Abstract
Urinary tract infections (UTI) are common and represent a substantial economic and public health burden. Roughly 80% of these infections are caused by a heterogeneous group of uropathogenic Escherichia coli (UPEC) strains. Antibiotics are standard therapy for UTI, but a rise in antibiotic resistance has complicated treatment, making the development of a UTI vaccine more urgent. Iron receptors are a promising new class of vaccine targets for UTI, as UPEC require iron to colonize the iron-limited host urinary tract and genes encoding iron acquisition systems are highly expressed during infection. Previously, three of six UPEC siderophore and heme receptors were identified as vaccine candidates by intranasal immunization in a murine model of ascending UTI. To complete the assessment of iron receptors as vaccine candidates, an additional six UPEC iron receptors were evaluated. Of the six vaccine candidates tested in this study (FyuA, FitA, IroN, the gene product of the CFT073 locus c0294, and two truncated derivatives of ChuA), only FyuA provided significant protection (P = 0.0018) against UPEC colonization. Intranasal immunization induced a robust and long-lived humoral immune response. In addition, the levels of FyuA-specific serum IgG correlated with bacterial loads in the kidneys [Spearman's rank correlation coefficient ρ(14) = −0.72, P = 0.0018], providing a surrogate of protection. FyuA is the fourth UPEC iron receptor to be identified from our screens, in addition to IutA, Hma, and IreA, which were previously demonstrated to elicit protection against UPEC challenge. Together, these iron receptor antigens will facilitate the development of a broadly protective, multivalent UTI vaccine to effectively target diverse strains of UPEC.
INTRODUCTION
The human urinary tract is one of the most-common sites for bacterial infection, second only to the respiratory tract (1). Most urinary tract infections (UTI) are caused when pathogenic bacteria, commonly found in the gastrointestinal tract, colonize the perineum and traverse the urethra to cause an infection in the bladder, clinically termed cystitis. Left untreated, cystitis can progress to pyelonephritis as colonizing bacteria ascend the ureters to cause a secondary infection in the kidneys (2). In severe cases, invading bacteria can breach epithelial and endothelial barriers in the kidney to gain access to the bloodstream, leading to systemic infection and sepsis, a serious and sometimes fatal complication (3).
While most UTIs seldom cause life-threatening or long-term health problems, the regularity with which they occur generates a substantial economic and public health burden (4). An estimated half of all women and 12% of men will experience a UTI in their lifetime, and almost a quarter of women who have one UTI will experience a second within 6 to 12 months (5). Commonly, these infections become recurrent, with an estimated three percent of women suffering from very frequent and often constant UTI (6). At the community level, frequent UTIs tax health care and financial resources, requiring over five million physician office visits, two million emergency room visits (7), and 500,000 hospitalizations annually in the United States (8), with associated annual costs estimated at $3.5 billion (9).
To prevent more-serious infection and speed recovery, patients with UTI are generally treated with a course of antibiotics, and individuals with recurrent infection may be prescribed antibiotics prophylactically (10). However, uropathogen resistance rates to first- and second-line antibiotic therapies are climbing steadily, which can complicate treatment and lead to therapeutic failure (11–13). For example, the resistance rate of community-acquired UTI isolates to the first-line antibiotic trimethoprim-sulfamethoxazole (TMP-SMX) currently exceeds 20% in most areas (11). Likewise, between 6 and 11% of community-acquired UTI isolates are resistant to the second-line fluoroquinolone agents ciprofloxacin and levofloxacin (14–16), and alarmingly, roughly 25% of catheter-associated UTIs are fluoroquinolone resistant (17). Multidrug resistance is also on the rise, so that now over 10% of cystitis isolates are resistant to at least three different classes of antimicrobial agents (15). Together, frequent and recurrent infection along with rising rates of antimicrobial resistance compromise effective long-term treatment for UTI, making the development of alternative management therapies for UTI essential.
Vaccine development represents a rational alternative approach to UTI prevention whereby the most common cause of UTI, uropathogenic Escherichia coli (UPEC), can be specifically targeted (18–20). UPEC represent a heterogeneous group of extraintestinal pathogenic E. coli strains that are responsible for roughly 75 to 80% of all uncomplicated, or community-acquired, UTIs (21) and an estimated 60% of complicated UTIs (22), or UTIs that occur in individuals where natural barriers to infection have been eroded by underlying conditions such as pregnancy or catheterization. Although UPEC strains can reside in the human gastrointestinal tract without causing disease, once in the urinary tract, they use an arsenal of virulence factors to colonize and survive in this alternative ecological niche, inducing a robust inflammatory immune response (23). While certain virulence factors can be more prevalent among UPEC strains, as of yet, no core set of virulence factors required for UPEC to cause UTI have been determined, making the identification of optimal UPEC vaccine targets a challenge (24).
Given that conventional vaccinology approaches targeting established UPEC virulence factors have yet to produce a commercially available vaccine for UTI, we undertook an alternative and unbiased, functional vaccinology approach to vaccine discovery that has been employed successfully against other bacterial pathogens, such as Streptococcus pneumoniae (25), Salmonella (26), and Neisseria meningitidis (27). By analyzing data compiled from a series of genomic (28–31), proteomic (32–35), and metabolic (36) screens, we were able to select UPEC antigens that fit criteria hypothesized to be desirable in a vaccine antigen, which we describe as PASivE: pathogen-specific, antigenic, surface-exposed, and in vivo expressed (37). Screening vaccine antigens for PASivE criteria ensured that the vaccine targets selected would not be expressed by commensal E. coli, would be accessible to and recognized by the host immune system, and would be highly expressed and likely important for UPEC pathogenesis during UTI. Of the 5,379 predicted proteins encoded by the prototype pyelonephritic UPEC strain CFT073, only six proteins, all involved in iron acquisition, met the rigorous PASivE criteria (38).
Of the vaccine candidates identified using PASivE criteria, three (Hma, IutA, and IreA) were found to protect against experimental UTI, establishing outer membrane iron receptors as a practical class of UPEC vaccine antigens (38). The purpose of this study was to complete the characterization of iron receptors as vaccine antigens by evaluating an additional six UPEC outer membrane iron receptors. Here, we describe the identification of the yersiniabactin receptor, FyuA, as a protective antigen that, following intranasal vaccination, elicits a sustained and robust serum IgG response that correlates with the protection of mice transurethrally challenged with UPEC.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The E. coli strains used in this study were cultured in Luria broth (LB; 10 g/liter tryptone, 5 g/liter yeast extract, 0.5 g/liter NaCl) at 37°C with aeration. E. coli strain 536 was isolated from a patient suffering from urinary tract infection (39), and E. coli strain CFT073 was isolated from the blood and urine of a hospitalized patient with acute pyelonephritis (40).
Plasmid construction.
The genes encoding the candidate vaccine antigens were PCR amplified from E. coli CFT073 genomic DNA {c0294, fitA, iroN, chuAM [encoding the middle fragment of ChuA, amino acid residues 260 to 493, hereinafter called ChuA(M)], and chuAC [encoding the C-terminal fragment of ChuA, amino acid residues 494 to 660, hereinafter called ChuA(C)]} or strain 536 genomic DNA (fyuA) using high-fidelity Platinum Taq polymerase (Invitrogen) and cloned into the XhoI and HindIII restriction sites of pBAD-myc-HisA (Invitrogen) to produce C-terminally tagged myc-His6× fusions. The resulting constructs were verified by sequencing.
Vaccine antigen preparation.
Recombinant protein expression was induced in E. coli TOP10 [FyuA-His6×, c0294-His6×, and ChuA(C)-His6×], E. coli BL21 [ChuA(M)-His6×], or E. coli C41(DE3) (IroN-His6× and FitA-His6×) cultured in Terrific broth (12 g/liter tryptone, 24 g/liter yeast extract, 4 ml/liter glycerol, 100 ml/liter filter-sterilized 0.17 M KH2PO4, and 0.72 M K2HPO4) at 37°C with aeration to an optical density at 600 nm (OD600) of 0.5 to 1.0 by the addition of 1 mM l-arabinose. Induced cultures were incubated at 37°C with aeration for 4 h before being harvested by centrifugation (8,000 × g for 10 min at 4°C).
Bacterial pellets were resuspended in 10 mM HEPES, pH 7, and 100 U Benzonase nuclease (Sigma-Aldrich). Bacterial suspensions were lysed by two passages through a French pressure cell (20,000 lb/in2), and the lysate was cleared by centrifugation (8,000 × g for 10 min at 4°C). Bacterial membranes and insoluble aggregates were separated from the cleared lysate by ultracentrifugation (112,000 × g for 30 min at 4°C) and solubilized in 5 ml 100 mM NaH2PO4, 10 mM Tris-HCl, 8 M urea, pH 8.0. His-tagged proteins were purified by affinity chromatography, using nickel-nitrilotriacetic acid agarose (Qiagen) under denaturing conditions according to the manufacturer's instructions. Eluted protein was renatured by four successive dialysis steps at 4°C (dialysis buffer 1 consisted of 100 mM NaH2PO4, 5 mM Tris, 6 M urea, 50 mM NaCl, pH 5.5; dialysis buffer 2 consisted of 100 mM NaH2PO4, 2.5 mM Tris, 4 M urea, 100 mM NaCl, 0.005% Zwittergent, pH 6.5; and dialysis buffer 3 consisted of 100 mM NaH2PO4, 2 M urea, 150 mM NaCl, 0.01% Zwittergent, pH 7.4) into a final solution containing 0.05% Zwittergent in phosphate-buffered saline (PBS), pH 7.4, and quantified using the bicinchoninic acid (BCA) protein assay (Pierce).
Vaccination.
Purified antigens were chemically cross-linked to cholera toxin (CT) (Sigma-Aldrich) at a ratio of 10:1 using N-succinimidyl 3-(2-pyridyldithio)propionate (SPDP) (Pierce) according to the manufacturer's recommendations. Cross-linked antigens were administered to 6- to 8-week-old female CBA/J mice intranasally at 20 μl/mouse (10 μl/nare). Animals received a primary dose on day 0 of 100 μg antigen cross-linked to 10 μg CT or 10 μg CT alone. Two booster immunizations of 25 μg antigen cross-linked to 2.5 μg CT or 2.5 μg CT alone were administered on days 7 and 14. When appropriate, mice were transurethrally challenged with a UPEC strain on day 21 as described below for the murine model of ascending UTI.
Murine model of ascending UTI.
Female CBA/J mice were inoculated transurethrally as previously described (41), with modifications. Bacteria were cultured overnight in LB at 37°C with aeration. The inoculating strain was harvested by centrifugation (3,500 × g for 30 min at 4°C) and resuspended in PBS to an OD600 of ≈4.0 (∼1 × 109 CFU/ml), and 50 μl of this suspension (108 CFU) was delivered to each mouse via a sterile 0.28-mm polyethylene catheter attached to an infusion pump (Harvard Apparatus) with a flow rate of 100 μl/min. The inoculum was quantified by plating dilutions onto LB agar. Forty-eight hours postinoculation (h.p.i.), the bladders and kidneys were removed from euthanized mice and homogenized in 3 ml PBS using a GLH homogenizer (Omni International). Using an Autoplate 4000 spiral plater (Spiral Biotech), tissue homogenates were plated onto LB agar. Colonies were enumerated with a QCount automated plate counter (Spiral Biotech), and the CFU/g tissue or CFU/ml urine determined (output). Protocols involving CBA/J mice complied with federal guidelines and the policies of the University of Michigan's Committee on Use and Care of Animals (UCUCA).
ELISA.
For the indirect enzyme-linked immunosorbent assay (ELISA), 50 μl of 10 μg/ml purified protein diluted in carbonate buffer (100 mM, pH 9.6) was coated onto 96-well enzyme immunoassay/radioimmunoassay (EIA/RIA) high-binding polystyrene plates (Costar number 9018; Corning) and incubated at 4°C overnight. Plates were washed by flooding all wells three times with wash buffer (0.05% Tween 20 in PBS) using an ELx405 microplate washer (Bio-Tek Instruments, Inc.). Nonspecific binding sites were blocked with SuperBlock blocking buffer (Pierce) as recommended by the manufacturer, and the plates were washed with wash buffer. The wells were coated with serum diluted 1:250 in blocking buffer or 50 μl undiluted urine and allowed to incubate for 1 h at 23°C. The plates were washed with wash buffer and coated with the secondary antibody, goat anti-mouse IgG (ab97265; Abcam) or goat anti-mouse IgA (ab97235; Abcam), diluted 1:10,000 in blocking buffer, and allowed to incubate at 23°C for 1 h. The plates were washed, and 50 μl of the substrate, 1-Step ultra TMB (3,3′,5,5′-tetramethylbenzidine)-ELISA (catalog number 34018; Thermo Scientific), was added and allowed to incubate at 23°C until color developed (≤20 min). Reactions were stopped by the addition of sulfuric acid, and the absorbance of each well was read with a μQuant plate reader (Bio-Tek Instruments, Inc.) at a wavelength of 450 nm.
Statistical analyses.
Graphing and statistical analyses were performed using Prism version 6 (GraphPad Software, Inc.) and R version 2.14.1 (R Development Core Team, 2011) (42). Significance was determined using the one- or two-tailed Mann-Whitney test where appropriate, and correlates of protection were determined using the two-tailed Spearman's rank test with linear regression to generate a best-fit line. Outliers determined to be three times the interquartile range by boxplot analysis were removed. All statistics were conducted using 95% confidence intervals where applicable.
RESULTS
Candidate antigen expression and purification.
In preparation for immunization, the genes for six vaccine antigens, FyuA, IroN, c0294, FitA, ChuA(M) (middle fragment of ChuA, amino acid residues 260 to 493), and ChuA(C) (C-terminal fragment of ChuA, amino acid residues 494 to 660) were cloned as His6× translational fusions, expressed, and purified under denaturing conditions as His6× affinity-tagged recombinant proteins. Bacterial cultures expressing recombinant vaccine antigens were lysed, and bacterial membrane proteins were harvested by ultracentrifugation. The pelleted membrane proteins were solubilized in 8 M urea and passed over a nickel-affinity column to enrich for vaccine antigen. Fractions with concentrated vaccine antigen were pooled and visualized by SDS-PAGE (Fig. 1). Urea was removed stepwise to allow outer membrane iron receptor vaccine antigens to refold by a series of dialysis steps to regain a beta-barrel configuration as has been demonstrated by circular dichroism (38).
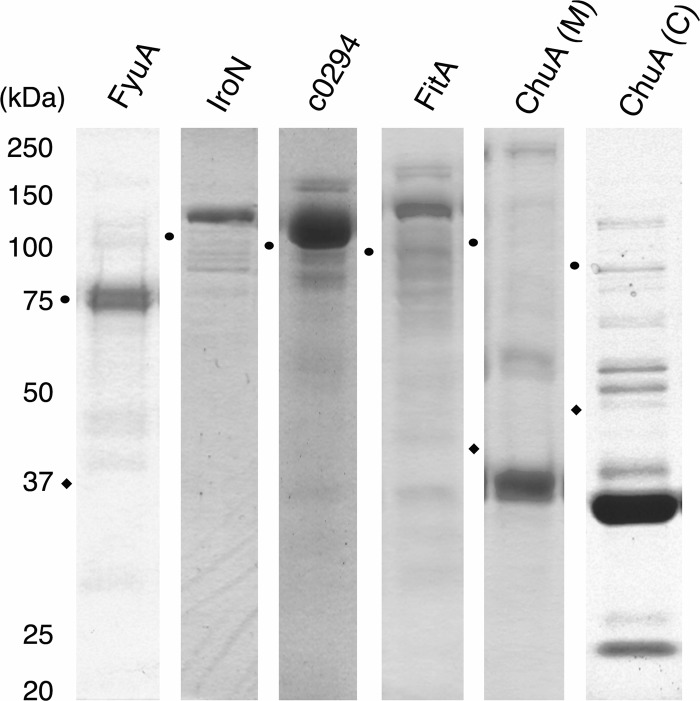
Fig 1.
Expression and purification of UPEC outer membrane iron receptors. Genes encoding outer membrane iron receptors were cloned from UPEC strains 536 and CFT073. Iron receptors were recombinantly expressed with a His tag and purified using immobilized nickel affinity chromatography. Purified protein fractions were separated by SDS-PAGE and stained with Invitrogen SimplyBlue safe stain. Predicted sizes of tagged proteins are as follows: FyuA, 77 kDa; IroN, 83 kDa; c0294, 82 kDa; FitA, 82 kDa, ChuA middle fragment [ChuA(M)], 30 kDa; ChuA C-terminal fragment [ChuA(C)], 22 kDa. Circle and diamond symbols indicate the locations of the 75-kDa and 37-kDa standard bands, respectively, for each individual gel.
Immunization with FyuA confers protection against pyelonephritis.
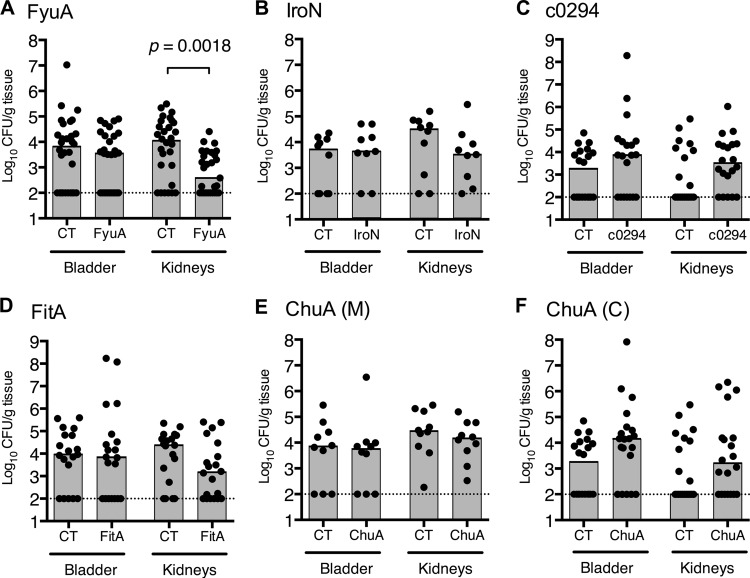
To induce a robust mucosal immune response, purified vaccine antigens were biochemically cross-linked to cholera toxin (CT) as an adjuvant, at a ratio of (10:1) (antigen/CT). Mice were immunized intranasally with either adjuvant-conjugated vaccine antigen (antigen-CT) or adjuvant alone (CT). Following primary immunization with 100 μg antigen cross-linked to 10 μg CT or 10 μg CT alone (day 0) and two booster doses of 25 μg antigen cross-linked to 2.5 μg CT or 2.5 μg CT alone (days 7 and 14), mice were transurethrally inoculated on day 21 with 1 × 108 CFU of UPEC. The prototypical pyelonephritis strain CFT073 was used as the challenge strain in all experiments, except when evaluating the vaccine antigen FyuA, where UPEC strain 536 was substituted as CFT073 does not express the siderophore receptor FyuA. The infection was allowed to progress for 48 h before the mice were euthanized and their bladders and kidneys removed. The organs were homogenized, and the UPEC bacterial load in the infected organs was quantified by determination of CFU (Fig. 2A to F). Of the six outer membrane iron receptor vaccine formulations tested, only the FyuA-based vaccine significantly protected the mice against experimental UTI (P = 0.0018), with vaccinated mice having a 29-fold decrease in median levels of UPEC kidney colonization in comparison to the levels of colonization in mice that only received adjuvant (Fig. 2A). While data for FyuA are pooled in Fig. 2A, vaccination with the antigen and control (CT alone) was carried out in three independent trials. In each case, FyuA-vaccinated mice had at least a 12-fold reduction (12.92, 95.65, and 14.18) in the median level of UPEC kidney colonization in comparison to mice given only adjuvant, with one of the three reductions being statistically significant (P = 0.2575, P = 0.0861, P = 0.0090). Although mice immunized with the IroN- and FitA-based vaccines also had reduced median levels of UPEC kidney colonization, neither reduction was statistically significant (P = 0.270 and P = 0.188, respectively) (Fig. 2B and D).
Fig 2.
Immunization with the yersiniabactin receptor FyuA protects against experimental pyelonephritis. Female CBA/J mice were intranasally vaccinated as described in the text with a primary dose of 100 μg purified protein cross-linked to 10 μg CT, followed by two boosts of 25 μg antigen cross-linked to 2.5 μg CT. One week following the final boost, animals were transurethrally inoculated with 1 × 108 CFU of E. coli 536 (A) or CFT073 (B to F), and colonization was measured 48 h.p.i. The numbers of animals per group are as follows. (A) CT, n = 30, and FyuA, n = 29, in three independent immunization experiments (for kidneys, P = 0.0430, 0.0045, and 0.1287). (B) CT, n = 10, and IroN, n = 9, in a single immunization experiment. (C) CT, n = 20, and c0294, n = 20, in two immunization experiments. (D) CT, n = 20, and FitA, n = 20, in two immunization experiments. (E) CT, n = 10, and ChuA(M), n = 10, in a single immunization experiment. (F) CT, n = 20, and ChuA(C), n = 20, in two immunization experiments. Symbols represent CFU/g tissue or CFU/ml urine of individual mice, and gray bars indicate median values. Dotted lines show the limit of detection (100 CFU/g) for this assay. Significance was determined using a two-tailed Mann-Whitney test. Only statistically significant differences are noted.
Immunized mice produce vaccine-specific serum IgG.
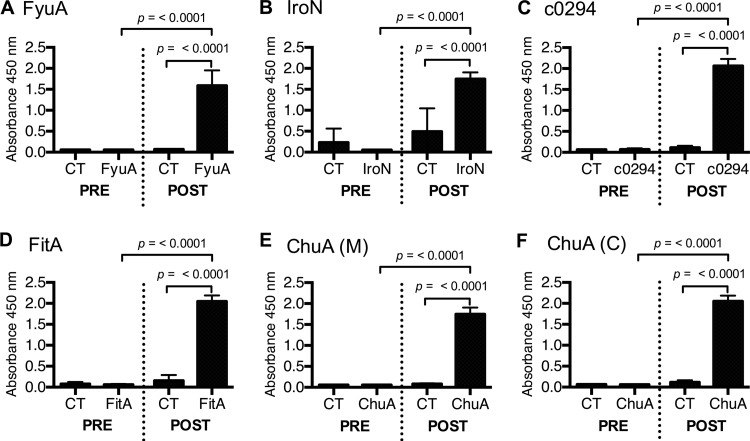
To verify that intranasal immunization with UPEC iron receptor-based vaccines induces a vaccine-specific humoral immune response, serum samples were taken from each mouse prior to the primary immunization (day 0) and again before UPEC challenge (day 21). The levels of vaccine antigen-specific serum IgG were quantified via indirect ELISA (Fig. 3A to F). All vaccine formulations induced a robust, antigen-specific serum IgG response following intranasal immunization, confirming that the failure of some vaccine antigens to significantly reduce median UPEC colonization levels was not due to the insufficient induction of a humoral immune response (Fig. 3B to F).
Fig 3.
Intranasal immunization with the yersiniabactin receptor FyuA and all other antigens induces significant antigen-specific serum IgG expression in mice. Serum was collected from mice immunized with antigen-CT or CT prior to immunization (PRE) and after immunization but before UPEC challenge (POST). Samples were plated in antigen-coated plates and probed for antigen-specific IgG via indirect ELISA. Absorbance reflects relative quantity of serum IgG. Each experimental group consisted of 20 individual mice from two separate immunization experiments. Error bars indicate the means ± standard deviations. Significance was determined using a one-tailed Mann-Whitney test.
Immunized mice secrete vaccine-specific IgA in urine.
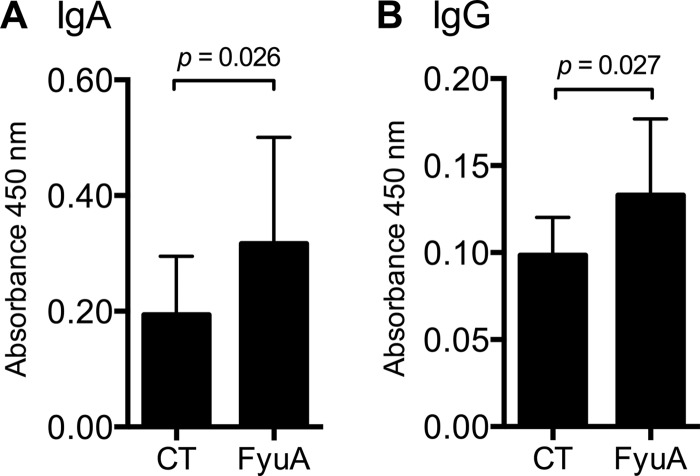
To evaluate the humoral immune response at the site of UPEC colonization, urine samples were collected from individual mice following a course of immunization with either CT or FyuA-CT, and the levels of FyuA-specific urinary IgA and IgG were quantified by indirect ELISA (Fig. 4A and B). Intranasal immunization with the FyuA-based vaccine induced statistically significant levels of urinary IgA and IgG in comparison to the levels in CT-immunized mice (Fig. 4A and B).
Fig 4.
Mice immunized intranasally with the yersiniabactin receptor FyuA produce FyuA-specific urinary antibodies. Urine collected from mice immunized with FyuA-CT (FyuA) or CT was plated on FyuA-coated plates and probed for FyuA-specific IgA (A) or IgG (B) antibodies via indirect ELISA. Absorbance reflects relative quantity of immunoglobulin. Each group (CT or FyuA) consisted of 10 individual mice from a single immunization experiment. Error bars indicate the means ± standard deviations. Significance was determined using a one-tailed Mann-Whitney test.
Vaccine-specific serum antibodies correlate with UPEC bacterial load.
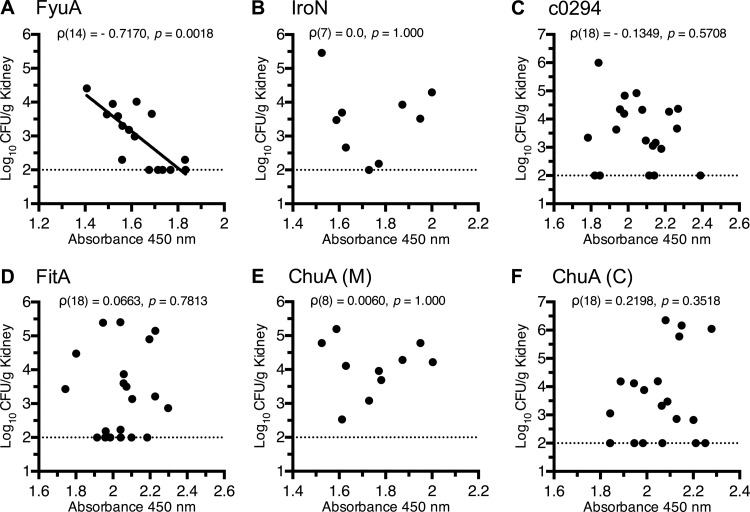
Almost all current vaccines block infection by inducing pathogen-specific antibodies in the serum or mucosa, and if a serological correlate of protection can be identified from a humoral immune response, it can provide a valuable tool to evaluate vaccine efficacy and design (43). Given our hypothesis that vaccine-mediated protection against UTI is dependent on a robust humoral immune response, it was important to determine whether the antibody levels in vaccinated mice correlated with the UPEC bacterial load in the urinary tract following transurethral challenge. To evaluate whether we could identify a serological correlate of protection for any of the antigens, we performed a Spearman's rank analysis comparing the levels of vaccine-specific serum IgG and the amount of UPEC bacterial load (CFU/g kidney) for each vaccine antigen using data from each vaccinated mouse (Fig. 5A to F). Only in the group of mice immunized with the protective vaccine antigen, FyuA, did a reduction of UPEC bacterial colonization of the kidney strongly correlate with the levels of vaccine-specific serum IgG [Spearman's rank, ρ(14) = −0.72, P = 0.0018] (Fig. 5A). The levels of FyuA-specific urinary IgA or serum IgG did not significantly correlate with the UPEC bacterial load in the bladder (data not shown).
Fig 5.
A correlation between vaccine-specific serum IgG titers and reduced bacterial counts is observed only in mice immunized with the protective vaccine. Normalized kidney CFU values from immunized and E. coli-challenged mice are plotted against their respective vaccine-specific serum IgG levels as measured by indirect ELISA, where absorbance at 450 nm reflects the relative quantity of vaccine-specific serum IgG. Dotted lines indicate the limit of detection (100 CFU/g kidney tissue) for the immunization assay. Correlative significance was determined using a two-tailed Spearman's rank correlation, and the best-fit line was determined by linear regression; the best-fit line is shown only when there is a statistically significant correlation (P < 0.05). (A) The results of linear regression for FyuA are as follows: R2 = 0.60, F(1,14) = 20.6, P = 0.005.
Immunization with FyuA generates long-lived plasma cells.
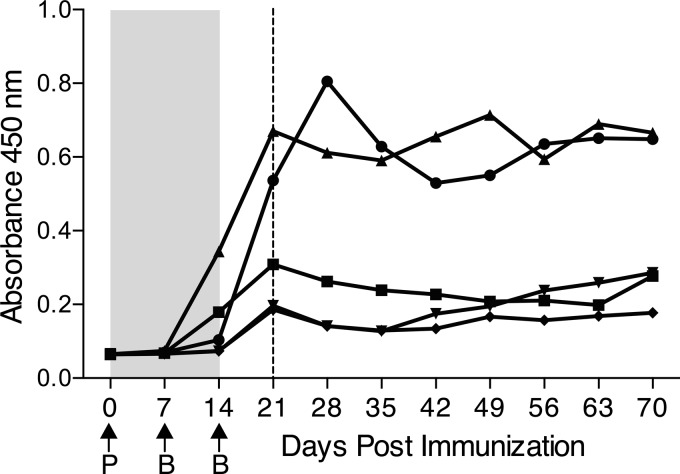
Upon initial antigen exposure, naive B cells can progress through two different paths of cell development, differentiating into either short- or long-lived plasma cells. Short-lived plasma cells mediate the initial humoral response to an antigen by secreting large amounts of antibodies; they typically appear 3 to 6 days after immunization and disappear after 2 weeks through programed cell death (44). Naive B cells that differentiate into long-lived plasma cells move to the bone marrow after maturation and maintain serological memory by continuing to secrete antibodies long after the initial infection, thereby providing a critical first line of defense against reinfection (44). To determine if vaccination with UPEC iron receptors has the potential to provide long-term immunity through the generation of long-lived plasma cells, we monitored vaccine-specific serum antibody levels for several weeks after immunization, to the point when serum antibody production could no longer be attributed to short-lived plasma cells. Per our defined dose and vaccination schedule, we intranasally immunized five female CBA/J mice with CT-cross-linked FyuA and obtained weekly serum samples during the course of the immunization and for 8 weeks following the last vaccine antigen booster dose. At the time when a transurethral challenge with UPEC would ordinarily be conducted, FyuA-specific serum IgG was at or near its highest level (Fig. 6). Near-peak levels of FyuA-specific serum antibodies were maintained for at least 70 days after the initial vaccination and at least 56 days after the last booster dose (Fig. 6). Given that the half-life for serum IgG subtypes averages 21 days or less, the sustained antibody response at 70 days in the absence of additional boosting suggests that immunization with FyuA generated FyuA-specific long-lived plasma cells.
Fig 6.
Immunization with the yersiniabactin receptor FyuA generates long-lived plasma cells. Five female CBA/J mice were intranasally vaccinated as described in the text with a primary dose (P) of 100 μg purified FyuA cross-linked to 10 μg CT, followed by two booster doses (B) of 25 μg FyuA cross-linked to 2.5 μg CT. Weekly serum samples were taken from mice (prior to same-day immunizations), and FyuA-specific serum IgG levels were quantified via indirect ELISA. The shaded area indicates the immunization period, and arrows indicate when vaccine was administered. The dashed line indicates the time point when mice would ordinarily be challenged with UPEC to evaluate vaccine efficacy in a vaccine trial.
DISCUSSION
UTIs are persistent in the general community and among hospitalized patients and, with rising rates of antibiotic resistance, are becoming increasingly more difficult to treat. The development of an effective UTI vaccine to lessen this substantial public health burden would be enormously beneficial to the population at large. Previously, we identified iron acquisition proteins, including siderophore and heme receptors, as potent vaccine antigens that can protect the urinary tract from UPEC colonization. In a systematic screen, we tested six outer membrane components of distinct iron acquisition systems of E. coli CFT073 and identified three antigens that protect against infection (IreA, IutA, and Hma) when used for intranasal vaccination (38). Having recognized iron acquisition proteins as a defined target for vaccination, we describe here the evaluation of an additional six iron acquisition proteins for vaccination to prevent UTI.
Of the six candidate antigens described here, only the yersiniabactin receptor, FyuA, stimulated a protective response, whereas IroN, FitA, the ChuA fragments, and the gene product of the strain CFT073 locus c0294 did not. FyuA-immunized mice elicited a vaccine-specific humoral immune response that strongly correlated with the levels of UPEC kidney colonization after transurethral challenge with 108 CFU of E. coli strain 536. This humoral response was maintained for at least 8 weeks following the final antigen exposure, providing evidence for the generation of long-lived, vaccine-specific plasma cells and demonstrating that an FyuA-based vaccine has the potential to provide robust and long-term protection against UTI. Not surprisingly, the other five antigens tested also elicited strong serum antibody responses following intranasal immunization; however, none significantly protected mice from UPEC challenge or induced a correlative humoral immune response. Given the harsh denaturing conditions required for the purification of antigens, it is unlikely that the immune response we observed after immunization was the result of lipopolysaccharide (LPS) contamination in our vaccine preparations. When we tested our vaccine preparations previously, we detected no contaminating LPS by limulus amebocyte lysis assay (38). In addition, given that five of the six vaccine antigens failed to protect against infection, it is highly unlikely that the protection observed for one antigen could be the result of contaminating LPS.
Why five of these antigens failed to provide protection despite inducing high levels of vaccine-specific antibodies is unclear. It is unlikely that all the vaccine targets are equally accessible by the host's humoral immune system, and possible differences in the abundance or exposure of vaccine targets on the bacterial surface may offer an explanation for the observed differences in vaccine efficacy. In addition, although we hypothesize that bacterial clearance is mediated by pathogen opsonization or neutralization, another possible mechanism may be the generation of antibodies that bind to the receptors and inhibit their function. That is, a successful protective antigen might have to generate antibodies that both opsonize bacteria and fix complement, as well as prevent the uptake of the cognate siderophore, in this case, yersiniabactin. This hypothesis awaits testing for loss-of-function (iron uptake) studies in vaccinated mice.
Clearly, variations in the mouse model, immunization route, adjuvant, antigen preparation and dose, challenge strain and inoculum dose, and challenge method all have an impact on the evaluation of vaccine efficacy. For example, in previous studies, denatured IroN delivered subcutaneously with Freund's complete adjuvant to BALB/c mice protected against lethal challenge with the extraintestinal pathogenic E. coli (ExPEC) strain S26 delivered intraperitoneally (45) or, if administered without adjuvant, protected BALB/c mice from kidney colonization by ExPEC strain CP9 delivered intravenously (46). The candidate antigen FitA, when administered intraperitoneally with Freund's complete adjuvant or Freund's incomplete adjuvant to CD1 mice, protected against lethal challenge from ExPEC strains CFT073 and IH3034 delivered intraperitoneally and ExPEC strain 536 delivered intravascularly (47). Such differences in experimental design between our studies and those of others may account for the differences we observe in protection for IroN and FitA antigens.
During our previous UPEC vaccine antigen screen (38), we tested the potential of the vaccine antigen ChuA to protect against experimental UTI, but only 19 out of 30 ChuA-immunized mice survived immunization. To more clearly evaluate the potential of ChuA as a UPEC vaccine candidate, we designed and evaluated truncated derivatives of ChuA. Although none of the mice immunized with truncated derivatives of ChuA experienced toxic effects during the course of immunization, as we had observed for intact ChuA, neither truncated ChuA derivative (M or C) significantly protected mice from transurethral challenge. Although it is possible that individual immunogenic epitopes of ChuA could have been disrupted by expressing only fragments of the protein, the results presented here are in accordance with our previous study (38) using whole renatured ChuA and with work done by Durant and colleagues evaluating denatured ChuA as an ExPEC vaccine antigen (45).
The identification of FyuA as a protective vaccine antigen against UPEC adds a fourth antigen, in addition to Hma, IreA, and IutA identified previously (38), that can be included in an intranasal vaccine to prevent UTI. Epidemiological studies and gene expression data indicate that fyuA is highly expressed during UTI in women (48), is prevalent among UPEC strains, being carried on the high-pathogenicity island (49, 50), and is an important fitness factor during experimental UTI (51). In addition, protection from infection after FyuA immunization has been demonstrated in alternative models of ExPEC infection. Subcutaneous immunization with denatured FyuA significantly protected BALB/c mice against lethal challenge by the ExPEC strain S26 (45), and a multiepitope vaccine containing a fragment of FyuA protected mice from intraperitoneal challenge by CFT073 in the liver and spleen (52). Indeed, given that many bacterial pathogens require a source of iron to cause infection, FyuA is critical for the virulence of other enteric pathogens, such as Yersinia (53) and Klebsiella (54) species.
Interestingly, immunization with FyuA provided protection in a tissue-specific manner, significantly reducing UPEC infection in the kidneys but not in the bladder. Tissue-specific protection has been observed before for antigens Hma, which also only protected in the kidney, and IreA, which only protected in the bladder, and provokes questions regarding the role of these iron receptors during infection and whether differences in iron receptor expression or function between organ sites may account for the observed differences in tissue-specific protection. In addition, although we found the level of FyuA-specific serum IgG to be a surrogate of protection after immunization, it is still unclear whether the level of FyuA-specific IgG mediates clearance of the urinary tract or whether alternative mechanisms or components of the adaptive immune response, such as urinary IgA or cellular immunity, are providing UPEC clearance. These hypotheses await testing by passive protection and adoptive transfer assays in a mouse model of UTI.
Clearly, targeting bacterial iron acquisition systems represents a rational approach to UPEC vaccine development. The four protective iron receptor vaccine antigens identified in our combined screens, Hma, IreA, IutA (38), and FyuA (described here), are highly expressed during infection and are more prevalent among uropathogenic strains (in 69%, 20%, 65%, and 87% of strains, respectively) than among fecal commensal strains (in 17%, 17%, 17%, and 59% of strains, respectively) (32, 55). Because no single antigen is present in all UPEC strains and absent from all commensal strains, targeting a single iron receptor is unlikely to yield the broad level of immunity required for an effective UTI vaccine. However, by combining the identified antigens, we can develop an iron receptor-based multiepitope vaccine with the potential to provide broad protection against the heterogeneous UPEC population.
ACKNOWLEDGMENTS
We thank the University of Michigan Center for Statistical Consultation and Research for assistance with the manuscript.
This work was supported in part by Public Health Service grants AI43365 and AI059722 from the National Institutes of Health.
Footnotes
Published ahead of print 24 June 2013
REFERENCES
- 1.Schappert SM, Rechtsteiner EA. 2008. Ambulatory medical care utilization estimates for 2006. Natl. Health Stat. Report Aug 6:1–29 [PubMed] [Google Scholar]
- 2.Muhldorfer I. 2001. Emerging bacterial pathogens. Preface. Contrib. Microbiol. 8:XI–XIV [PubMed] [Google Scholar]
- 3.Mehnert-Kay SA. 2005. Diagnosis and management of uncomplicated urinary tract infections. Am. Fam. Physician 72:451–456 [PubMed] [Google Scholar]
- 4.Foxman B. 2002. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am. J. Med. 113(Suppl 1A):5S–13S [DOI] [PubMed] [Google Scholar]
- 5.Foxman B, Brown P. 2003. Epidemiology of urinary tract infections: transmission and risk factors, incidence, and costs. Infect. Dis. Clin. North Am. 17:227–241 [DOI] [PubMed] [Google Scholar]
- 6.Foxman B, Gillespie B, Koopman J, Zhang L, Palin K, Tallman P, Marsh JV, Spear S, Sobel JD, Marty MJ, Marrs CF. 2000. Risk factors for second urinary tract infection among college women. Am. J. Epidemiol. 151:1194–1205 [DOI] [PubMed] [Google Scholar]
- 7.Schappert SM, Rechtsteiner EA. 2011. Ambulatory medical care utilization estimates for 2007. Vital Health Stat. 13:1–38 [PubMed] [Google Scholar]
- 8.Hall MJ, DeFrances CJ, Williams SN, Golosinskiy A, Schwartzman A. 2010. National Hospital Discharge Survey: 2007 summary. Natl. Health Stat Report Oct 26:1–20, 24 [PubMed] [Google Scholar]
- 9.Litwin MS, Saigal CS, Yano EM, Avila C, Geschwind SA, Hanley JM, Joyce GF, Madison R, Pace J, Polich SM, Wang M. 2005. Urologic Diseases in America Project: analytical methods and principal findings. J. Urol. 173:933–937 [DOI] [PubMed] [Google Scholar]
- 10.Kodner CM, Thomas Gupton EK. 2010. Recurrent urinary tract infections in women: diagnosis and management. Am. Fam. Physician 82:638–643 [PubMed] [Google Scholar]
- 11.Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, Moran GJ, Nicolle LE, Raz R, Schaeffer AJ, Soper DE. 2011. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin. Infect. Dis. 52:e103–e120 [DOI] [PubMed] [Google Scholar]
- 12.Moura A, Nicolau A, Hooton T, Azeredo J. 2009. Antibiotherapy and pathogenesis of uncomplicated UTI: difficult relationships. J. Appl. Microbiol. 106:1779–1791 [DOI] [PubMed] [Google Scholar]
- 13.Foxman B, Ki M, Brown P. 2007. Antibiotic resistance and pyelonephritis. Clin. Infect. Dis. 45:281–283 [DOI] [PubMed] [Google Scholar]
- 14.Gordon KA, Jones RN, SENTRY Participant Groups (Europe, Latin America, North America) 2003. Susceptibility patterns of orally administered antimicrobials among urinary tract infection pathogens from hospitalized patients in North America: comparison report to Europe and Latin America. Results from the SENTRY Antimicrobial Surveillance Program (2000). Diagn. Microbiol. Infect. Dis. 45:295–301 [DOI] [PubMed] [Google Scholar]
- 15.Schito GC, Naber KG, Botto H, Palou J, Mazzei T, Gualco L, Marchese A. 2009. The ARESC study: an international survey on the antimicrobial resistance of pathogens involved in uncomplicated urinary tract infections. Int. J. Antimicrob. Agents 34:407–413 [DOI] [PubMed] [Google Scholar]
- 16.Zhanel GG, Hisanaga TL, Laing NM, DeCorby MR, Nichol KA, Weshnoweski B, Johnson J, Noreddin A, Low DE, Karlowsky JA, NAUTICA Group. Hoban DJ. 2006. Antibiotic resistance in Escherichia coli outpatient urinary isolates: final results from the North American Urinary Tract Infection Collaborative Alliance (NAUTICA). Int. J. Antimicrob. Agents 27:468–475 [DOI] [PubMed] [Google Scholar]
- 17.Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, Fridkin SK, National Healthcare Safety Network Team, Participating National Healthcare Safety Network Facilities 2008. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006-2007. Infect. Control Hosp. Epidemiol. 29:996–1011 [DOI] [PubMed] [Google Scholar]
- 18.Brumbaugh AR, Mobley HL. 2012. Preventing urinary tract infection: progress toward an effective Escherichia coli vaccine. Expert Rev. Vaccines 11:663–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russo TA, Johnson JR. 2006. Extraintestinal isolates of Escherichia coli: identification and prospects for vaccine development. Expert Rev. Vaccines 5:45–54 [DOI] [PubMed] [Google Scholar]
- 20.Dobrindt U, Hacker J. 2008. Targeting virulence traits: potential strategies to combat extraintestinal pathogenic E. coli infections. Curr. Opin. Microbiol. 11:409–413 [DOI] [PubMed] [Google Scholar]
- 21.Foxman B. 2010. The epidemiology of urinary tract infection. Nat. Rev. Urol. 7:653–660 [DOI] [PubMed] [Google Scholar]
- 22.Nicolle LE, AMMI Canadian Guidelines Committee 2005. Complicated urinary tract infection in adults. Can. J. Infect. Dis. Med. Microbiol. 16:349–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson JR. 1991. Virulence factors in Escherichia coli urinary tract infection. Clin. Microbiol. Rev. 4:80–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson JR, Kuskowski MA, Gajewski A, Soto S, Horcajada JP, Jimenez de Anta MT, Vila J. 2005. Extended virulence genotypes and phylogenetic background of Escherichia coli isolates from patients with cystitis, pyelonephritis, or prostatitis. J. Infect. Dis. 191:46–50 [DOI] [PubMed] [Google Scholar]
- 25.Giefing C, Meinke AL, Hanner M, Henics T, Bui MD, Gelbmann D, Lundberg U, Senn BM, Schunn M, Habel A, Henriques-Normark B, Ortqvist A, Kalin M, von Gabain A, Nagy E. 2008. Discovery of a novel class of highly conserved vaccine antigens using genomic scale antigenic fingerprinting of pneumococcus with human antibodies. J. Exp. Med. 205:117–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee SJ, Liang L, Juarez S, Nanton MR, Gondwe EN, Msefula CL, Kayala MA, Necchi F, Heath JN, Hart P, Tsolis RM, Heyderman RS, MacLennan CA, Felgner PL, Davies DH, McSorley SJ. 2012. Identification of a common immune signature in murine and human systemic Salmonellosis. Proc. Natl. Acad. Sci. U. S. A. 109:4998–5003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pizza M, Scarlato V, Masignani V, Giuliani MM, Arico B, Comanducci M, Jennings GT, Baldi L, Bartolini E, Capecchi B, Galeotti CL, Luzzi E, Manetti R, Marchetti E, Mora M, Nuti S, Ratti G, Santini L, Savino S, Scarselli M, Storni E, Zuo P, Broeker M, Hundt E, Knapp B, Blair E, Mason T, Tettelin H, Hood DW, Jeffries AC, Saunders NJ, Granoff DM, Venter JC, Moxon ER, Grandi G, Rappuoli R. 2000. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science 287:1816–1820 [DOI] [PubMed] [Google Scholar]
- 28.Lloyd AL, Rasko DA, Mobley HL. 2007. Defining genomic islands and uropathogen-specific genes in uropathogenic Escherichia coli. J. Bacteriol. 189:3532–3546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen SL, Hung CS, Xu J, Reigstad CS, Magrini V, Sabo A, Blasiar D, Bieri T, Meyer RR, Ozersky P, Armstrong JR, Fulton RS, Latreille JP, Spieth J, Hooton TM, Mardis ER, Hultgren SJ, Gordon JI. 2006. Identification of genes subject to positive selection in uropathogenic strains of Escherichia coli: a comparative genomics approach. Proc. Natl. Acad. Sci. U. S. A. 103:5977–5982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Snyder JA, Haugen BJ, Buckles EL, Lockatell CV, Johnson DE, Donnenberg MS, Welch RA, Mobley HL. 2004. Transcriptome of uropathogenic Escherichia coli during urinary tract infection. Infect. Immun. 72:6373–6381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bahrani-Mougeot FK, Buckles EL, Lockatell CV, Hebel JR, Johnson DE, Tang CM, Donnenberg MS. 2002. Type 1 fimbriae and extracellular polysaccharides are preeminent uropathogenic Escherichia coli virulence determinants in the murine urinary tract. Mol. Microbiol. 45:1079–1093 [DOI] [PubMed] [Google Scholar]
- 32.Hagan EC, Mobley HL. 2007. Uropathogenic Escherichia coli outer membrane antigens expressed during urinary tract infection. Infect. Immun. 75:3941–3949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walters MS, Mobley HL. 2009. Identification of uropathogenic Escherichia coli surface proteins by shotgun proteomics. J. Microbiol. Methods 78:131–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alteri CJ, Mobley HL. 2007. Quantitative profile of the uropathogenic Escherichia coli outer membrane proteome during growth in human urine. Infect. Immun. 75:2679–2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vigil PD, Alteri CJ, Mobley HL. 2011. Identification of in vivo-induced antigens including an RTX family exoprotein required for uropathogenic Escherichia coli virulence. Infect. Immun. 79:2335–2344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henderson JP, Crowley JR, Pinkner JS, Walker JN, Tsukayama P, Stamm WE, Hooton TM, Hultgren SJ. 2009. Quantitative metabolomics reveals an epigenetic blueprint for iron acquisition in uropathogenic Escherichia coli. PLoS Pathog. 5:e1000305. 10.1371/journal.ppat.1000305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sivick KE, Mobley HL. 2009. An “omics” approach to uropathogenic Escherichia coli vaccinology. Trends Microbiol. 17:431–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alteri CJ, Hagan EC, Sivick KE, Smith SN, Mobley HL. 2009. Mucosal immunization with iron receptor antigens protects against urinary tract infection. PLoS Pathog. 5:e1000586. 10.1371/journal.ppat.1000586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hacker J, Knapp S, Goebel W. 1983. Spontaneous deletions and flanking regions of the chromosomally inherited hemolysin determinant of an Escherichia coli O6 strain. J. Bacteriol. 154:1145–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mobley HL, Green DM, Trifillis AL, Johnson DE, Chippendale GR, Lockatell CV, Jones BD, Warren JW. 1990. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect. Immun. 58:1281–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson DE, Lockatell CV, Hall-Craigs M, Mobley HL, Warren JW. 1987. Uropathogenicity in rats and mice of Providencia stuartii from long-term catheterized patients. J. Urol. 138:632–635 [DOI] [PubMed] [Google Scholar]
- 42.R Development Core Team 2011. R: a language and environment for statistical computing, 2.14.1 R Foundation for Statistical Computing, Vienna, Austria [Google Scholar]
- 43.Plotkin SA. 2010. Correlates of protection induced by vaccination. Clin. Vaccine Immunol. 17:1055–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elgueta R, de Vries VC, Noelle RJ. 2010. The immortality of humoral immunity. Immunol. Rev. 236:139–150 [DOI] [PubMed] [Google Scholar]
- 45.Durant L, Metais A, Soulama-Mouze C, Genevard JM, Nassif X, Escaich S. 2007. Identification of candidates for a subunit vaccine against extraintestinal pathogenic Escherichia coli. Infect. Immun. 75:1916–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Russo TA, McFadden CD, Carlino-MacDonald UB, Beanan JM, Olson R, Wilding GE. 2003. The siderophore receptor IroN of extraintestinal pathogenic Escherichia coli is a potential vaccine candidate. Infect. Immun. 71:7164–7169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moriel DG, Bertoldi I, Spagnuolo A, Marchi S, Rosini R, Nesta B, Pastorello I, Corea VA, Torricelli G, Cartocci E, Savino S, Scarselli M, Dobrindt U, Hacker J, Tettelin H, Tallon LJ, Sullivan S, Wieler LH, Ewers C, Pickard D, Dougan G, Fontana MR, Rappuoli R, Pizza M, Serino L. 2010. Identification of protective and broadly conserved vaccine antigens from the genome of extraintestinal pathogenic Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 107:9072–9077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hagan EC, Lloyd AL, Rasko DA, Faerber GJ, Mobley HL. 2010. Escherichia coli global gene expression in urine from women with urinary tract infection. PLoS Pathog. 6:e1001187. 10.1371/journal.ppat.1001187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johnson JR, Johnston B, Kuskowski MA, Nougayrede JP, Oswald E. 2008. Molecular epidemiology and phylogenetic distribution of the Escherichia coli pks genomic island. J. Clin. Microbiol. 46:3906–3911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schubert S, Picard B, Gouriou S, Heesemann J, Denamur E. 2002. Yersinia high-pathogenicity island contributes to virulence in Escherichia coli causing extraintestinal infections. Infect. Immun. 70:5335–5337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garcia EC, Brumbaugh AR, Mobley HL. 2011. Redundancy and specificity of Escherichia coli iron acquisition systems during urinary tract infection. Infect. Immun. 79:1225–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wieser A, Romann E, Magistro G, Hoffmann C, Norenberg D, Weinert K, Schubert S. 2010. A multiepitope subunit vaccine conveys protection against extraintestinal pathogenic Escherichia coli in mice. Infect. Immun. 78:3432–3442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fetherston JD, Kirillina O, Bobrov AG, Paulley JT, Perry RD. 2010. The yersiniabactin transport system is critical for the pathogenesis of bubonic and pneumonic plague. Infect. Immun. 78:2045–2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lawlor MS, O'Connor C, Miller VL. 2007. Yersiniabactin is a virulence factor for Klebsiella pneumoniae during pulmonary infection. Infect. Immun. 75:1463–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spurbeck RR, Dinh PC, Jr, Walk ST, Stapleton AE, Hooton TM, Nolan LK, Kim KS, Johnson JR, Mobley HL. 2012. Escherichia coli isolates that carry vat, fyuA, chuA, and yfcV efficiently colonize the urinary tract. Infect. Immun. 80:4115–4122 [DOI] [PMC free article] [PubMed] [Google Scholar]