Abstract
Background
CMV infections have been linked to vasculopathies like atherosclerosis and Scleroderma. CMV infects vascular endothelium with intermittent shedding of the virus and the development of latency.
Methods
We adopted a model of arteritis, developed by Presti et al. (1998), triggered by murine cytomegalovirus (MCMV) infection. Our studies focused on neointima formation. Groups of mice include: 1) immunocompetent 129S, 2) immunocompetent 129S receiving whole body irradiation and MCMV, 3) IFN-γR-/- receiving MCMV, and 4) IFN-γR-/- receiving MCMV and whole body irradiation.
Results
Mice were inoculated with MCMV (5 x 104 or 1 x 105 PFU's) by i.p. injection; hearts and abdominal aortas were collected and histopathology evaluated. Infected immunocompetent animals exhibited widespread perivascular inflammation, which subsided by 8 weeks. Intimal pathology was not observed in any control group. Immunocompetent animals receiving MCMV and irradiation developed mild to moderate intimal lesions associated with medial and adventitial inflammation. IFN-γR-/- mice infected for 4 months and receiving whole body irradiation 2 months after infection developed pathology characterized by extensive adventitial and medial infiltrate and significant neointima, suggesting that infection and immunosuppression were co-requisites of neointima formation. Immunohistochemical analysis revealed myofibroblasts as a major component of neointima. The disease is characterized by up-regulation of growth factors (TGF-β1, PDGF-A and B). Apoptosis was detected in the intimal layer of affected aortas. Active proliferation of myofibroblasts and infiltrating cells was also detected.
Conclusion
These results indicate that CMV infections may lead to intimal injury that results in the formation of neointima characteristic of autoimmune vasculopathies.
Editorial note
A commentary on this article can be found at http://www.biomedcentral.com/1471-8219/2/5
Introduction
Cardiovascular diseases, a major health concern in industrialized countries [1-4], include vasculopathies such as atherosclerosis [5] and autoimmune vascular diseases such as lupus [6], graft versus host disease [7], and systemic sclerosis [8]. Many factors are involved in the development and progression of these diseases including lifestyle (diet, smoking, and lack of physical exercise); genes, and environment [1-4]. Also, infectious agents, including both bacterial (Chlamydia) [9,10], and viral (CMV) [11,12], have been implicated. CMV, a herpes virus, causes chronic asymptomatic infections in immunocompetent individuals, termed latency [13]. However, in situations of immunocompromise, CMV is reactivated, which in many cases leads to organ failure and death [13]. Epidemiological reports indicate that chronic CMV infections in humans may play an important role in pathogenesis of vascular diseases such as atherosclerosis [14] and systemic sclerosis [11,12]. In addition, a recent report [15] revealed that SSc autoantibodies bind to CMV late protein UL94 and induce apoptosis in endothelial cells therefore implicating molecular mimicry as a potential mechanism accounting for the link between SSc and CMV.
Here we report that MCMV infections of gene-targeted mice lacking IFN-γR and subjected to whole body irradiation develop vascular lesions that over 4 months progress to severe vasculopathy characterized by significant neointima formation, a prominent feature of autoimmune vasculopathies in humans. Furthermore, imunohistochemical analyses indicate the presence of significant lymphohistiocytic infiltrate in the adventitia of affected arteries containing both T and B-lymphocytes. Neointima stained positive for both -smooth muscle actin and PCNA indicating proliferation of smooth muscle cells possibly mediated by growth factors TGF-β1, PDGF-A and PDGF-B, while TUNEL indicated apoptosis in the intimal layer in affected arteries.
Materials and methods
Mice
All experiments described in this study confirm with "The guide for the Care and Use of Laboratory Animals" published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). CD-1 mice used for generating the MCMV stock were purchased from Charles River Laboratories (Wilmington, MA). Experimental groups included: 1) adult, immunocompetent 129S mice, and 2) adult B6,129S IFN-γR-/- mice, both obtained from Jackson Laboratories (Bar Harbor, MN). Food and water were provided ad libitum. All mice were housed in hepa filtered cages in the approved animal facility and monitored daily for the development of clinical manifestations of infection.
Preparation of MCMV stock and infection protocol
MCMV strain Smith stock was purchased from American Type Tissue Collection (Rockville, MD) This virus stock had been propagated in SC-1 cells (mouse embryo fibroblast cell line). To enhance pathogenicity, the virus was passed three times in adult immunocompetent CD-1 mice, which were infected with MCMV by i.p. injection with 5 x 105 plaque forming units (pfu). Two weeks after infection mice were sacrificed, salivary glands were collected, and virus stock was prepared as a 10% weight/volume homogenate. Concentration of virus in these homogenates was determined by a standard plaque assay on an infected 3T-12 fibroblast cell line. Final MCMV stock contained 4.2 x 106 PFUs/ml of salivary gland homogenate. Control animals were infected with the same concentration of salivary gland homogenate obtained from control uninfected CD-1 mice.
Experimental protocol
Two months old mice were injected i.p. with either MCMV or control salivary gland homogenate. Starting at day 15 after infection and for as long as 6 months of age, mice were sacrificed on a biweekly basis by i.p. injection of sodium nembutal. Hearts and abdominal aortas were collected and histopathological and immunohistochemical analyses were performed. To both accelerate the development of vascular pathology and to deepen the immunocompromised state, these mice were irradiated with a sublethal dose of gamma radiation (300 Rads) at 4 months of age. Tissue sections were coded and scoring was performed in a blinded fashion.
Histology
H&E, Movat trichrome, and Verhoeff van Gieson, staining was performed on 4 μm sections of aortas fixed for 16 hours in neutral buffered formalin (10%), and embedded in paraffin.
Antibodies
Polyclonal antibodies specific for CD4, CD8, γ/δ-TCR, TGF-β1, PDGF-A, PDGF-B, MCP-1 and polyclonal antibody to PCNA were purchased from Santa Cruz Biotechnology Inc., (Santa Cruz, CA). Polyclonal antibody to B-lymphocyte marker (B-220) was purchased from Caltag Laboratories (Burlingame, CA). Monoclonal antibody specific for α-smooth muscle actin (clone 1A4) was purchased from Sigma (St. Louis, MO).
Immunohistochemical analysis
Tissues were collected, fixed in formalin, and embedded in paraffin. After deparaffinizing and two 5-minute washes in PBS, the sections were blocked for 20 minutes with a solution of 2% unconjugated goat anti-mouse IgG in 1 x PBS. Indicated dilutions of the primary antibodies were added to the slides and incubated for 2 hours at room temperature followed by two 5-minute washes with PBS. Biotinylated secondary IgG diluted 1:100 was added to the slides and incubated for 30 minutes. After two additional 5-minute PBS washes, the tissue sections were treated with a 3% solution of hydrogen peroxide to quench endogenous peroxidase activity. After two additional 5-minute washes, the slides were incubated for 30 minutes in the solution containing the avidin-biotin-peroxidase complex. Diaminobenzidine/H2O2 substrate was allowed to react with the peroxidase labeled tissue sections for 20 minutes. After two 1-minute rinses in distilled water, the tissue sections were counterstained with hematoxilin, cleared and dehydrated by successive gradations through 70%, 95%, and 100% ethanol followed by a final passage in xylene. The slides were mounted and analyzed by bright field microscopy.
Tunel
Animals were sacrificed and tissues collected and processed as described for immunohistochemistry. TUNEL assay was performed utilizing a kit for in situ detection of apoptosis, Oncogene, (Cambridge, MA), according to manufacturer's instructions. Briefly, 5 μm sequential sections were deparaffinized and fixed with 4% paraformaldehyde for 20 minutes at room temperature followed by 30 minute wash in PBS. After a 5 minute rinse with PBS, 50 μl of TUNEL reaction mixture was added to tissue sections and incubated in humidified chamber for 60 minutes at 37°C followed by three 5 minute rinses in PBS and directly analyzed under the microscope.
Results
Neointima formation triggered by MCMV infection
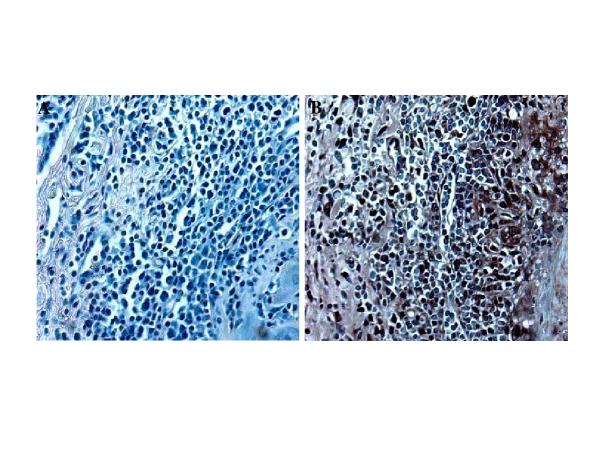
Both immunocompetent 129S and immunodeficient IFN-γR-/- mice were infected by i.p. injection at age 2 months with either 1 x 105 or 5 x 104 pfus of MCMV stock. At 1 x 105 pfus/mouse, 90% of IFN-γR-/- mice died with necrotizing hepatitis between days 5-10. Reducing the dose of MCMV to 5 x 104 pfu resulted in lower mortality rates (30%). In the immunocompetent group no deaths occurred at either dose of MCMV. Surviving mice that received either dose of virus developed perivascular mononuclear cellular infiltrates in a number of organs including lungs, kidneys, and salivary glands. Vascular disease in the IFN-γR-/- group of mice began with development of an inflammatory infiltrate in the adventitia of aortas 3-4 weeks after infection in 30% of animals (Table 1) (Fig. 1A). This infiltrate was comprised predominantly of lymphohistiocytic cells (Fig. 1A). The vascular disease progressed over a time course of 4 months and affected between 50 and 65 % of animals (Table 1). In the group of IFN-γR-/- mice receiving sublethal (300 Rads) whole body irradiation 2 months after infection all examined animals exhibited vascular pathology (Table 1). Animals appeared healthy and all survived whole body irradiation. In the immunocompetent group of animals these infiltrates were cleared by 8 weeks after infection and from that point these animals were histologically indistinguishable from the uninfected group. On the contrary, in the IFN-γR-/- mice infected with MCMV, vascular disease progressed to the formation of neointima in aortas by 4 months of age, (Fig. 1B,C,D). Verhoeff-van Gieson staining indicated the focal destruction of aortic elastic fibrils in addition to intimal thickening (Fig. 1D) Controls in experiments using IFN-γR-/- mice included: 1) uninfected animals, 2) mice that were exposed only to the whole body irradiation and 3) mice that received only MCMV. Histopathological analysis of abdominal aortas collected from transgenic, infected and irradiated animals revealed severe vasculitis characterized by significant inflammatory infiltrate in the adventitia and media (Table 2), (Fig. 2). At the same time hearts showed no signs of disease (data not shown). Verhoeff-van Gieson (Fig. 2C) and Movat pentachrome (Fig. 2D) staining indicated the focal destruction of elastic tissue, as well as potential trafficking of the infiltrating cells through these breaks in the elastica. The extent of vascular damage in all mice from this group was similar. The most striking feature of the MCMV induced vascular disease was the formation of neointima in aortas of MCMV infected immunodeficient mice after irradiation (Table 2), (Fig 2). At the same time there was no vascular pathology in any of the control groups (Table 2) (Fig 2A). Verhoeff-van Gieson staining of aortas collected from IFN-γR-/- mice 30 days after irradiation (not shown) and IFN-γR-/- mice 60 days after irradiation (Fig. 2) indicated that the intimal vascular lesion induced by MCMV infection was characterized by collagen deposition and smooth muscle proliferation. We utilized monoclonal antibody specific for α-SMA, a marker for myofibroblast cells, and found that neointima seen in the aortas of MCMV infected experimental animals contained myofibroblasts. (Fig. 3).
Table 1.
Overview of the time course and incidence of vascular pathology in IFN-γR-/- mice
| Age (months) | ||||||
| Experimental group | 2.5 | 3.0 | 3.5 | 4.0* | 4.5 | 6.0 |
| IFN-γR-/-MCMV+IRR | - | - | - | - | 6/6 | 8/8 |
| IFN-γR-/- MCMV | 0/6 | 2/6 | 4/6 | 4/8 | 4/6 | 5/8 |
| Controls ¶ | - | 0/6 | - | 0/8 | - | 0/4 |
Mice were infected at the age of 2 months. * = Irradiation. Results are presented as number of animals affected / total number of animals in the group. ¶ = 129S, immunocompetent mice infected with MCMV.
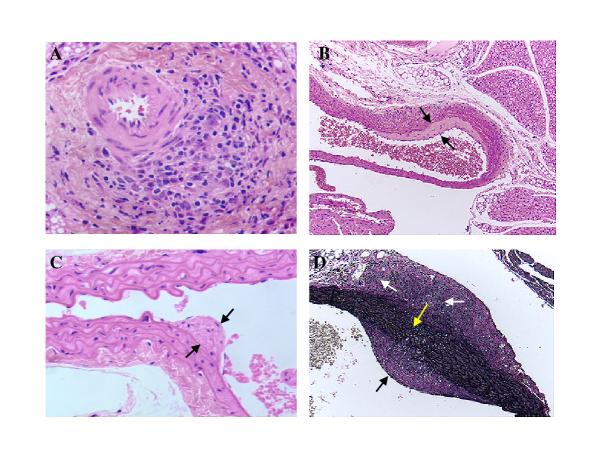
Figure 1.
Beginning of arterial wall thickening induced by infecting IFN-γR-/- mice with MCMV. A. H&E staining of small aortic branch 21 days after MCMV infection showing focal lymphohistiocytic adventitial infiltrate. B and C. Beginning of intimal thickening 2 months after MCMV infection (H&E staining) Spaces between black arrows indicates intimal thickening. D. Verhoeff-van Gieson staining showing small breaks in the elastic tissue of aorta 2 months after MCMV infection. White arrows indicate focal adventitial inflammation and fibrosis, black arrow indicates intimal thickening, yellow arrow points to small breaks in the elastic tissue. Objective magnification x 20
Table 2.
Overview of experimental groups of mice and seveverity of vascular disease.
| Lesions* | |||||
| Genetic | MCMV | Intima | Media | Adventitia | |
| IFN-γR-/- | + | Genetic | ++++ | ++++ | |
| IFN-γR-/- | + | - | ++ | ++ | ++ |
| Wild type | + | + | + | + | + |
| Wild type | + | - | 0 | 0 | 0 |
| Controls¶ | - | -/+ | 0 | 0 | 0 |
MCMV = MCMV infection 4 months, IRR = whole body irradiation * = 0 – 4 + semi quantitative visual estimate. + = mild adventitial mononuclear cell infiltrate, no changes in the media or intima. ++ = significant adventitial inflammation also extending to the media, mild intimal thickening, +++ = in addition to adventitial and medial inflammation formation of neointima. ++++ = severe obliteration due to neointima formation in abdominal aorta, severe inflammation of adventitia and media characterized by breaks in the elastica. ¶ = wild type or IFN-γR-/- 129S mice receiving salivary gland homogenate from uninfected animals, + IRR in half.
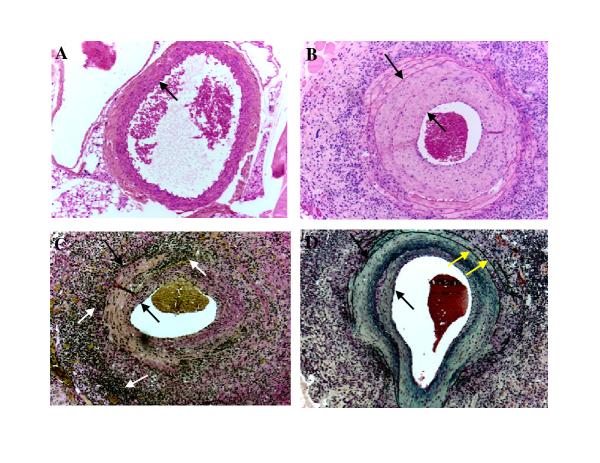
Figure 2.
Neointima formation in the aortas of MCMV infected IFN-γR-/- mice 2 months after whole body irradiation. A. H&E staining of abdominal aorta collected from uninfected IFN-γR-/- mouse 2 months after whole body irradiation. Arrow points to the intima. B. H&E staining of a large abdominal vessel collected from MCMV infected IFN-γR-/- mouse 4 months after infection and 2 months after whole body irradiation. Space between arrows indicates the thickness of the neointima C. Verhoeff-van Gieson stain of a large abdominal vessel collected from MCMV infected IFN-γR-/- mouse 4 months after infection and 2 months after whole body irradiation. Verhoeff-van Gieson stain: muscle (brown-yellow), collagen (red), cell cytoplasm (yellow), nuclei (blue-black). Focal transmural necrotizing vasculitis is seen. . Space between black arrows indicate intimal thickening and white arrows indicate focal adventitial inflammation and fibrosis. D. Movat staining of a large abdominal vessel collected from MCMV infected IFN-γ R-/- mouse 4 months after infection and 2 months after whole body irradiation. Movat stain: elastica (black), collagen and reticular fibers (yellow), mucosubstance (blue-green), red blood cells (red). Space between black arrows indicates the thickness of the neointima. Yellow arrows indicate normal external and internal elastica. Objective magnification x 20.
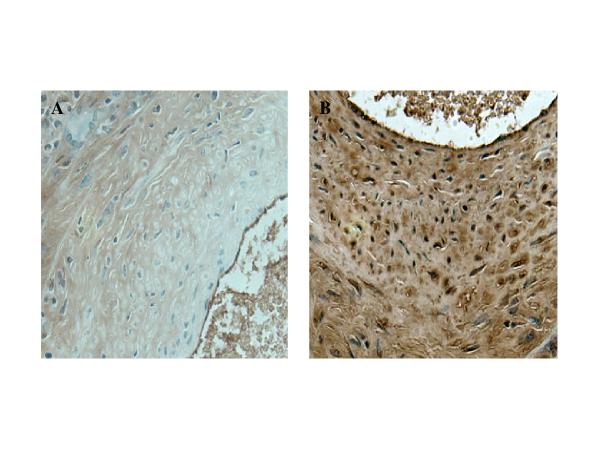
Figure 3.
Immunohistochemistry of neointima. A. Negative control for α-SMA staining (primary antibody omitted). B. Section of the aorta collected from MCMV infected IFN-γR-/- mouse 4 months after infection and 2 months after whole body irradiation were stained with monoclonal anti α-SMA. Objective magnification x 40
Immunohistochemical characterization of immune response to MCMV infection
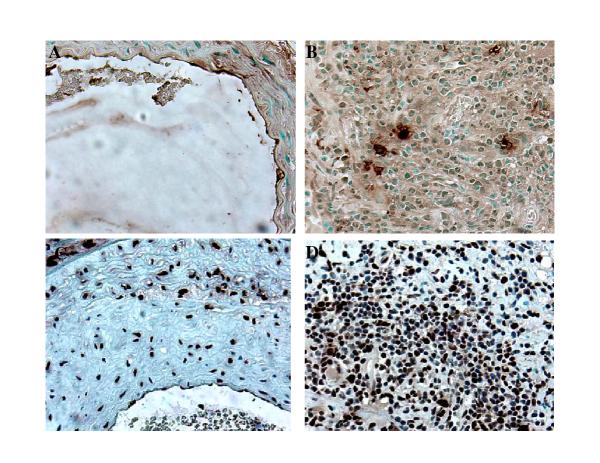
Immunohistochemical characterization of the inflammatory infiltrate seen in the adventitia and media of the affected aortas revealed both helper T-lymphocytes (CD4+) (Fig. 4B) and cytotoxic T-lymphocytes (CD8+) (Fig. 4C) throughout the lesion. Since one of the characteristics of autoimmune vasculopathies is the appearance of high numbers of γ/δ-TCR [16] bearing cells, we studied this cell type in our model of vascular disease. Results from these experiments revealed a large number of γ/δ-TCR bearing lymphocytes throughout the lesion (Fig. 4D). We also investigated the presence of B-lymphocytes in the vascular lesion of MCMV infected mice. While T-lymphocytes were identified throughout the lesion these cells were mostly found in small follicles (Fig. 4A).
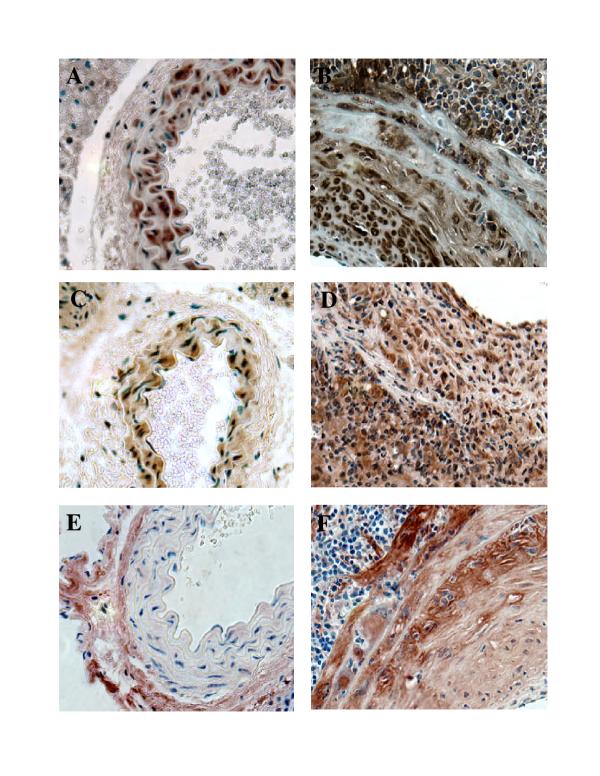
Figure 4.
Characterization of inflammatory infiltrate. Aortas collected from MCMV infected IFN-γR-/- mice 4 months after infection and 2 months after whole body irradiation. A. Immunostaining specific for B-lymphocytes (B220+). B. Staining specific for helper T-lymphocytes (CD4+). C. Staining specific for cytotoxic T-lymphocytes (CD8+). D. Immunostaining specific for γ/δ T-cell receptor bearing T-lymphocytes (γ/δ-TCR+). Objective magnification x 40
Investigation of the role of cytokines and growth factors
Growth factors possibly responsible for the neointimal proliferation were studied based on their putative importance in autoimmune vasculopathies in humans. Immunohistochemical analysis indicated a significant overproduction of TGF-β1, PDGF-A, and PDGF-B in the MCMV induced intimal lesion (Fig. 5). While TGF-β 1 and PDGF-A immunoreactivity in affected arteries is localized to all three layers (intima, media, adventitia) (Fig. 5B and 5D), PDGF-B staining is localized to the media and neointima (Fig. 5F). MCP-1, a chemokine that has been implicated in pathogenesis of several autoimmune vascular diseases including SSc, was noted in the adventitia and media of affected arteries (Fig. 6). We did not detect MCP-1 positive staining in neointima (data not shown).
Figure 5.
Investigation of the role of growth factors in the pathogenesis of MCMV induced neointima formation. A,C,E abdominal aortas collected from control uninfected IFN-γR-/- mice 2 months after whole body irradiation. B,D,F aortas collected from MCMV infected IFN-γR-/- mice 4 months after infection and 2 months after whole body irradiation.A&B staining specific for TGF-β1. C & D staining specific for PDGF-A. E & F staining specific for PDGF-B. Objective magnification x 40
Figure 6.
Immunohistochemical investigation of the expression of MCP-1. A. Negative control for MCP-1 (primary antibody omitted). B. Section of the aorta collected from MCMV infected IFN-γR-/- mouse 4 months after infection and 2 months after whole body irradiation were stained with polyclonal antibody specific for MCP-1. Objective magnification x 40
Role of apoptosis and proliferation
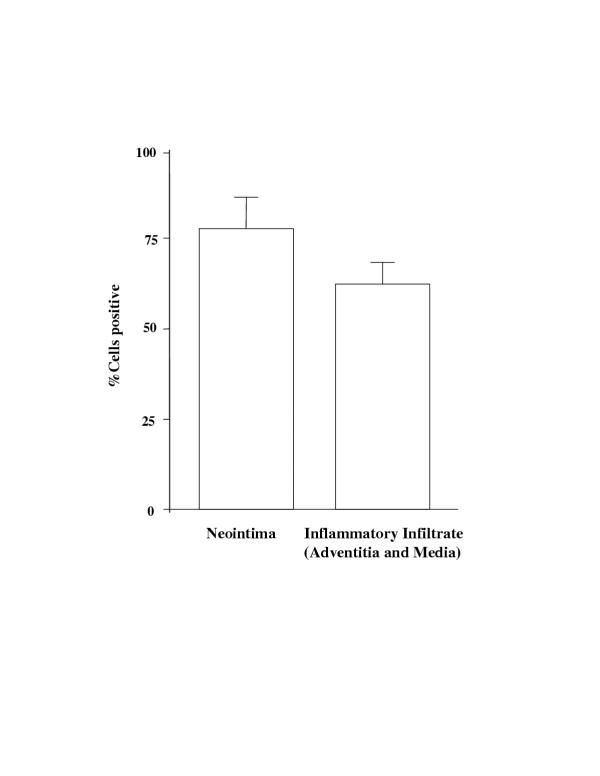
Further, we investigated the role of apoptosis and proliferation in vascular pathology triggered by MCMV infection of immunosuppressed mice. In situ TUNEL revealed positive staining in the intimal layer of affected aortas (Fig 7A). In addition, a small portion of infiltrating inflammatory cells stained positive by TUNEL (Fig. 7B). Smooth muscle/myofibroblast cells forming the neointima did not reveal any cells positive by TUNEL (Fig. 7A). Immunohistochemical staining for PCNA was used to identify cell proliferation. The majority of cells in the neointima (Fig. 7C and Fig. 8), as well as infiltrating inflammatory cells (Fig. 7D and FIG. 8), exhibit immunoreactivity for PCNA, indicating active proliferation.
Figure 7.
Investigation of apoptosis and proliferation in the pathogenesis of MCMV induced neointima formation. A and B. TUNEL performed on the aorta collected from MCMV infected IFN-γR-/- mouse 4 months after infection and 2 months after whole body irradiation. C. and D. Immunostaining specific for PCNA. A. C. Neointima.B. D. inflammatory infiltrate. Objective magnification x 40
Figure 8.
Morphometric analysis of PCNA positive staining. Both neointima and adventitia/media were analyzed by averaging cell numbers from three selected high power view fields (objective magnification 40X). Data were collected from three individual animals and are expressed as % positive cells ± SD.
Discussion
The major finding of this study is the formation of neointima associated with MCMV infection in immunocompromised mice. The disease described in our study resembles pathological processes seen in other autoimmune vasculopathies. However, it is important to note that while there are undoubtedly autoimmune aspects of atherosclerosis, this issue is still under debate [17]. The formation of neointima characteristic of the vasculopathy in autoimmune diseases by this approach has, to our knowledge, not been previously reported. While MCMV vasculitis in IFN-γR-/- mice has been previously reported [18], that study does not focus on intimal proliferation. In our experimental conditions we were able to increase yield from 62% affected animals in IFN-γR-/- mice infected with MCMV to 100% by including whole body irradiation of chronically infected IFN-γR-/- mice (Table 1). In addition, a striking increase in the severity of intimal lesions was noted in irradiated mice (Table 2). We propose that there are two possible explanations for the increase in both the number of affected animals, as well as increase in the severity of vascular disease in MCMV infected IFN-γR-/- mice receiving whole body irradiation treatment. First, sublethal irradiation additionally immunosuppresses the animals allowing unlimited replication of MCMV which may lead to direct viral damage. Second, the immune system after recovering from effects of radiation (reconstitution) contains large number of MCMV specific CTLs that may be capable of inducing intimal pathology while attempting to clear the infection.
CMV infections have been implicated in a number of vascular diseases. CMV invades the vascular endothelium with the development of both latency and intermittent shedding of the virus to distal tissues, often by detachment of infected endothelial cells. In immunocompetent individuals CMV infections are of chronic or latent type and are usually not associated with clinically detectable disease. In immunocompromised patients such as neonates, transplant recipients and patients with AIDS, CMV infections are a leading cause of organ failure and death. Nonetheless, the mechanisms of CMV reactivation and induction of vascular lesions are not well understood. Clinical and epidemiological studies strongly suggest the association of CMV infection in the progression of numerous vascular diseases, including atherosclerosis [16-20], rapidly progressive coronary artery disease, endothelialitis in heart transplant recipients [21-24], coronary restenosis [25,26], and inflammatory aortic disease [27,28] as well as SSc [11,12]. We attempted to reproduce the endpoint of such diseases by using immunosupression and CMV infection, allowing us to investigate cellular and molecular events leading to this pathology. While SSc is an autoimmune disease affecting microvasculature, some recent studies also described increased incidence of vascular pathology in the large arterial vessels of SSc patients [29-31]. However, it is still unclear if macro vascular pathology is a primary feature of SSc or an indirect effect of some of the downstream events related to this disease.
Inoculation of immunocompetent mice with MCMV causes persistent productive infection in salivary glands followed by latency [32,33]. This latency is characterized by the presence of the viral genome whereas infectious viral particles could not be detected. In addition it was possible to reactivate virus either in vivo or in vitro from tissue explants. While there are several lines of evidence to suggest that the immune system, or its absence, plays a role in MCMV reactivation from latency [34,35] the mechanism is not known. Latent CMV in many organs can be reactivated from its dormant state by several means of immunosuppression, including transmission from donors to immunosuppressed recipients of transplants, and blood transfusion.
Prior studies describe development of MCMV associated vascular lesions in large arterial vessels of mice. The i.p. injection of suckling mice induces vascular lesions in the aorta and pulmonary artery, which are characterized by cellular infiltrates containing T-lymphocytes [36]. A more recent study describes the development of MCMV induced vascular lesions in the aorta of both immunocompetent mice and those lacking IFN-γ responsiveness where IFN-γ is determined to be a major antiviral factor preventing the long term persistence of aortic vascular lesions [18]. The present study focused on neointima formation, which was seen only in immunocompromised mice. Our results indicated presence of both CD4+ helper, and CD8+cytotoxic T-lymphocytes in the adventitia of the affected arteries. Significant portion of these T-lymphocytes are γ/δ-TCR+ This subpopulation of lymphocytes has been identified as part of the pathogenesis of autoimmune diseases. For example, it has been shown that γ/δ-TCR+ lymphocytes from SSc patients recognize and lyse endothelial cells at a significantly higher rate then γ/δ cells isolated from control subjects [16]. Also, a recent study indicates that γ/δ-TCR+ bearing lymphocytes play an important role in immune response of immunosuppressed transplant recipients to CMV infection [37]. NK cells, natural antibodies, and complement, potentially important in controlling CMV infection, await further study.
Growth factors TGF-β, and PDGF are contributing factors in vascular diseases. TGF-β upregulates α-smooth muscle actin gene transcription [38]. Since we identified significant α-smooth muscle actin expression in our model and this coincided with the expression of TGF-β, it is possible that TGF-β mediates α-smooth muscle actin expression in our model. The elevated expression of PDGF in our model is also significant since the expression of these growth factors by endothelial cells can be induced by direct immune cell – endothelial cell interaction, promoting smooth muscle cell migration and proliferation in atherosclerosis [39]. CMV, as well as other viruses, induces expression of a number of cytokines and growth factors in cells in vitro, including TGF-β, a profibrogenic cytokine [40,41]. MCP-1 is a member of the β-chemokine (C-C) family [42] and this chemokine has been implicated in pathogenesis of SSc [43]. Our results (Fig. 6) indicate that this chemokine may play a role in recruitment of inflammatory cells to CMV infected vasculature.
We investigated the role of apoptosis in pathogenesis of MCMV induced intimal lesion since apoptosis of virally infected cells is one of the mechanisms by which host may limit the virus spread. The presence of apoptotic T-lymphocytes has been reported to be a feature of MCMV infection [44]. Apoptosis of vascular endothelium induced by immune effectors has been described in both SSc patient vessels as well as those of UCD chickens (one of the animal models for SSc) [45]. Recently, it has been reported that IgG autoantibodies from SSc patients bind CMV late protein UL94 and this induces apoptosis in human endothelial cells [15]. We detected the presence of apoptotic cells in the intimal layer as well as in inflammatory infiltrate of the affected aortas (Fig. 7A &7B). In the future we will ask if apoptosis in our model is induced by anti-endothelial autoantibodies, MCMV infection of endothelium, or is induced and/or perpetuated by inflammatory cells. Apoptosis of vascular endothelium may be the trigger that initiates the uncontrolled repair mechanism characterized by the proliferation of smooth muscle cells/myofibroblasts, leading to the development of neointima in MCMV induced vascular disease.
The development of CMV induced vasculopathies is a major cause of morbidity and mortality in immunosuppressed patients. CMV infections have been identified as important mediator of organ failure and death in transplant recipients and AIDS patients. Although the role of CMV infection in pathogenesis of vascular diseases in immunocompetent patients is less clear, epidemiological studies strongly suggest that chronic or latent infections may accelerate the development of diseases such as atherosclerosis and SSc. In fact, it is fair to hypothesize that several diseases thought to be of "idiopathic" origin may be triggered by chronic infections with CMV and other infectious agents in the genetically susceptible host. Animal models, such as those described in this paper, will allow us to better understand the role that viral infections play in the pathogenesis of vascular diseases. It is crucial to identify factors responsible for contributing to the development of vascular pathology in order to develop better preventive as well as treatment modalities.
Competing interests
None declared
Abbreviations
CMV = cytomegalovirus, MCMV = murine cytomegalovirus, MCP-1 = monocyte chemoattractant protein-1, PCNA = proliferating cell nuclear antigen, PDGF = platelet derived growth factor, SSc = systemic sclerosis, TGF-β1 = transforming growth factor-β1, TUNEL = terminal TdT-mediated dUTP nick end labeling, α-SMA = α-smooth muscle actin
Pre-publication history
The pre-publication history for this paper can be accessed here:
http://www.biomedcentral.com/content/backmatter/1471-2474-2-3-b1.pdf
Commentary
A commentary on this article can be found at:
Acknowledgments
Acknowledgements
The authors wish to thank Dr. Jean-Michel Goust for helpful discussions, and Margaret Romano for the preparation of histological sections.
Work supported by:Medical University of South Carolina Institutional Research Grant(DH),RGK Foundation gift(ECL);Kate and Claiborne Johnson gift (ECL).
Contributor Information
Damir Hamamdzic, Email: hamamdd@musc.edu.
Russell A Harley, Email: harleyr@musc.edu.
Debra Hazen-Martin, Email: hazenmad.GWS1.CL@musc.edu.
E Carwile LeRoy, Email: leroyc@musc.edu.
References
- Bouchard C. Physical inactivity. Can J Cardiol. 1999;15 Suppl G:89G–92G. [PubMed] [Google Scholar]
- Paradis G, Fodor JG. Diet and the prevention of cardiovascular diseases. Can J Cardiol. 1999;15 Suppl G:81G–88G. [PubMed] [Google Scholar]
- Reeder B, Bouchard C. Obesity and cardiovascular diseases. Can J Cardiol. 1999;15 Suppl G:69G–72G. [PubMed] [Google Scholar]
- Pickering T. Cardiovascular pathways: socioeconomic status and stress effects on hypertension and cardiovascular function. Ann N Y Acad Sci. 1999;896:262–277. doi: 10.1111/j.1749-6632.1999.tb08121.x. [DOI] [PubMed] [Google Scholar]
- Fuster V. Human lesion studies. Ann N Y Acad Sci. 1997;811:207–24. doi: 10.1111/j.1749-6632.1997.tb52003.x. [DOI] [PubMed] [Google Scholar]
- Moder KG, Miller TD, Tazelaar HD. Cardiac involvement in systemic lupus erythematosus. Mayo Clin Proc. 1999;74:275–284. doi: 10.4065/74.3.275. [DOI] [PubMed] [Google Scholar]
- Cook NS, Zerwes HG, Rudin M, Beckmann N, Schuurman HJ. Chronic graft loss: dealing with the vascular alterations in solid organ transplantation. Transplant Proc. 1998;30(5):2413–2418. doi: 10.1016/S0041-1345(98)00703-9. [DOI] [PubMed] [Google Scholar]
- Kahaleh MB, LeRoy EC. Autimmunity and vascular involvement in systemic sclerosis (SSc). Autoimmunity. 1999;31:195–214. doi: 10.3109/08916939908994064. [DOI] [PubMed] [Google Scholar]
- Yamashita K, Ouchi K, Shirai M, Gondo T, Nakazawa T, Ito H. Distribution of Chlamydia pneumoniae infection in the atherosclerotic carotid artery. Stroke. 1998;29(4):773–778. doi: 10.1161/01.str.29.4.773. [DOI] [PubMed] [Google Scholar]
- Muhlestein JB. Bacterial infections and atherosclerosis. J Investig Med. 1998;46(8):396–402. [PubMed] [Google Scholar]
- Pandey JP, LeRoy EC. Human cytomegalovirus and the vasculopathies of autoimmune diseases (especially scleroderma), allograft rejection, and coronary restenosis. Arthritis Rheum. 1998;41:10–15. doi: 10.1002/1529-0131(199801)41:1<10::AID-ART2>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Neidhart M, Kuchen S, Distler O, Bruhlman P, Michel BA, Gay RE, Gay S. Increased serum levels of antibodies against human cytomegalovirus and prevalence of autoantibodies in systemic sclerosis. Arthritis Rheum. 1999;42:389–392. doi: 10.1002/1529-0131(199902)42:2<389::AID-ANR23>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Mocarski ES. Cytomegalo viruses and their Replication. Fields Virology Edited by Fields BN et al Pennsylvania, Lippincott-Raven, 1996. pp. 2447–2491.
- Nieto FJ, Adam E, Sorlie P, Farzadegan H, Melnick JL, Constock GW, Szklo M. Cohort study of Cytomegalovirus infection as a risk factor for carotid intimal-medial thickening, a measure of subclinical atherosclerosis. Circulation. 1996;94:922–927. doi: 10.1161/01.cir.94.5.922. [DOI] [PubMed] [Google Scholar]
- Lunardi C, Bason C, Navone R, Millo E, Damonte G, Corrocher R, Puccetti A. Systemic sclerosis immunoglobulin G antibodies bind the human cytomegalovims late protein UL94 and induce apoptosis in human endothelial cells. Nat Med. 2000;6(10):1183–1186. doi: 10.1038/80533. [DOI] [PubMed] [Google Scholar]
- Kahaleh MB, Fan PS, Otsuka T. Gammadelta receptor bearing T cells in scleroderma: enhanced interaction with vascular endothelial cells in vitro. Clin Immunol. 1999;1(2):188–195. doi: 10.1006/clim.1999.4694. [DOI] [PubMed] [Google Scholar]
- Shoenfeld Y, Sherer Y, Harats D. Atherosclerosis as an infectious, inflammatory and autoimmune disease. Trends Immunol. 2001;22(6):293–295. doi: 10.1016/S1471-4906(01)01922-6. [DOI] [PubMed] [Google Scholar]
- Presti RM, Pollock JL, Dal Canto AJ, O'Guin AK, Virgin IV HW. Interferon γ regulates acute and latent murine cytomegalovirus infection and chronic disease of the great vessels. J Exp Med. 1998;188:577–588. doi: 10.1084/jem.188.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorlie PD, Adam E, Melnick SL, Folsom A, Skelton T, Chambless LE, Barnes R, Melnick JL. Cytomegalovirus/herpesvirus and carotid atherosclerosis: the ARIC study. J Med Virol. 1994;42:33–37. doi: 10.1002/jmv.1890420107. [DOI] [PubMed] [Google Scholar]
- Melnick JL, Adam E, DeBakey ME. Cytomegalovirus and atherosclerosis. Bioessays. 1995;17:899–903. doi: 10.1002/bies.950171012. [DOI] [PubMed] [Google Scholar]
- Koskinen P, Lemstrom K, Bruggeman C, Lautenschlager I, Hayry P. Acute cytomegalovirus infection induces subendothelial inflammation (endothelialitis) in the allograft vascular wall. A possible linkage with enhanced allograft atherosclerosis. Am J Pathol. 1994;144:41–50. [PMC free article] [PubMed] [Google Scholar]
- Dummer S, Lee A, Breinig MK, Kormos Ho RM, Griffth B. Investigation of cytomegalovirus infection as a risk factor for coronary atherosclerosis in the explanted hearts of patients undergoing heart transplantation. J Med Virol. 1994;44:305–309. doi: 10.1002/jmv.1890440316. [DOI] [PubMed] [Google Scholar]
- McDonald K, Rector TS, Braunlin EA, Kubo SH, Olivari NT. Association of coronary artery disease in cardiac transplant recipients with cytomegalovirus infection. Am J Cardiol. 1989;64:359–362. doi: 10.1016/0002-9149(89)90535-3. [DOI] [PubMed] [Google Scholar]
- Grattan MT, Moreno-Cabral CE, Stames VA, Oyer PE, Stinson EB, Shumway NE. Cytomegalovirus infection is associated with cardiac allograft rejection and atherosclerosis. JAMA. 1989;261:3561–3566. doi: 10.1001/jama.261.24.3561. [DOI] [PubMed] [Google Scholar]
- Speir E, Modali R, Huang ES, Leon MB, Shawl F, Finkel T, Epstein SE. Potential role of human cytomegalovirus and p53 interaction in coronary restenosis. Science. 1994;265:391–394. doi: 10.1126/science.8023160. [DOI] [PubMed] [Google Scholar]
- Zhou YF, Leon MB, Waclawiw MA, Popma JJ, Yu ZX, Finkel T, Epstein SE. Association between prior cytomegalovirus infection and the risk ofrestenosis after coronary atherectomy. N Engl J Med. 1996;335:624–630. doi: 10.1056/NEJM199608293350903. [DOI] [PubMed] [Google Scholar]
- Yonemicu Y, Nakagawa K, Tanaka S, Mori R, Sugimachi K, Sueishi K. In situ detection of frequent and active infection of human cytomegalovirus in inflammatory abdominal aortic aneurysms: possible pathogenic role in sustained chronic inflammatory reaction. Lab Invest. 1996;74:723–736. [PubMed] [Google Scholar]
- Tanaka S, Komori K, Okadome K, Sugimachi K, Mori R. Detection of active cytomegalovirus infection in inflammatory aortic aneurysms with RNA polymerase chain reaction. J Vasc Surg. 1994;20:235–243. doi: 10.1016/0741-5214(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Hafner J, Schneider E, Burg G, Cassina PC. Management of leg ulcers in patients with rheumatoid arthritis or systemic sclerosis: the importance of concomitant arterial and venous disease. J Vasc Surg. 2000;32(2):322–329. doi: 10.1067/mva.2000.106942. [DOI] [PubMed] [Google Scholar]
- Ho M, Veale D, Eastmond C, Nuki G, Belch J. Macrovascular disease and systemic sclerosis. Ann Rheum Dis. 2000;59(1):39–43. doi: 10.1136/ard.59.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford L, Englert H, Gover J, Bertouch J. Distribution of macro vascular disease in scleroderma. Ann Rheum Dis. 1998;57(8):476–479. doi: 10.1136/ard.57.8.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock JL, Virgin HW. Latency, without persistence, of murine cytomegalovirus in spleen and kidney. J Virol. 1995;69:1762–1768. doi: 10.1128/jvi.69.3.1762-1768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz S, Steffens HP, Mayer A, Harris JR, Reddehase MJ. Latency versus persistence of intermittent recurrences: evidence for latent state of murine cytomegalovirus in the lungs. J Virol. 1997;71:2980–2987. doi: 10.1128/jvi.71.4.2980-2987.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo D, Armstrong JA, Ho M. Activation of latent murine cytomegalovirus infection: cocultivation, cell transfer, and the effect of immunosuppression. J Infect Dis. 1978;138:890–896. doi: 10.1093/infdis/138.6.890. [DOI] [PubMed] [Google Scholar]
- Balthesen M, Messerle M, Reddehase MJ. Lungs are a major organ site of cytomegalovirus latency and recurrence. J Virol. 1993;67:5360–5366. doi: 10.1128/jvi.67.9.5360-5366.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangler CA, Baker SE, Kariuki Njenga M, Chia SH. Murine cytomegalovirus associated arteritis. Vet Pathol. 1995;32:127–133. doi: 10.1177/030098589503200205. [DOI] [PubMed] [Google Scholar]
- Dechanet J, Merville P, Lim A, Retiere C, Pitard V, Lafarge X, Michelson S, Meric C, Hallet MM, Kourilsky P, Potaux L, Bonneville M, Moreau JF. Implication of gammadelta T cells in the human immune response to cytomegalovirus. J Clin Invest. 1999;103(10):1437–1449. doi: 10.1172/JCI5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautmann MB, Madsen CS, Owens GK. A transforming growth factor beta (TGFbeta) control element drives TGFbeta-induced stimulation of smooth muscle alpha-actin gene expression in concert with two CArG elements. J Biol Chem. 1997; 272(16):10948–56. doi: 10.1074/jbc.272.16.10948. [DOI] [PubMed] [Google Scholar]
- Funayama H, Ikeda U, Takahashi M, Sakata Y, Kitagawa S, Takahashi Y, Masuyama J, Furukawa Y, Miura Y, Kano S, Matsuda M, Shimada K. Human monocyte-endothelial cell interaction induces platelet-derived growth factor expression. Cardiovasc Res. 1998;37(1):216–224. doi: 10.1016/S0008-6363(97)00224-1. [DOI] [PubMed] [Google Scholar]
- Michelson S, Alcami J, Kim SJ, Danielpour D, Bachelerie F, Picard L, Bessia C, Paya C, Virelizier JL. Human cytomegalovirus infection induces transcription and secretion of transforming growth factor beta 1. J Virol. 1994;68(9):5730–5737. doi: 10.1128/jvi.68.9.5730-5737.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taichman RS, Nassiri MR, Reilly MJ, Ptak RG, Emerson SG, Drach JC. Infection and replication of human cytomegalovirus in bone marrow stromal cells: effects on the production of IL-6, MIP-1alpha, and TGF-beta1. Bone Marrow Transplant. 1997;19(5):471–480. doi: 10.1038/sj.bmt.1700685. [DOI] [PubMed] [Google Scholar]
- Van Coillie E, Van Damme J, Opdenakker G. The MCP/eotaxin subfamily of CC chemokines. Cytokine Growth Factor Rev. 1999;10:61–86. doi: 10.1016/S1359-6101(99)00005-2. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Sato S, Takehara K. Augmented production of chemokines (monocyte chemotactic protein-1 (MCP-1), macrophage inflammatory protein-1 alpha (MIP-lalpha) and MIP-lbeta) in patients with systemic sclerosis: MCP-1 and MIP-lalpha may be involved in the development of pulmonary fibrosis. Clin Exp Immunol. 1999;117(1):159–165. doi: 10.1046/j.1365-2249.1999.00929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Sumichika H, Hamano S, He X, Minamishima Y, Kimura G, Nomoto K. Induction of apoptosis of T cells by infecting mice with murine cytomegalo virus. J Virol. 1995;69:4769–4775. doi: 10.1128/jvi.69.8.4769-4775.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgonc R, Gruschwitz MS, Dietrich H, Recheis H, Gershwin ME, Wick G. Endothelial cell apoptosis is a primary pathogenic event underlying skin lesions in avian and human scleroderma. J Clin Invest. 1996;98:785–792. doi: 10.1172/JCI118851. [DOI] [PMC free article] [PubMed] [Google Scholar]