Abstract
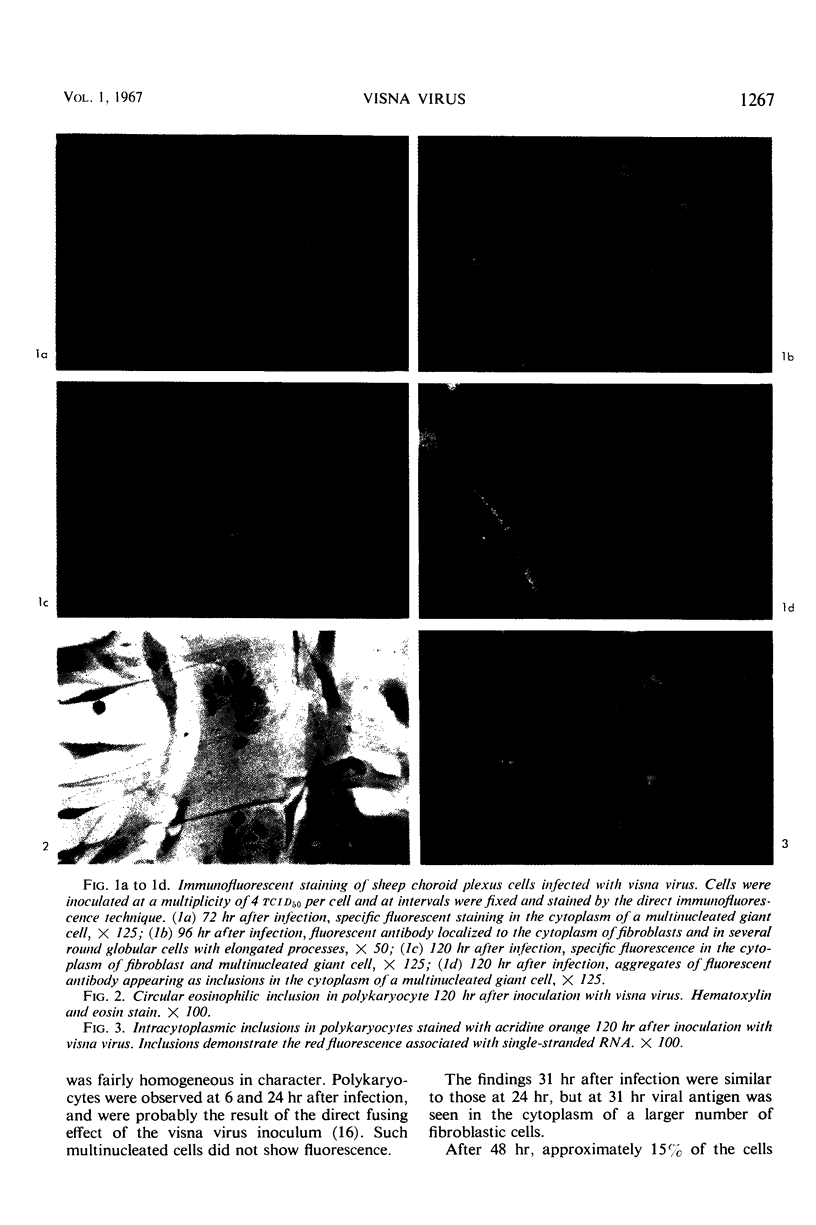
Sequential morphological changes occurring in sheep choroid plexus cells infected with visna virus were studied by direct immunofluorescence, acridine orange, and hematoxylin and eosin staining methods. Specific immunofluorescence was first detected in the perinuclear cytoplasm of solitary cells 24 hr after infection. As the infection progressed, viral antigen appeared in an increasing number of cells, and rounded globular cells with long slender processes harboring intense fluorescence were seen. Nuclear fluorescence was not observed in infected monolayers. Polykaryocytes formed within 6 hr after inoculation due to the direct cell-fusing effect of the virus inoculum did not show specific fluorescence. Viral antigen was found, however, in the cytoplasm of multinucleated giant cells in cover slips harvested after new infective virus had been released, and later in the course of infection circular fluorescent inclusions were seen in the cytoplasm of polykaryocytes. Comparable eosinophilic inclusions were observed in hematoxylin and eosin preparations, and acridine orange staining of infected monolayers demonstrated similar inclusions which fluoresced with the color characteristic of single-stranded nucleic acid and were susceptible to digestion with ribonuclease. Visna virus appears to be a ribonucleic acid virus which replicates in the cytoplasm.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BABLANIAN R., EGGERS H. J., TAMM I. STUDIES ON THE MECHANISM OF POLIOVIRUS-INDUCED CELL DAMAGE. I. THE RELATION BETWEEN POLIOVIRUS,-INDUCED METABOLIC AND MORPHOLOGICAL ALTERATIONS IN CULTURED CELLS. Virology. 1965 May;26:100–113. doi: 10.1016/0042-6822(65)90030-9. [DOI] [PubMed] [Google Scholar]
- Brown E. R., Buinauskas P., Schwartz S. O. Immunofluorescent antibody studies of a murine leukemia virus. J Bacteriol. 1966 Oct;92(4):978–982. doi: 10.1128/jb.92.4.978-982.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussell R. H., Karzon D. T. Canine distemper virus in ferret, dog and bovine kidney cell cultures. Arch Gesamte Virusforsch. 1965;17(2):163–202. doi: 10.1007/BF01267904. [DOI] [PubMed] [Google Scholar]
- CHANY C., COOK M. K. [A virus-induced factor producing the formation of syncytium in cell culture]. Ann Inst Pasteur (Paris) 1960 Jun;98:920–924. [PubMed] [Google Scholar]
- COONS A. H., KAPLAN M. H. Localization of antigen in tissue cells; improvements in a method for the detection of antigen by means of fluorescent antibody. J Exp Med. 1950 Jan 1;91(1):1–13. doi: 10.1084/jem.91.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compans R. W., Holmes K. V., Dales S., Choppin P. W. An electron microscopic study of moderate and virulent virus-cell interactions of the parainfluenza virus SV5. Virology. 1966 Nov;30(3):411–426. doi: 10.1016/0042-6822(66)90119-x. [DOI] [PubMed] [Google Scholar]
- Duc-Nguyen H., Rosenblum E. N. Immuno-electron microscopy of the morphogenesis of mumps virus. J Virol. 1967 Apr;1(2):415–429. doi: 10.1128/jvi.1.2.415-429.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENDERS J. F., PEEBLES T. C. Propagation in tissue cultures of cytopathogenic agents from patients with measles. Proc Soc Exp Biol Med. 1954 Jun;86(2):277–286. doi: 10.3181/00379727-86-21073. [DOI] [PubMed] [Google Scholar]
- FRANKLIN R. M. A cytochemical description of the multiplication of mengovirus in L-929 cells. J Cell Biol. 1962 Jan;12:1–15. doi: 10.1083/jcb.12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOMATOS P. J., TAMM I., DALES S., FRANKLIN R. M. Reovirus type 3: physical characteristics and interaction with L cells. Virology. 1962 Jul;17:441–454. doi: 10.1016/0042-6822(62)90139-3. [DOI] [PubMed] [Google Scholar]
- GRESSER I., ENDERS J. F. Cytopathogenicity of mumps virus in cultures of chick embryo and human amnion cells. Proc Soc Exp Biol Med. 1961 Aug-Sep;107:804–807. doi: 10.3181/00379727-107-26761. [DOI] [PubMed] [Google Scholar]
- Gomatos P. J., Tamm I. THE SECONDARY STRUCTURE OF REOVIRUS RNA. Proc Natl Acad Sci U S A. 1963 May;49(5):707–714. doi: 10.1073/pnas.49.5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harter D. H., Choppin P. W. Cell-fusing activity of visna virus particles. Virology. 1967 Feb;31(2):279–288. doi: 10.1016/0042-6822(67)90172-9. [DOI] [PubMed] [Google Scholar]
- Holmes K. V., Choppin P. W. On the role of the response of the cell membrane in determining virus virulence. Contrasting effects of the parainfluenza virus SV5 in two cell types. J Exp Med. 1966 Sep 1;124(3):501–520. doi: 10.1084/jem.124.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe C., Morgan C., de Vaux St Cyr C., Hsu K. C., Rose H. M. Morphogenesis of type 2 parainfluenza virus examined by light and electron microscopy. J Virol. 1967 Feb;1(1):215–237. doi: 10.1128/jvi.1.1.215-237.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelloff G., Vogt P. K. Localization of avian tumor virus group-specific antigen in cell and virus. Virology. 1966 Jul;29(3):377–384. doi: 10.1016/0042-6822(66)90213-3. [DOI] [PubMed] [Google Scholar]
- LEPINE P., CHANY C., DROZ B., ROBBE-FOSSAT F. Cytopathogenic effect of two newly recognized myxovirus strains: mechanism of syncytial formation. Ann N Y Acad Sci. 1959 Jul 21;81:62–72. doi: 10.1111/j.1749-6632.1959.tb49295.x. [DOI] [PubMed] [Google Scholar]
- MAYOR H. D., HILL N. O. Acridine orange staining of a single-stranded DNA bacteriophage. Virology. 1961 Jun;14:264–266. doi: 10.1016/0042-6822(61)90202-1. [DOI] [PubMed] [Google Scholar]
- MILOVANOVIC M. V., ENDERS J. F., MITUS A. Cultivation of measles virus in human amnion cells and in developing chick embryo. Proc Soc Exp Biol Med. 1957 May;95(1):120–127. doi: 10.3181/00379727-95-23140. [DOI] [PubMed] [Google Scholar]
- NOYES W. F. Development of Rous sarcoma virus antigens in cultured chick embryo cells. Virology. 1960 Nov;12:488–492. doi: 10.1016/0042-6822(60)90170-7. [DOI] [PubMed] [Google Scholar]
- OSATO T., MIRAND E. A., GRACE J. T., Jr PROPAGATION AND IMMUNOFLUORESCENT INVESTIGATIONS OF FRIEND VIRUS IN TISSUE CULTURE. Nature. 1964 Jan 4;201:52–54. doi: 10.1038/201052a0. [DOI] [PubMed] [Google Scholar]
- Osato T., Mirand E. A., Grace J. T., Jr Hemadsorption and immunofluorescence of Friend virus in cell culture. Proc Soc Exp Biol Med. 1965 Aug-Sep;119(4):1187–1191. doi: 10.3181/00379727-119-30410. [DOI] [PubMed] [Google Scholar]
- PLOWRIGHT W., FERRIS R. D. Studies with rinderpest virus in tissue culture. I. Growth and cytopathogenicity. J Comp Pathol. 1959 Apr;69(2):152–172. doi: 10.1016/s0368-1742(59)80015-1. [DOI] [PubMed] [Google Scholar]
- Payne F. E., Solomon J. J., Purchase H. G. Immunofluorescent studies of group-specific antigen of the avian sarcoma-leukosis viruses. Proc Natl Acad Sci U S A. 1966 Feb;55(2):341–349. doi: 10.1073/pnas.55.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUCKLE G. Studies with measles virus. I. Propagation in different tissue culture systems. J Immunol. 1957 May;78(5):330–340. [PubMed] [Google Scholar]
- SIGURDSSON B., PALSSON P. A. Visna of sheep; a slow, demyelinating infection. Br J Exp Pathol. 1958 Oct;39(5):519–528. [PMC free article] [PubMed] [Google Scholar]
- SIGURDSSON B., PALSSON P., GRIMSSON H. Visna, a demyelinating transmissible disease of sheep. J Neuropathol Exp Neurol. 1957 Jul;16(3):389–403. doi: 10.1097/00005072-195707000-00010. [DOI] [PubMed] [Google Scholar]
- STOKER M. G. Mode of intercellular transfer of herpes virus. Nature. 1958 Nov 29;182(4648):1525–1526. doi: 10.1038/1821525a0. [DOI] [PubMed] [Google Scholar]
- THORMAR H. A COMPARISON OF VISNA AND MAEDI VIRUSES. I. PHYSICAL, CHEMICAL AND BIOLOGICAL PROPERTIES. Res Vet Sci. 1965 Jan;6:117–129. [PubMed] [Google Scholar]
- THORMAR H. An electron microscope study of tissue cultures infected with visna virus. Virology. 1961 Aug;14:463–475. doi: 10.1016/0042-6822(61)90339-7. [DOI] [PubMed] [Google Scholar]
- THORMAR H. The growth cycle of visna virus in monolayer cultures of sheep cells. Virology. 1963 Mar;19:273–278. doi: 10.1016/0042-6822(63)90064-3. [DOI] [PubMed] [Google Scholar]
- Thormar H. Observations on visna virus-infected cell cultures stained with acridine orange. Acta Pathol Microbiol Scand. 1966;68(1):54–58. doi: 10.1111/apm.1966.68.1.54. [DOI] [PubMed] [Google Scholar]
- VOGT P. K., RUBIN H. Localization of infectious virus and viral antigen in chick fibroblasts during successive stages of infection with Rous sarcoma virus. Virology. 1961 Apr;13:528–544. doi: 10.1016/0042-6822(61)90284-7. [DOI] [PubMed] [Google Scholar]
- Yoshida K., Smith K. L., Pinkel D. Studies of murine leukemia viruses. I. Detection of Moloney and Rauscher leukemia viruses by indirect immunofluorescence. Proc Soc Exp Biol Med. 1966 Jan;121(1):72–81. doi: 10.3181/00379727-121-30701. [DOI] [PubMed] [Google Scholar]






