Abstract
Nanoscale titanium dioxide (TiO2) is increasingly used in consumer goods and is entering waste streams, thereby exposing and potentially affecting environmental microbes. Protozoans could either take up TiO2 directly from water and sediments or acquire TiO2 during bactivory (ingestion of bacteria) of TiO2-encrusted bacteria. Here, the route of exposure of the ciliated protozoan Tetrahymena thermophila to TiO2 was varied and the growth of, and uptake and accumulation of TiO2 by, T. thermophila were measured. While TiO2 did not affect T. thermophila swimming or cellular morphology, direct TiO2 exposure in rich growth medium resulted in a lower population yield. When TiO2 exposure was by bactivory of Pseudomonas aeruginosa, the T. thermophila population yield and growth rate were lower than those that occurred during the bactivory of non-TiO2-encrusted bacteria. Regardless of the feeding mode, T. thermophila cells internalized TiO2 into their food vacuoles. Biomagnification of TiO2 was not observed; this was attributed to the observation that TiO2 appeared to be unable to cross the food vacuole membrane and enter the cytoplasm. Nevertheless, our findings imply that TiO2 could be transferred into higher trophic levels within food webs and that the food web could be affected by the decreased growth rate and yield of organisms near the base of the web.
INTRODUCTION
With the increased production and use of manufactured nanomaterials, there are concerns regarding their environmental buildup and possible ecological impacts (1, 2). One highly produced nanomaterial is nanoscale titanium dioxide (TiO2), which is found in a wide range of impermanent consumer products such as household paints (3, 4), cosmetics (5), medicinal ointments (6), and food products (7). On the basis of the projected increase in its production (8), manufactured TiO2 will increasingly enter the environment, where it could impact ecosystem health (9), including food web interactions between protozoan predators and bacterial prey that are important in nutrient cycling (1).
Many manufactured nanomaterials (10), including TiO2 (11) and either cadmium telluride (CdTe) (12) or cadmium selenide (CdSe) (13) quantum dots (QDs), have been shown to impact bacterial cell membranes and interfere with bacterial population growth (12, 13). In experiments with planktonic Pseudomonas aeruginosa growing in the presence of CdSe QDs, cell membranes were disrupted by reactive oxygen species, resulting in QDs entering cells (13–15). In a trophic-transfer experiment, we showed that intracellular CdSe QDs in P. aeruginosa prey were transferred into the ciliated protozoan predator Tetrahymena thermophila and biomagnified in the process (15). In another investigation, carbon nanotubes were directly ingested by these protozoans, which interfered with bactivory (ingestion of bacteria) (16), but trophic transfer was not studied. Thus, at least two scenarios of protozoan exposure to manufactured nanomaterials have been reported: protozoan predation upon bacteria with already internalized nanomaterials and direct ingestion of nanomaterials from the aqueous environment.
A third, potentially common, scenario for nanomaterial exposure to protozoans is via ingestion of nanomaterial-coated bacteria. While there are various potential nanomaterial impacts on bacteria according to nanomaterial properties, as well as exposure conditions (10), many nanomaterials such as TiO2 are observed to adsorb to cell membranes, e.g., of Escherichia coli (11) and P. aeruginosa (17). In fact, since nanomaterials are often larger than typical bacterial cell wall openings (18), external envelope adsorption may be the prevalent mode of manufactured nanomaterial association with bacteria. Given this likelihood, questions arise concerning protozoan predation of such bacteria, i.e., if it occurs and whether bacterially sorbed nanomaterials, including TiO2, can be transferred into protozoans during bactivory of nanomaterial-encrusted bacteria. Information about the trophic transfer of bacterially adsorbed TiO2, with possible TiO2 biomagnification in protozoa, will provide a better understanding of the potential impacts of manufactured TiO2 on base food web interactions.
In this study, we investigated (i) whether TiO2 that is adsorbed to P. aeruginosa is transferred to T. thermophila during protozoan bactivory and (ii) the possible effects on protozoan growth. The condition of T. thermophila grown with TiO2-encrusted P. aeruginosa is compared to that of direct uptake of TiO2, i.e., by T. thermophila grown in bacterium-free, nutrient-rich medium that is amended with TiO2.
MATERIALS AND METHODS
Strains, media, and nanomaterials.
The bacterial strain used in this study was P. aeruginosa PG201, a Gram-negative organism studied previously (13, 15, 17) and maintained at −80°C. As described before (15), the protozoan strain was T. thermophila SB210E. The medium used for P. aeruginosa cultivation was Luria Bertani (LB) broth or agar. The medium used for T. thermophila cultivation was either super-nutrient-rich medium (1% SSP [1% proteose peptone, 0.1% yeast extract, 0.2% dextrose, 75 μM Fe EDTA]) for growth experiments involving direct TiO2 exposure or starvation medium (Dryl's medium [2 mM sodium citrate, 2 mM NaH2PO4 · H2O, 1 mM Na2HPO4, 1.5 mM CaCl2, pH 7.4]) for trophic-transfer experiments.
The nanomaterial was dry powder industrial P25 Aeroxide TiO2 (Evonik, Parsippany, NJ) (75% anatase and 25% rutile) (19), which was stored in the dark at room temperature prior to use. As described by Horst et al. (17), the industrial TiO2 used in these experiments is highly heterogeneous, with equivalent particle diameters ranging from 6.4 to 73.8 nm, where 75% of the particles are between 15 and 60 nm (average diameter, 37.5 nm). The electrophoretic mobility (zeta potential) of this TiO2 varies with aqueous chemistry (20) and in LB medium is −17.9 mV (17). All other chemicals were reagent grade or better (Sigma Chemical or Fisher Scientific). Nanopure water (pH 6.9, 18.2 MΩ-cm) was used to prepare media and nanomaterial suspensions.
Preparation of P. aeruginosa and T. thermophila inocula.
The bacterial inoculum was initially streaked from frozen stock (−80°C, preserved in 70% LB plus 30% [vol/vol] glycerol) onto LB agar and cultivated in the dark (12 h, 30°C). Following incubation, several discrete colonies were dispersed into 4 ml of LB broth to serve as the liquid inoculum.
T. thermophila cells maintained axenically by passaging every 3 weeks in nutrient medium at room temperature were inoculated into standard (10 cm by 15 mm) sterile plastic petri dishes containing 10 ml SSP and grown in a humidity chamber without shaking (17 h, 30°C), similarly to before (15). After reaching mid to late exponential phase, the culture was centrifuged (1,470 × g, 40 s). The cell pellet was washed once with Dryl's medium, and cells were resuspended to a concentration of ca. 650,000 ml−1 in 10 ml of Dryl's medium in petri dishes and then starved (17 h, 30°C) in humidity chambers. The starved T. thermophila cells were centrifuged, washed in Dryl's medium, and resuspended in either SSP or Dryl's medium for growth experiments, including trophic-transfer studies (Dryl's medium only).
P. aeruginosa cultivation in liquid medium.
P. aeruginosa was cultured in LB broth to determine the effects of TiO2 exposure on growth and the amount of TiO2 adsorbed to the bacteria; identical P. aeruginosa cultivation conditions were used to generate prey for trophic-transfer experiments. Cultivation was performed similarly to before (17), i.e., in the dark in 96-well plates (flat-bottom polystyrene with clear bottom and sides; Corning Incorporated) with 200 μl of LB broth (with or without 0.1 mg TiO2 ml−1) per well and inoculation with exponential-phase P. aeruginosa. For the TiO2 treatments, dry TiO2 powder was preweighed, added to sterile LB broth, vortexed (10 min), dispensed into wells, and inoculated with P. aeruginosa. As before, TiO2 handled in this way initially formed large agglomerates that dispersed during P. aeruginosa population growth because of the adsorption of TiO2 onto P. aeruginosa surfaces (17). Each treatment (including uninoculated controls) was prepared in triplicate. The multiwell plates were incubated (30°C, 200 rpm) in a Synergy HT Multi-Mode microplate reader (Biotek Instruments, Winooski, VT) equipped with a xenon lamp set to measure optical density at 600 nm (OD600) regularly over time.
P. aeruginosa cultures for use in T. thermophila feeding (trophic-transfer) experiments were prepared in glass flasks containing 400 ml of LB broth with or without 0.1 mg TiO2 ml−1 and 25 μl of P. aeruginosa inoculum. The flasks were incubated in the dark at 30°C while shaking at 200 rpm until the OD600 reached 0.4 (ca. 10 h). The flask contents were dispensed (40-ml aliquots) into 10 50-ml tubes for centrifugation (12,000 × g, 10 min), and the supernatants were discarded. Each pellet was washed once with Dryl's medium to remove LB medium constituents and resuspended in 20 ml of sterile Dryl's medium. The washed cells for each treatment were combined, and triplicate (60-ml) aliquots from each flask were transferred into separate, sterile glass flasks from which 1 ml was removed for fixation and cell counting. The experiment was repeated identically to provide P. aeruginosa cells for imaging by environmental scanning electron microscopy (ESEM). The remaining cell suspensions, which, according to the above steps, consisted of a washed 2× concentration of the harvested culture, were used for T. thermophila feeding experiments. It was determined, by assessing the initial mass of TiO2 administered to the P. aeruginosa culture versus the TiO2 observed to be sorbed onto these bacteria (by ESEM; see the supplemental material), that all of the TiO2 administered was sorbed to the bacteria at the start of the trophic-transfer experiment. Thus, there was no need to separate unbound TiO2 from bacterially sorbed TiO2 during culture washing.
T. thermophila growth without P. aeruginosa prey.
T. thermophila cells were cultivated in either SSP or Dryl's medium in the absence of P. aeruginosa prey to determine the effects of direct TiO2 exposure on T. thermophila growth rates and yields. In the control cultures (without TiO2), starved T. thermophila cells were suspended in 12 ml of either Dryl's or SSP medium in petri dishes. Where used, TiO2 was first sonicated (10 W, 20 min, Branson 8210; Branson, Danbury, CT) and diluted to a final concentration of 0.10 mg ml−1 in 12 ml of either Dryl's or SSP medium, and then T. thermophila inocula were dispensed into the TiO2 suspensions. In the control and TiO2 treatments, the starting culture represented a 100× dilution of starved inocula to 6,500 cells ml−1. Each inoculated culture (i.e., each medium with or without TiO2) was established in triplicate. Abiotic controls consisted of uninoculated medium with or without TiO2. T. thermophila cells were cultured in the dark at 30°C in a humidity chamber without agitation. The cultures were sampled over time for cell counting and observation of live-cell characteristics under a dissecting microscope. Cultivation was repeated within 1 day as described above but with enough replicates to allow for sacrificing of entire cultures at 2, 8, 16, and 22 h for use in Nomarski and electron microscopy.
T. thermophila growth with P. aeruginosa prey.
T. thermophila cells were cultured in Dryl's medium with P. aeruginosa to determine growth rates and yields when bacterial prey, with or without preadsorbed TiO2, was the only food source. In the control T. thermophila growth experiment, control P. aeruginosa cells (cultured without TiO2 for 10 h) were suspended in Dryl's medium as described above. Starved T. thermophila cells were added to achieve a final density of ca. 6,500 ml−1 as described before (15). The trophic-transfer and control growth experiments were conducted identically, except that P. aeruginosa cells used in trophic-transfer studies had presorbed TiO2. Six individual replicate experiments were prepared—three each for the control and trophic-transfer experiments. All other cultivation conditions and approaches, including additional experimental replication for microscopy, were identical to the procedures used for T. thermophila cultured in SSP medium (without P. aeruginosa).
Cell counting by light microcopy.
P. aeruginosa cells for counting were fixed with glutaraldehyde (2.5%, vol/vol) and stored at 4°C briefly (less than 24 h). Fixed cells were then stained with SYBR gold (Life Technologies, Carlsbad, CA) and counted by epifluorescence microscopy (Nikon E800, ×1,000 total magnification) as described before (13).
T. thermophila population growth was assessed for all of the cultures (with or without, P. aeruginosa prey or TiO2) by counting the cells in a 100-μl sample (fixed with 5 μl formaldehyde) periodically (2, 4, 8, 16, and 22 h) with a hemocytometer. Duplicate counts were averaged for each time point. For differential interference contrast (Nomarski) imaging of T. thermophila morphology, 1.5 ml of each T. thermophila culture was fixed with glutaraldehyde to a final concentration of ∼2.5% (vol/vol). An aliquot (50 μl) was imaged at 200× magnification by Nomarski bright-field microscopy. T. thermophila cells were also observed periodically during population growth for overall morphology and swimming behavior (without fixing) with a dissecting microscope.
Electron microscopy and X-ray microanalysis.
Electron microscopy was used to image TiO2 on P. aeruginosa and within T. thermophila cells. TiO2 uptake into T. thermophila cells was quantified by digitally analyzing electron micrographs. This, in turn, allowed calculations of TiO2 bioconcentration and biomagnification.
Imaging by ESEM was used to confirm that TiO2 was adsorbing to P. aeruginosa cell membranes as described before (17). The washed bacteria were imaged directly on a carbon-coated aluminum stub with an FEI Co. XL30 field emission gun (FEG) environmental scanning electron microscope (Philips Electron Optics, Eindhoven, The Netherlands) at a 20-kV accelerating voltage with a gaseous secondary electron detector in the wet mode (4 torr) and a 10.3-mm working distance. This method had been independently confirmed previously (by cryoscanning electron microscopy and by dynamic light scattering of P. aeruginosa cells) to reliably enable visualization of P. aeruginosa cells retaining a tightly adsorbed TiO2 coating on cellular outer membranes (17).
Imaging by scanning transmission electron microscopy (STEM) was used for high-magnification observations of T. thermophila, including internalized TiO2. To prepare T. thermophila cells for STEM imaging and analysis, 10 ml of each T. thermophila culture was fixed with glutaraldehyde to a final concentration of ∼2.5% (vol/vol) and then refrigerated (4°C) until embedding. The 10-ml fixed samples were prepared for embedding by centrifugation (10,000 × g, 10 min), followed by three sequential washings with 500 μl of nanopure water. The cell pellet was combined with 50 μl of 2% Noble agar and extruded as an agar worm; this was followed by optional staining (1 h) with 2% OsO4 (15). Both stained and unstained specimens were prepared. The stained and unstained specimens were then sequentially dehydrated with 25%, 50%, 75%, and 2× 100% ethanol at intervals of 15 min. The dehydrated samples were infused with 1:1 ethanol-acetone and 100% acetone at 15-min intervals, followed by a final 8-h infusion of 1:1 acetone-resin at 4°C. The specimen agar worms were transferred into molds with 100% Eponate 12 resin and dried (60°C, 24 h) (15). The samples in resin blocks were sectioned with an ultramicrotome with a 45° Diatome diamond knife. The ultrathin sections (60 nm) were deposited on Formvar-coated 200-mesh copper grids (15). Imaging was performed with an FEI XL30 FEG environmental scanning electron microscope (Philips Electron Optics, Eindhoven, The Netherlands) at a 30-kV accelerating voltage with a STEM detector in high-vacuum mode at an ∼6.7-mm working distance. All images were dark field with minimal digital postprocessing.
ESEM was also used to evaluate TiO2 distribution within T. thermophila cultured in trophic-transfer experiments. Selective embedded samples were thick sectioned (5 μm) and deposited onto sticky carbon dots (Ted Pella, Redding, CA) adhering to 100% silicon slides (1 by 1 cm; Electron Microscopy Sciences, Hatfield, PA) (21). The thick sections were imaged by ESEM (as described above) in the wet mode (4 torr) and at a 10.3-mm working distance at a 20-kV accelerating voltage with a backscatter secondary electron detector.
To confirm the presence and location of elemental titanium (Ti) in thick sections, energy-dispersive X-ray spectroscopy (EDS) of selected areas of either 1-μm2 (ESEM) or 100-nm2 (STEM, data not included) areas was performed with an X-ray spectrometer (15 s at 25.6 μS) with a sapphire Super Ultra-Thin Window (EDAX, Inc., Mahwah, NJ) on an FEI Nano600 FEG microscope (15). Genesis analytical software (EDAX, Inc.) was used in conventional atomic number, absorption, and fluorescence (ZAF) correction mode to analyze the spectra (15), i.e., to obtain atomic mass percentages for titanium along with additional elements (C, O, Si, P, S, Cl, K, Ca) (22).
X-ray microscopy.
X-ray microscopy was used to visualize the TiO2 metal (Ti) distribution in T. thermophila cells. A resin-embedded thick section (50 μm) of T. thermophila cells (harvested after 22 h of growth on TiO2-encrusted P. aeruginosa) was mounted to a thin wire for imaging with an Xradia UltraXRM-L200 3-D (Xradia, Inc., Pleasanton, CA) microscope with a quasimonochromatic operating photon energy of 8 keV, configured with a 65-μm isotropic field of view at a 150-nm spatial resolution (23). The Zernike phase-contrast imaging mode was used to enhance the visualization of TiO2 within T. thermophila food vacuoles (24). A tomography series was created from transmission X-ray images collected at 0.2° steps across a 180° rotation range with a 4-min integration time per image. The Xradia XMReconstructor software package was used to reconstruct the tomography series into a stack of tomograms corresponding to virtual slices inside the specimen by the filtered back projection reconstruction technique. The Xradia XM3DViewer software assembled these tomograms into various renderings to visualize the area imaged in both planar and three-dimensional (3D) volumetric formats.
Calculation of TiO2 mass adsorbed to P. aeruginosa.
The mass of TiO2 adsorbed to P. aeruginosa cells was calculated by assuming, and then confirming via ESEM images, that all of the TiO2 administered became adsorbed to P. aeruginosa bacterial cells during growth. The mass of TiO2 per P. aeruginosa cell was divided by the calculated cell volume and the dry cell mass to estimate the volume- and mass-based concentrations of TiO2 delivered with prey cells during T. thermophila predation of TiO2-encrusted P. aeruginosa (see the supplemental material). The volumetric concentration of TiO2 on P. aeruginosa cells was used to calculate the biomagnification factor (BMF) associated with TiO2 trophic transfer from P. aeruginosa into T. thermophila. Note that since the TiO2 nanoparticles administered here greatly exceed the size of typical bacterial cell envelope openings (18) and since TiO2 thus was only extracellularly adsorbed onto P. aeruginosa cells, there was no basis for the calculation of a bioconcentration factor (BCF) for P. aeruginosa uptake of TiO2.
Quantitative analysis of T. thermophila food vacuoles.
For T. thermophila cells grown either directly with TiO2 (SSP medium) or with TiO2-encrusted P. aeruginosa (in Dryl's medium), T. thermophila food vacuole geometries and the amounts of TiO2 that accumulated in food vacuoles were measured by quantitative analysis of STEM images. The image measurements, made with the Measurement Tool in Adobe Photoshop, were of the food vacuole diameters and areas and also the areas in the food vacuoles that were TiO2 dense as evidenced by image brightness. Between three and eight T. thermophila cells and between 9 and 23 food vacuoles were analyzed at each time point. The number of visible food vacuoles per cell varied, as this depended on the plane of the section created when specimens embedded in random orientations were microtomed for STEM imaging. At each time point, the diameters of up to seven randomly selected food vacuoles were measured for each image. Because the food vacuoles appeared to be circular, the area of each was calculated by assuming circular geometry. The areas of the same food vacuoles were then measured directly in Adobe Photoshop with the Area Measurement Tool. The directly measured areas were compared to the calculated areas to determine if the assumption of circular geometry, and thus spherical geometry for volume calculations, was legitimate.
With the Area Select and Measurement Tools in Photoshop, the area of TiO2 filling within each of the same food vacuoles was measured as described above after adjusting the image brightness to retain only the bright, electron-dense (TiO2-enriched) regions of each food vacuole. The total measured bright area was typically irregularly shaped and often consisted of multiple irregular subareas. Still, the total measured area was converted to a circular geometry so that a volume could be calculated by assuming spherical geometry. The assumption of spherical geometry is justified by the observation that, in sections of many cells at random angles, food vacuoles always showed a nearly circular cross section. The equivalent diameter (Dti; see Fig. S1 in the supplemental material) of a circle whose area was equivalent to the measured area, ATi (see Fig. S1), was calculated by using the equation for a circle. Relating the equations for a circle and a sphere to one another, the volume of TiO2 filling was calculated as V = 4/3 r ATi, where the radius r equals DTi/2. The volume calculation thus required the assumption that the TiO2 in food vacuoles propagated depthwise into and out of the plane of the imaged embedded specimen section. Since this assumption would likely generate an overestimate of TiO2 in the food vacuoles, calculations of bioconcentration and biomagnification were also likely overestimates. The percentage by volume of the food vacuole that was filled with TiO2 was then calculated (see Fig. S1 and Table S1 in the supplemental material). The mass of TiO2 per food vacuole was also calculated by multiplying the TiO2 volume of each food vacuole by the TiO2 density of 3.97 g cm−3 (17). The mass of TiO2 per T. thermophila cell was estimated by assuming that there were seven TiO2-containing food vacuoles (at all of the sampling points) per cell, on the basis of a 3D X-ray volume rendering (see Movie S1 in the supplemental material). The T. thermophila cellular concentration of TiO2 was then estimated by assuming a constant cell volume of 7,042 μm3, as measured before (15). These measured and calculated variables are summarized in Table S1 and shown as a graphical scheme in Fig. S1 in the supplemental material.
Bioconcentration and biomagnification calculations.
BCFs of TiO2 in T. thermophila cells grown in SSP medium were calculated at 2, 8, 16, and 22 h by dividing the cellular TiO2 concentration by the TiO2 concentration in the medium. There were two key assumptions in making the T. thermophila BCF calculations. The first was that TiO2 in T. thermophila cells was only within the food vacuoles. This was confirmed by inspection of STEM images and EDS data acquired in T. thermophila cytoplasm external to the food vacuoles. The second was that the medium TiO2 concentration was decreased from the initial concentration by the amount of TiO2 accumulated in the T. thermophila population at each time point.
The BMFs at 2, 8, 16, and 22 h for T. thermophila cells feeding on TiO2-encrusted P. aeruginosa cells were calculated by dividing the T. thermophila cellular TiO2 mass concentration by the P. aeruginosa cellular TiO2 mass concentration, where both cellular mass concentrations were on a dry cell mass basis (25, 26).
Quantitative assessment of TiO2 within T. thermophila populations.
The amounts of TiO2 within whole T. thermophila populations at each time point were calculated by combining the T. thermophila cell concentrations with the TiO2 masses in food vacuoles (and therefore T. thermophila cells) that were measured by quantitative image analysis. Mass values were calculated at each time point by multiplying the TiO2 mass per T. thermophila cell by the population size (the number of T. thermophila cells per milliliter of culture) and by 12 ml (culture volume in T. thermophila growth experiments). Percentages were calculated at each time point by dividing the masses by the total TiO2 administered in the 12 ml of T. thermophila culture medium (1.2 × 109 pg for SSP, 2.16 × 109 pg for Dryl's medium plus TiO2-encrusted P. aeruginosa) and then multiplying each fraction by 100.
Additional data and statistical analyses.
Exponential growth rate constants for P. aeruginosa and T. thermophila cells were calculated from the slopes of the best-fit (by linear regression) lines through log-transformed OD600 (P. aeruginosa) or cell count (T. thermophila) time course data for each replicate in each growth experiment with Microsoft Excel 2010 software. Statistical analyses of the replicate results were performed with SPSS 12.0.1 (SPSS Inc., Chicago, IL) or Microsoft Excel 2010 software. Means were compared by the Student t test or by one-way analysis of variance.
RESULTS
Growth and morphology of P. aeruginosa.
Planktonic P. aeruginosa grew exponentially with or without TiO2 (see Fig. S2 in the supplemental material). The specific growth rates averaged 0.97 ± 0.11 h−1 and 0.97 ± 0.06 h−1 in the control and TiO2 treatments, respectively; these values did not significantly differ (t test, P = 0.50), which indicated that TiO2 was not growth inhibitory and did not interfere with OD600 measurements. ESEM micrographs of P. aeruginosa cells cultivated with TiO2 showed extracellular bright accumulations on the cells, confirming the adsorption of electron-dense Ti onto their cell membranes (see Fig. S3 in the supplemental material).
T. thermophila growth and motility.
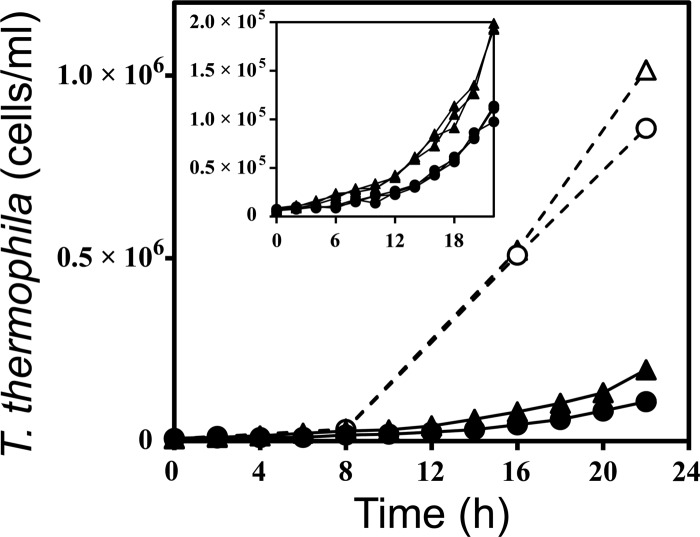
T. thermophila cells grew rapidly in rich (SSP) medium with or without TiO2 (Fig. 1). Exponential growth occurred over 16 h; the associated T. thermophila specific growth rate averaged 0.26 ± 0.003 h−1 and was the same with or without TiO2 (P = 0.17). The maximum cell counts (22 h; Fig. 1) were approximately 19% lower when TiO2 was present than in the control, and the difference was significant (P = 0.00). As expected, there was no growth in Dryl's medium in the absence of P. aeruginosa as prey, with or without TiO2 (data not shown).
Fig 1.
Growth curves of T. thermophila cultured in rich (SSP) medium without P. aeruginosa prey (open symbols, dashed lines) or in Dryl's medium with bacterial prey (closed symbols, solid lines). Triangles (△, ▲) and circles (○, ●) represent control treatment (without TiO2) and treatment with TiO2, respectively. Each point is the average of three independent replicates. Standard-error bars are smaller than the symbols and thus not visible. The inset graph shows the individual replicate growth curves for the six Dryl's medium (with prey) cultures. The symbols in the inset represent the same treatments as in the main graph.
By staining and direct counting, the initial concentration of P. aeruginosa cells in T. thermophila trophic-transfer experiments was 3.6 ×109 ml−1. The growth of the T. thermophila predator population was exponential over 22 h (Fig. 1; see Fig. S4 in the supplemental material), and the associated specific growth rate of the protozoans consuming control prey (i.e., no adsorbed TiO2) averaged 0.14 ± 0.00 h−1 (n = 3). This specific growth rate was 7% higher than the average specific growth rate of the protozoans eating prey that had preadsorbed TiO2 (0.13 ± 0.00 h−1, n = 3), and the difference between the two treatments was significant (P = 0.03). Growth of T. thermophila with TiO2-encrusted P. aeruginosa resulted in a 45% reduction in the 22-h protozoan cell count compared to that of T. thermophila with control P. aeruginosa (P = 0.00; Fig. 1). As expected, growth of T. thermophila in Dryl's medium when P. aeruginosa prey was the exclusive food source was slower than in SSP, with or without TiO2 (Fig. 1). The final (22-h) protozoan cell count in Dryl's medium with P. aeruginosa was lower by approximately 81% without TiO2 (P = 0.00) and by approximately 89% with TiO2 (P = 0.00) than that in rich (SSP) medium (no bacteria). When considering the initial counts of P. aeruginosa prey (4.3 ×1010 cells, i.e., 3.6 ×109 cells ml−1 in 12 ml; see the supplemental material) and assuming a yield of 2 ×10−5 T. thermophila cells per P. aeruginosa cell as described before (15), the maximum expected T. thermophila population size was 8.6 ×105 cells per culture or 7.2 ×104 cells ml−1. This is a slightly lower T. thermophila cell concentration than that measured in this experiment at 22 h (Fig. 1), suggesting that the T. thermophila populations in this trophic-transfer experiment were likely in late exponential phase by 22 h.
Light and Nomarski microscopy of control T. thermophila cells grown in SSP showed normal, vigorously swimming cells. Observations of the protozoans grown in the trophic-transfer experiments with P. aeruginosa showed physically normal T. thermophila cells swimming comparatively slower than cells growing in SSP. The swimming characteristics, grazing behavior, and physical appearance of T. thermophila cells remained similar throughout the 22-h experiment in all of the treatments. Thus, no obvious signs of TiO2-induced morbidity of T. thermophila cells were observed under light microscopy in any of the treatments.
T. thermophila uptake of TiO2 in rich medium.
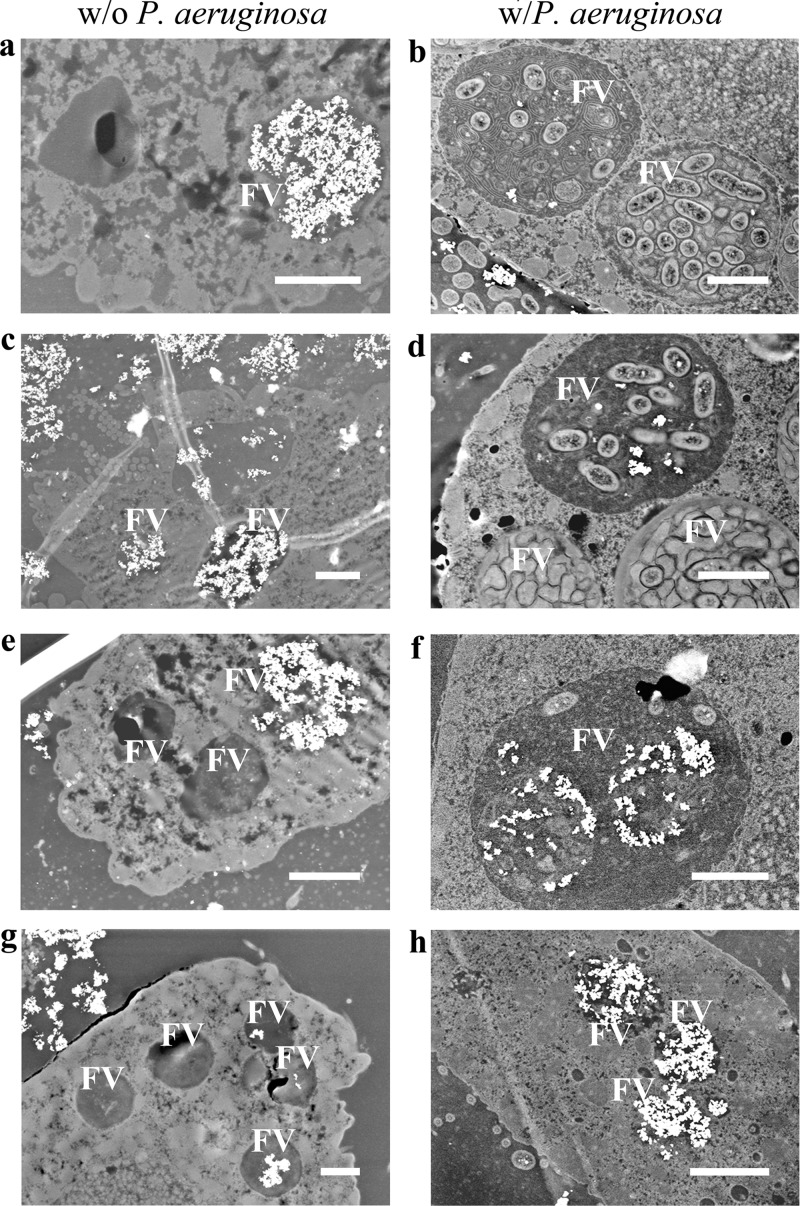
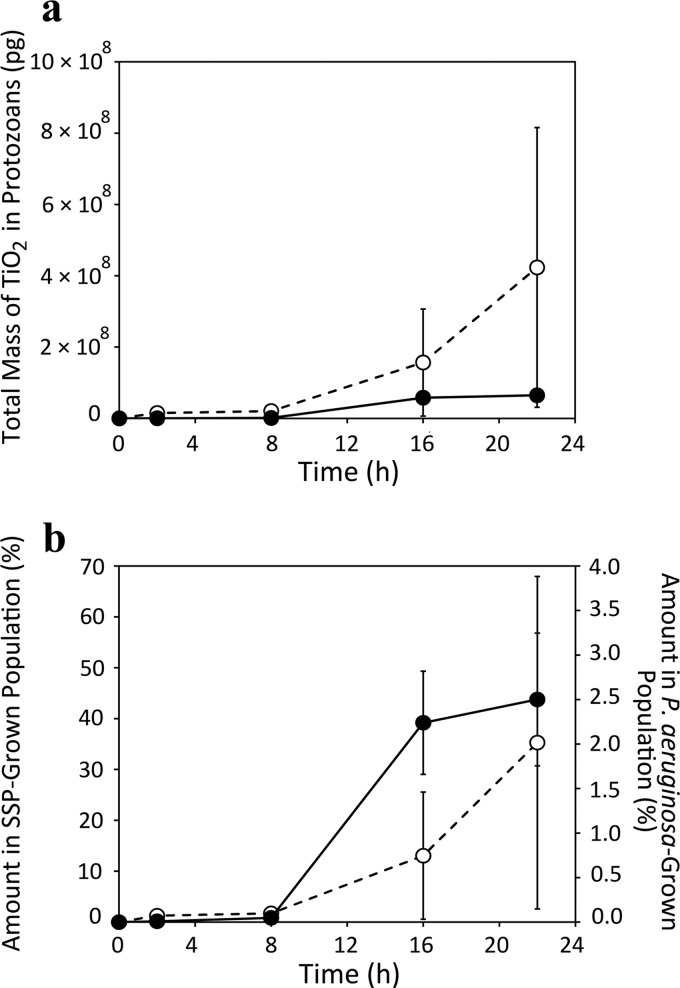
For T. thermophila grown in SSP medium with TiO2, the bright white spots in the unstained specimen STEM images show the presence of TiO2 in food vacuoles at all of the time points, although there are temporal variations in TiO2 accumulation (Fig. 2). The 2- and 8-h samples showed abundant TiO2 accumulations in food vacuoles (Fig. 2a and c), indicating that T. thermophila was engulfing TiO2 along with organic nutrients from the SSP medium (see Fig. S5 in the supplemental material). The 16- and 22-h samples showed smaller amounts of TiO2 in food vacuoles (Fig. 2e and g). These trends are supported by the data obtained via quantitative image analysis (Fig. 3a; see Table S2 in the supplemental material). The TiO2 available for consumption in the medium surrounding T. thermophila cells was apparent at 22 h (upper left corner, Fig. 2g) and also at 16 h (not shown). Consistent with the images of food vacuoles over time (Fig. 2), the calculated food vacuole volumes increased between 2 and 8 h, decreased at 16 h, and then increased again at 22 h (Fig. 3a; see Table S2). The TiO2 mass per T. thermophila cell was highest at 2 h and then decreased until 16 to 22 h (Fig. 3a; see Table S4). The percent filling of food vacuoles by TiO2 declined in a similar pattern (Fig. 3a). Still, although the overall TiO2 mass per T. thermophila cell declined over time (Fig. 3a; see Table S4), the total TiO2 in the T. thermophila population increased over time while the population size grew (Fig. 4). The final TiO2 mass within the entire population was 0.42 mg (Fig. 4, top), which was ca. 35% of the total initial TiO2 mass administered (Fig. 4, bottom).
Fig 2.
Dark-field STEM micrographs of unstained thin sections of T. thermophila cells grown in rich medium (SSP) in the presence of TiO2 (0.1 mg ml−1) without (w/o) bacteria (left column) or in Dryl's medium with (w) TiO2-encrusted P. aeruginosa prey (right column). T. thermophila cells are shown after 2 h (a, b), 8 h (c, d), 16 h (e, f), and 22 h (g, h) of growth, where 16 and 22 h were during rapid growth (Fig. 1). Food vacuoles (FV) are indicated. The brighter spots are indicative of higher-molecular-weight elements, in this case, Ti associated with TiO2. Scale bars, 2 μm.
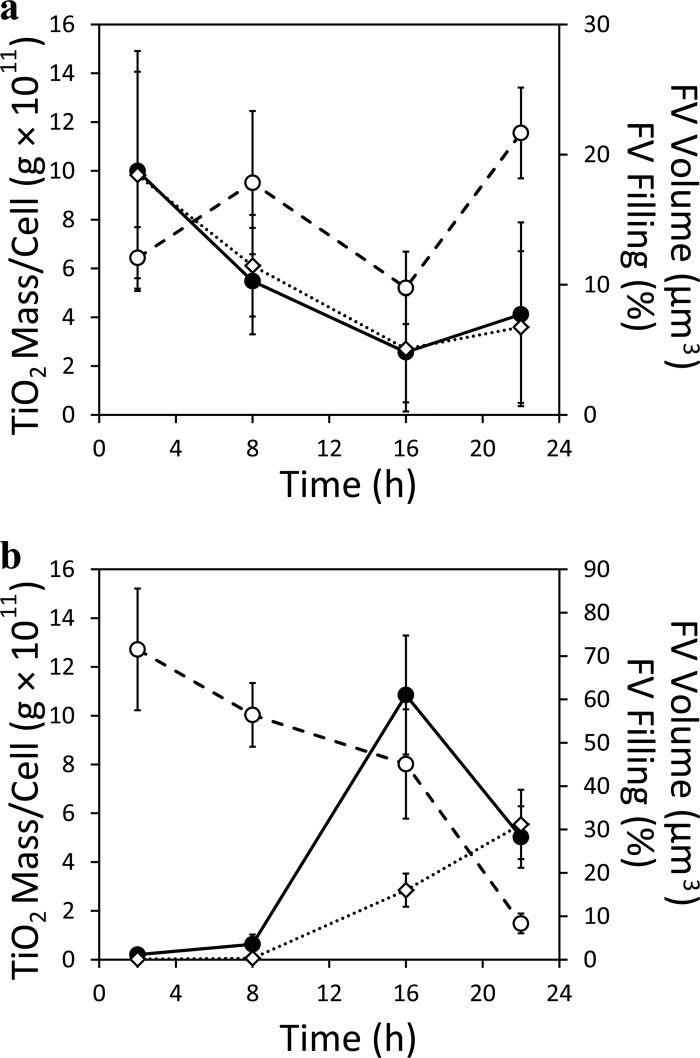
Fig 3.
Time course during T. thermophila growth (Fig. 1) of mean food vacuole (FV) volume (○, dashed lines), measured average percent food vacuole volume filled with TiO2 (♢, dotted lines), and TiO2 mass per T. thermophila cell (●, solid lines). (a) T. thermophila grown in rich (SSP) medium with 0.1 mg ml−1 TiO2 but without P. aeruginosa bacterial prey. (b) T. thermophila grown by feeding exclusively on TiO2-encrusted P. aeruginosa cells. The patterns are consistent with those observed in electron micrographs (Fig. 2). The plotted values are tabulated in Tables S2 to S5 in the supplemental material.
Fig 4.
Total TiO2 mass (a) and percentage of total administered TiO2 (b) within whole T. thermophila populations at each time point during growth. Dashed line (○), SSP-grown population; solid line (●), population grown in Dryl's medium with P. aeruginosa. Points are average values (n = 3). Error bars are the result of propagating errors according to standard methods.
T. thermophila uptake of TiO2 from P. aeruginosa prey.
STEM images, acquired at 2, 8, 16, and 22 h, of unstained samples of T. thermophila grown exclusively by feeding on TiO2-encrusted P. aeruginosa in Dryl's medium showed increasing amounts of TiO2 in food vacuoles over time (Fig. 2). Normal digestion of P. aeruginosa cells in T. thermophila was indicated by the few observable P. aeruginosa cells in food vacuoles. Greater TiO2 amounts, with still fewer undigested P. aeruginosa cells in food vacuoles, were observed at 16 h than at earlier time points (Fig. 2f). ESEM imaging showed TiO2 bound to P. aeruginosa cell membranes within a T. thermophila food vacuole at 16 h (see Fig. S6 in the supplemental material); control (i.e., not TiO2 exposed) treatments did not exhibit this brightness (not shown). The EDS spectrum (see Fig. S6) confirmed the presence of titanium. At 22 h, most food vacuoles appeared to be filled with TiO2 and there were very few undigested P. aeruginosa cells (Fig. 2h). X-ray microscopy images of a thick section of a T. thermophila cell cultured to 22 h with TiO2-encrusted P. aeruginosa cells as prey show, consistent with the STEM image (Fig. 2h), food vacuoles filled with TiO2 mainly near the oral apparatus (see Fig. S7 and Movie S1 in the supplemental material). Nomarski images taken at 22 h also show numerous dark food vacuoles, illustrating that they contained TiO2 (see Fig. S8).
The volume fraction of TiO2 in food vacuoles varied with time when T. thermophila grown in SSP with TiO2 was compared with T. thermophila grown with TiO2-encrusted P. aeruginosa (Fig. 3). Initially (2 h), the food vacuoles of T. thermophila that were fed TiO2-encrusted P. aeruginosa contained little TiO2 (Fig. 3b; see Table S3). Over time, however, the amount increased (Fig. 3b; see Table S3). Essentially, the food vacuole-filling patterns were reversed for T. thermophila grown in TiO2-amended SSP and T. thermophila grown with TiO2-encrusted P. aeruginosa (Fig. 3; see Table S2 versus S3).
The average food vacuole volume decreased rather consistently between 2 and 22 h for T. thermophila cells feeding exclusively on TiO2-encrusted P. aeruginosa (Fig. 3b; see Table S3), while the volume percent of food vacuoles filled with TiO2 increased (Fig. 3b). The calculated mass of TiO2 per T. thermophila cell increased between 2 and 16 h for T. thermophila feeding on TiO2-encrusted P. aeruginosa but then dropped at 22 h to less than half of the 16-h value (Fig. 3b; see Table S5). Although T. thermophila population growth still appeared to be exponential between 16 and 22 h (Fig. 1; see Fig. S4), the total TiO2 in this predatory T. thermophila population peaked at 16 h and was 0.06 mg at 22 h (Fig. 4, top). This final mass of TiO2 in the T. thermophila population represented less than 3% of the starting TiO2 mass associated with P. aeruginosa-encrusted cells (Fig. 4, bottom).
Quantitative assessment of TiO2 bioconcentration and biomagnification.
The mass of TiO2 adsorbed per P. aeruginosa cell was determined to be 0.06 pg (see Section 1.2 of the supplemental material). On a dry cell mass basis and on a cell volume basis, this is equivalent to 0.33 pg of TiO2/pg of TiO2-encrusted dry P. aeruginosa cells and 0.00015 mg of TiO2/ml of P. aeruginosa cells, respectively. All of the TiO2 administered in the trophic-transfer growth medium was adsorbed to P. aeruginosa cells (see the supplemental material).
For T. thermophila grown in rich medium with TiO2, the cellular mass concentration of TiO2 (Fig. 3a; see Table S4) declined from 2 to 22 h. During the growth time course, the cellular TiO2 made up 5% or less of the T. thermophila cell mass (see Table S4). The T. thermophila BCFs, calculated by dividing the T. thermophila cellular TiO2 mass concentration by the initial SSP medium TiO2 concentration, were 142, 78, 40, and 98 at 2, 8, 16, and 22 h, respectively (see Table S4). By these BCFs, TiO2 was bioconcentrated in T. thermophila cells apparently only in the food vacuoles (Fig. 2) and not in the growth medium. However, its bioconcentration varied over time (see Table S4), as evidenced by the initially TiO2-enriched and then TiO2-depleted and enriched-again food vacuoles over time (Fig. 2 and 3a; see Table S2).
In T. thermophila grown by predation of TiO2-encrusted P. aeruginosa, the T. thermophila cellular mass concentration of TiO2 (Fig. 3b; see Table S5) increased from 2 and 8 h to 16 h and then decreased at 22 h. In the 2- to 22-h growth period, the TiO2 mass per T. thermophila cell was up to 5% of the T. thermophila mass (see Table S5). When taking into account the cellular concentration of TiO2 on TiO2-encrusted P. aeruginosa prey (0.06 pg of TiO2/pg of TiO2-encrusted P. aeruginosa cells; see the supplemental material), the BMF was much less than 1, i.e., with a low of 0.003 at 2 h and a high of 0.16 at 16 h (see Table S5). Thus, TiO2 was far more concentrated on P. aeruginosa cells than in T. thermophila cells. The low BMFs are partly explained by the volumetric dilution of initially bacterially associated TiO2 into the much larger protozoan cells (i.e., 7,042 μm3 for a T. thermophila cell versus 0.4 μm3 for a bare P. aeruginosa cell; see Section 1.3 of the supplemental material) and also by the observation that TiO2 stayed within food vacuoles and was not distributed into the T. thermophila cytoplasm (Fig. 2). The localization of ingested TiO2 to food vacuoles within T. thermophila also limited biomagnification, as food vacuoles ultimately excrete their contents into the medium. Assuming that the yield of T. thermophila from predation upon control P. aeruginosa was ca. 2 × 10−5 protozoans per P. aeruginosa cell, as described before (15), the maximum potential mass of TiO2 in the T. thermophila population would be (1.95 ×105 T. thermophila cells) × (2 × 105 P. aeruginosa cells/T. thermophila cell) × (0.06 pg of TiO2/P. aeruginosa cell) = 0.23 ×1010 pg or 2.33 mg of TiO2. This maximum potential TiO2 mass delivered into the T. thermophila population after 22 h of predation of TiO2-encrusted P. aeruginosa is very close to the total TiO2 initially associated with P. aeruginosa in the trophic-transfer experiment (2.6 mg; see Section 2 of the supplemental material). Clearly, since a much smaller mass of TiO2 was in the T. thermophila population by 22 h (0.06 mg, Fig. 4), ingestion of TiO2 via predation of TiO2-encrusted P. aeruginosa had to have been accompanied by extensive TiO2 excretion from T. thermophila food vacuoles. Thus, although food vacuoles were smaller and more TiO2 filled over time (Fig. 3b), which may have contributed to a lower growth rate (Fig. 1), TiO2 biomagnification did not occur in predatory T. thermophila, owing to localization of TiO2 limited to T. thermophila food vacuoles, a cellular volumetric dilution effect, and ongoing excretion of TiO2 back into the medium.
DISCUSSION
Given the prevalence and increasing use of nanoscale TiO2 in consumer goods (8), it is potentially concerning that nanoscale TiO2—a relatively insoluble manufactured nanomaterial—will accumulate in the environment, where microbial processes contributing to nutrient cycling could be impacted. Concentrations of nanoscale titanium were recently reported to be up to 6% in aquatic sediments near the outlet of a major wastewater treatment plant outfall (27). In the present study, a model protozoan, T. thermophila, was exposed to lower concentrations of TiO2 via one of two routes, direct ingestion from surrounding aqueous medium or predation of TiO2-encrusted bacterial prey, in this case, P. aeruginosa that had previously been shown to extracellularly adsorb TiO2 (17). The results showed that the effects of TiO2 on T. thermophila could vary with the exposure route.
Protozoans could be exposed to nanoscale TiO2 in the environment via either medium or prey, and our results suggest TiO2 uptake in either case. Protozoans accumulate nutrients by swimming through the water column and grazing along the solid-water interface (28), feeding on bacteria, inert materials, and other dead or decaying debris small enough to enter the oral apparatus (29, 30). Here, TiO2 was ingested indiscriminately by T. thermophila. This was evidenced by the observation that the amount of nanomaterial taken up was quantitatively similar to the mass expected on the basis of the numbers of bacteria in the food vacuoles. For example, an 8-h food vacuole shows around 10 bacterial cells (Fig. 2d). This number of cells would have carried approximately 0.6 pg of TiO2 (10 cells, each with 0.06 pg TiO2; see Section 1 of the supplemental material) into the food vacuole, which is comparatively very close to the average mass of TiO2 per food vacuole (0.9 pg; see Table S3). This indicates that there was no preferential feeding, e.g., of sparsely versus heavily coated P. aeruginosa cells. While there are prior examples of preferential feeding by protozoa, including an acidophilic protozoan that preferentially consumed Acidithiobacillus ferrooxidans over Leptospirillum ferrooxidans (31, 32) and marine protozoa that preferentially grazed on larger allochthonous bacteria even within a larger population of small autochthonous bacteria (33), other results are more similar to ours, i.e., showing predator-prey behavior with bacterially sorbed or free-floating particles and no preferential feeding with either inert beads (34) or CdSe ZnS-capped QD nanoparticles (35).
In a prior study conducted similarly, the trophic transfer of CdSe QDs resulted in significant toxicity to T. thermophila at the cellular and population levels, owing to the inherent chemical toxicity of the nanomaterials and the translocation of the QDs past food vacuoles and into the T. thermophila cytoplasm (15). In contrast, there were no obvious toxic cellular effects (as indicated by normal T. thermophila morphology and motility) of nanoscale TiO2. Rather, effects were restricted to population growth. While the exact mechanism was not elucidated in this study, TiO2 uptake into food vacuoles could displace nutrients and thereby create a nutrient-limiting condition that has an effect at the population scale, i.e., slightly slowing population growth for predatory T. thermophila exclusively fed TiO2-encrusted P. aeruginosa. A consequence of this occurrence in nature could be a negative effect on the predator-prey equilibrium, which has inherent ecosystem level implications. This consideration would not have been predicted by the lack of observed toxicity to individual cells.
The potential for nanomaterial uptake into organisms and enhanced transfer of nanomaterials in food chains, i.e., bioaccumulation and biomagnification, is also of interest in the ecotoxicology of nanomaterials (15, 36). In a prior study, Cd associated with CdSe QDs was biomagnified during trophic transfer from P. aeruginosa to T. thermophila (15), suggesting that bacteria with their bioconcentrated nanomaterials could initiate biomagnification at the base of food webs. Nanomaterial uptake into bacteria has been demonstrated for gold (37), silver (38), cerium dioxide (CeO2) (39), and CdSe QDs (13), as well as clays and other nanomaterials that tend to aggregate in nature (40, 41). However, here, while T. thermophila fed upon TiO2-encrused P. aeruginosa and thereby ingested TiO2, there was no quantitative biomagnification. In an analogous study of zebrafish that were ingesting TiO2 either directly or by predation upon TiO2-contaminated daphnids, the BCFs and BMFs were in the ranges of those reported here (42). In this study, since TiO2 in T. thermophila cells was confined within food vacuoles, there was little opportunity for nanoscale TiO2 to be magnified in these protozoans. With other nontoxic nanoscale metals, biomagnification does not occur since the predator-prey interactions facilitate metal recycling. For example, many iron-encrusted microbial communities are continually being grazed on by protozoa (43, 44) and the iron is ultimately utilized in protozoan and bacterial processes (45, 46) as an alternate Fe source that increases productivity and nitrogen uptake in food webs (47–49). Protozoa also engulf manganese-encrusted bacteria, which contributes to the geochemical cycling of Mn during normal protozoan bacterial digestion (50). Bioaccumulated thallium adsorbed to bacteria that are preyed upon by protozoa contributes to geochemical cycling through the oxidation of thermodynamically stable Tl(I) to more abundant Tl(III) (51). Here, the rather insoluble (20) TiO2 nanomaterial was ingested and egested, as was the protozoan's food, and thus a greater proportion of the overall TiO2 in the cultures was within the protozoans as their population size increased. Still, although biomagnification did not occur in our study, TiO2 uptake was not beneficial to T. thermophila and in fact was slightly harmful on the population scale.
The ever-increasing use of manufactured TiO2 increases the potential for population level impacts on organisms at the lowest trophic levels, which are fundamental to the overall health of ecosystems. Here, TiO2 adsorbed to P. aeruginosa prey, which is a likely common association of manufactured nanomaterials with bacteria, was ingested along with P. aeruginosa during T. thermophila predation. In both regimens of TiO2 intake (trophic transfer and directly via medium) by T. thermophila, the main effects were the presence of TiO2 in the T. thermophila population and a reduced population growth rate and yield. An implication is that, by their uptake into and growth effects on microbial food chains, nanomaterials could impact higher trophic levels irrespective of biomagnification.
Supplementary Material
ACKNOWLEDGMENTS
This research was funded primarily by the National Science Foundation and the Environmental Protection Agency under cooperative agreement DBI-0830117. Any opinions, findings, and conclusions or recommendations expressed in this material are ours and do not necessarily reflect the views of either the National Science Foundation or the Environmental Protection Agency. This work has not been subjected to Environmental Protection Agency review, and no official endorsement should be inferred.
ESEM and STEM were partly performed in the Micro-Environmental Imaging and Analysis Facility at the University of California Santa Barbara (www.bren.ucsb.edu/facilities/MEIAF/) under National Science Foundation awards BES-9977772 and DBI-0216480. Funding to E.O. was provided by National Science Foundation award MCB-1025069.
Footnotes
Published ahead of print 12 July 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01680-13.
REFERENCES
- 1.Klaine SJ, Alvarez PJJ, Batley GE, Fernandes TF, Handy RD, Lyon DY, Mahendra S, McLaughlin MJ, Lead JR. 2008. Nanomaterials in the environment: behavior, fate, bioavailability, and effects. Environ. Toxicol. Chem. 27:1825–1851 [DOI] [PubMed] [Google Scholar]
- 2.Lawton J. 2008. Novel materials in the environment: the case of nanotechnology. Royal Commission on Environmental Pollution, London, United Kingdom [Google Scholar]
- 3.Kaegi R, Ulrich A, Sinnet B, Vonbank R, Wichser A, Zuleeg S, Simmler H, Brunner S, Vonmont H, Burkhardt M, Boller M. 2008. Synthetic TiO2 nanoparticle emission from exterior facades into the aquatic environment. Environ. Pollut. 156:233–239 [DOI] [PubMed] [Google Scholar]
- 4.Weir A, Westerhoff P, Fabricius L, Hristovski K, von Goetz N. 2012. Titanium dioxide nanoparticles in food and personal care products. Environ. Sci. Technol. 46:2242–2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nohynek GJ, Antignac E, Re T, Toutain H. 2010. Safety assessment of personal care products/cosmetics and their ingredients. Toxicol. Appl. Pharmacol. 243:239–259 [DOI] [PubMed] [Google Scholar]
- 6.Choksi AN, Poonawalla T, Wilkerson MG. 2010. Nanoparticles: a closer look at their dermal effects. J. Drugs Dermatol. 9:475–481 [PubMed] [Google Scholar]
- 7.Phillips LG, Barbano DM. 1997. The influence of fat substitutes based on protein and titanium dioxide on the sensory properties of lowfat milks. J. Dairy Sci. 80:2726–2731 [DOI] [PubMed] [Google Scholar]
- 8.Robichaud CO, Uyar AE, Darby MR, Zucker LG, Wiesner MR. 2009. Estimates of upper bounds and trends in nano-TiO2 production as a basis for exposure assessment. Environ. Sci. Technol. 43:4227–4233 [DOI] [PubMed] [Google Scholar]
- 9.Boxall AB, Tiede K, Chaudhry Q. 2007. Engineered nanomaterials in soils and water: how do they behave and could they pose a risk to human health? Nanomedicine 2:919–927 [DOI] [PubMed] [Google Scholar]
- 10.Suresh AK, Pelletier DA, Doktycz MJ. 2013. Relating nanomaterial properties and microbial toxicity. Nanoscale 5:463–474 [DOI] [PubMed] [Google Scholar]
- 11.Mileyeva-Biebesheimer ON, Zaky A, Gruden CL. 2010. Assessing the impact of titanium dioxide and zinc oxide nanoparticles on bacteria using a fluorescent-based cell membrane integrity assay. Environ. Eng. Sci. 27:329–335 [Google Scholar]
- 12.Dumas EM, Ozenne V, Mielke RE, Nadeau JL. 2009. Toxicity of CdTe quantum dots in bacterial strains. IEEE Trans. Nanobioscience 8:58–64 [DOI] [PubMed] [Google Scholar]
- 13.Priester JH, Stoimenov PK, Mielke RE, Webb SM, Ehrhardt C, Zhang JP, Stucky GD, Holden PA. 2009. Effects of soluble cadmium salts versus CdSe quantum dots on the growth of planktonic Pseudomonas aeruginosa. Environ. Sci. Technol. 43:2589–2594 [DOI] [PubMed] [Google Scholar]
- 14.Kloepfer JA, Mielke RE, Wong MS, Nealson KH, Stucky G, Nadeau JL. 2003. Quantum dots as strain- and metabolism-specific microbiological labels. Appl. Environ. Microbiol. 69:4205–4213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Werlin R, Priester JH, Mielke RE, Krämer S, Jackson S, Stoimenov PK, Stucky GD, Cherr GN, Orias E, Holden PA. 2011. Biomagnification of cadmium selenide quantum dots in a simple experimental microbial food chain. Nat. Nanotechnol. 6:65–71 [DOI] [PubMed] [Google Scholar]
- 16.Ghafari P, St-Denis CH, Power ME, Jin X, Tsou V, Mandal HS, Bols NC, Tang X. 2008. Impact of carbon nanotubes on the ingestion and digestion of bacteria by ciliated protozoa. Nat. Nanotechnol. 3:347–351 [DOI] [PubMed] [Google Scholar]
- 17.Horst AM, Neal AC, Mielke RE, Sislian PR, Suh WH, Mädler L, Stucky GD, Holden PA. 2010. Dispersion of TiO2 nanoparticle agglomerates by Pseudomonas aeruginosa. Appl. Environ. Microbiol. 76:7292–7298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demchick P, Koch AL. 1996. The permeability of the wall fabric of Escherichia coli and Bacillus subtilis. J. Bacteriol. 178:768–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohno T, Sarukawa K, Tokieda K, Matsumura M. 2001. Morphology of a TiO2 photocatalyst (Degussa, P-25) consisting of anatase and rutile crystalline phases. J. Catal. 203:82–86 [Google Scholar]
- 20.Keller AA, Wang HT, Zhou DX, Lenihan HS, Cherr G, Cardinale BJ, Miller R, Ji ZX. 2010. Stability and aggregation of metal oxide nanoparticles in natural aqueous matrices. Environ. Sci. Technol. 44:1962–1967 [DOI] [PubMed] [Google Scholar]
- 21.Kirk S, Skepper J, Donald AM. 2009. Application of environmental scanning electron microscopy to determine biological surface structure. J. Microsc. 233:205–224 [DOI] [PubMed] [Google Scholar]
- 22.Douglas S, Abbey W, Mielke R, Conrad P, Kanik I. 2008. Textural and mineralogical biosignatures in an unusual microbialite from Death Valley, California. Icarus 193:620–636 [Google Scholar]
- 23.Priester JH, Ge Y, Mielke RE, Horst AM, Moritz SC, Espinosa K, Gelb J, Walker SL, Nisbet RM, An Y-J, Schimel JP, Palmer RG, Hernandez-Viezcas JA, Zhao L, Gardea-Torresdey JL, Holden PA. 2012. Soybean susceptibility to manufactured nanomaterials with evidence for food quality and soil fertility interruption. Proc. Natl. Acad. Sci. U. S. A. 109:E2451–E2456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tkachuk A, Duewer F, Cui H, Feser M, Wang S, Yun W. 2007. X-ray computed tomography in Zernike phase contrast mode at 8 keV with 50-nm resolution using Cu rotating anode X-ray source. Z. Kristallogr. 222:650–655 [Google Scholar]
- 25.Luoma SN, Rainbow PS. 2008. Metal contamination in aquatic environments: science and lateral management. Cambridge University Press, Cambridge, United Kingdom [Google Scholar]
- 26.Arnot JA, Gobas F. 2006. A review of bioconcentration factor (BCF) and bioaccumulation factor (BAF) assessments for organic chemicals in aquatic organisms. Environ. Rev. 14:257–297 [Google Scholar]
- 27.Luo ZX, Wang ZH, Li QZ, Pan QK, Yan CZ, Liu F. 2011. Spatial distribution, electron microscopy analysis of titanium and its correlation to heavy metals: occurrence and sources of titanium nanomaterials in surface sediments from Xiamen Bay, China. J. Environ. Monit. 13:1046–1052 [DOI] [PubMed] [Google Scholar]
- 28.Güde H. 1979. Grazing by protozoa as selection factor for activated-sludge bacteria. Microb. Ecol. 5:225–237 [DOI] [PubMed] [Google Scholar]
- 29.Sherr EB, Sherr BF. 2002. Significance of predation by protists in aquatic microbial food webs. Antonie Van Leeuwenhoek 81:293–308 [DOI] [PubMed] [Google Scholar]
- 30.Pernthaler J. 2005. Predation on prokaryotes in the water column and its ecological implications. Nature Rev. Microbiology 3:537–546 [DOI] [PubMed] [Google Scholar]
- 31.McGinness S, Johnson DB. 1992. Grazing of acidophilic bacteria by a flagellated protozoan. Microb. Ecol. 23:75–86 [DOI] [PubMed] [Google Scholar]
- 32.Johnson DB. 1995. Acidophilic microbial communities: candidates for bioremediation of acidic mine effluents. Int. Biodeterior. Biodegradation 35:41–58 [Google Scholar]
- 33.Menon P, Becquevort S, Billen G, Servais P. 1996. Kinetics of flagellate grazing in the presence of two types of bacterial prey. Microb. Ecol. 31:89–101 [DOI] [PubMed] [Google Scholar]
- 34.Boenigk J, Matz C, Jurgens K, Arndt H. 2001. Confusing selective feeding with differential digestion in bacterivorous nanoflagellates. J. Eukaryot. Microbiol. 48:425–432 [DOI] [PubMed] [Google Scholar]
- 35.Holbrook RD, Murphy KE, Morrow JB, Cole KD. 2008. Trophic transfer of nanoparticles in a simplified invertebrate food web. Nat. Nanotechnol. 3:352–355 [DOI] [PubMed] [Google Scholar]
- 36.Hou WC, Westerhoff P, Posner JD. 2013. Biological accumulation of engineered nanomaterials: a review of current knowledge. Environ. Sci. Processes Impacts 15:103–122 [DOI] [PubMed] [Google Scholar]
- 37.Deplanche K, Macaskie LE. 2008. Biorecovery of gold by Escherichia coli and Desulfovibrio desulfuricans. Biotechnol. Bioeng. 99:1055–1064 [DOI] [PubMed] [Google Scholar]
- 38.Sondi I, Salopek-Sondi B. 2004. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 275:177–182 [DOI] [PubMed] [Google Scholar]
- 39.Limbach LK, Bereiter R, Mueller E, Krebs R, Gaelli R, Stark WJ. 2008. Removal of oxide nanoparticles in a model wastewater treatment plant: influence of agglomeration and surfactants on clearing efficiency. Environ. Sci. Technol. 42:5828–5833 [DOI] [PubMed] [Google Scholar]
- 40.Lead JR, Davison W, Hamilton-Taylor J, Buffle J. 1997. Characterizing colloidal material in natural waters. Aquat. Geochem. 3:213–232 [Google Scholar]
- 41.Guo L, Santschi PH. 2007. Ultrafiltration and its applications to sampling and characterisation of aquatic colloids, p 159–221 In Wilkinson KJ, Lead JR. (ed), Environmental colloids and particles: behaviour, separation and characterisation. John Wiley & Sons Ltd., West Sussex, United Kingdom [Google Scholar]
- 42.Zhu XS, Wang JX, Zhang XZ, Chang Y, Chen YS. 2010. Trophic transfer of TiO2 nanoparticles from daphnia to zebrafish in a simplified freshwater food chain. Chemosphere 79:928–933 [DOI] [PubMed] [Google Scholar]
- 43.Gillan DC, Ribesse J, de Ridder C. 2004. The iron-encrusted microbial community of Urothoe poseidonis (Crustacea, Amphipoda). J. Sea Res. 52:21–32 [Google Scholar]
- 44.Gillan DC, de Ridder C. 1997. Morphology of a ferric iron-encrusted biofilm forming on the shell of a burrowing bivalve (Mollusca). Aquat. Microb. Ecol. 12:1–10 [Google Scholar]
- 45.Chase Z, Price NM. 1997. Metabolic consequences of iron deficiency in heterotrophic marine protozoa. Limnol. Oceanogr. 42:1673–1684 [Google Scholar]
- 46.Wilson ME, Britigan BE. 1998. Iron acquisition by parasitic protozoa. Parasitol. Today 14:348–353 [DOI] [PubMed] [Google Scholar]
- 47.Kudo I, Noiri Y, Cochlan WP, Suzuki K, Aramaki T, Ono T, Nojiri Y. 2009. Primary productivity, bacterial productivity and nitrogen uptake in response to iron enrichment during the SEEDS II. Deep-Sea Res. II 56:2755–2766 [Google Scholar]
- 48.Christaki U, Obernosterer I, Van Wambeke F, Veldhuis M, Garcia N, Catala P. 2008. Microbial food web structure in a naturally iron-fertilized area in the Southern Ocean (Kerguelen Plateau). Deep-Sea Res. II 55:706–719 [Google Scholar]
- 49.Vogel C, Fisher NS. 2009. Trophic transfer of Fe, Zn and Am from marine bacteria to a planktonic ciliate. Mar. Ecol. Prog. Ser. 384:61–68 [Google Scholar]
- 50.Zeiner CA, Lion LW, Shuler ML, Ghirose WC, Hay A. 2006. Cycling of biogenic Mn-oxides in a model microbial predator-prey system. Geomicrobiol. J. 23:37–43 [Google Scholar]
- 51.Twining BS, Twiss MR, Fisher NS. 2003. Oxidation of thallium by freshwater plankton communities. Environ. Sci. Technol. 37:2720–2726 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.