Abstract
Highly pure (>95%) terminally differentiated neurons derived from pluripotent stem cells appear healthy at 2 weeks after infection with varicella-zoster virus (VZV), and the cell culture medium contains no infectious virus. Analysis of the healthy-appearing neurons revealed VZV DNA, transcripts, and proteins corresponding to the VZV immediate early, early, and late kinetic phases of replication. Herein, we further characterized virus in these neuronal cells, focusing on (i) transcription and expression of late VZV glycoprotein C (gC) open reading frame 14 (ORF14) and (ii) ultrastructural features of virus particles in neurons. The analysis showed that gC was not expressed in most infected neurons and gC expression was markedly reduced in a minority of VZV-infected neurons. In contrast, expression of the early-late VZV gE glycoprotein (ORF68) was abundant. Transcript analysis also showed decreased gC transcription compared with gE. Examination of viral structure by high-resolution transmission electron microscopy revealed fewer viral particles than typically observed in cells productively infected with VZV. Furthermore, viral particles were more aberrant, in that most capsids in the nuclei lacked a dense core and most enveloped particles in the cytoplasm were light particles (envelopes without capsids). Together, these results suggest a considerable deficiency in late-phase replication and viral assembly during VZV infection of neurons in culture.
INTRODUCTION
Varicella-zoster virus (VZV) is an exclusively human neurotropic alphaherpesvirus. Primary infection usually produces varicella (chickenpox), after which virus becomes latent in ganglionic neurons along the entire neuraxis, revealing a unique nonlytic relationship of VZV with neurons, in contrast to productive infection of cells derived from other organs (1). Initial attempts to explore the VZV-neuron relationship were done with human ganglionic cells cultured in vitro and experimentally infected with VZV. While both neurons and nonneuronal cells became infected, productive infection of nonneuronal cells resulted in destruction of the entire culture (2, 3). Later studies in which VZV-infected human fetal fibroblasts were cocultivated with explanted human fetal dorsal root ganglia indicated that neurons were resistant to apoptosis (4). Recently, heterogeneous cultures derived from human embryonic stem cells that contained neurons were infected with VZV, but again productive infection ensued, presumably due to the presence of a significant proportion of nonneuronal cells (5). When differentiated human neural stem cells that were >90% neurons based on immunostaining were infected with VZV, no cytopathic effect developed, and VZV DNA, virus-specific transcripts, and proteins were detected in healthy-appearing neurons (6). Similarly, weeks after VZV infection of cultures containing >95% terminally differentiated human neurons derived from pluripotent stem cells, the neurons appeared healthy, and the tissue culture medium did not contain infectious virus (7); none of the cells stained positive for glial fibrillary acidic protein; although identification of the minority of the cells that were not tubulin positive is unknown, no cytopathic effect was seen in the cultures after infection with VZV. Once again, the nonproductively infected induced pluripotent stem cell neurons contained VZV DNA, transcripts, and proteins corresponding to VZV immediate early, early, and some late genes; no markers of the apoptotic caspase cascade were found. Herein, we further examined these nonproductively infected neurons to determine whether diminished production of virion components and/or final assembly of complete virions play a role in limiting productive virus infection.
MATERIALS AND METHODS
Neuronal cells and VZV infection.
iCell neurons (Cellular Dynamics International, Madison, WI) were thawed, seeded at 75,000 to 100,000 cells/cm2 onto cell culture surfaces freshly coated with laminin, and maintained in iCell complete maintenance medium at 37°C in 5% CO2. The neuron culture medium (50 to 75% of total volume) was changed three times each week. Human fetal lung (HFL) fibroblasts were cultured in Dulbecco's minimum essential medium (DMEM) containing 100 U/ml penicillin and 10 μg/ml streptomycin and supplemented with 10% fetal bovine serum (FBS). Tissue culture plates (6-well) (Corning, Tewksbury, MA) were coated with 1 ml poly-l-ornithine solution (Sigma, St. Louis, MO) for 1 to 2 h at room temperature, washed twice with sterile water, and coated with 3 ml of laminin (3.3 μg/ml) (Sigma) for at least 2 h at 37°C in an incubator. Neurons (∼1 × 106) were infected with VZV (Zostavax; Merck, Whitehouse Station, NJ) at 1,000 to 3,000 PFU in iCell complete maintenance medium. HLFs were similarly infected with VZV (1,000 to 3,000 PFU) and maintained in DMEM supplemented with 10% FBS.
RNA analysis.
Total RNA was extracted from infected neurons at 14 days postinfection (d.p.i.) and from HFL cells at the height of cytopathic effect (CPE) (5 to 7 d.p.i.) using the mirVANA microRNA (miRNA) extraction kit (Ambion, Austin, TX). Briefly, cells were lysed in lysis buffer (Ambion) using a rubber policeman. Any visible cell clumps were homogenized using a 17- to 26-gauge needle with a 1-ml syringe (12 to 20 strokes) and incubated on ice for 10 min. RNA (200 ng in 100 μl PCR-grade water [Teknova, Hollister, CA]) was DNase treated using a Turbo-DNA-free kit (Ambion). Briefly, 50-μl samples were incubated with 1.5 μl DNase for 30 min, followed by addition of fresh DNase (1.5 μl) and incubated for an additional 30 min at 37°C. DNase was inactivated with inactivation buffer (Ambion) for 5 min at room temperature with mixing. Sequestered DNase was pelleted by centrifugation at 10,000 rpm for 5 min at 4°C, and the supernatant was transferred to a new tube. cDNA synthesis was completed using the Transcriptor first-strand cDNA synthesis kit (Roche, Indianapolis, IN). Anchored oligo-[dT]18 primer was added and annealed for 10 min at 65°C to ensure high conversion of mRNA to cDNA. After primer annealing, deoxynucleoside triphosphates (dNTPs), reverse transcription reaction buffer, and RNase inhibitor were added. Samples were divided into 2 aliquots (with and without reverse transcriptase [RT]) to monitor residual VZV DNA. cDNA was stored at −80°C. Both RT+ and RT− samples were used for real-time PCR analysis. VZV primers and probes (Table 1), Taq Universal probe SuperScript ROX mix (Bio-Rad, Hercules, CA), and PCR-grade water in a final volume of 20 μl were combined with 2 μl cDNA and added to MicroAmp 96-well plates. Quantitative real-time PCR (qPCR) was conducted with VZV-specific primers (IDT, Coralville, IA) on an Applied Biosystems 7500 fast PCR system. RT− and RT+ samples were amplified along with wild-type VZV DNA standard (106 to 100 genome copies). cDNA was denatured at 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and 60°C for 30 s.
Table 1.
Primers and probes for real-time PCR in this study
| Name | Protein | Sequence |
Size (bp) | ||
|---|---|---|---|---|---|
| Forward primer | Reverse primer | Probe (FAM)a | |||
| ORF14 | gC | 5′ GGATGCATAGGGTTGCGATAA 3′ | 5′ TGCATCTACCTACGCCACTA 3′ | 5′ CCTTGGGACATTGGGTCTTATCGCA 3′ | 107 |
| ORF62 | IE62 | 5′ CCTTGGAAACCACATGATCGT 3′ | 5′ AGCAGAAGCCTCCTCGACAA 3′ | 5′ TGCAACCCGGGCGTCCG 3′ | 79 |
| ORF68 | gE | 5′ GTACATTTGGAACATGCGCG 3′ | 5′ TCCACATATGAAACTCAGCCC 3′ | 5′ AAAACAAGAAACCCTACGCCCGC 3′ | 140 |
FAM, 6-carboxyfluorescein.
Confocal and electron microscopy.
Coated coverslips for neuronal cultures were obtained from BD Biosciences (San Jose, CA). Cells were fixed in 4% paraformaldehyde for 20 min at room temperature, washed three times in phosphate-buffered saline (PBS), permeabilized in 0.3% Triton for 10 min, rinsed three times with PBS, and either stained or stored at 4°C in PBS. Cells were blocked with 5% nonfat dry milk in PBS, incubated with primary antibodies (mouse anti-VZV gC or anti-VZV gE) for 2 h at room temperature, washed three times with PBS, and further incubated with Alexa Fluor GAM 488-conjugated anti-mouse antibody for 1 h. Hoechst H33342 was used to stain cell DNA. Images of the labeled infected cells were collected on a laser-scanning confocal microscope as described previously (8). A three-dimensional (3D) image of a single infected cell was generated from a z-stack using Imaris software as described previously (9). At 14 d.p.i., neurons in 6-well cell culture plates were fixed in 2.5% glutaraldeyde for 30 min at room temperature, further fixed with 1% OsO4–2.5% potassium ferrocyanide for 1 h, washed three times in deionized water, and stained with 4% uranyl acetate for 1 h as described previously (10). Samples were then dehydrated in an ethanol-water series (25% to 100% ethanol) and embedded in Epon12 before polymerization at high temperature. The resulting Epon plastic was cut, and thin sections (80-nm) were placed onto slot grids. After staining with uranium acetate and lead citrate, cells were examined with a Jeol 1230 transmission electron microscope (TEM).
RESULTS
Ultrastructure of VZV-infected cultured neurons at low magnification.
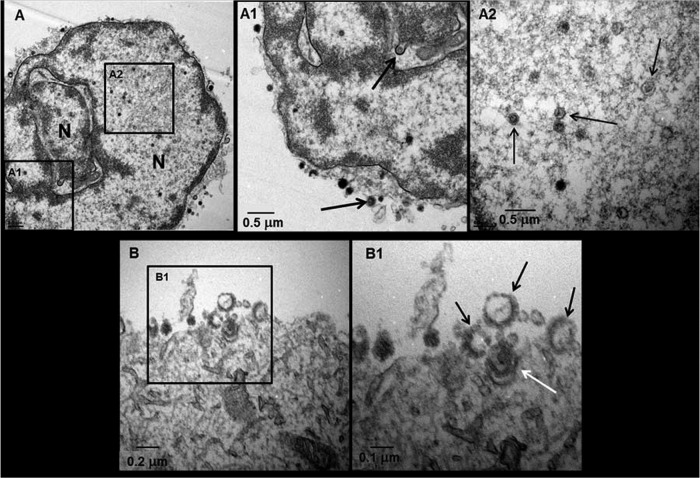
Examination of VZV-infected neurons using low-magnification transmission electron microscope (TEM) revealed viral particles in both the nuclei (Fig. 1A) and cytoplasm (Fig. 1B) of the cells. Viral capsids were seen egressing into the outer nuclear membrane as they exited the nucleus en route to the cytoplasm, with multiple viral particles on the cell surface (Fig. 1A1). Some viral capsids had either no DNA core or an aberrant DNA core (Fig. 1A2). VZV particles in the cytoplasm are shown in panel B. Enveloped particles within vacuoles near and on the outer cell membrane after exocytosis were also detected (Fig. 1B1).
Fig 1.
Ultrastructure of VZV particles in infected neurons. A VZV-infected neuron with a multilobed nucleus exhibited viral particles and capsids (A). A viral capsid was seen egressing from the nucleus (A1, top arrow), and viral particles were seen on the cell surface (A1, bottom arrow). Multiple capsids were seen in the nucleus (A2, arrows). Among virus particles seen on the cell surface (B), four were near the cell surface (B1), three of which were light particles lacking a DNA core (black arrows); the fourth particle was a complete virion (white arrow).
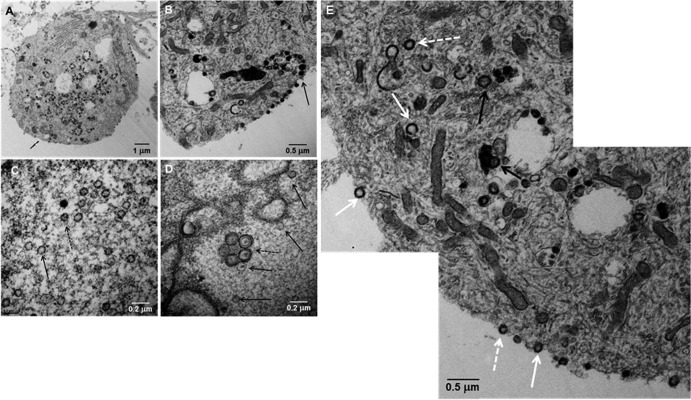
Fig 2.
Transmission electron microscopy of VZV-infected neurons. A cytoplasmic section of one VZV-infected neural cell body exhibited a large number of cytoplasmic viral particles with a smaller number on the cell surface (A). A section of a different infected neuron exhibited indistinct viral particles egressing onto the surface of the cell (B; arrow). At high magnification, 23 viral capsids were seen in a section of an infected cell nucleus (C), of which 7 or 8 contained a DNA core (dashed arrow), while another viral capsid lacked DNA (solid arrow). A vesicle of enveloped viral particles (D) was seen in the nucleus (dashed arrow) with several viral capsids (solid arrows). A montage of the cytoplasm and cell surface of a VZV-infected neuron showed viral particles without capsids and viral DNA (solid white arrows), viral particles with capsids but not viral DNA (dashed white arrows), and complete viral particles with capsid and DNA (black arrows) (E).
Unusual ultrastructure features of VZV particles in neurons.
Unlike during productive infection, a remarkably high number of L particles (i.e., envelopes with no capsid) were detected in neuronal cells (Fig. 2A). Most L particles were observed within large cytoplasmic vacuoles, although some egressed onto the outer cell membrane (Fig. 2B); many capsids in the nuclei contained little or no viral DNA (Fig. 2C). A remarkable finding was the presence of a vacuole containing 5 partially enveloped viral particles within the nucleus (Fig. 2D). Based on extensive earlier TEM analyses (11), these partially enveloped particles, with little or no DNA core, appear to represent capsids that exit the inner nuclear membrane but then traffic back into the nucleus rather than continuing to egress through the outer nuclear membrane. Similarly, herpes simplex virus 1 (HSV-1) capsids lacking a DNA core are rarely transported into the cytoplasm for envelopment (12). Ultrastructural analysis of VZV-infected neurons revealed (i) enveloped capsids lacking a DNA core, (ii) complete virions (enveloped capsids with DNA), and (iii) L particles (an envelope with no capsid) (Fig. 2E). The absence of a double wall in the vacuoles containing viral particles in the cytoplasm indicated they were not autophagosomes, as observed in nonneuronal cells (13).
Expression of VZV gC and gE in infected neurons.
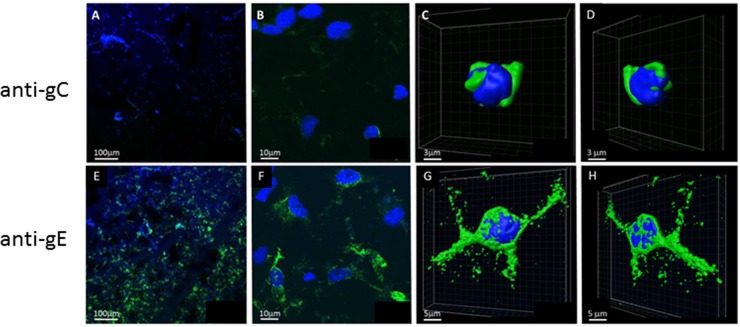
Based on the hypothesis that the late kinetic phase of VZV replication is delayed, we searched for expression of an early-late glycoprotein (gamma-1; gE) and a true late glycoprotein (gamma-2; gC) in infected neuronal cells (Fig. 3). Ten images of infectious foci examined by confocal immunohistochemistry revealed VZV gE in 68% of 300 infected cells. Virtually 100% of cells near the center of an infectious focus were VZV gE positive, while fewer were positive on the boundary of the focus. The gC-positive foci were smaller and more difficult to detect (Fig. 3A and B) than the gE-positive foci (Fig. 3E and F); in six images of infectious foci, less than 10% of cells were gC positive and often with only a single gC-positive cell within a field. At higher magnification, VZV gC was detected mainly in the perinuclear endoplasmic reticulum (ER) (Fig. 3C and D), while VZV gE was detected throughout the cytoplasm of infected cells (Fig. 3G and H), suggesting comparatively restricted gC biosynthesis. Three-dimensional images (Fig. 3 C, D, G, and H) showed the remarkable differences in distribution of gE and gC in VZV-infected neurons.
Fig 3.
Expression of VZV glycoproteins C and E in VZV-infected neurons. VZV-infected neurons were fixed at 14 d.p.i. and stained with anti-VZV gC antibody (A to D), anti-VZV gE antibody (E to H), and Hoechst H33342 as described in the text. Images were collected on a laser-scanning confocal microscope at 100× (A and E) and 900× (B and F). The 3D images of single infected cells were generated from a z-stack using Imaris software (C, D, G, and H). Note the markedly reduced gC staining (A and B) compared to the abundant gE staining (E and F) in infected neurons. gC staining was restricted to the perinuclear endoplasmic reticulum (C and D), in contrast to extensive cytoplasmic gE staining (G and H).
Relative transcription in VZV-infected human neurons versus fibroblasts.
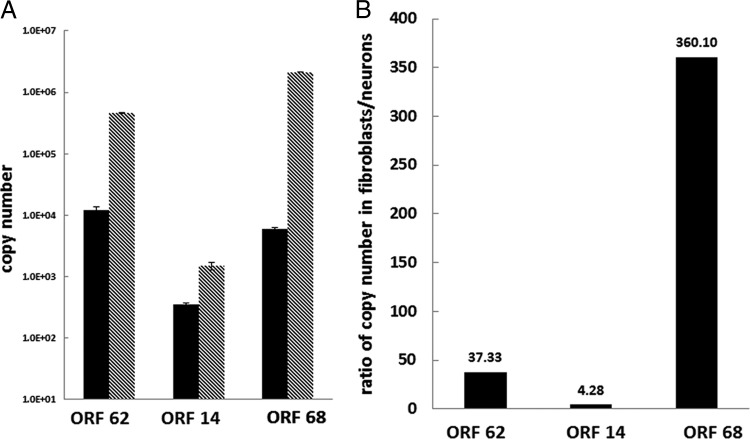
To test whether the relative expression of gC and gE in infected neurons reflected smaller amounts of the cognate transcript, infected neurons and HFL fibroblasts were analyzed by qPCR for expression of VZV open reading frame 14 (ORF14) (gC) and VZV ORF68 (gE), in addition to the immediate early transactivator ORF62; all transcripts were detected in virus-infected neurons at 14 d.p.i. and in VZV-infected HFL cells at the height of VZV-induced CPE (Fig. 4). When examined in equal amounts of cDNA synthesized from RNA extracted from both cell types, VZV ORF62, -14, and -68 transcripts were more abundant in productively infected HFL cells than in nonproductively infected neurons (Fig. 4A). The relative VZV transcript abundance was ORF68 > ORF62 > ORF14 in HLF cells, compared to ORF62 > ORF68 > ORF14 in neurons. The abundance of each VZV transcript in HFL cells compared to neurons ranged from 360 (ORF68) to 4 (ORF 14) (Fig. 4B). Overall, reduced transcription of true late VZV gC correlated with its reduced translation.
Fig 4.
VZV mRNA transcripts in VZV-infected neurons and fibroblasts. The abundance of all VZV transcripts was decreased in neurons (black bars) compared to fibroblasts (gray) (A). The ratio of VZV transcripts expressed in fibroblasts and neurons is shown in panel B.
DISCUSSION
Early studies established that VZV infection in the typical exanthem of varicella and herpes zoster led to the assembly of enumerable prototypic herpesviral particles, each containing an envelope surrounding a capsid with a dense core. Electron micrographs of virions from vesicle fluid are reviewed by Grose (14). Earlier studies by other laboratories failed to find a similarly high proportion of prototypic viral particles in VZV-infected cells in tissue culture (15, 16). Ultrastructural examination of VZV particles in cultures of human melanoma cells by both scanning electron microscopy (SEM) and TEM (11) revealed syncytia resulting in large toroidal structures consisting of multiple nuclei underlying large areas of plasma membrane. Along the surface of the outer cell membrane, VZV particles egressed in long parallel pathways called “viral highways” (17). Within infected cell nuclei, large numbers of viral capsids were readily detected, some forming regular crystalline arrays. Various DNA patterns within viral capsids were seen (18). Since the DNA pattern within each nucleus is not always characteristic of that associated with a complete double-stranded herpesvirus genome, some DNA cores likely represent encapsidation of incomplete VZV genomes. Particles within the cytoplasm were particularly divergent in their appearance, often containing envelopes of various thicknesses; most of these particles were housed within vacuoles of various diameters. SEM examination of particles within viral highways in infected melanoma cells revealed that >80% of egressed particles lacked a capsid and thus were called L particles. Importantly, the particle/PFU ratio was 40,000:1 (10).
Most recently, differentiated human neurons infected with VZV were found to contain viral DNA, transcripts, and proteins in the absence of a cytopathic effect (7). These virological features differ from those known to exist in human ganglia latently infected with VZV, where virus particles are not seen in infected neurons. While valid comparison between VZV-infected neurons in vitro and latently infected human ganglia is precluded by the relatively short viability of cultured neurons after primary infection needed for such analyses (months to years), the cells provide a useful in vitro system to determine how nonlytic infection of neurons is maintained. VZV particles in these neuronal cells did not resemble prototypic complete virions. Moreover, the population of viral particles within cultured neurons was even more aberrant than that seen in productively infected human melanoma cells. In particular, subpopulations of L particles and enveloped empty capsids were abundant, and empty capsids were readily identified in infected cell nuclei. The abundance of enveloped empty capsids contrasts with herpes simplex virus (HSV) assembly, in which empty capsids rarely exit the nucleus (12).
The presence of aberrant virus particles in VZV-infected neurons in tissue culture is typified by the differential expression of an early-late gene and a late-late gene, respectively, coding for VZV gE and gC. We found VZV gE in >90% of infected neurons, as previously shown for VZV gene 63 and the gE gene (6) as well as for the VZV genes 62, 63, and 29, as well as the VZV genes coding for thymidine kinase (TK), gE, and gH (7), whereas VZV gC was only rarely detected in infected neurons and then only in the perinuclear ER regions. The low abundance of VZV gC protein in infected neurons was paralleled by low levels of true late VZV gC transcripts compared to transcription of the VZV IE62 gene and the early-late VZV gE gene in infected neurons, suggesting that low VZV gC abundance in infected neurons is due to a block in virus transcription rather than translation. Reduced transcription of VZV gC compared to that of VZV IE62 and VZV gE in neurons and fibroblasts is consistent with previous observations of reduced VZV gC transcription in human melanoma cells (8, 19). While VZV gC production is significantly reduced in both virus-infected neurons and nonneuronal cells in tissue culture, VZV gC is nevertheless abundant in vesicles (20). Because VZV in tissue culture is highly cell associated (10), while VZV in vesicles is cell free (21), it is possible VZV gC facilitates virus egress. Alternatively, reduced gC expression may be a marker of limited true late VZV replications in these neuronal cells.
Finally, the nonpermissive nature of human neurons to VZV was also found in primary human fetal brain cell cultures infected with HSV, in which astrocytes, but not neurons, developed a virus-induced cytopathic effect and HSV-1 antigen expression (22).
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants AG032958 (R.J.C., and D.G.), AG006127 (D.G.), NS082228 (R.J.C.), and AI89716 (C.G.) from the National Institutes of Health.
We thank Marina Hoffman for editorial review and Lori DePriest for manuscript preparation.
Footnotes
Published ahead of print 26 June 2013
REFERENCES
- 1.Weller TH, Witton HM, Bell EJ. 1958. The etiologic agents of varicella and herpes zoster; isolation, propagation, and cultural characteristics in vitro. J. Exp. Med. 108:843–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilden DH, Wroblewska Z, Kindt V, Warren KG, Wolinsky JS. 1978. Varicella-zoster virus infection of human brain cells and ganglion cells in tissue culture. Arch. Virol. 56:105–117 [DOI] [PubMed] [Google Scholar]
- 3.Wigdahl B, Rong BL, Kinney-Thomas E. 1986. Varicella-zoster virus infection of human sensory neurons. Virology 152:384–399 [DOI] [PubMed] [Google Scholar]
- 4.Hood C, Cunningham AL, Slobedman B, Boadle RA, Abendroth A. 2003. Varicella-zoster virus-infected human sensory neurons are resistant to apoptosis, yet human foreskin fibroblasts are susceptible: evidence for a cell-type-specific apoptotic response. J. Virol. 77:12852–12864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Markus A, Grigoryan S, Sloutskin A, Yee MB, Zhu H, Yang IH, Thakor NV, Sarid R, Kinchington PR, Goldstein RS. 2011. Varicella-zoster virus (VZV) infection of neurons derived from human embryonic stem cells: direct demonstration of axonal infection, transport of VZV, and productive neuronal infection. J. Virol. 85:6220–6233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pugazhenthi S, Nair S, Velmurugan K, Liang Q, Mahalingam R, Cohrs RJ, Nagel MA, Gilden D. 2011. Varicella-zoster virus infection of differentiated human neural stem cells. J. Virol. 85:6678–6686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu X, Seitz S, Pointon T, Bowlin JL, Cohrs RJ, Jonjic S, Haas J, Wellish M, Gilden D. 2013. Varicella zoster virus infection of highly pure terminally differentiated human neurons. J. Neurovirol. 19:75–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Storlie J, Jackson W, Hutchinson J, Grose C. 2006. Delayed biosynthesis of varicella-zoster virus glycoprotein C: upregulation by hexamethylene bisacetamide and retinoic acid treatment of infected cells. J. Virol. 80:9544–9556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peters GA, Tyler SD, Carpenter JE, Jackson W, Mori Y, Arvin AM, Grose C. 2012. The attenuated genotype of varicella-zoster virus includes an ORF0 transitional stop codon mutation. J. Virol. 86:10695–10703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carpenter JE, Henderson EP, Grose C. 2009. Enumeration of an extremely high particle-to-PFU ratio for varicella-zoster virus. J. Virol. 83:6917–6921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harson R, Grose C. 1995. Egress of varicella-zoster virus from the melanoma cell: a tropism for the melanocyte. J. Virol. 69:4994–5010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vlazny DA, Kwong A, Frenkel N. 1982. Site-specific cleavage/packaging of herpes simplex virus DNA and the selective maturation of nucleocapsids containing full-length viral DNA. Proc. Natl. Acad. Sci. U. S. A. 79:1423–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carpenter JE, Jackson W, Benetti L, Grose C. 2011. Autophagosome formation during varicella-zoster virus infection following endoplasmic reticulum stress and the unfolded protein response. J. Virol. 85:9414–9424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grose C. 1994. Varicella zoster virus infections, p 117–185 In Glaser R, Jones JF. (ed). Herpesvirus infections, Marcel Dekker, New York, NY [Google Scholar]
- 15.Achong BG, Meurisse EV. 1968. Observations on the fine structure and replication of varicella virus in cultivated human amnion cells. J. Gen. Virol. 3:305–308 [DOI] [PubMed] [Google Scholar]
- 16.Gershon A, Cosio L, Brunell PA. 1973. Observations on the growth of varicella-zoster virus in human diploid cells. J. Gen. Virol. 18:21–31 [DOI] [PubMed] [Google Scholar]
- 17.Padilla JA, Nii S, Grose D. 2003. Imaging of the varicella zoster virion in the viral highways: comparison with herpes simplex viruses 1 and 2, cytomegalovirus, pseudorabies virus, and human herpes viruses 6 and 7. J. Med. Virol. 70(Suppl 1):S103–S110 [DOI] [PubMed] [Google Scholar]
- 18.Grose C, Harson R, Beck S. 1995. Computer modeling of prototypic and aberrant nucleocapsids of varicella-zoster virus. Virology 214:321–329 [DOI] [PubMed] [Google Scholar]
- 19.Storlie J, Carpenter JE, Jackson W, Grose C. 2008. Discordant varicella-zoster virus glycoprotein C expression and localization between cultured cells and human skin vesicles. Virology 382:171–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grose C. 2010. Autophagy during common bacterial and viral infections of children. Pediatr. Infect. Dis. J. 29:1040–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arvin AM. 2001. Varicella-zoster virus: molecular virology and virus-host interactions. Curr. Opin. Microbiol. 4:442–449 [DOI] [PubMed] [Google Scholar]
- 22.Kennedy PG, Clements BG, Brown SM. 1983. Differential susceptibility of human neural cell types in culture to infection with herpes simplex virus. Brain 106:101–119 [DOI] [PubMed] [Google Scholar]