Abstract
Trypanosoma brucei gambiense, a parasitic protozoan belonging to kinetoplastids, is the main etiological agent of human African trypanosomiasis (HAT), or sleeping sickness. One major characteristic of this disease is the dysregulation of the host immune system. The present study demonstrates that the secretome (excreted-secreted proteins) of T. b. gambiense impairs the lipopolysaccharide (LPS)-induced maturation of murine dendritic cells (DCs). The upregulation of major histocompatibility complex class II, CD40, CD80, and CD86 molecules, as well as the secretion of cytokines such as tumor necrosis factor alpha, interleukin-10 (IL-10), and IL-6, which are normally released at high levels by LPS-stimulated DCs, is significantly reduced when these cells are cultured in the presence of the T. b. gambiense secretome. Moreover, the inhibition of DC maturation results in the loss of their allostimulatory capacity, leading to a dramatic decrease in Th1/Th2 cytokine production by cocultured lymphocytes. These results provide new insights into a novel efficient immunosuppressive mechanism directly involving the alteration of DC function which might be used by T. b. gambiense to interfere with the host immune responses in HAT and promote the infectious process.
INTRODUCTION
Human African trypanosomiasis (HAT), or sleeping sickness, is caused by Trypanosoma brucei gambiense and Trypanosoma brucei rhodesiense, extracellular eukaryotic flagellate parasites transmitted by tsetse flies (Glossina spp.). Infection with T. b. gambiense gives rise to the chronic form of sleeping sickness in West and Central Africa, whereas infection with T. b. rhodesiense results in the acute form of sleeping sickness in East Africa. If left untreated, both forms of the disease are fatal (1). Treatment of the disease is difficult and may have potentially life-threatening side effects (1–6). To date, no prophylactic chemotherapy and no prospect of a vaccine are in view. Therefore, novel therapeutic targets for fighting the parasite are urgently needed. This requires a clear understanding of the different factors involving host characteristics and the intrinsic properties of the parasite, such as its ability to escape the host immune system.
Trypanosomes of the T. brucei group cause many immune system disorders. The most striking biological phenomenon is the ability of the trypanosome to keep changing its surface antigenic coat (variant surface glycoprotein [VSG]) (7), which allows evasion of the host's defense mechanisms by inducing polyclonal activation of B cells (8). Moreover, during trypanosomiasis, nonspecific polyclonal activation of B and T lymphocytes is associated with immunosuppression, facilitating parasite evasion, survival, and dissemination (8, 9).
A class of proteins that is particularly noteworthy with respect to immunomodulation is the excreted-secreted proteins (ESPs), among which several were analyzed individually and were shown to be involved in various aspects of the virulence process or pathogenesis (10–13). For instance, trypanosome-secreted proteins induce macrophage arginase, a hallmark of macrophage alternative activation (12), indicating their potency as paracrine modulators.
The initial recognition of an invading pathogen by antigen-presenting cells (APCs) is crucial in regulating innate and adaptive immune responses. Given their unique and essential function in the initiation of the acquired immune response (14), dendritic cells (DCs) have been demonstrated to be the primary cell population responsible for activating naive VSG-specific Th-cell responses in a tissue-specific manner (15). DC maturation is associated with increased expression of major histocompatibility complex (MHC) and other costimulatory molecules (CD40, CD80, CD86) that enhance the proliferation of naive T cells, while DC activation is related to the production of inflammatory cytokines that regulate T-cell differentiation (16). Interestingly, resistance to trypanosome infection was associated with upregulation of costimulatory molecules, secretion of interleukin-12 (IL-12), and presentation of VSG peptides to T cells in vivo (15). However, tumor necrosis factor alpha (TNF-α)- and nitric oxide (NO) synthase 2-producing DCs are responsible for trypanosome infection-associated pathogenesis (17), but the regulation of DC populations appears to be even more complex, since T. brucei VSG or TNF-α was shown to induce partial maturation of DCs, generating a default Th2-cell differentiation response, whereas lipopolysaccharide (LPS)-induced full maturation of the same DCs was shown to generate a Th1-cell response (18).
To better understand the mechanisms that underlie the host immune system disorders primed through DC population modulation in a T. b. gambiense infection, we investigated the effects of the secretomes of two different T. b. gambiense strains on LPS-induced maturation and activation of murine DCs. We chose the model of DCs stimulated by LPS, a Toll-like receptor 4 (TLR4) ligand that has been shown to be a saliva component modulating the host innate immune response through a vector bite in different models (19–21), to dissect the effect of the trypanosome's secretome in the context of tsetse fly transmission. Our ex vivo data show that T. b. gambiense secretomes impair the LPS-induced maturation of DCs and inhibit the DC-mediated secretion of proinflammatory cytokines required to activate the host immune system to fight the parasite.
MATERIALS AND METHODS
Mice and rats.
Female BALB/c (H-2d) and C57BL/6 (H-2b) mice aged 6 to 8 weeks and male Wistar rats aged 6 to 12 weeks were purchased from Charles River Laboratories (L'Arbresle, France). All animal experiments were conducted according to Centre de Coopération Internationale en Recherche Agronomique pour le Développement (CIRAD) institutional guidelines; the protocols were submitted to and approved by the Ethics Committee on Animal Experimentation at the CIRAD, Montpellier, France.
Trypanosomes and secretomes.
Trypanosome bloodstream strains FEO (ITMAP 1893 MHOM R) and OK (ITMAP 1841) (22, 23) were used for the experiments. The parasites were intraperitoneally injected into rats. When their multiplication reached the logarithmic growth stage, the parasites were purified from blood by chromatography on a DEAE-cellulose column, as previously described (24). The parasites were resuspended at a concentration of 200 × 106 cells/ml in a secretion buffer (Ringer lactate; glucose, 0.6%; KCl, 0.4%; NaHCO3, 0.125%; l-glutamine, 2 mM; minimal essential medium nonessential amino acids, pH 8) (12) and incubated at 37°C in 5% CO2 for 2 h. Every 15 min, parasite viability was controlled by microscopic examination and quantified by flow cytofluorometric analysis using the DNA intercalant propidium iodide (PI), as recommended by the manufacturer (Immunotech, Marseille, France). Parasite viability was always high (in general, nearly 98%) and remained constant for 2 h. In addition, only one peptide of VSG supported by a very low peptide score [36] was sequenced by tandem mass spectrometry (MS/MS) in secretomes (25). In mass spectrometry, proteins supported by only one peptide are not considered valid. This means that VSG was not identified in the secretome fractions used for the experiments. One may conclude that the extracellular proteins present in the trypanosome incubation medium are really secreted proteins and not proteins released into the medium after lysis of the parasites. At the end of the experiment, the reaction was stopped by centrifugation of parasites at 1,000 × g for 10 min. The supernatant was collected and filtered on a 0.2-μm-pore-size filter and immediately aliquoted and frozen at −80°C until use. The protein concentration was determined by the Bradford dye binding procedure (Bio-Rad, Marnes-la-Coquette, France).
One-dimensional electrophoretic analysis.
Proteins from the different secretome samples (FEO and OK strains) were heated at 100°C for 2 min and spun for 5 min at 14,000 × g prior to separation by one-dimensional (1-D) sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were separated on 24- by 18-cm Tricine-SDS-polyacrylamide gels (12% acrylamide) (26). After migration, the gels were fixed and proteins were visualized by Coomassie brilliant blue R-250. Images of the gels were taken with a high-resolution scanner (Amersham Biosciences).
Generation of mouse BMDCs and spleen cells.
The tissue culture medium R5 consists of RPMI 1640 medium (Perbio Science, Brebières, France) supplemented with 10% heat-inactivated fetal bovine serum (Perbio Science), 2 mM l-glutamine, 0.1 mM nonessential amino acids, 50 μM β-mercaptoethanol (Sigma-Aldrich Chimie, Lyon, France), 100 IU/ml penicillin, 100 μg/ml streptomycin, and 1 mM sodium pyruvate (Life Technologies). Immature bone marrow-derived dendritic cells (BMDCs) were obtained by flushing the tibias and femurs of BALB/c mice with RPMI 1640 medium and passing bone marrow through a nylon membrane (cell strainer; Dutscher, Cergy, France) as described by Inaba et al. (27). After red blood cell lysis with ammonium chloride, T and B cells were depleted using mouse pan-T and pan-B Dynabeads (Life Technologies, Saint-Aubin, France), and monocytes were removed by adherence for 3 h at 37°C under 5% CO2 in R5 medium. Nonadherent cells (5 × 105 cells/ml) were collected and further cultured for differentiation in 24-well plates for 6 days in R5 medium containing 1,000 IU/ml recombinant mouse granulocyte-macrophage colony-stimulating factor (rmGM-CSF) and 1,000 IU/ml recombinant mouse interleukin-4 (rmIL-4) (R&D Systems, Lille, France). At day 7, the cell population contained at least 70% CD11c+ cells, determined by flow cytometry analysis using a FACSCalibur flow cytometer (BD Biosciences, Le Pont de Claix, France), and these were considered differentiated cells. These cells were collected and placed for maturation (106 cells/ml) in 24-well plates containing R5 medium supplemented with either 10 ng/ml LPS (in the presence or absence of 10 μg/ml polymyxin B preincubated for 1 h at 37°C with LPS), 5 μg/ml T. b. gambiense secretome, or both for 24 h at 37°C. Control cells were cultured in R5 medium alone or in R5 medium supplemented with secretion medium.
Spleen cells were obtained by splenectomy of C57BL/6 mice and spleen dissociation in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (Perbio Science, Brebières, France), 100 IU/ml penicillin, and 100 μg/ml streptomycin (Life Technologies). After passage through a nylon membrane (cell strainer; Dutscher), red blood cells were lysed with ammonium chloride. Spleen cell viability was assessed by the trypan blue dye exclusion test and was over 97%. Spleen cells were further cocultured with BMDCs in the mixed lymphocyte reaction (MLR).
Cell phenotyping and FACS analysis.
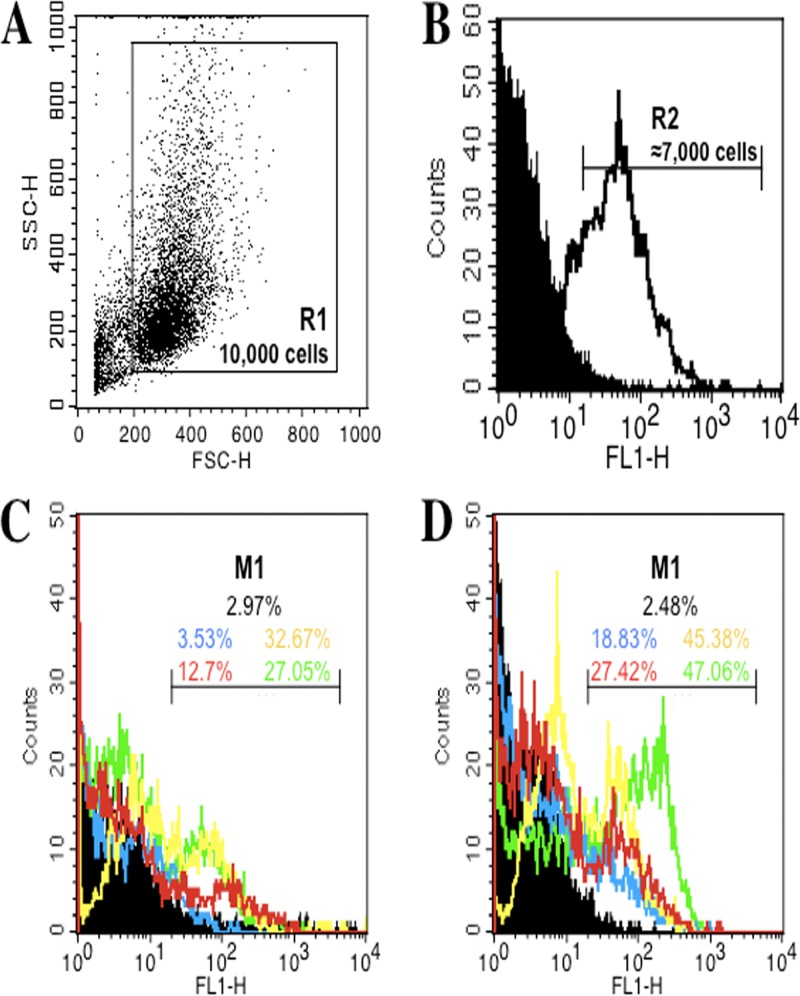
All monoclonal antibodies (MAbs) used for cell phenotyping by flow cytometry were purchased from Becton, Dickinson-Pharmingen (Le Pont de Claix, France). Briefly, immunostaining was performed using MAbs conjugated to fluorescein isothiocyanate (FITC) or phycoerythrin (PE), anti-CD40-FITC (3/23, rat IgG2a), anti-CD80-FITC (B7-1/16-10A1, hamster IgG2), anti-CD86-FITC (B7-2/GL1, rat IgG2a), anti-CD11c-FITC (HL3, hamster IgG1), anti-major histocompatibility complex class II (anti-MHC-II)-PE (I-Ek, mouse IgG2a), and control isotypes (rat IgG2a, hamster IgG1 and IgG2, mouse IgG2a). After stimulation with either LPS, the T. b. gambiense secretome, or both, BMDCs from BALB/c mice were collected in phosphate-buffered saline (PBS; Life Technologies) containing 0.1% bovine serum albumin (BSA; Life Technologies) and 0.1% NaN3 (Sigma-Aldrich Chimie) and incubated with MAbs for 30 min at 4°C. After two rounds of washing in PBS-BSA-NaN3, 10,000 cells were analyzed per sample in triplicate. Fluorescence-activated cell sorting (FACS) data were collected and analyzed on a FACSCalibur flow cytometer (BD Biosciences) using CellQuest Pro software. The results are expressed as the percentage of cells gated in the peak of fluorescence (M1) for each cell surface marker histogram plot.
Mixed lymphocyte reaction.
BMDCs from BALB/c mice stimulated with LPS, the T. b. gambiense secretome, or both were used as stimulator cells, and spleen cells from C57BL/6 mice were used as responder cells. Stimulator BMDCs were incubated for 5 days at 37°C under 5% CO2 with allogeneic spleen cells in 96-well plates containing 0.2 ml R5 medium per well at a 1:30 stimulator/responder cell ratio (control wells contained unstimulated BMDCs). After centrifugation, the supernatant was collected and kept at −80°C for cytokine production measurement. Cell proliferation was evaluated by a nonradioactive enzyme-linked immunosorbent assay (ELISA) technique using a monoclonal antibody against 5-bromo-2-deoxyuridine (BrdU) (28), which was added for 18 h on day 4, and revealed by an enzyme-conjugated second antibody, according to the manufacturer's instructions (BrdU cell proliferation detection kit III; Boehringer-Mannheim, Germany). The absorbance at 405 nm was read in an ELISA plate reader (Labsystems Multiscan EX). Proliferative responses were expressed as splenocyte proliferation indexes, which represent the ratio between the mean ± standard deviation (SD) of the optical densities obtained with stimulated BMDCs (LPS in the presence or absence of trypanosome secretome) and the mean ± standard deviation of the optical densities obtained with unstimulated cultured BMDCs.
Cytokine quantification.
IL-10, TNF-α, and IL-6 in culture supernatants (50 μl) of BMDCs exposed for 48 h to the following stimuli were quantified using a BD cytometric bead array (CBA) mouse inflammation kit (BD Biosciences): LPS (10 ng/ml) with secretion medium or T. b. gambiense secretome (5 μg/ml). The IL-2, IL-4, IL-5, gamma interferon (IFN-γ), and TNF-α in the culture supernatants (50 μl) of the allogeneic MLR assays were quantified after 5 days using a BD CBA mouse T helper (Th1/Th2) cytokine kit (BD Biosciences). Data were acquired by flow cytometry (FACSCalibur) using two-color detection and analyzed by BD Bioscience cytometric bead array software according to the manufacturer's instructions (BD Biosciences).
Statistical analyses.
Data are presented as the mean ± standard deviation of triplicates from at least two independent experiments. Statistical significance was analyzed using the Student t test; P values are indicated for each histogram.
RESULTS
One-dimensional electrophoresis protein profiling of the secretomes.
The profiles generated by the electrophoretic separation of the secretome proteins from the two bloodstream parasite strains (OK and FEO) are shown in Fig. 1. A high number of bands were separated, and these corresponded to proteins with molecular masses ranging from 7 to 150 kDa.
Fig 1.
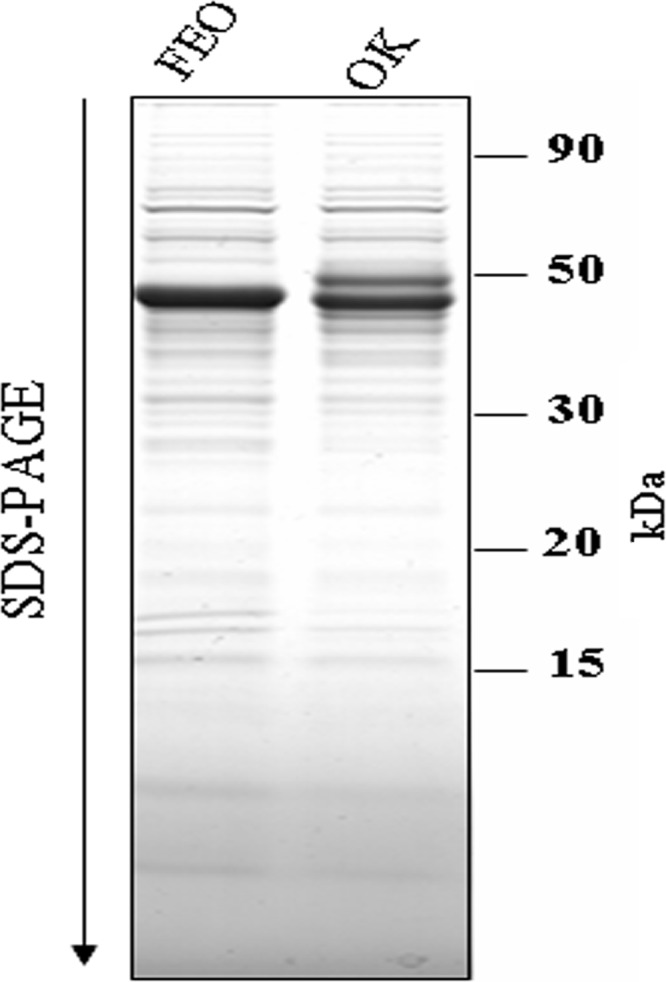
Protein profiles of two different T. brucei gambiense strains. Coomassie blue-stained SDS-polyacrylamide gel showing (from right to left) molecular size markers and secretomes from two Trypanosoma strains, OK and FEO.
Visual observation of the 1-D gels showed similarities, as well as some quantitative and qualitative differences, between the secretome profiles of the two parasite strains. Protein identifications have previously been investigated, and proteins were classified into 12 functional categories, including the folding and degradation, nucleotide metabolism, and hypothetical protein categories (25).
The T. b. gambiense secretome inhibits CD40 and MHC-II expression in BMDCs.
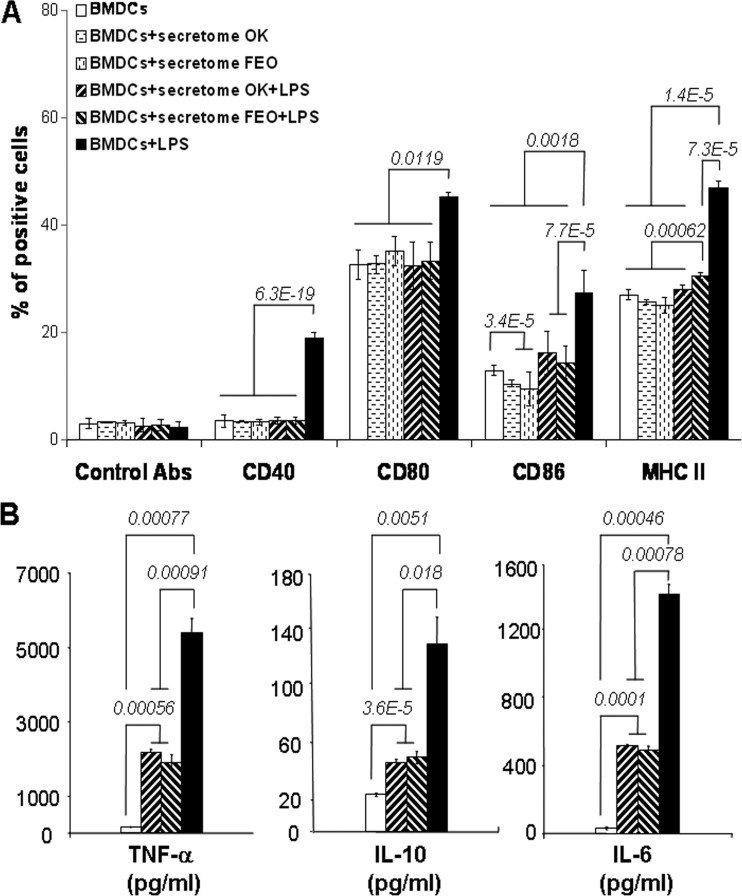
Since maturation is an essential process during which DCs acquire optimal immunostimulatory properties, we examined the effect of the T. b. gambiense secretome, a paracrine immunomodulating compartment of trypanosomes, on the response of BMDCs to LPS, which has previously been described to be a major stimulus for cytokine production and upregulation of surface molecule expression (29, 30). Figure 2 depicts the analytical strategy followed for phenotyping the mouse BMDCs differentiated in culture, which were first gated in R1 (n = 10,000 cells) according to cell size and granulometry (forward scatter/side scatter [FSC/SSC] dot plot; Fig. 2A); these cells were then gated in R2 according to positivity for CD11c (a cell surface marker expressed by murine DCs) (Fig. 2B). This gated cell population (n ≈ 7,000 cells) was then analyzed for MHC class II molecule and CD40, CD80, and CD86 costimulatory molecule expression. The levels of all phenotypic markers were increased upon LPS stimulation, as shown by the change in the fluorescence intensity of the corresponding markers in the M1 statistical interval (Fig. 2C and D), which was then used to calculate the percentage of cells positive for each experimental condition. Contrary to LPS, the secretion medium or the secretomes of T. b. gambiense alone, as well as polymyxin B, had no effect on the levels of expression of BMDC MHC class II molecules or the costimulatory molecules (CD40, CD80, and CD86), similar to control BMDCs cultured in R5 medium alone (Fig. 3A and Table 1; see Fig. S1 in the supplemental material). We next evaluated the effect of T. b. gambiense secretomes on DC maturation by looking for phenotypic changes in BMDCs exposed to LPS. LPS alone markedly induced the expression of MHC class II and all costimulatory molecules (CD40, CD80, and CD86) by BMDCs compared to that by control BMDCs (Fig. 3A). In contrast, a dramatic inhibition of the LPS-induced maturation of BMDCs was observed in the presence of T. b. gambiense secretomes according to the levels of expression of the CD40, CD80, CD86, and MHC class II molecules (Fig. 3A). The inhibitory effect was similar to that of polymyxin B (see Fig. S1 in the supplemental material), a well-described chelator of LPS-type endotoxin (31). Interestingly, whereas the inhibitory effect of the T. b. gambiense secretome on the expression of CD40 and CD80 was total, there were statistically significant slight differences in the effect of the secretome on the expression of CD86 and MHC class II molecules depending on the strain of T. b. gambiense from which the secretome originated. The secretomes of the OK and FEO strains exhibited a weak direct inhibitory effect on CD86 expression by BMDCs but seemed to be less efficient than the other markers in inhibiting LPS-induced CD86 expression (Fig. 3A). Moreover, the secretome of the OK strain showed a better inhibitory effect on MHC class II molecule expression than the secretome of the FEO strain (Fig. 3A).
Fig 2.
Strategy for phenotyping by expression of surface activation markers of mouse BMDCs using flow cytometry analysis (FACSCalibur flow cytometer and CellQuest Pro software; Becton, Dickinson). DCs differentiated in culture from mouse bone marrow myeloid cells were first gated according to cell size and granulometry (A; gate R1, n = 10,000 cells) and were then gated for CD11C (marker of differentiated murine DCs) positivity (B; gate R2, >70% positive cells). Differentiated DCs gated in R2 were further analyzed by immunostaining for levels of expression of CD40 (blue), CD80 (yellow), CD86 (red), and MHC class II (green) molecules (the respective control isotypes are in black) without (C) or with (D) stimulation by LPS. The percentages of positive cells were calculated as the change in fluorescence intensity (M1) for each experimental condition presented in Fig. 3 and Fig. S1 in the supplemental material.
Fig 3.
T. b. gambiense secretomes modulate the phenotype of immature BMDCs. (A) After stimulation with LPS in the presence or absence of the T. b. gambiense secretome, BMDCs from BALB/c mice were collected in PBS containing 0.1% bovine serum albumin and 0.1% NaN3 and incubated with MAbs for 30 min at 4°C. BMDCs gated for CD11c positivity (R2; Fig. 2) were analyzed for changes in CD40, CD80, CD86, and MHC class II molecule expression levels (mean ± SD of the percentage of cells gated in M1; Fig. 2; Table 1). (B) TNF-α, IL-10, and IL-6 in culture supernatants of BMDCs exposed to the following stimuli for 48 h were quantified using a BD CBA mouse inflammation kit: LPS with or without T. b. gambiense secretomes. Data were acquired by flow cytometry (FACSCalibur), were analyzed by BD Bioscience cytometric bead array software according to the manufacturer's instructions, and are presented as the mean ± SD cytokine concentration (pg/ml; Table 2). For both panels A and B, cells in culture medium alone (BMDCs) were used as a negative control, and those supplemented with LPS were used as a positive control. Data are presented as the mean ± SD of triplicates from three independent experiments. Statistical significance was analyzed using the Student t test; P values are indicated for each histogram.
Table 1.
Changes in levels of CD40, CD80, CD86, and MHC-II molecule expression by CD11c+ BMDCs
| Stimulator | Mean % ± SD BMDCs gated in M1 expressinga: |
||||
|---|---|---|---|---|---|
| Control Ab | CD40 | CD80 | CD86 | MHC-II | |
| BMDCs | 2.97 ± 0.91 | 3.53 ± 1.17 | 32.67 ± 2.82 | 12.7 ± 0.93 | 27.05 ± 1.02 |
| BMDCs + OK secretome | 3.32 ± 0.16 | 3.43 ± 0.11 | 32.74 ± 1.72 | 10.45 ± 0.64 | 25.71 ± 0.42 |
| BMDCs + FEO secretome | 3.1 ± 0.43 | 3.27 ± 0.54 | 35.17 ± 2.85 | 9.54 ± 3.05 | 25.12 ± 1.47 |
| BMDCs + OK secretome + LPS | 2.63 ± 1.21 | 3.57 ± 0.58 | 32.47 ± 4.22 | 16.19 ± 3.99 | 28 ± 0.98 |
| BMDCs + FEO secretome + LPS | 2.7 ± 1.05 | 3.59 ± 0.61 | 33.47 ± 3.42 | 14.43 ± 3.06 | 30.59 ± 0.59 |
| BMDCs + LPS | 2.48 ± 0.78 | 18.83 ± 1.17 | 45.38 ± 0.78 | 27.42 ± 4.16 | 47.06 ± 1.18 |
Percentage of cells gated in M1 in Fig. 2.
The T. b. gambiense secretome inhibits cytokine production by BMDCs.
To confirm the inhibitory effect of the T. b. gambiense secretome on DC maturation, the cytokine profile of stimulated BMDCs was determined. At first, the secretomes of T. b. gambiense alone had no effect on BMDC cytokine production, with the levels of production being similar to those for the control BMDCs cultured in R5 medium alone (data not shown). Immature BMDCs from BALB/c mice stimulated with LPS displayed increased expression of TNF-α, IL-10, and IL-6 (Fig. 3B and Table 2). Interestingly, the LPS stimulation was significantly inhibited in the presence of T. b. gambiense secretomes, as indicated by a significant decrease in the production of TNF-α (2-fold, P < 0.001), IL-10 (2-fold, P < 0.02), and IL-6 (3-fold, P < 0.001) (Fig. 3B). The inhibitory effect of the T. b. gambiense secretome was independent of the parasite strain considered (Fig. 3B). Taken together with phenotyping data, these data show that the T. b. gambiense secretome inhibits DC functional maturation.
Table 2.
Concentrations of cytokines secreted by CD11c+ BMDCs
| Stimulator | Mean concn (pg/ml) ±SD |
||
|---|---|---|---|
| TNF-α | IL-10 | IL-6 | |
| BMDCs | 235.1 ± 5.01 | 23.03 ± 3.01 | 24.67 ± 2.52 |
| BMDCs + OK secretome + LPS | 2,176.47 ± 117.65 | 44.62 ± 3.07 | 525 ± 15 |
| BMDCs + FEO secretome + LPS | 1,941.18 ± 176.47 | 50.26 ± 0.03 | 475 ± 25 |
| BMDCs + LPS | 5,352.94 ± 352.94 | 128.23 ± 21.51 | 1,400 ± 75 |
The T. b. gambiense secretome inhibits the allostimulatory capacity of BMDCs and Th1/Th2 cytokine production by cocultured lymphocytes.
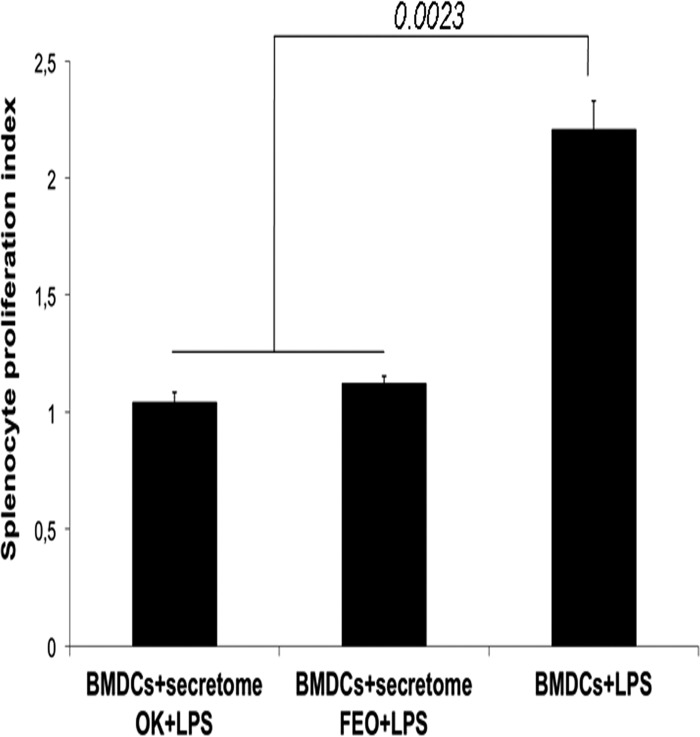
The function of DCs can be further characterized by an ability to stimulate alloreactive (naive) spleen cells (32). A mixed lymphocyte reaction (MLR) was performed using BALB/c mouse BMDCs stimulated with LPS in the presence or absence of the T. b. gambiense secretomes as stimulator cells and cocultured spleen cells from C57BL/6 mice as responder cells. BDMCs stimulated with LPS alone induced spleen cell proliferation, as indicated by a splenocyte proliferation index higher than 2 (Fig. 4). In contrast, whatever the secretome of T. b. gambiense considered, the secretome completely abolished the allostimulatory capacity of LPS-stimulated BMDCs, as indicated by a splenocyte proliferation index of 1 (Fig. 4).
Fig 4.
Ability of differentiated BMDCs to stimulate allogeneic T cells. BMDCs from BALB/c mice cultured in medium alone or incubated with LPS in the presence or absence of T. b. gambiense secretomes were used as stimulator cells, and spleen cells from C57BL/6 mice were used as responder cells. The proliferative response of splenocytes was measured by ELISA after BrdU incorporation and is expressed as the cell proliferation index. Data are presented as the mean ± SD of triplicates from two independent experiments. Statistical significance was analyzed using the Student t test; P values are indicated for each histogram.
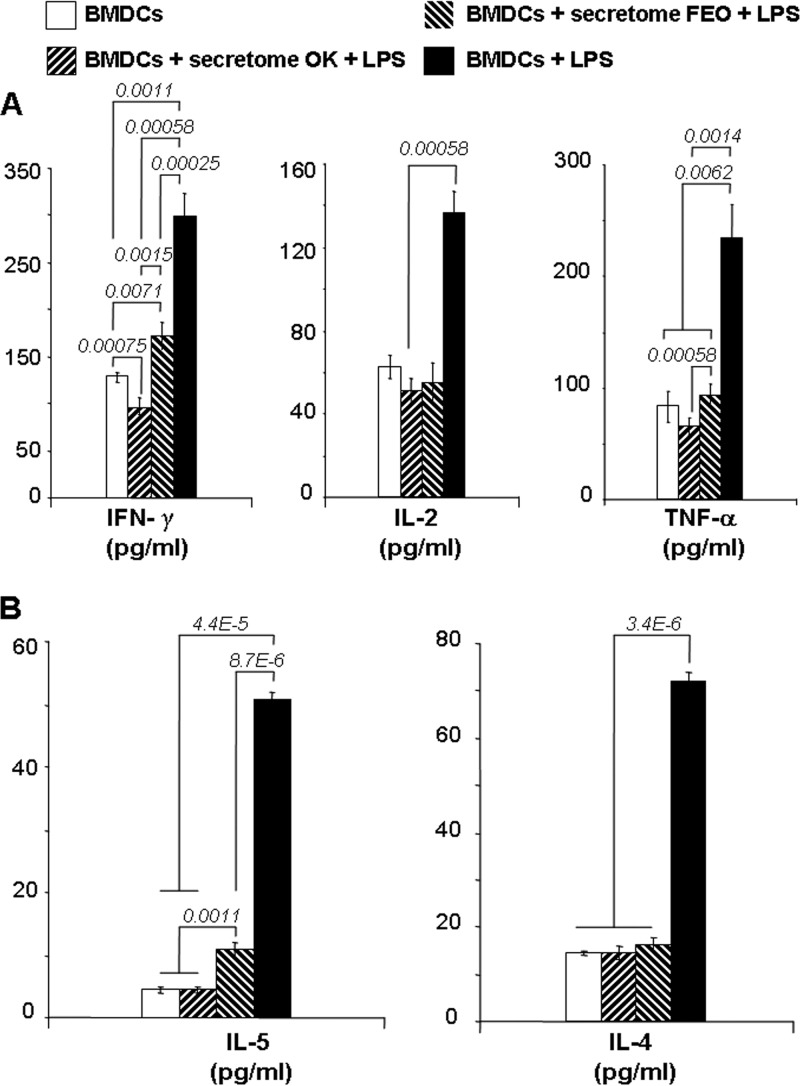
To confirm the impairment of BMDC immunomodulating properties, Th1 and Th2 cytokines in culture supernatants from C57BL/6 mouse spleen cells stimulated with BALB/c mouse BMDCs exposed to LPS in the presence or absence of the T. b. gambiense secretomes were quantified at 48 h. The pattern of Th1 cytokine (IFN-γ, IL-2, and TNF-α) expression by C57BL/6 mouse spleen cells was dramatically inhibited in cells stimulated by BMDCs exposed to LPS in the presence of T. b. gambiense secretomes (Fig. 5A and Table 3). A 2-fold inhibition of IL-2 production by allogeneic splenocytes was observed for cells cocultured with BMDCs stimulated with LPS in the presence of T. b. gambiense secretomes compared to the level of production for cells stimulated with LPS alone (Fig. 5A). For IFN-γ and TNF-α production, inhibition differed according to the T. b. gambiense secretome: the inhibitory effect of the FEO strain's secretome was less efficient than that of the OK strain's secretome (Fig. 5A).
Fig 5.
T. brucei secretomes inhibit Th1/Th2 cytokine production. IFN-γ, IL-2, and TNF-α (A) and IL-4 and IL-5 (B) in the culture supernatants of the allogeneic MLR assays were quantified after 5 days using a BD CBA mouse T helper (Th1/Th2) cytokine kit. Data were acquired by flow cytometry (FACSCalibur), were analyzed by BD Bioscience cytometric bead array software according to the manufacturer's instructions, and are presented as the mean ± SD cytokine concentration (pg/ml; Table 3). Cells in culture medium alone (BMDCs) were used as a negative control, and those supplemented with LPS were used as a positive control. Data are presented as the mean ± SD of triplicates from four independent experiments. Statistical significance was analyzed using the Student t test; P values are indicated for each histogram.
Table 3.
Concentrations of cytokines secreted by splenocytes in MLR
| Stimulator | Mean concn (pg/ml) ± SD |
||||
|---|---|---|---|---|---|
| IFN-γ | IL-2 | TNF-α | IL-5 | IL-4 | |
| BMDCs | 127.26 ± 6.02 | 62.17 ± 5.01 | 83.33 ± 12.96 | 5 ± 0.5 | 14.44 ± 0.25 |
| BMDCs + OK secretome + LPS | 97.07 ± 8.01 | 52 ± 5 | 62.45 ± 10.91 | 5 ± 0.4 | 14.35 ± 1.3 |
| BMDCs + FEO secretome + LPS | 170.41 ± 15.02 | 55 ± 9 | 91.82 ± 5.09 | 11.42 ± 1.01 | 15.74 ± 1.75 |
| BMDCs + LPS | 300 ± 20 | 137.17 ± 10.01 | 231.27 ± 31.01 | 51.25 ± 1.25 | 71.47 ± 1.51 |
In the same way, Th2 cytokine production (IL-4 and IL-5) by allogeneic splenocytes was significantly inhibited (about 4-fold) by T. b. gambiense secretomes (P < 5E−5) (Fig. 5B). IL-4 production was fully inhibited in cells cocultured with BMDCs stimulated by LPS in the presence of the T. b. gambiense secretome, whatever the strain considered (Fig. 5B). For IL-5 production, the inhibitory effect induced by the FEO strain's secretome was not complete (P ≈ 0.001) compared to that induced by the OK strain's secretome (Fig. 5B).
DISCUSSION
The inoculation of African trypanosomes into their mammalian hosts triggers a series of events involving, primarily, innate immunity and, secondarily, specific immunity. In experimental models, immunosuppression was attributed to polyclonal B-cell activation induced by continuous complex antigenic variation through VSG switching to circumvent host antibody targeting, as well as the generation of suppressor T cells or a defect in the T-cell population due to a loss of macrophage functions (33). Macrophages, which are APCs, were also shown to participate in immunosuppression, reflected by the failure of T cells from infected hosts to proliferate in response to specific or mitogenic stimulation (34–38). Like other professional APCs, DCs also play a central role in antigen presentation and activation of naive T cells, and they both can initiate and modulate immune responses as a function of exogenous stimuli (39). Alteration of DC function is one of the various strategies developed by malignant cells to escape immune elimination (40). In a trypanosome infection, DCs play a crucial role in the activation of Th-cell responses, with DCs being the primary cell population inducing a VSG-specific response in naive T cells (15). However, VSG induces partial maturation of DCs, leading to a Th2-cell response that does not exert antitrypanosome activity (18). Recently, natural killer T cells and regulatory T cells have been demonstrated to influence the outcome of a trypanosome infection (41–43). Moreover, trypanosomes are also capable of reducing B lymphopoiesis and selectively destroying B-cell populations (44, 45). The previous short description of immunological disorders induced by trypanosomes makes one believe that the trypanosome has developed a strategy of communication through paracrine mediators to dysregulate immune functions in a discrete manner, in addition to the powerful mechanism of VSG switching. This is why in the present study we investigated the effects of the T. b. gambiense secretome on both maturation of DCs and their immunomodulating functions. We demonstrated that the trypanosome secretome inhibited functional maturation and activation of BMDCs and impaired promotion of the Th-cell response in the mouse model. The secretome exerted suppressive activity on LPS-stimulated DCs by inhibiting the expression of CD40, CD80, CD86, and MHC class II molecules, as well as allostimulatory activity.
We chose stimulation by LPS not only because it is a well-established biological interaction tool in fundamental immunology but also because it seems to participate in the trypanosome's strategy to enslave the immune system of the host. During natural infection, the presence of bacteria on the mammal's skin or in the digestive tract of the insect vector (46–49) might provide exogenous LPS through a tsetse fly bite of the mammal's skin, and the exogenous LPS may further participate in host immune system dysregulation. For instance, it has been proven that the level of LPS-binding protein (LBP) increased in the plasma of mice infected by Trypanosoma brucei, which can enhance the cellular responses of the infected host to LPS (50). Interestingly, in the expression site containing the serum resistance-associated (SRA) gene, there is an expression site-associated gene (ESAG5) that indicated a possible relationship to the LBP family (51). These elements argue in favor of the hypothesis that the trypanosome uses LPS to immunomodulate the cells of the host immune system to its advantage.
The extracellular location of African trypanosomes involves strong molecular cross talk with host immune cells, which occurs through cell-cell contact or excreted-secreted factors exhibiting immunomodulatory properties. In previous experiments aiming at identifying the trypanosome's secretome components (26), we could assign 50% of the proteins to three major categories: protein folding and degradation, nucleotide metabolism, and unassigned functions. Forty percent of the remaining proteins fell into one of five categories: carbohydrate metabolism, amino acid metabolism, protein synthesis, signaling, and cell cycle and cell organization. Moreover, the secretome appeared to be unexpectedly rich in various peptidases covering more than 10 peptidase families or subfamilies, among which some could play a role in pathology (52–54). Even though the functions of the secretome identified are not involved in parasite physiology, previous evidence exists for their potential involvement in mechanisms of enslavement of the host. For instance, a paracrine factor synthesized by trypanosomes was described to trigger IFN-γ production by CD8+ T cells and act as a growth factor (55). IFN-γ also induces classical macrophage activation that leads to production of cytotoxic factors, such as nitric oxide (NO) and reactive oxygen species (ROS), which are trypanostatic but at the same time enhance immunosuppression and alter the blood-brain barrier (8). Few T. brucei-derived factors that modulate the innate immune response have been identified. The glycosylphosphatidylinositol anchor of VSG, CpG oligodeoxynucleotides, and the trypanosome-suppressive immunomodulating factor (TSIF) induce TNF- and NO-secreting M1 cells (56–58). These factors might favor the control of parasite development in the early stage of infection; however, their continuous release in the late stage of infection can maintain inflammation and induce tissue pathogenicity. It was well described that the production of IFN-γ by polarized T cells contributed to early host resistance during infection and that VSG-specific T-cell responses acted through the activation of macrophages to produce trypanocidal factors such as reactive oxygen intermediates, reactive nitrogen intermediates, and TNF-α (38, 59, 60). In addition to a sequential effect of IFN-γ, it is also noteworthy that soluble VSG can inhibit the responsiveness of macrophages to IFN-γ (61) and that this inhibition could be a strategy to limit the immunopathological effects induced by the antitrypanosome inflammatory response (62). We also demonstrated that soluble factors from T. brucei induced arginase expression and activity and antagonized NO synthase (NOS)-mediated conversion of l-arginine into NO in infected mice. Arginase induction favors polyamine and trypanothione synthesis, promoting trypanosome growth (12, 63–65).
We now add a new property to the trypanosome's secretome, which is the capacity to block the maturation of differentiated DCs through impairment of expression of surface markers (CD40, CD80, CD86, and MHC class II molecules). These are key markers of cellular phenotypic maturation and are required for dendritic cells to efficiently activate the immune response (14); in particular, CD40 has emerged as a key pathway for signaling the functions of B cells, monocytes, and DCs (66). Recently, it was proven that blocking the CD40 pathway did not alter the expression of MHC and costimulatory molecules but significantly reduced the production of inflammatory cytokines (67). This is similar to our observation that T. brucei secretome-triggered BMDCs inhibit LPS-stimulated production of TNF-α and IL-6 proinflammatory cytokines, as well as IL-10. These cytokines are key mediators of Th1 and Th2 responses (16). Since proinflammatory cytokines are important to a proper innate immunity and costimulatory molecules are directly involved in the induction of an acquired immune response, our results suggest that T. brucei-secreted proteins may help the parasite survive in the human host by downregulating maturation of DCs to weaken the reactivity of the immune system against infection. To address how T. b. gambiense secretomes may influence the development of immune responses, we examined their influence on the DC-dependent differentiation of naive CD4 cells into Th1 and Th2 cells. DCs pulsed with the secretomes of two strains showed little to no capacity to support the differentiation of naive CD4 lymphocytes into IFN-γ-, IL-2-, or TNF-α-producing Th1 cells or into IL-5- or IL-4-producing Th2 cells. Consequently, the suppressive activities of the T. b. gambiense secretomes on antigen-presenting DCs altered the lymphocytic response. If we postulate that secretome molecules can immunosuppress the allostimulatory capacity of DCs, they can further inhibit the cytokine production capacities of responder spleen cells. Moreover, as we had only 70% differentiated DCs in our experiments, we can postulate that among the remaining 30% of cells, some cells could be myeloid cell-derived suppressor cells (MDSCs), which exhibit no specific marker, which leaves their suppressor activity as their only hallmark function (68). This supposed effect might at least modify cytokine production. Nevertheless, our findings are limited to those obtained with the secretome without the trypanosome in an ex vivo context, and the effect seems to be much more complex in vivo. For instance, a default Th2-cell differentiation pathway independent of the surface receptors and signaling pathways involved was demonstrated to be induced by DCs matured under suboptimal inflammatory conditions. The data also indicated that quantitative differences in DC maturation might direct Th2- versus Th1-cell responses, since suboptimally matured inflammatory DCs induce Th2-cell maturation by default, whereas fully mature DCs induce Th1-cell maturation (18). Similar changes in surface markers and cytokine profiles have also been reported for Trypanosoma cruzi and Leishmania, which, in addition to African trypanosomes, present intracellular stages in the infected host. Indeed, secretion of the cytokines IL-12, TNF-α, and IL-6 as well as the upregulation of HLA-DR and CD40 molecules was significantly reduced after T. cruzi infection. The same effects could be induced by T. cruzi-conditioned medium, indicating that at least these inhibitory effects were mediated by soluble factors released by T. cruzi (69). Comparable results were found in Leishmania-infected BMDCs, which showed decreased CD40, CD86, and MHC-II expression and loss of their capacity to induce T-cell proliferation (70). This fact points to the importance of trypanosome secreted proteins at the time of infection to quickly switch off the proinflammatory process and establish infection. In this way, a blockade of the CD40 costimulatory pathway is one of the most successful means of inhibiting an alloreactive T-cell response, as demonstrated for allografts (71), a strategy which could be compared to that used by the parasite. The same impairment of the CD4+ T-cell response was, curiously, described in aging, resulting in susceptibility to infections and cancer (72). The same phenotypic parameters, lower levels of MHC-peptide complex expression and reduced CD40 levels (72), were observed, as if trypanosome infection induced preaging of the host immune system.
Through this pioneering study, we demonstrated that the T. b. gambiense secretome contains key factors that inhibit LPS-induced DC maturation as well as the cytokine production and allostimulatory ability of DCs. The microvesicular mode of export was hypothesized to present several advantages in comparison to the classical secretory pathway: it may deliver an avalanche of new epitopes that overwhelm the host immune system or that allow communication between trypanosomes themselves by exchanging receptors in the form of nonprotein cytosolic compounds or even, potentially, genomic information (25). In addition, it could provide the means to address the functional consequences of immune downregulation for the survival of parasites and/or the regulation of immunopathogenesis. Finally, the identification of the factor(s) in the T. b. gambiense secretome and of the DC receptor(s) involved might uncover new therapeutic targets that may be used to fight against sleeping sickness.
Supplementary Material
ACKNOWLEDGMENTS
This investigation received financial support from the Institut de Recherche pour le Développement (IRD), Centre de Coopération Internationale en Recherche Agronomique pour le Développement (CIRAD).
We acknowledge Pascale Louis-Plencé (INSERM) for precious help and fruitful discussions.
Footnotes
Published ahead of print 24 June 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00125-13.
REFERENCES
- 1.Stich A, Abel PM, Krishna S. 2002. Human African trypanosomiasis. Br. Med. J. 325:203–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pépin J, Milord F. 1994. The treatment of human African trypanosomiasis. Adv. Parasitol. 33:1–47 [DOI] [PubMed] [Google Scholar]
- 3.Legros D, Ollivier G, Gastellu-Etchegorry M, Paquet C, Burri C, Jannin J, Buscher P. 2002. Treatment of human African trypanosomiasis—present situation and needs for research and development. Lancet Infect. Dis. 2:437–440 [DOI] [PubMed] [Google Scholar]
- 4.Burri C, Brun R. 2003. Eflornithine for treatment of human African trypanosomiasis. Parasitol. Res. 90(Suppl 1):S49–S52 [DOI] [PubMed] [Google Scholar]
- 5.Kennedy PGE. 2004. Human African trypanosomiasis of the CNS: current issues and challenges. J. Clin. Invest. 113:496–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Priotto G, Pinoges L, Fursa IB, Burke B, Nicolay N, Grillet G, Hewison C, Balasegaram M. 2008. Safety and effectiveness of first line eflornithine for Trypanosoma brucei gambiense sleeping sickness in Sudan: cohort study. Br. Med. J. 336:705–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horn D, McCulloch R. 2010. Molecular mechanisms underlying the control of antigenic variation in African trypanosomes. Curr. Opin. Microbiol. 13:700–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vincendeau P, Bouteille B. 2006. Immunology and immunopathology of African trypanosomiasis. An. Acad. Bras. Cienc. 78:645–665 [DOI] [PubMed] [Google Scholar]
- 9.Diffley P. 1983. Trypanosomal surface coat variant antigen causes polyclonal lymphocyte activation. J. Immunol. 131:1983–1986 [PubMed] [Google Scholar]
- 10.Okenu DMN, Opara KN, Nwuba RI, Nwagwu M. 1999. Purification and characterisation of an extracellular released protease of Trypanosoma brucei. Parasitol. Res. 85:424–428 [DOI] [PubMed] [Google Scholar]
- 11.Lonsdale-Eccles JD, Grab DJ. 2002. Trypanosome hydrolase and the blood-brain barrier. Trends Parasitol. 18:17–19 [DOI] [PubMed] [Google Scholar]
- 12.Holzmuller P, Biron DG, Courtois P, Koffi M, Bras-Goncalves R, Daulouède S, Solano P, Cuny G, Vincendeau P, Jamonneau V. 2008. Virulence and pathogenicity patterns of Trypanosoma brucei gambiense field isolates in experimentally infected mouse: differences in host immune response modulation by secretome and proteomics. Microbes Infect. 10:79–86 [DOI] [PubMed] [Google Scholar]
- 13.Grébaut P, Chuchana P, Brizard JP, Demettre E, Seveno M, Bossard G, Jouin P, Vincendeau P, Bengaly Z, Boulangé A, Cuny G, Holzmuller P. 2009. Identification of total and differentially expressed excreted-secreted proteins from Trypanosoma congolense strains exhibiting different virulence and pathogenicity. Int. J. Parasitol. 39:1137–1150 [DOI] [PubMed] [Google Scholar]
- 14.Banchereau J, Steinman J. 1998. Dendritic cells and the control of immunity. Nature 392:245–252 [DOI] [PubMed] [Google Scholar]
- 15.Dagenais TR, Freeman BE, Demick KP, Paulnock DM, Mansfield JM. 2009. Processing and presentation of variant surface glycoprotein molecules to T cells in African trypanosomiasis. J. Immunol. 183:3344–3355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobayashi T, Walsh PT, Walsh MC, Speirs KM, Chiffoleau E, King CG, Hancock WW, Caamano JH, Hunter CA, Scott P, Turka LA, Choi Y. 2003. TRAF6 is a critical factor for dendritic cell maturation and development. Immunity 19:353–363 [DOI] [PubMed] [Google Scholar]
- 17.Bosschaerts T, Guilliams M, Stijlemans B, Morias Y, Engel D, Tacke F, Hérin M, De Baetselier P, Beschin A. 2010. Tip-DC development during parasitic infection is regulated by IL-10 and requires CCL2/CCR2, IFN-gamma and MyD88 signaling. PLoS Pathog. 6:e1001045. 10.1371/journal.ppat.1001045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pletinckx K, Stijlemans B, Pavlovic V, Laube R, Brandl C, Kneitz S, Beschin A, De Baetselier P, Lutz MB. 2011. Similar inflammatory DC maturation signatures induced by TNF or Trypanosoma brucei antigens instruct default Th2-cell responses. Eur. J. Immunol. 41:3479–3494 [DOI] [PubMed] [Google Scholar]
- 19.Wasserman HA, Singh S, Champagne DE. 2004. Saliva of the yellow fever mosquito, Aedes aegypti, modulates murine lymphocyte function. Parasite Immunol. 26:295–306 [DOI] [PubMed] [Google Scholar]
- 20.Menezes MJ, Costa DJ, Clarêncio J, Miranda JC, Barral A, Barral-Netto M, Brodskyn C, de Oliveira CI. 2008. Immunomodulation of human monocytes following exposure to Lutzomyia intermedia saliva. BMC Immunol. 9:12. 10.1186/1471-2172-9-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brake DK, Wikel SK, Tidwell JP, Pérez de León AA. 2010. Rhipicephalus microplus salivary gland molecules induce differential CD86 expression in murine macrophages. Parasit. Vectors 3:103. 10.1186/1756-3305-3-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paindavoine P, Zampetti-Bosseler F, Coquelet H, Pays E, Steinert M. 1989. Different allele frequencies in Trypanosoma brucei brucei and Trypanosoma brucei gambiense populations. Mol. Biochem. Parasitol. 32:61–71 [DOI] [PubMed] [Google Scholar]
- 23.Tait A, Babiker EA, Le Ray D. 1984. Enzyme variation in Trypanosoma brucei spp. I. Evidence for the sub-speciation of Trypanosoma brucei gambiense. Parasitology 89:311–326 [DOI] [PubMed] [Google Scholar]
- 24.Lanham SM, Godfrey DG. 1970. Isolation of salivarian trypanosomes from man and other mammals using DEAE-cellulose. Exp. Parasitol. 28:521–534 [DOI] [PubMed] [Google Scholar]
- 25.Geiger A, Hirtz C, Bécue T, Bellard E, Centeno D, Gargani D, Rossignol M, Cuny G, Peltier JB. 2010. Exocytosis and protein secretion in Trypanosoma. BMC Microbiol. 10:20. 10.1186/1471-2180-10-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schägger H, Von Jagow G. 1987. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166:368–379 [DOI] [PubMed] [Google Scholar]
- 27.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. 1992. Generation of large numbers of DC from mouse bone marrow cultures supplemented with GM-CSF. J. Exp. Med. 176:1693–1702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huong PL, Kolk AH, Eggelte TA, Verstijnen CP, Gilis H, Hendriks JT. 1991. Measurement of antigen specific lymphocyte proliferation using 5-bromo-deoxyuridine incorporation. An easy and low cost alternative to radioactive thymidine incorporation. J. Immunol. Methods 140:243–248 [DOI] [PubMed] [Google Scholar]
- 29.McKenzie JL, Prickett TC, Hart DN. 1989. Human dendritic cells stimulate allogeneic T cells in the absence of IL-1. Immunology 67:290–297 [PMC free article] [PubMed] [Google Scholar]
- 30.Kopydlowski KM, Salkowski CA, Cody MJ, van Rooijen N, Major J, Hamilton TA, Vogel SN. 1999. Regulation of macrophage chemokine expression by lipopolysaccharide in vitro and in vivo. J. Immunol. 163:1537–1544 [PubMed] [Google Scholar]
- 31.Bhor VM, Thomas CJ, Surolia N, Surolia A. 2005. Polymyxin B: an ode to an old antidote for endotoxic shock. Mol. Biosyst. 1:213–222 [DOI] [PubMed] [Google Scholar]
- 32.Muraille E, Andris F, Pajak B, Wissing KM, De Smedt T, Desalle F, Goldman M, Alegre ML, Urbain J, Moser M, Leo O. 1999. Downregulation of antigen-presenting cell functions after administration of mitogenic anti-CD3 monoclonal antibodies in mice. Blood 94:4347–4357 [PubMed] [Google Scholar]
- 33.Gasbarre LC, Hug K, Louis J. 1981. Murine T lymphocyte specificity for African trypanosomes. II. Suppression of the T lymphocyte proliferative response to Trypanosoma brucei by systemic trypanosome infection. Clin. Exp. Immunol. 45:165–172 [PMC free article] [PubMed] [Google Scholar]
- 34.Schleifer KW, Mansfield JM. 1993. Suppressor macrophages in African trypanosomiasis inhibit T cell proliferative responses by nitric oxide and prostaglandins. J. Immunol. 151:5492–5503 [PubMed] [Google Scholar]
- 35.Darji A, Sileghem M, Heremans H, Brys L, De Baetselier P. 1993. Inhibition of T-cell responsiveness during experimental infections with Trypanosoma brucei: active involvement of endogenous gamma interferon. Infect. Immun. 61:3098–3102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mabbott NA, Coulson PS, Smythies LE, Wilson RA, Sternberg JM. 1998. African trypanosome infections in mice that lack the interferon-γ receptor gene: nitric oxide-dependent and -independent suppression of T-cell proliferative responses and the development of anaemia. Immunology 94:476–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Darji A, Beschin A, Sileghem M, Heremans H, Brys L, De Baetselier P. 1996. In vitro simulation of immunosuppression caused by Trypanosoma brucei: active involvement of gamma interferon and tumor necrosis factor in the pathway of suppression. Infect. Immun. 64:1937–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Magez S, Radwanska M, Beschin A, Sekikawa K, De Baetselier P. 1999. Tumor necrosis factor alpha is a key mediator in the regulation of experimental Trypanosoma brucei infections. Infect. Immun. 67:3128–3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dresch C, Leverrier Y, Marvel J, Shortman K. 2012. Development of antigen cross-presentation capacity in dendritic cells. Trends Immunol. 33:381–388 [DOI] [PubMed] [Google Scholar]
- 40.Michielsen AJ, Hogan AE, Marry J, Tosetto M, Cox F, Hyland JM, Sheahan KD, O'Donoghue DP, Mulcahy HE, Ryan EJ, O'Sullivan JN. 2011. Tumour tissue microenvironment can inhibit dendritic cell maturation in colorectal cancer. PLoS One 6:e27944. 10.1371/journal.pone.0027944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guilliams M, Oldenhove G, Noel W, Hérin M, Brys L, Loi P, Flamand V, Moser M, De Baetselier P, Beschin A. 2007. African trypanosomiasis: naturally occurring regulatory T cells favor trypanotolerance by limiting pathology associated with sustained type 1 inflammation. J. Immunol. 179:2748–2757 [DOI] [PubMed] [Google Scholar]
- 42.Wei G, Tabel H. 2008. Regulatory T cells prevent control of experimental African trypanosomiasis. J. Immunol. 180:2514–2521 [DOI] [PubMed] [Google Scholar]
- 43.Namangala B, Yokoyama N, Ikehara Y, Taguchi O, Tsujimura K, Sugimoto C, Inoue N. 2008. Effect of CD4+CD25+ T cell-depletion on acute lethal infection of mice with Trypanosoma congolense. J. Vet. Med. Sci. 70:751–759 [DOI] [PubMed] [Google Scholar]
- 44.Radwanska M, Guirnalda P, De Trez C, Ryffel B, Black S, Magez S. 2008. Trypanosomiasis-induced B cell apoptosis results in loss of protective anti-parasite antibody responses and abolishment of vaccine-induced memory responses. PLoS Pathog. 4:e1000078. 10.1371/journal.ppat.1000078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bockstal V, Guirnalda P, Caljon G, Goenka R, Telfer JC, Frenkel D, Radwanska M, Magez S, Black SJ. 2011. T. brucei infection reduces B lymphopoiesis in bone marrow and truncates compensatory splenic lymphopoiesis through transitional B-cell apoptosis. PLoS Pathog. 7:e1002089. 10.1371/journal.ppat.1002089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mediannikov O, Audoly G, Diatta G, Trape JF, Raoult D. 2012. New Rickettsia sp. in tsetse flies from Senegal. Comp. Immunol. Microbiol. Infect. Dis. 35:145–150 [DOI] [PubMed] [Google Scholar]
- 47.Geiger A, Fardeau ML, Grebaut P, Vatunga G, Josénando T, Herder S, Cuny G, Truc P, Ollivier B. 2009. First isolation of Enterobacter, Enterococcus, and Acinetobacter spp. as inhabitants of the tsetse fly (Glossina palpalis palpalis) midgut. Infect. Genet. Evol. 9:1364–1370 [DOI] [PubMed] [Google Scholar]
- 48.Geiger A, Fardeau ML, Falsen E, Ollivier B, Cuny G. 2010. Serratia glossinae sp. nov., isolated from the midgut of the tsetse fly Glossina palpalis gambiensis. Int. J. Syst. Evol. Microbiol. 60:1261–1265 [DOI] [PubMed] [Google Scholar]
- 49.Geiger A, Fardeau ML, Njiokou F, Joseph M, Asonganyi T, Ollivier B, Cuny G. 2011. Bacterial diversity associated with populations of Glossina spp. from Cameroon and distribution within the Campo sleeping sickness focus. Microb. Ecol. 62:632–643 [DOI] [PubMed] [Google Scholar]
- 50.Ngure RM, Eckersall PD, Mungatana NK, Mburu JN, Jennings FW, Burke J, Murray M. 2009. Lipopolysaccharide binding protein in the acute phase response of experimental murine Trypanosoma brucei brucei infection. Res. Vet. Sci. 86:394–398 [DOI] [PubMed] [Google Scholar]
- 51.Barker AR, Wickstead B, Gluenz E, Gull K. 2008. Bioinformatic insights to the ESAG5 and GRESAG5 gene families in kinetoplastid parasites. Mol. Biochem. Parasitol. 162:112–122 [DOI] [PubMed] [Google Scholar]
- 52.Anosa VO, Isoun TT. 1976. Serum proteins, blood and plasma volumes in experimental Trypanosoma vivax infections of sheep and goats. Trop. Anim. Health Prod. 8:14–19 [DOI] [PubMed] [Google Scholar]
- 53.Pierotti A, Dong KW, Glucksman MJ, Orlowski M, Roberts JL. 1990. Molecular cloning and primary structure of rat testes metalloendopeptidase EC 3.4.24.15. Biochemistry 29:10323–10329 [DOI] [PubMed] [Google Scholar]
- 54.Brandenberger G, Buguet A, Spiegel K, Stanghellini A, Muanga G, Bogui P, Dumas M. 1996. Disruption of endocrine rhythms in sleeping sickness with preserved relationship between hormonal pulsatility and the REM-NREM sleep cycles. J. Biol. Rhythms 11:258–267 [DOI] [PubMed] [Google Scholar]
- 55.Bakhiet M, Olsson T, Edlund C, Hojeberg B, Holmberg K, Lorentzen J, Kristensson K. 1993. A Trypanosoma brucei brucei-derived factor that triggers CD8+ lymphocytes to interferon-gamma secretion: purification, characterization and protective effects in vivo by treatment with a monoclonal antibody against the factor. Scand. J. Immunol. 37:165–178 [DOI] [PubMed] [Google Scholar]
- 56.Magez S, Stijlemans B, Radwanska M, Pays E, Ferguson MA, De Baetselier P. 1998. The glycosyl-inositol-phosphate and dimyristoylglycerol moieties of the glycosylphosphatidylinositol anchor of the trypanosome variant-specific surface glycoprotein are distinct macrophage-activating factors. J. Immunol. 160:1949–1956 [PubMed] [Google Scholar]
- 57.Leppert BJ, Mansfield JM, Paulnock DM. 2007. The soluble variant surface glycoprotein of African trypanosomes is recognized by a macrophage scavenger receptor and induces I kappa B alpha degradation independently of TRAF6-mediated TLR signaling. J. Immunol. 179:548–556 [DOI] [PubMed] [Google Scholar]
- 58.Gómez-Rodríguez J, Stijlemans B, De Muylder G, Korf H, Brys L, Berberof M, Darji A, Pays E, De Baetselier P, Beschin A. 2009. Identification of a parasitic immunomodulatory protein triggering the development of suppressive M1 macrophages during African trypanosomiasis. J. Infect. Dis. 200:1849–1860 [DOI] [PubMed] [Google Scholar]
- 59.Daulouède S, Bouteille B, Moynet D, De Baetselier P, Courtois P, Lemesre JL, Buguet A, Cespuglio R, Vincendeau P. 2001. Human macrophage tumor necrosis factor (TNF)-alpha production induced by Trypanosoma brucei gambiense and the role of TNF-alpha in parasite control. J. Infect. Dis. 183:988–991 [DOI] [PubMed] [Google Scholar]
- 60.Drennan MB, Stijlemans B, Van den Abbeele J, Quesniaux VJ, Barkhuizen M, Brombacher F, De Baetselier P, Ryffel B, Magez S. 2005. The induction of a type 1 immune response following a Trypanosoma brucei infection is MyD88 dependent. J. Immunol. 175:2501–2509 [DOI] [PubMed] [Google Scholar]
- 61.Coller SP, Mansfield JM, Paulnock DM. 2003. Glycosylinositolphosphate soluble variant surface glycoprotein inhibits IFN-gamma-induced nitric oxide production via reduction in STAT1 phosphorylation in African trypanosomiasis. J. Immunol. 171:1466–1472 [DOI] [PubMed] [Google Scholar]
- 62.Stijlemans B, Guilliams M, Raes G, Beschin A, Magez S, De Baetselier P. 2007. African trypanosomosis: from immune escape and immunopathology to immune intervention. Vet. Parasitol. 148:3–13 [DOI] [PubMed] [Google Scholar]
- 63.Fairlamb AH, Cerami A. 1992. Metabolism and functions of trypanothione in the Kinetoplastida. Annu. Rev. Microbiol. 46:695–729 [DOI] [PubMed] [Google Scholar]
- 64.Gobert AP, Daulouede S, Lepoivre M, Boucher JL, Bouteille B, Buguet A, Cespuglio R, Veyret B, Vincendeau P. 2000. l-Arginine availability modulates local nitric oxide production and parasite killing in experimental trypanosomiasis. Infect. Immun. 68:4653–4657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Duleu S, Vincendeau P, Courtois P, Semballa S, Lagroye I, Daulouede S, Boucher JL, Wilson KT, Veyret B, Gobert AP. 2004. Mouse strain susceptibility to trypanosome infection: an arginase-dependent effect. J. Immunol. 172:6298–6303 [DOI] [PubMed] [Google Scholar]
- 66.O'Sullivan B, Thomas R. 2003. CD40 and dendritic cell function. Crit. Rev. Immunol. 23:83–107 [DOI] [PubMed] [Google Scholar]
- 67.Ferrer IR, Liu D, Pinelli DF, Koehn BH, Stempora LL, Ford ML. 2012. CD40/CD154 blockade inhibits dendritic cell expression of inflammatory cytokines but not costimulatory molecules. J. Immunol. 189:4387–4395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haile LA, Greten TF, Korangy F. 2012. Immune suppression: the hallmark of myeloid derived suppressor cells. Immunol. Invest. 41:581–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Van Overtvelt L, Vanderheyde N, Verhasselt V, Ismaili J, De Vos L, Goldman M, Willems F, Vray B. 1999. Trypanosoma cruzi infects human dendritic cells and prevents their maturation: inhibition of cytokines, HLA-DR, and costimulatory molecules. Infect. Immun. 67:4033–4040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Figueiredo AB, Serafim TD, Marques-da-Silva EA, Meyer-Fernandes JR, Afonso LC. 2012. Leishmania amazonensis impairs DC function by inhibiting CD40 expression via A2B adenosine receptor activation. Eur. J. Immunol. 42:1203–1215 [DOI] [PubMed] [Google Scholar]
- 71.Larsen CP, Elwood ET, Alexander DZ, Ritchie SC, Hendrix R, Tucker-Burden C, Cho HR, Aruffo A, Hollenbaugh D, Linsley PS, Winn KJ, Pearson TC. 1996. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature 381:434–438 [DOI] [PubMed] [Google Scholar]
- 72.Pereira LF, de Souza AP, Borges TJ, Bonorino C. 2011. Impaired in vivo CD4+ T cell expansion and differentiation in aged mice is not solely due to T cell defects: decreased stimulation by aged dendritic cells. Mech. Ageing Dev. 132:187–194 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.