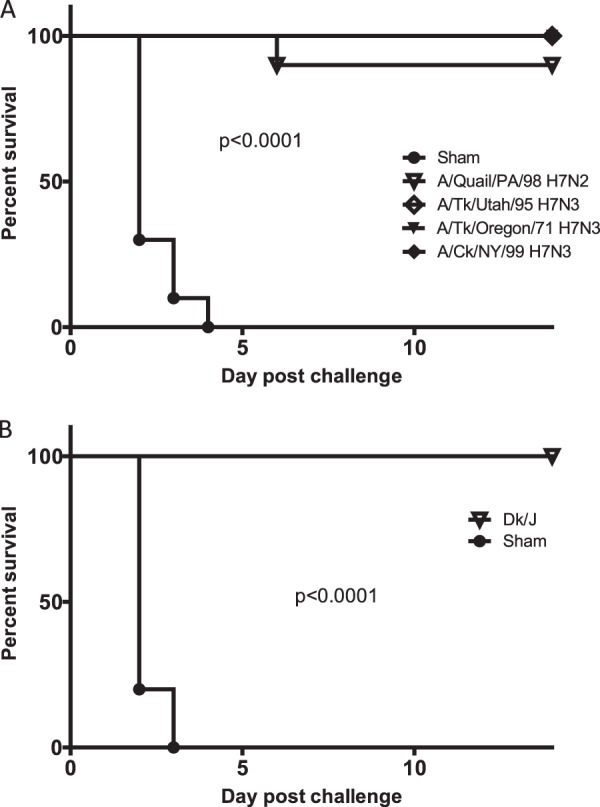
Kaplan-Meier survival plots for challenge experiments I and II. (A) Percent survival versus day postchallenge with A/Ck/Jalisco/CPA1/2012 H7N3 HPAI virus for groups vaccinated with U.S. isolates A/turkey/Utah/24721-10/1995 (H7N3), A/turkey/Oregon/1971 (H7N3), A/chicken/New York/12273-11/1999 (H7N3), and A/quail/Pennsylvania/20304/1998 (H7N2). (B) Birds vaccinated with A/Cinnamon Teal/Mexico/2817/2006 (H7N3) and challenged with A/chicken/Jalisco/CPA1/2012. Statistical significance in survival curves between the sham-vaccinated and vaccinated groups was determined by the log-rank (Mantel-Cox) test (P < 0.05).
Fig 5.
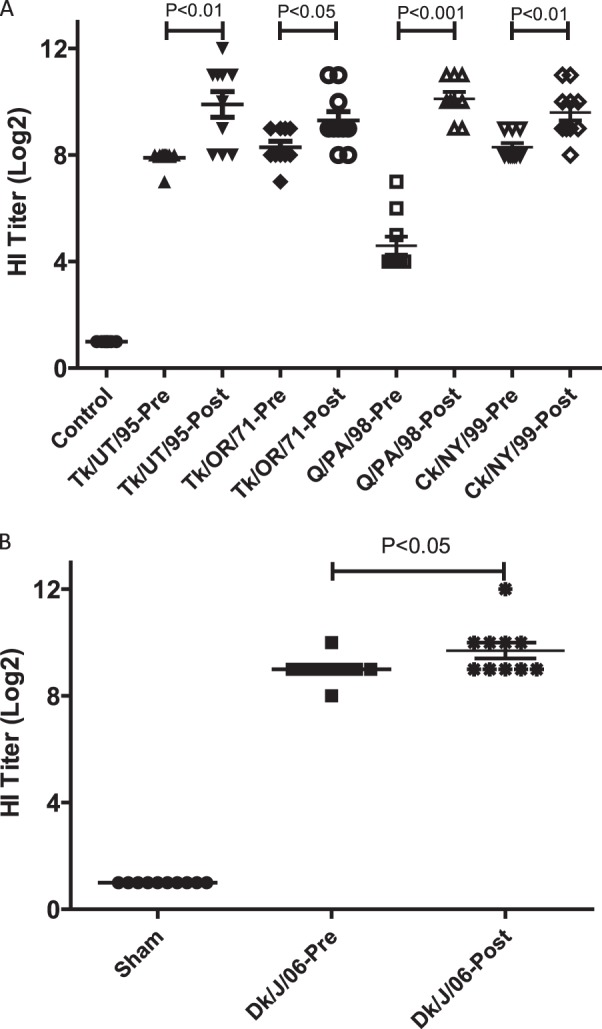
Individual (log2) pre- and postchallenge HI titers for groups in experiments I (A) and II (B). Birds in experiment I were vaccinated with a single dose at 7 weeks of age and challenged at 10 weeks of age. Serum was taken from survivors at 2 weeks p.c. Birds in experiment II received a single vaccination at 2 weeks of age and were challenged at 5 weeks of age. Serum was taken from survivors at 2 weeks p.c. Statistical significance between mean titers was determined by ANOVA using Tukey's multiple-comparison test (P < 0.05).
Fig 6.
Isolation of the Ck/J/12 H7N3 HPAI virus from chickens in experiment I. Birds were vaccinated with a single dose containing 512 HA units of inactivated Tk/UT/95, Tk/OR/71, Q/PA/98, or Ck/NY/99 virus at 7 weeks of age and challenged with Ck/J/12 virus at 10 weeks of age. Oropharyngeal (A and C) and cloacal (B and D) swab specimens were taken on days 2 (A and B) and 4 (C and D) postchallenge. Viral titers are expressed as the log10 number of EID50s per ml. The lower limit of detection was 0.9 log10 EID50 per ml.
Protection conferred by vaccination using the Mexican H7N3 LPAI virus.
Sham-vaccinated birds demonstrated 100% mortality by day 3 p.c. when challenged with Ck/J/12 H7N3 HPAI virus (Fig. 4b). Eight of these chickens died or were euthanized at day 2 p.c., and two died at day 3 p.c., with the MDT for the group being 2.2 days p.c. In contrast, birds receiving the CT/MX/06 vaccine were 100% protected from mortality when challenged at 3 weeks postvaccination. Postvaccination titers of antibody to the challenge virus were evaluated prior to challenge. Median antibody titers were determined by the HI assay to be 9 log2 units in the CT/MX/06-vaccinated group, whereas no antibodies were detected in the sham-vaccinated birds (Fig. 5b). Following challenge, antibody titers in CT/MX/06-vaccinated birds increased to approximately 10 log2 units, which was significantly different from the prechallenge titers. Not unexpectedly, both the incidence and the level of viral shedding were significantly reduced in the CT/MX/06-vaccinated birds. Nine out of 10 sham-vaccinated birds shed detectable levels of virus by the oropharyngeal route at day 2 p.c., and 7/10 birds shed through the cloacal route (Fig. 7). In sham-vaccinated birds, the mean levels of viral shedding in oropharyngeal and cloacal swabs were determined to be 104.5 EID50s/ml and 102.3 EID50s/ml, respectively. In contrast, 1/10 and 0/10 CT/MX/06-vaccinated birds shed virus on day 2 by the oropharyngeal and cloacal routes, respectively, with the one positive swab specimen containing 100.9 EID50/ml. On day 4 p.c., 2/10 and 1/10 CT/MX/06-vaccinated birds shed virus by the oral and cloacal routes, respectively, with all titers being 100.9 EID50/ml (data not shown).
Fig 7.
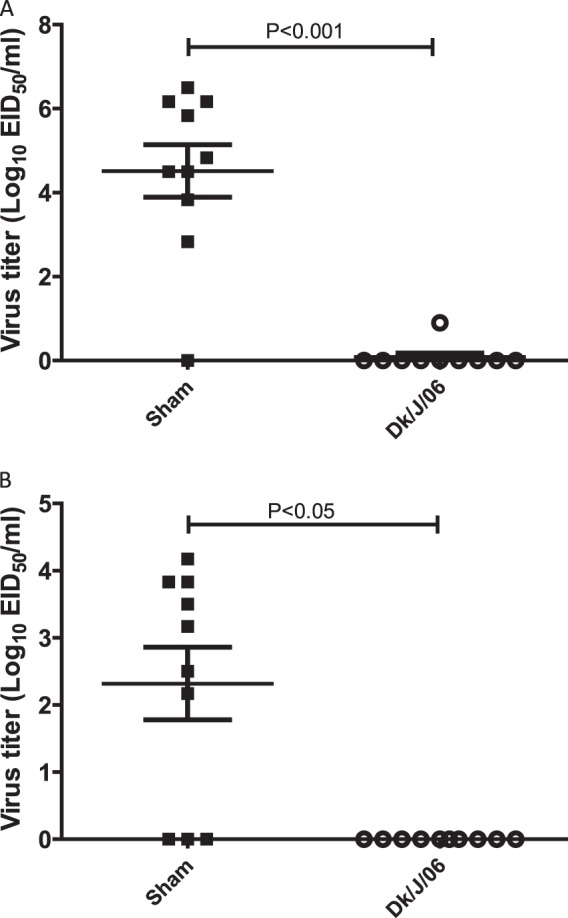
Isolation of the Ck/J/12 H7N3 HPAI virus in chickens in experiment II. Birds were vaccinated with a single dose containing 512 HA units of inactivated CT/MX/06 at 2 weeks of age and challenged with Ck/J/12 at 5 weeks of age. Oral (A) and cloacal (B) swab specimens were taken on day 2 postchallenge. Viral titers are expressed as the log10 number of EID50s per ml. The lower limit of detection was 0.9 log10 EID50 per ml.
DISCUSSION
On 21 June 2012, an immediate notification of the isolation of H7N3 HPAI virus in poultry in Jalisco, Mexico, was submitted to the World Organization for Animal Health (International Office of Epizootics). The virus was primarily isolated from egg-laying commercial birds, with no backyard birds and a few wild birds being infected. Although the origins of the original index case remain unknown, H7N3 LPAI virus had been isolated from wild birds in previous years in Canada, Mexico, and the United States, leading to the conclusions that this was a wild-bird-origin LPAI virus transmitted to poultry and mutating to an HPAI virus (16–18, 27). While the outbreak has been contained to that region, no information regarding the circulation of H7N3 LPAI virus in poultry prior to detection of the HPAI virus is available. To date, greater than 11 million birds have died and another 10 million were destroyed during the event. Approximately 166 million doses of inactivated H7N3 vaccine have been applied to poultry to curb the outbreak (16). The surveillance and epidemiological investigation yielded greater than 81,000 samples for testing from the region, with 44 positive H7N3 HPAI virus isolations being from commercial layers and 2 being from wild birds (a common grackle [Quiscalus quiscula] and a barn swallow [Hirundo rustica]). The outbreak was thought to have been contained with no new outbreaks for almost 4 months, but new outbreaks in poultry were reported in January 2013, indicating a need for further control and surveillance (28).
In these studies, the Mexican 2012 H7N3 HPAI virus produced severe clinical disease, lesions, and high mortality, similar to the findings reported for other HPAI viruses (29–32). However, respiratory signs were more prominent than previously described for other HPAI viruses. The Asian lineage H5N1 HPAI viruses have a major vascular endothelial cell tropism, and generalized circulatory collapse is considered the most important mechanism involved in production of disease and sudden death. Other HPAI viruses, including the 1994 Australian H7N3, 1999-2000 Italian H7N1, and 1991 England H5N1 avian influenza viruses, also showed vascular endothelial tropism inducing cell death and subsequent production of edema and necrosis (33–39). In our study, strong staining for virus antigen was observed in the vascular endothelial cells in the nasal epithelium, trachea, comb, and eyelid, with the consequent edema and hemorrhage. However, virus staining in the vascular endothelium of other organs and tissues was infrequent. This restricted differential tropism for endothelial cells was also observed with another H7 HPAI virus of the H7N7 subtype (39). In the severely edematous wattle skin of chickens affected in this outbreak, most endothelial cells contained virus antigen, while in all other tissues, virus antigen was detected in only a few endothelial cells (39). In our study, the extensive virus replication in the vascular endothelial cells and epithelial cells of the upper respiratory tract of the chickens also explains the severe respiratory signs observed in the infected birds, including the copious nasal discharge and the resulting gasping, dyspnea, and suffocation.
The Ck/J/12 virus had a hemagglutinin insert that led to the highly pathogenic phenotype. All characterized HP H7 viruses have insertions of 2 to 10 additional amino acids at the cleavage site. The mechanism for these introductions appears to be the result of homologous or nonhomologous recombination (8, 15, 40, 41). The Ck/J/12 virus had an insert of 24 nucleotides, or 8 amino acids (aa), that was consistent with the sequences of viruses responsible for recent HP H7 virus outbreaks from Canada (8-aa insert) and Chile (10-aa insert) (8, 15). The source of the insert at the cleavage site for H7 viruses appears to be the host's (chicken's) 28S rRNA on the basis of the 100% sequence homology. The source of the insert of the previously described Canadian and Chilean viruses was from genes from the same virus, the matrix and nucleoprotein genes, respectively. However, a laboratory-derived mutant that had an HP phenotype with an insert of 28S rRNA has also been described (40).
Vaccines for AIV and their field application can be an effective tool within a comprehensive control program that includes education, enhanced biosecurity (including quarantine, cleaning and disinfection, movement controls for poultry, etc.), diagnostics and surveillance, elimination of infected poultry, and potentially decreased poultry susceptibility (i.e., after vaccination) (42). One of the goals of this study was to evaluate both Mexican and U.S. H7 LPAI viruses as potential vaccines for control of the H7N3 HPAI virus. Administration of a single dose of inactivated vaccine to birds conferred immunity against lethal challenge with the Mexican H7 HPAI virus. While all vaccines protected against challenge, the Q/PA/98 isolate offered the least protection, which prior to challenge correlated with decreased HI titers against the challenge virus when using the challenge virus as the antigen in the test. This group also demonstrated the highest incidence of shedding among all vaccinated groups. Since the HA contents of all vaccines were equal, differences in protection, titers, and shedding cannot be attributed to differences in antigen load in the vaccine. The HA sequence of this vaccine isolate shared the least similarity to the HA sequence of the challenge virus, corroborated by the low antigenic relatedness, and it is known that greater genetic similarity between HAs of vaccine and field viruses results in reduced mortality and shedding (43–45). Thus, minor antigenic differences between the isolates likely contributed to decreased protection in this group. We have previously demonstrated that inactivated H5 vaccines provided protection and reduced shedding when the HA sequences differed by up to 13% (46–48). In those studies, the isolates used were collected over a 38-year period, yet they still provided protection against recent HPAI virus. More recently, Abbas et al. also demonstrated that many different H7 isolates from different countries could provide protection against different Pakistani H7N3 HPAI viruses (49). Taken together, our results support previous findings that vaccines better matched to field isolates provide increased protection and decrease the risk of transmission by limiting virus shedding.
The efficacy of these vaccines was tested at 21 days postvaccination, which is typical for experimental vaccines to allow adaptive immune stimulation. Although the birds affected in the outbreak were laying hens (egg type), we also tested broiler (meat-type) birds with the U.S. isolate vaccines to determine if there was a difference in response on the basis of the type of chicken involved. The results demonstrated that three of the isolates, including the two USDA master seeds previously used as part of a vaccine bank, protected against challenge with this isolate. Although we tested only a single dose, layer-type birds typically require booster vaccinations with inactivated vaccines because they live longer. We have previously demonstrated that multiple vaccine applications reduced virus shedding in turkeys challenged with H7N2 LPAI virus (22). Whether the vaccines constructed here induce protective immunity at times earlier than 3 weeks remains to be determined, as does the duration of immunity.
In summary, we have described the pathobiological characteristics of the new HPAI virus Ck/J/12 in chickens and determined its tissue tropism. Unlike H5N1 HPAI virus, the H7N3 virus induced stronger respiratory signs. In addition, the viral tropism was less specific for the vascular endothelium, but the virus did produce a systemic infection with damage to multiple visceral organs. Sequence analysis clearly determined that this virus is of North American lineage and contains a previously reported insertion of host 28S rRNA at the HA cleavage site (40). Finally, the majority of vaccinated birds were shown to be protected from clinical signs and death and had reduced viral shedding. Thus, the approved H7 vaccine master seed isolates can be produced for use in poultry to provide effective options in the event of additional HPAI H7N3 virus outbreaks.
ACKNOWLEDGMENTS
We thank Aniko Zsak, Diane Smith, Scott Lee, Michele Edenfield, and Suzanne DeBois for excellent technical assistance. In addition, we acknowledge Roger Brock and Ronald Graham for animal care assistance.
This research was supported by funding from USDA, ARS, CRIS project 6612-32000-062-00D.
Footnotes
Published ahead of print 12 June 2013
REFERENCES
- 1. Cox NJ, Subbarao K. 2000. Global epidemiology of influenza: past and present. Annu. Rev. Med. 51:407–421 [DOI] [PubMed] [Google Scholar]
- 2. Ada GL, Jones PD. 1986. The immune response to influenza infection. Curr. Top. Microbiol. Immunol. 128:1–54 [DOI] [PubMed] [Google Scholar]
- 3. Fouchier RA, Munster V, Wallensten A, Bestebroer TM, Herfst S, Smith D, Rimmelzwaan GF, Olsen B, Osterhaus AD. 2005. Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. J. Virol. 79:2814–2822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lambkin R, Dimmock NJ. 1996. Longitudinal study of an epitope-biased serum haemagglutination-inhibition antibody response in rabbits immunized with type A influenza virions. Vaccine 14:212–218 [DOI] [PubMed] [Google Scholar]
- 5. Webby RJ, Webster RG. 2003. Are we ready for pandemic influenza? Science 302:1519–1522 [DOI] [PubMed] [Google Scholar]
- 6. Halvorson DA, Kelleher CJ, Senne DA. 1985. Epizootiology of avian influenza: effect of season on incidence in sentinel ducks and domestic turkeys in Minnesota. Appl. Environ. Microbiol. 49:914–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hirst M, Astell CR, Griffith M, Coughlin SM, Moksa M, Zeng T, Smailus DE, Holt RA, Jones S, Marra MA, Petric M, Krajden M, Lawrence D, Mak A, Chow R, Skowronski DM, Tweed SA, Goh S, Brunham RC, Robinson J, Bowes V, Sojonky K, Byrne SK, Li Y, Kobasa D, Booth T, Paetzel M. 2004. Novel avian influenza H7N3 strain outbreak, British Columbia. Emerg. Infect. Dis. 10:2192–2195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Suarez DL, Senne DA, Banks J, Brown IH, Essen SC, Lee CW, Manvell RJ, Mathieu-Benson C, Moreno V, Pedersen JC, Panigrahy B, Rojas H, Spackman E, Alexander DJ. 2004. Recombination resulting in virulence shift in avian influenza outbreak, Chile. Emerg. Infect. Dis. 10:693–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Webster RG, Kawaoka Y, Bean WJ., Jr 1986. Molecular changes in A/chicken/Pennsylvania/83 (H5N2) influenza virus associated with acquisition of virulence. Virology 149:165–173 [DOI] [PubMed] [Google Scholar]
- 10. Wood GW, Banks J, McCauley JW, Alexander DJ. 1994. Deduced amino acid sequences of the haemagglutinin of H5N1 avian influenza virus isolates from an outbreak in turkeys in Norfolk, England. Arch. Virol. 134:185–194 [DOI] [PubMed] [Google Scholar]
- 11. Wood GW, McCauley JW, Bashiruddin JB, Alexander DJ. 1993. Deduced amino acid sequences at the haemagglutinin cleavage site of avian influenza A viruses of H5 and H7 subtypes. Arch. Virol. 130:209–217 [DOI] [PubMed] [Google Scholar]
- 12. Max V, Herrera J, Moreira R, Rojas H. 2007. Avian influenza in Chile: a successful experience. Avian Dis. 51:363–365 [DOI] [PubMed] [Google Scholar]
- 13. Berhane Y, Hisanaga T, Kehler H, Neufeld J, Manning L, Argue C, Handel K, Hooper-McGrevy K, Jonas M, Robinson J, Webster RG, Pasick J. 2009. Highly pathogenic avian influenza virus A (H7N3) in domestic poultry, Saskatchewan, Canada, 2007. Emerg. Infect. Dis. 15:1492–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pasick J, Berhane Y, Hooper-McGrevy K. 2009. Avian influenza: the Canadian experience. Rev. Sci. Tech. 28:349–358 [DOI] [PubMed] [Google Scholar]
- 15. Pasick J, Handel K, Robinson J, Copps J, Ridd D, Hills K, Kehler H, Cottam-Birt C, Neufeld J, Berhane Y, Czub S. 2005. Intersegmental recombination between the haemagglutinin and matrix genes was responsible for the emergence of a highly pathogenic H7N3 avian influenza virus in British Columbia. J. Gen. Virol. 86:727–731 [DOI] [PubMed] [Google Scholar]
- 16. OIE 2012. Update on highly pathogenic avian influenza in animals (type H5 and H7). OIE, Paris, France: http://www.oie.int/animal-health-in-the-world/update-on-avian-influenza/2012 [Google Scholar]
- 17. Wainwright S, Trevennec C, Claes F, Vargas-Terán M, Martin V, Lubrotha J. 2012. Highly pathogenic avian influenza in Mexico (H7N3). Food and Agriculture Organization, Rome, Italy: http://www.fao.org/docrep/016/an395e/an395e.pdf [Google Scholar]
- 18. Cuevas-Domínguez EA, González-Guzmán S, Quintana-López JA, Loza-Rubio E, González-Rebeles C, García-Espinosa G. 2009. Detection of orthomyxovirus H7N3 in waterfowl from the State of Mexico. REDVET-Rev. Electron. Vet. 10, no 4 [Google Scholar]
- 19. Senne DA. 2008. Virus propagation in embryonating eggs, p 204–208 In Dufour-Zavala L. (ed), A laboratory manual for the isolation, identification and characterization of avian pathogens, vol 5 American Association of Avian Pathologists, Athens, GA [Google Scholar]
- 20. King DJ. 1991. Evaluation of different methods of inactivation of Newcastle disease virus and avian influenza virus in egg fluids and serum. Avian Dis. 35:505–514 [PubMed] [Google Scholar]
- 21. Pedersen JC. 2008. Hemagglutination-inhibition test for avian influenza virus subtype identification and the detection and quantitation of serum antibodies to the avian influenza virus. Methods Mol. Biol. 436:53–66 [DOI] [PubMed] [Google Scholar]
- 22. Tumpey TM, Kapczynski DR, Swayne DE. 2004. Comparative susceptibility of chickens and turkeys to avian influenza A H7N2 virus infection and protective efficacy of a commercial avian influenza H7N2 virus vaccine. Avian Dis. 48:167–176 [DOI] [PubMed] [Google Scholar]
- 23. Swayne DE, Senne DA, Beard CW. 1996. Avian influenza, p 150–155 In Swayne DE. (ed), A laboratory manual for the isolation and identification of avian pathogens, 4th ed American Association of Avian Pathologists, Kennett Square, PA [Google Scholar]
- 24. Pantin-Jackwood MJ, Swayne DE. 2007. Pathobiology of Asian highly pathogenic avian influenza H5N1 virus infections in ducks. Avian Dis. 51:250–259 [DOI] [PubMed] [Google Scholar]
- 25. Perdue ML, Latimer J, Greene C, Holt P. 1994. Consistent occurrence of hemagglutinin variants among avian influenza virus isolates of the H7 subtype. Virus Res. 34:15–29 [DOI] [PubMed] [Google Scholar]
- 26. Perkins LE, Swayne DE. 2001. Pathobiology of A/chicken/Hong Kong/220/97 (H5N1) avian influenza virus in seven gallinaceous species. Vet. Pathol. 38:149–164 [DOI] [PubMed] [Google Scholar]
- 27. Liechti R, Gleizes A, Kuznetsov D, Bougueleret L, Le Mercier P, Bairoch A, Xenarios I. 2010. OpenFluDB, a database for human and animal influenza virus. Database (Oxford) 2010:baq004. 10.1093/database/baq004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. OIE 2013. Update on highly pathogenic avian influenza in animals (type H5 and H7). OIE, Paris, France: http://www.oie.int/animal-health-in-the-world/update-on-avian-influenza/2013 [Google Scholar]
- 29. Pantin-Jackwood MJ, Swayne DE. 2009. Pathogenesis and pathobiology of avian influenza virus infection in birds. Rev. Sci. Tech. 28:113–136 [PubMed] [Google Scholar]
- 30. Swayne DE. 1997. Pathobiology of H5N2 Mexican avian influenza virus infections of chickens. Vet. Pathol. 34:557–567 [DOI] [PubMed] [Google Scholar]
- 31. Swayne DE, Pantin-Jackwood M. 2006. Pathogenicity of avian influenza viruses in poultry. Dev. Biol. (Basel) 124:61–67 [PubMed] [Google Scholar]
- 32. Swayne DE, Slemons RD. 2008. Using mean infectious dose of high- and low-pathogenicity avian influenza viruses originating from wild duck and poultry as one measure of infectivity and adaptation to poultry. Avian Dis. 52:455–460 [DOI] [PubMed] [Google Scholar]
- 33. Capua I, Terregino C, Cattoli G, Mutinelli F, Rodriguez JF. 2003. Development of a DIVA (differentiating infected from vaccinated animals) strategy using a vaccine containing a heterologous neuraminidase for the control of avian influenza. Avian Pathol. 32:47–55 [DOI] [PubMed] [Google Scholar]
- 34. Hooper PT. 1989. Lesions in chickens experimentally infected with 1985 H7N7 avian influenza virus. Aust. Vet. J. 66:155–156 [DOI] [PubMed] [Google Scholar]
- 35. Hooper PT, Russell GW, Selleck PW, Stanislawek WL. 1995. Observations on the relationship in chickens between the virulence of some avian influenza viruses and their pathogenicity for various organs. Avian Dis. 39:458–464 [PubMed] [Google Scholar]
- 36. Kobayashi Y, Horimoto T, Kawaoka Y, Alexander DJ, Itakura C. 1996. Neuropathological studies of chickens infected with highly pathogenic avian influenza viruses. J. Comp. Pathol. 114:131–147 [DOI] [PubMed] [Google Scholar]
- 37. Kobayashi Y, Horimoto T, Kawaoka Y, Alexander DJ, Itakura C. 1996. Pathological studies of chickens experimentally infected with two highly pathogenic avian influenza viruses. Avian Pathol. 25:285–304 [DOI] [PubMed] [Google Scholar]
- 38. Mo IP, Brugh M, Fletcher OJ, Rowland GN, Swayne DE. 1997. Comparative pathology of chickens experimentally inoculated with avian influenza viruses of low and high pathogenicity. Avian Dis. 41:125–136 [PubMed] [Google Scholar]
- 39. van Riel D, van den Brand JM, Munster VJ, Besteboer TM, Fouchier RA, Osterhaus AD, Kuiken T. 2009. Pathology and virus distribution in chickens naturally infected with highly pathogenic avian influenza A virus (H7N7) during the 2003 outbreak in The Netherlands. Vet. Pathol. 46:971–976 [DOI] [PubMed] [Google Scholar]
- 40. Khatchikian D, Orlich M, Rott R. 1989. Increased viral pathogenicity after insertion of a 28S ribosomal RNA sequence into the haemagglutinin gene of an influenza virus. Nature 340:156–157 [DOI] [PubMed] [Google Scholar]
- 41. Orlich M, Gottwald H, Rott R. 1994. Nonhomologous recombination between the hemagglutinin gene and the nucleoprotein gene of an influenza virus. Virology 204:462–465 [DOI] [PubMed] [Google Scholar]
- 42. Swayne DE, Kapczynski D. 2008. Strategies and challenges for eliciting immunity against avian influenza virus in birds. Immunol. Rev. 225:314–331 [DOI] [PubMed] [Google Scholar]
- 43. Kapczynski DR, Swayne DE. 2009. Influenza vaccines for avian species. Curr. Top. Microbiol. Immunol. 333:133–152 [DOI] [PubMed] [Google Scholar]
- 44. Lee YJ, Sung HW, Choi JG, Lee EK, Jeong OM, Kwon YK, Kwon JH, Song CS, Kimd JH. 2007. Effects of homologous and heterologous neuraminidase vaccines in chickens against H5N1 highly pathogenic avian influenza. Avian Dis. 51:476–478 [DOI] [PubMed] [Google Scholar]
- 45. Swayne DE, Perdue ML, Beck JR, Garcia M, Suarez DL. 2000. Vaccines protect chickens against H5 highly pathogenic avian influenza in the face of genetic changes in field viruses over multiple years. Vet. Microbiol. 74:165–172 [DOI] [PubMed] [Google Scholar]
- 46. Swayne DE, Beck JR, Garcia M, Stone HD. 1999. Influence of virus strain and antigen mass on efficacy of H5 avian influenza inactivated vaccines. Avian Pathol. 28:245–255 [DOI] [PubMed] [Google Scholar]
- 47. Swayne DE, Beck JR, Perdue ML, Beard CW. 2001. Efficacy of vaccines in chickens against highly pathogenic Hong Kong H5N1 avian influenza. Avian Dis. 45:355–365 [PubMed] [Google Scholar]
- 48. Swayne DE, Garcia M, Beck JR, Kinney N, Suarez DL. 2000. Protection against diverse highly pathogenic H5 avian influenza viruses in chickens immunized with a recombinant fowlpox vaccine containing an H5 avian influenza hemagglutinin gene insert. Vaccine 18:1088–1095 [DOI] [PubMed] [Google Scholar]
- 49. Abbas MA, Spackman E, Fouchier R, Smith D, Ahmed Z, Siddique N, Sarmento L, Naeem K, McKinley ET, Hameed A, Rehmani S, Swayne DE. 2011. H7 avian influenza virus vaccines protect chickens against challenge with antigenically diverse isolates. Vaccine 29:7424–7429 [DOI] [PubMed] [Google Scholar]