Abstract
Francisella tularensis is a highly infectious intracellular bacterium that causes the zoonotic infection tularemia. While much literature exists on the host response to F. tularensis infection, the vast majority of work has been conducted using attenuated strains of Francisella that do not cause disease in humans. However, emerging data indicate that the protective immune response against attenuated F. tularensis versus F. tularensis type A differs. Several groups have recently reported that interleukin-17 (IL-17) confers protection against the live vaccine strain (LVS) of Francisella. While we too have found that IL-17Rα−/− mice are more susceptible to F. tularensis LVS infection, our studies, using a virulent type A strain of F. tularensis (SchuS4), indicate that IL-17Rα−/− mice display organ burdens and pulmonary gamma interferon (IFN-γ) responses similar to those of wild-type mice following infection. In addition, oral LVS vaccination conferred equivalent protection against pulmonary challenge with SchuS4 in both IL-17Rα−/− and wild-type mice. While IFN-γ was found to be critically important for survival in a convalescent model of SchuS4 infection, IL-17 neutralization from either wild-type or IFN-γ−/− mice had no effect on morbidity or mortality in this model. IL-17 protein levels were also higher in the lungs of mice infected with the LVS rather than F. tularensis type A, while IL-23p19 mRNA expression was found to be caspase-1 dependent in macrophages infected with LVS but not SchuS4. Collectively, these results demonstrate that IL-17 is dispensable for host immunity to type A F. tularensis infection, and that induced and protective immunity differs between attenuated and virulent strains of F. tularensis.
INTRODUCTION
Francisella tularensis is a highly infectious, Gram-negative facultative intracellular bacterium that causes the zoonotic infection tularemia. The type and severity of tularemia depends on the strain, dose, and route of infection (1). F. tularensis subspecies tularensis (type A) and holarctica (type B) cause the majority of human cases, with subspecies tularensis being more virulent (1). Inhalation of F. tularensis results in respiratory or pneumonic tularemia and can present anything from a mild pneumonia to an acute infection with high fever, malaise, chills, cough, delirium, and pulse-temperature dissociation (1). Untreated respiratory forms of tularemia due to type A strains have mortality rates of >30% (2), while antibiotic treatment can decrease this number to approximately 2% (3). The high infectivity (10 to 50 microorganisms) (4) and mortality of F. tularensis infections have led to the weaponization of the organism, including the introduction of antibiotic resistance, by several nations (3). In addition, no vaccines are currently licensed to prevent tularemia. Due to these concerns, F. tularensis has been determined to be a Category A Bioterrorism agent by the CDC.
Although a live vaccine strain (LVS) derived from F. tularensis subspecies holarctica was created over 50 years ago, questions remain regarding its efficacy and possible reversion to virulence, and it is not licensed for human use (1). LVS is attenuated in humans but retains dose- and route-dependent virulence for mice (5), although it is not as virulent as wild-type A and B strains. As LVS causes disease in mice, it has been studied extensively as a model intracellular pathogen (6). However, emerging evidence suggests that in vitro and in vivo immune responses differ between type A Francisella and the LVS (7–10). In addition, immunotherapeutic strategies that confer potent protection against pulmonary LVS infection only confer partial or negligible protection against pulmonary infection with type A Francisella (6, 11, 12). Due to the differences in induced and protective immune responses between attenuated and virulent F. tularensis subspecies, it is important to study the immune response to virulent strains of F. tularensis that cause disease in humans in order to determine truly protective correlates of immunity with relevance to human disease.
Interleukin-17 (IL-17) is a proinflammatory cytokine that can confer protection against a variety of extracellular and intracellular bacterial pathogens (13–15). While no literature exists on the role of IL-17 during infection with virulent F. tularensis, several earlier studies with F. tularensis LVS indicated a role in protection. One study showed that IL-12p35-deficient mice, as well as wild-type mice, are capable of resolving infection; however, IL-12p40-deficient mice are much more susceptible to disease, suggesting that IL-23 (a heterodimer of IL-23p19 and IL-12p40), which can induce IL-17 production, is important in resolving F. tularensis infections (39). It was also shown that intranasal LVS infection leads to increased IL-17 expression in the bronchoalveolar lavage fluid of infected mice (16), and the infection of human peripheral blood mononuclear cells (PBMCs) with LVS in vitro also induces IL-17 responses (17). In addition, the infection of human PBMCs in vitro with either Francisella novicida or type A F. tularensis SchuS4 was shown to induce IL-23p19 mRNA (18). Taken together, these results suggested a protective role for IL-17 during F. tularensis infections. Indeed, during the course of our studies, several groups demonstrated that IL-17 was important for protection in C57BL/6 mice during pulmonary infection with F. tularensis LVS; however, no investigation was made on the role of IL-17 during infection with type A F. tularensis (19–21). Here, we show that IL-17 receptor-deficient mice are impaired in their ability to control F. tularensis LVS infection; however, IL-17 was found to be dispensable for immunity to type A F. tularensis SchuS4.
MATERIALS AND METHODS
Bacterial strains, culture conditions, and mice.
F. tularensis SchuS4 or LVS was cultured in modified Mueller-Hinton (MMH) broth (0.025% ferric pyrophosphate, 2% IsoVitaleX, and 0.1% glucose) at 37°C with constant shaking overnight, aliquoted into 1-ml samples, frozen at −80°C, and thawed just before use as previously described (22). Titers of frozen stocks were determined by enumerating viable bacteria from serial dilutions plated on MMH agar (0.025% ferric pyrophosphate, 2% IsoVitaleX, 0.1% glucose, and 0.025% fetal bovine serum). The number of viable bacteria in frozen stock vials varied by less than 5% over a 12-month period. These stocks were used to generate cultures for F. tularensis SchuS4 or LVS infection studies. All experiments with F. tularensis SchuS4 were performed in biosafety level 3 (BSL-3) facilities at Montana State University or the University of Missouri.
Mice.
Six-week-old female C57BL/6 or BALB/c mice were purchased from Charles River Laboratories. A breeding colony of gamma interferon−/− (IFN-γ−/−) mice (The Jackson Laboratory, Bar Harbor, ME) on a BALB/c background was maintained at Montana State University. IL-17Rα−/− mice on a C57BL/6 background were a gift from Amgen (Seattle, WA). All mice were housed in sterile microisolator cages in the laboratory animal resources facility at Montana State University or the University of Missouri and were provided with sterile water and food ad libitum. All animal care and experimental procedures were in accordance with institutional and federal policies for animal health and well-being.
Mouse infection.
For intratracheal (i.t.) infections, mice were anesthetized with isoflurane gas, and a 22-gauge blunted needle was passed through the oral pharynx into the trachea. A 50-μl volume of phosphate-buffered saline (PBS) containing ∼5,000 CFU of F. tularensis LVS or ∼10 to 150 CFU of SchuS4 then was injected into the lung. For oral vaccination with F. tularensis LVS, mice were orally gavaged with 1 × 108 CFU of F. tularensis LVS in 200 μl PBS (after neutralizing stomach acidity by oral gavage with 200 μl of a 50% saturated sodium bicarbonate solution) (23). Francisella inocula were prepared by the dilution of frozen stock of known titer into PBS. In some experiments, mice were treated intraperitoneally (i.p.) on days 1 to 9 postinfection with gentamicin (10 mg/kg of body weight; Sigma-Aldrich, St. Louis, MO) in order to extend the time to mortality (24). For survival experiments, mice were monitored for morbidity and mortality twice daily for up to 28 days, at which time survivors were euthanized. In some experiments, IL-17 was depleted in vivo via the intraperitoneal (i.p.) administration of 0.25 mg of anti-IL-17A monoclonal antibody (MAb) (clone 17F3; BioXCell, Lebanon, NH) on days −0, 1, and 7 in relation to infection, while control mice received rat IgG, as we described previously (15). We have found this regimen of IL-17 depletion to enhance the susceptibility of wild-type mice to infection with F. tularensis LVS (unpublished observation).
ELISA and CFU determination.
In some experiments, mice were sacrificed at various times postinfection for the detection of CFU or cytokines in lung and/or spleen homogenates. For CFU determination, mouse organs were homogenized in sterile PBS, and homogenates were serially diluted and plated on MMH plates. The plates then were incubated at 37°C for 48 h, at which time CFU were enumerated. For enzyme-linked immunosorbent assay (ELISA), mouse lungs were homogenized, centrifuged for 10 min at 10,000 × g, and then sterilized through a 0.1-μm filter. IL-17A concentrations in these lung filtrates were determined using the Ready-Set-Go IL-17A ELISA kit from eBioscience (San Diego, CA) according to the manufacturer's instructions.
Isolation and infection of murine peritoneal macrophages.
Peritoneal macrophages were isolated as previously described (25). Briefly, mice were given a single intraperitoneal injection of 1.0 ml of expired thioglycolate medium (Difco, Detroit, MI), and 3 days later, the peritoneum of each mouse was washed with RPMI 1640 (Gibco BRL-Life Technologies [Life Technologies], Grand Island, NY) containing 2% fetal calf serum (Life Technologies) without antibiotics. Peritoneal cells were washed twice in the same medium without antibiotics and allowed to adhere overnight to 24-well microtiter plates. Macrophages (1 ×106/ml) were infected with F. tularensis LVS or SchuS4 (30 bacteria:1 macrophage) for 2 h at 37°C and 5% CO2. Macrophages were then washed with PBS and fresh RPMI 1640 complete medium (CM) containing 10% fetal bovine serum, and 50 μg/ml gentamicin (Sigma) was added and cells incubated for 30 min at 37°C and 5% CO2 to kill extracellular bacteria. Cells were then washed twice with PBS, and then fresh CM without antibiotics was added to the wells for the remainder of the experiment (this is considered the 0-h time point). For time points of >8 h, gentamicin was added to the wells for the last 45 min of incubation (26). To enumerate intracellular bacteria, cells were washed three times with PBS and then lysed with sterile 1% saponin in PBS (27). Serial logarithmic dilutions of macrophage lysates were then performed and plated in triplicate onto MMH agar for incubation at 37°C and 5% CO2 for 2 to 3 days. In some cases, macrophages were treated overnight prior to infection with 50 μM caspase-1 inhibitor YVAD-cmk (Cayman Chemical, Ann Arbor, MI) or dimethylsulfoxide (DMSO) as a vehicle control (28). YVAD-cmk or DMSO was maintained in the wells throughout the experiments, other than during washes.
Extraction of RNA and qRT-PCR analysis of F. tularensis-infected murine macrophages and lungs.
F. tularensis-infected murine macrophages or lungs were homogenized and lysed in Tri reagent (Qiagen, Valencia, CA). RNA was then isolated according to the manufacturer's instructions prior to being further purified via extraction with an RNeasy Mini kit (Qiagen). cDNA was generated using the Superscript III first-strand synthesis system (Life Technologies, Grand Island, NY). Primers for immune-related genes (IFN-γ, IL-17, IL-1β, IL-23p19, IL-12p40, and IL-18), along with β-actin (endogenous control), were designed using the PrimerQuest application from Integrated DNA Technologies. Relative specific mRNA was quantified by measuring SYBR green incorporation during real-time quantitative reverse transcription-PCR (qRT-PCR) (performed in triplicate for each sample) using the comparative threshold method (29).
Statistical analysis.
Statistical differences between two groups were determined using Student's t test with the significance set at P < 0.05. For comparison between three or more groups, analysis was done by one-way analysis of variance (ANOVA) followed by Tukey's multiple-comparison test with significance determined at P < 0.05. For in vivo studies, significance in survival was assessed using log-rank analysis with significance set at P < 0.05.
RESULTS
IL-17 receptor-deficient mice are impaired in innate immunity to F. tularensis LVS but not F. tularensis SchuS4.
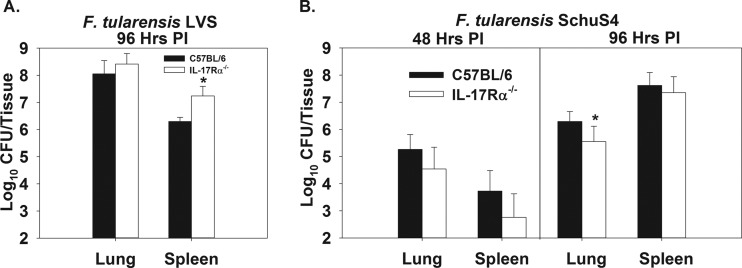
To investigate innate immunity to Francisella, C57BL/6 or IL-17Rα−/− mice (on a C57BL/6 background) were infected i.t. with 5,000 CFU of F. tularensis LVS or 50 CFU of F. tularensis SchuS4. These doses of Francisella were chosen because we have found them to induce 100% lethality. We found that at 96 h postinfection, IL-17Rα−/− mice were significantly more susceptible to colonization of the spleen by F. tularensis LVS than were wild-type mice (Fig. 1A). In addition, we found that at time points >144 h postinfection, IL-17Rα−/− mice had higher splenic and pulmonary levels of LVS than did C57BL/6 mice, and they succumbed to infection more quickly (data not shown). However, IL-17Rα−/− mice infected with SchuS4 were unimpaired in their ability to control bacterial colonization of the lung and spleen at 48 and 96 h postinfection in relation to C57BL/6 mice. IL-17Rα−/− mice actually displayed a slight but statistically significant reduction in pulmonary colonization by SchuS4 compared to wild-type animals; however, this was only observed at 96 h postinfection (Fig. 1B).
Fig 1.
IL-17Rα−/− mice are impaired in primary immunity to F. tularensis LVS but not virulent F. tularensis SchuS4. C57BL/6 or IL-17Rα−/− mice on a C57BL/6 background (IL-17Rα−/−) were infected intratracheally (i.t.) with 5,000 CFU of F. tularensis LVS (A) or 50 CFU of F. tularensis SchuS4 (B). At 48 or 96 h postinfection, lung and splenic bacterial burdens were determined. Error bars represent standard deviations (SD). *, P < 0.05 compared to C57BL/6 mice infected with the same strain of Francisella at the same time point. Data shown in panel A and at 48 h in panel B were calculated by averaging the values from individual mice pooled from 2 independent experiments (10 mice/group). Data shown for 96 h in panel B was calculated by averaging the values from individual mice pooled from 5 independent experiments (20 mice/group).
IL-17Rα−/− mice display an unimpaired IFN-γ response during pulmonary infection with F. tularensis SchuS4.
Work by others showed that IL-17 was required for the induction of an IFN-γ response in the lungs of mice infected with F. tularensis LVS (21), whereas in a parasite infection model, IL-17Rα−/− mice were found to have an exaggerated IFN-γ response (30). Therefore, to investigate the effect of impaired IL-17 signaling on the induction of IFN-γ during infection with virulent F. tularensis, wild-type C57BL/6 or IL-17Rα−/− mice were infected i.t. with 50 CFU of F. tularensis SchuS4. At 48 and 96 h after infection, RNA was extracted from the lungs of infected animals, and qRT-PCR was performed to assess the induction of IL-17 and IFN-γ mRNA relative to that of mock-infected animals. We found that IL-17Rα−/− mice displayed a similar level of IFN-γ and IL-17 mRNA induction at both time points in relation to C57BL/6 mice (Fig. 2). At 96 h postinfection, it was also observed that IFN-γ mRNA was more potently induced than IL-17 in the lungs of both wild-type and IL-17Rα−/− mice (Fig. 2).
Fig 2.
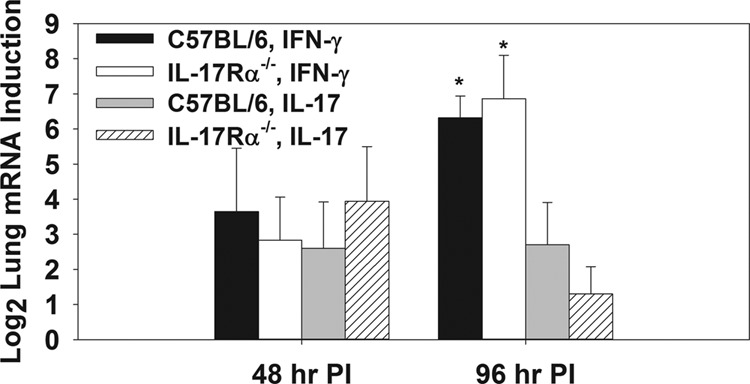
IL-17Rα−/− mice display an unimpaired IFN-γ response during pulmonary infection with F. tularensis SchuS4. C57BL/6 or IL-17Rα−/− mice (n = 4 to 5/group) were infected intratracheally (i.t.) with 50 CFU of F. tularensis SchuS4. At 48 or 96 h postinfection, RNA was extracted from the lungs and qRT-PCR was performed to determine the log2 induction of IFN-γ and IL-17 mRNA in infected mice compared to that of mock-infected mice (normalized to β-actin). Error bars represent SD. *, P < 0.05 compared to IL-17 induction by the same strain of mouse at the same time point.
IFN-γ, but not IL-17, is required for protection against virulent F. tularensis infection in a convalescent model of tularemia.
It has been suggested that during infection with F. tularensis LVS, the role of IL-17 is more important in convalescence (19). In addition, the protective role of IL-17 in bacterial infection models has been proposed to be either dependent on IFN-γ (21) or enhanced in the absence of IFN-γ (15). Therefore, we neutralized IL-17 in both wild-type and IFN-γ−/− mice in a convalescent model of tularemia. BALB/c or IFN-γ−/− mice were depleted of IL-17 via neutralizing MAb (or treated with rat IgG as a control) and treated i.p. with 10 mg/kg gentamicin on days 1 to 9 postinfection with 50 CFU of F. tularensis SchuS4 (24). Morbidity and mortality were recorded over time. IL-17 neutralization had no effect on morbidity in wild-type animals, as mice lost weight at a similar rate starting around day five postinfection (Fig. 3A). Mortality was also unaffected in wild-type mice by IL-17 neutralization, as survival times did not differ (Fig. 3B). We found that while IFN-γ had no effect on weight loss (Fig. 3A), it was critically important for survival in this model (Fig. 3B). Similar to what we observed in wild-type animals, depletion of IL-17 from IFN-γ−/− mice did not affect weight loss or survival (Fig. 3A and B).
Fig 3.
IFN-γ, but not IL-17, is required for protection against virulent F. tularensis infection in a convalescent model of tularemia. BALB/c or IFN-γ−/− mice (BALB/c background) (n = 10/group) were treated i.p. with 0.25 mg of IgG or anti-IL-17 MAb on days −1, 0, and 7 relative to i.t. infection with 50 CFU of F. tularensis SchuS4. Mice were also treated i.p. with 10 mg/kg gentamicin on days 1 to 9. Surviving mice were weighed (A) and monitored for morbidity and mortality (B). Error bars depict standard errors of the means (SEM). *^, P < 0.05 compared to mice of the same genotype treated with IgG.
IL-17Rα is dispensable for oral LVS vaccine-induced immunity against pulmonary challenge with virulent F. tularensis.
To assess the role of IL-17 during a protective, vaccine-induced immune response against virulent F. tularensis, C57BL/6 or IL-17Rα−/− mice were immunized twice orally with 1 × 108 CFU of F. tularensis LVS or with PBS as a control at 2 and 4 weeks prior to i.t. challenge with 10 CFU of F. tularensis SchuS4. We used an oral route of vaccination, as both strains of mice survived oral vaccination with LVS, whereas IL-17Rα−/− mice were found to succumb to a lower level of pulmonary LVS infection than were wild-type mice (data not shown). We found that oral LVS infection was able to provide partial protection against a low-dose pulmonary challenge with F. tularensis SchuS4, as measured by delayed weight loss (Fig. 4A) and delayed mortality/increased survival (Fig. 4B). However, the absence of IL-17Rα−/− did not render LVS-vaccinated animals (or vehicle-treated animals) more susceptible to weight loss and lethality induced by SchuS4 infection (Fig. 4A and B). In fact, LVS-vaccinated IL-17Rα−/− mice lost slightly less weight early after challenge than wild-type animals (Fig. 4A); however, this modest effect was not pursued further.
Fig 4.
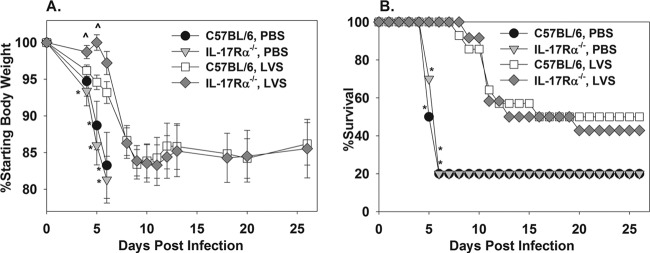
IL-17Rα is dispensable for oral LVS vaccine-induced immunity against pulmonary challenge with virulent F. tularensis. C57BL/6 or IL-17Rα−/− mice (n = 10 to 14/group) were treated orally with PBS or 1 × 108 CFU of F. tularensis LVS on 28 and 14 days before i.t. infection with 10 CFU of F. tularensis SchuS4. Body weights (A) and survival (B) were recorded. Mice that succumbed to infection were assigned a body weight of 75%. Error bars depict SEM. *, P < 0.05 compared to mice of the same genotype that were vaccinated with LVS. ^, P < 0.05 compared to LVS-vaccinated C57BL/6 mice.
LVS-infected mice have higher pulmonary levels of IL-17 than mice infected with SchuS4.
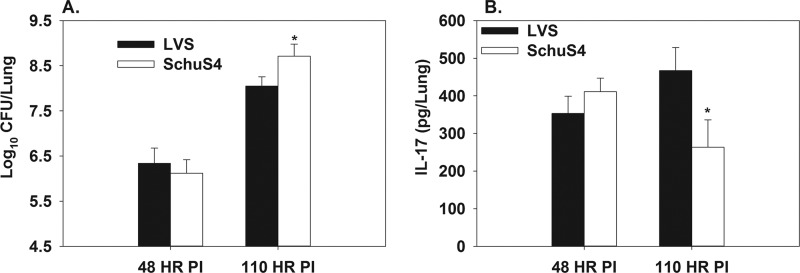
To determine if F. tularensis LVS and SchuS4 differentially induce IL-17, we infected C57BL/6 mice i.t. with 5,000 CFU of LVS or 50 CFU of SchuS4. At 96 h after infection, we found that IL-17 mRNA levels were >3-fold higher in animals infected with LVS than in SchuS4-infected animals. However, using these infectious doses, we found that LVS CFU levels were ∼10-fold higher in the lung than what was observed in SchuS4-infected animals at this time point (data not shown). To achieve similar levels of SchuS4 and LVS in the lung, we used a higher dose of SchuS4 and compared bacterial colonization and IL-17 induction in the lung by infection with LVS (5,000 CFU i.t.) and SchuS4 (150 CFU i.t.) at 48 and 110 h. At 48 h postinfection, mice infected with LVS or SchuS4 displayed similar pulmonary bacterial burdens and IL-17 protein levels (Fig. 5A and B). However, at 110 h after infection, LVS-infected mice had higher pulmonary levels of IL-17 than did SchuS4-infected mice, despite lower levels of LVS in the lung (Fig. 5A and B), indicating that enhanced IL-17 mRNA or protein levels in LVS-infected mice are not dependent on bacterial burden.
Fig 5.
LVS-infected mice have higher pulmonary levels of IL-17 than mice infected with SchuS4 at 110 but not 48 h postinfection. C57BL/6 mice (n = 5 group) were infected i.t. with 5,000 CFU of LVS or 150 CFU of SchuS4. At 48 and 110 h postinfection, lungs were homogenized and bacterial burdens were determined by colony counts (A), and IL-17 levels were determined by ELISA (B). Error bars depict SD. *, P < 0.05 compared to LVS-infected C57BL/6 mice.
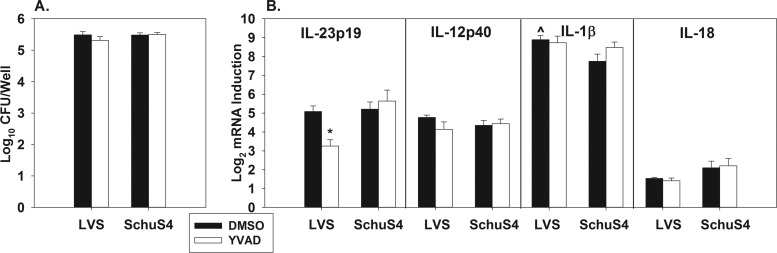
IL-23p19 induction by LVS, but not SchuS4, is caspase-1 dependent in vitro.
To investigate the mechanism by which LVS induces a stronger IL-17 response than SchuS4, we queried the role of caspase-1 in the induction of IL-23p19 mRNA. Caspase-1 is an inflammatory caspase that mediates the release of bioactive IL-1β and IL-18 proteins (28). This is of interest, because IL-1 can induce IL-23 (31) and caspase-1-dependent IL-1 can synergize with IL-23 to enhance IL-17 responses in vivo (32). Therefore, to study the role of caspase-1 in the induction of IL-23p19 mRNA, peritoneal macrophages were infected with LVS or SchuS4 and treated with the caspase-1 inhibitor YVAD-cmk or DMSO as a vehicle control. Twenty h after infection, some wells containing macrophages were lysed and intracellular bacteria enumerated, whereas RNA was extracted from other macrophage wells to determine the relative induction of IL-23p19, IL-23p40, IL-1β, and IL-18 mRNAs via qRT-PCR in relation to mock-infected macrophages. YVAD-cmk was not found to have an effect on the intracellular replication of either Francisella strain (Fig. 6A). Caspase-1 inhibition also did not have an effect on IL-1β or IL-18 mRNA expression (Fig. 6B), which would be expected, as caspase-1 is involved in the processing and release of bioactive IL-1β and IL-18 proteins rather than mRNA expression. Likewise, IL-12p40 mRNA expression was unaffected by YVAD-cmk (Fig. 6B). However, caspase-1 inhibition reduced mRNA expression of IL-23p19 by ∼4-fold in LVS-infected but not SchuS4-infected macrophages (Fig. 6B). In addition, IL-1β mRNA levels were found to be slightly but significantly higher in vehicle-treated macrophages infected with LVS in relation to vehicle-treated, SchuS4-infected macrophages (Fig. 6B).
Fig 6.
IL-23p19 induction by LVS, but not SchuS4, is caspase-1 dependent in vitro. Peritoneal macrophages (6 wells/group) from C57BL/6 mice were infected with F. tularensis LVS or SchuS4 at a multiplicity of infection of ∼30. Macrophages were also treated with the caspase-1 inhibitor YVAD-cmk or DMSO (vehicle control). Twenty h after infection, intracellular bacterial CFU were quantified (A), and mRNA expression of immune-related genes was determined via qRT-PCR (plotted as log2 induction relative to mock-infected macrophages) (B). Error bars depict SEM. *, P < 0.05 compared to DMSO-treated macrophages infected with the same strain of Francisella. ^, P < 0.05 compared to macrophages treated with DMSO and infected with SchuS4. Data are representative of 2 to 3 independent experiments.
DISCUSSION
In the last 10 years there has been a marked increase in Francisella research, in part due to the bioweapon potential of virulent strains of Francisella tularensis (5). While much research has been conducted on immune responses to Francisella, much of the work has been limited to using attenuated strains of Francisella (BSL-2) that do not cause disease in humans. While these BSL-2 stains are easier to work with from a time and cost perspective, emerging evidence indicates that the host response against attenuated and virulent strains differs (7–10), indicating that the study of virulent strains of F. tularensis is essential to determine correlates of protective immunity.
In this study, we investigated the role of IL-17 in tularemia due to findings by others that IL-17 and IL-23 (which can induce IL-17) are induced in vitro and in vivo following infection with attenuated strains of F. tularensis (16–18). Indeed, we did find that IL-17Rα−/− mice were more susceptible to LVS infection; however, during the course of our work, several groups reported similar findings (19–21), but no efforts were made by these groups to study the role of IL-17 during virulent F. tularensis infections. In contrast to our findings with LVS, we found that IL-17Rα was dispensable for protective innate immunity to SchuS4 infection. In fact, IL-17Rα−/− mice displayed a modest but statistically significant reduction in pulmonary SchuS4 colonization 96 h after infection compared to wild-type mice. These results suggest that IL-17 or IL-17F (which also signals through IL-17Rα) actually plays a detrimental role in pulmonary immunity; however, we did not pursue this effect due to the modest difference in colonization. Earlier studies with LVS also showed that lungs from IL-17−/− and IL-17Rα−/− mice displayed a markedly reduced capacity to produce IFN-γ mRNA and/or protein during infection than wild-type mice (21). However, in our studies with SchuS4, we did not find IL-17Rα−/− mice to be impaired in their ability to induce a pulmonary IFN-γ mRNA response. This differential requirement of IL-17 in the induction of an IFN-γ response during infection by the two different strains of Francisella may partially explain why IL-17Rα is required for a protective innate immune response against LVS but not SchuS4.
While we did not find IL-17 to be required for immunity to SchuS4 at 96 h postinfection, other reports on LVS suggested that IL-17 was more important during recovery from sublethal infection (19). However, because there is no sublethal dose of SchuS4 in mice, correlates of immunity required for survival from disease have been difficult to ascertain. To address the role of IL-17 during a longer time course of infection, we used a convalescent model of tularemia in which mice are treated with a suboptimal dose of gentamicin to extend the mean time to mortality following infection with SchuS4 (24). IFN-γ was found to be critically important for survival in this model, similar to what others have shown using other convalescent models of SchuS4 infection (5). However, IL-17 depletion had no effect on morbidity or mortality in wild-type mice, indicating that immune mediators other than IL-17 are required for the induction of a protective IFN-γ response during SchuS4 infection. In a mouse model of experimental brucellosis, we have found that the protective role of IL-17 is amplified in the absence of IFN-γ (15). However, IL-17 depletion had no effect on the morbidity and mortality of IFN-γ−/− mice infected with SchuS4, indicating that the role of IL-17 is not enhanced in these mice or that the function of IL-17 was masked by the critical role of IFN-γ.
While we did not find IL-17 to be a protective innate immune response against SchuS4 infection, in a Helicobacter pylori infection model, IL-17 can be dispensable or detrimental to innate immunity, whereas vaccination can induce a protective IL-17 response (33). In our work here, we show that oral LVS vaccination provides equivalent, partial protection against morbidity and mortality following pulmonary challenge with SchuS4 in both wild-type and IL-17Rα−/− mice. While we cannot exclude the possibility that IL-17 plays a limited role in controlling bacterial colonization in this model, or that other vaccination strategies may induce a protective IL-17 response, it appears that IL-17 is not absolutely required for a protective vaccine-mediated immune response against SchuS4 challenge.
Although IL-17 has been shown to be induced in the lungs of animals infected with LVS (16, 21), studies with SchuS4 have not found a potent induction of pulmonary IL-17 (5). To assess whether a differential induction of IL-17 by LVS versus SchuS4 was responsible for the contrasting role of IL-17 in immunity, we compared IL-17 levels in the lungs of mice infected with LVS or SchuS4. Despite higher bacterial loads of SchuS4, LVS-infected lungs had greater levels of IL-17 than did SchuS4-infected mice at 110 h (but not 48 h) after infection. This more potent induction of IL-17 by LVS, or perhaps suppression of IL-17 by SchuS4, may be related to the differential role of IL-17 in protection against the two strains.
Pulmonary administration of recombinant IL-17 (rIL-17) or rIL-23 can slightly enhance resistance to pulmonary LVS infection (19). However, while our experimental treatments and dosages were not exhaustive, we did not find pulmonary administration of rIL-17 to affect weight loss or colonization of the lung and spleen by SchuS4 (data not shown). IL-17 is also involved in the recruitment of neutrophils (21). Neutrophils have been shown to play a critical role against systemic challenge with LVS (34) and a partial role in controlling bacterial replication in mice challenged via aerosol with LVS (35). However, mice depleted of neutrophils show similar survival characteristics and bacterial burdens following i.n. challenge with SchuS4 (36). Therefore, the differential protection conferred by neutrophils against LVS versus SchuS4 infection may relate to the inability of IL-17 to protect against SchuS4.
One of the differences between LVS and type A F. tularensis infection in vivo is that LVS activates caspase-1, while type A F. tularensis activates caspase-3 (10). Caspase-1 and its upstream mediators have been shown to be required for immunity to attenuated strains of F. tularensis (37, 38). Caspase-1 is also required for the production of bioactive IL-1β and IL-18 proteins. As caspase-1 activation and IL-1β can enhance IL-23 and IL-17 induction in vitro and/or in vivo (28, 31, 32), we sought to determine if differential caspase activation by LVS and SchuS4 could result in different induction of immune responses. We found that while caspase-1 inhibition had no effect on the colonization of macrophages by LVS or SchuS4, IL-23p19 mRNA induction was reduced by caspase-1 inhibition in LVS- but not SchuS4-infected macrophages. Both LVS and SchuS4 also induced IL-12p40 and IL-1β mRNA responses. Therefore, as bioactive IL-1β (which requires caspase-1 processing) and IL-23 can synergize to induce IL-17 responses, it is possible that caspase-1 activation by LVS but not SchuS4 is required for the induction of a protective IL-17 response.
In this study, our results with LVS echo the work of others who showed that IL-17 was protective against attenuated strains of Francisella (19–21). However, we found that IL-17 was dispensable for innate, convalescent, and oral LVS vaccine-mediated protection against infection with virulent type A F. tularensis SchuS4. While studies with LVS have suggested that IL-17 is required for a protective IFN-γ immune response, IL-17 deficiency did not affect the induction of IFN-γ in SchuS4-infected mice. In addition, we found that caspase-1 activation was required for the induction of an IL-23p19 mRNA response in LVS- but not SchuS4-infected macrophages. Collectively, these studies emphasize that induced and protective immune responses differ between attenuated and virulent strains of F. tularensis, and that care must be taken when applying concepts obtained with attenuated strains of Francisella to infection with virulent strains. In addition, differential caspase activation by attenuated versus virulent F. tularensis may result in differential induction of immunity during tularemia and warrants further investigation.
ACKNOWLEDGMENTS
This work was supported by the Rocky Mountain Regional Center for Excellence in Bioterrorism and Emerging Infectious Diseases (NIH U54AI-65357) and NIH COBRE (GM 103500).
IL-17Rα−/− mice were a gift from Amgen.
Footnotes
Published ahead of print 17 June 2013
REFERENCES
- 1.Oyston PC. 2008. Francisella tularensis: unravelling the secrets of an intracellular pathogen. J. Med. Microbiol. 57:921–930 [DOI] [PubMed] [Google Scholar]
- 2.Allen LA, McCaffrey RL. 2007. To activate or not to activate: distinct strategies used by Helicobacter pylori and Francisella tularensis to modulate the NADPH oxidase and survive in human neutrophils. Immunol. Rev. 219:103–117 [DOI] [PubMed] [Google Scholar]
- 3.Dennis DT, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Friedlander AM, Hauer J, Layton M, Lillibridge SR, McDade JE, Osterholm MT, O'Toole T, Parker G, Perl TM, Russell PK, Tonat K. 2001. Tularemia as a biological weapon: medical and public health management. JAMA 285:2763–2773 [DOI] [PubMed] [Google Scholar]
- 4.Kirimanjeswara GS, Olmos S, Bakshi CS, Metzger DW. 2008. Humoral and cell-mediated immunity to the intracellular pathogen Francisella tularensis. Immunol. Rev. 225:244–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crane DD, Scott DP, Bosio CM. 2012. Generation of a convalescent model of virulent Francisella tularensis infection for assessment of host requirements for survival of tularemia. PLoS One 7:e33349. 10.1371/journal.pone.0033349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Troyer RM, Propst KL, Fairman J, Bosio CM, Dow SW. 2009. Mucosal immunotherapy for protection from pneumonic infection with Francisella tularensis. Vaccine 27:4424–4433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kingry LC, Troyer RM, Marlenee NL, Bielefeldt-Ohmann H, Bowen RA, Schenkel AR, Dow SW, Slayden RA. 2011. Genetic identification of unique immunological responses in mice infected with virulent and attenuated Francisella tularensis. Microbes Infect. 13:261–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crane DD, Warner SL, Bosio CM. 2009. A novel role for plasmin-mediated degradation of opsonizing antibody in the evasion of host immunity by virulent, but not attenuated, Francisella tularensis. J. Immunol. 183:4593–4600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bauler TJ, Chase JC, Bosio CM. 2011. IFN-β mediates suppression of IL-12p40 in human dendritic cells following infection with virulent Francisella tularensis. J. Immunol. 187:1844–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wickstrum JR, Bokhari SM, Fischer JL, Pinson DM, Yeh HW, Horvat RT, Parmely MJ. 2009. Francisella tularensis induces extensive caspase-3 activation and apoptotic cell death in the tissues of infected mice. Infect. Immun. 77:4827–4836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rozak DA, Gelhaus HC, Smith M, Zadeh M, Huzella L, Waag D, Adamovicz JJ. 2010. CpG oligodeoxyribonucleotides protect mice from Burkholderia pseudomallei but not Francisella tularensis Schu S4 aerosols. J. Immune Based Ther. Vaccines 8:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole LE, Mann BJ, Shirey KA, Richard K, Yang Y, Gearhart PJ, Chesko KL, Viscardi RM, Vogel SN. 2011. Role of TLR signaling in Francisella tularensis-LPS-induced, antibody-mediated protection against Francisella tularensis challenge. J. Leukoc. Biol. 90:787–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamada S, Umemura M, Shiono T, Tanaka K, Yahagi A, Begum MD, Oshiro K, Okamoto Y, Watanabe H, Kawakami K, Roark C, Born WK, O'Brien R, Ikuta K, Ishikawa H, Nakae S, Iwakura Y, Ohta T, Matsuzaki G. 2008. IL-17A produced by γδ T cells plays a critical role in innate immunity against Listeria monocytogenes infection in the liver. J. Immunol. 181:3456–3463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shibata K, Yamada H, Hara H, Kishihara K, Yoshikai Y. 2007. Resident δ+ γδT cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J. Immunol. 178:4466–4472 [DOI] [PubMed] [Google Scholar]
- 15.Clapp B, Skyberg JA, Yang X, Thornburg T, Walters N, Pascual DW. 2011. Protective live oral brucellosis vaccines stimulate Th1 and Th17 cell responses. Infect. Immun. 79:4165–4174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woolard MD, Hensley LL, Kawula TH, Frelinger JA. 2008. Respiratory Francisella tularensis live vaccine strain infection induces Th17 cells and prostaglandin E2, which inhibits generation of gamma interferon-positive T cells. Infect. Immun. 76:2651–2659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paranavitana C, Pittman P, Velauthapillai M, DaSilva L. 2008. Temporal cytokine profiling of Francisella tularensis-infected human peripheral blood mononuclear cells. J. Microbiol. Immunol. Infect. 41:192–199 [PubMed] [Google Scholar]
- 18.Butchar JP, Rajaram MV, Ganesan LP, Parsa KV, Clay CD, Schlesinger LS, Tridandapani S. 2007. Francisella tularensis induces IL-23 production in human monocytes. J. Immunol. 178:4445–4454 [DOI] [PubMed] [Google Scholar]
- 19.Markel G, Bar-Haim E, Zahavy E, Cohen H, Cohen O, Shafferman A, Velan B. 2010. The involvement of IL-17A in the murine response to sub-lethal inhalational infection with Francisella tularensis. PLoS One 5:e11176. 10.1371/journal.pone.0011176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cowley SC, Meierovics AI, Frelinger JA, Iwakura Y, Elkins KL. 2010. Lung CD4− CD8− double-negative T cells are prominent producers of IL-17A and IFN-γ during primary respiratory murine infection with Francisella tularensis live vaccine strain. J. Immunol. 184:5791–5801 [DOI] [PubMed] [Google Scholar]
- 21.Lin Y, Ritchea S, Logar A, Slight S, Messmer M, Rangel-Moreno J, Guglani L, Alcorn JF, Strawbridge H, Park SM, Onishi R, Nyugen N, Walter MJ, Pociask D, Randall TD, Gaffen SL, Iwakura Y, Kolls JK, Khader SA. 2009. Interleukin-17 is required for T helper 1 cell immunity and host resistance to the intracellular pathogen Francisella tularensis. Immunity 31:799–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bosio CM, Dow SW. 2005. Francisella tularensis induces aberrant activation of pulmonary dendritic cells. J. Immunol. 175:6792–6801 [DOI] [PubMed] [Google Scholar]
- 23.Skyberg JA, Thornburg T, Kochetkova I, Layton W, Callis G, Rollins MF, Riccardi C, Becker T, Golden S, Pascual DW. 2012. IFN-γ-deficient mice develop IL-1-dependent cutaneous and musculoskeletal inflammation during experimental brucellosis. J. Leukoc. Biol. 92:375–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sutherland MD, Goodyear AW, Troyer RM, Chandler JC, Dow SW, Belisle JT. 2012. Post-exposure immunization against Francisella tularensis membrane proteins augments protective efficacy of gentamicin in a mouse model of pneumonic tularemia. Vaccine 30:4977–4982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skyberg JA, Thornburg T, Rollins M, Huarte E, Jutila MA, Pascual DW. 2011. Murine and bovine γδ T cells enhance innate immunity against Brucella abortus infections. PLoS One 6:e21978. 10.1371/journal.pone.0021978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skyberg JA, Rollins MF, Holderness JS, Marlenee NL, Schepetkin IA, Goodyear A, Dow SW, Jutila MA, Pascual DW. 2012. Nasal Acai polysaccharides potentiate innate immunity to protect against pulmonary Francisella tularensis and Burkholderia pseudomallei infections. PLoS Pathog. 8:e1002587. 10.1371/journal.ppat.1002587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chalabaev S, Anderson CA, Onderdonk AB, Kasper DL. 2011. Sensitivity of Francisella tularensis to ultrapure water and deoxycholate: implications for bacterial intracellular growth assay in macrophages. J. Microbiol. Methods 85:230–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lalor SJ, Dungan LS, Sutton CE, Basdeo SA, Fletcher JM, Mills KH. 2011. Caspase-1-processed cytokines IL-1β and IL-18 promote IL-17 production by γδ and CD4 T cells that mediate autoimmunity. J. Immunol. 186:5738–5748 [DOI] [PubMed] [Google Scholar]
- 29.Schmittgen TD, Livak KJ. 2008. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3:1101–1108 [DOI] [PubMed] [Google Scholar]
- 30.Tosello Boari J, Amezcua Vesely MC, Bermejo DA, Ramello MC, Montes CL, Cejas H, Gruppi A, Acosta Rodriguez EV. 2012. IL-17RA signaling reduces inflammation and mortality during Trypanosoma cruzi infection by recruiting suppressive IL-10-producing neutrophils. PLoS Pathog. 8:e1002658. 10.1371/journal.ppat.1002658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris KM, Fasano A, Mann DL. 2008. Cutting edge: IL-1 controls the IL-23 response induced by gliadin, the etiologic agent in celiac disease. J. Immunol. 181:4457–4460 [DOI] [PubMed] [Google Scholar]
- 32.Dungan LS, Mills KH. 2011. Caspase-1-processed IL-1 family cytokines play a vital role in driving innate IL-17. Cytokine 56:126–132 [DOI] [PubMed] [Google Scholar]
- 33.Kabir S. 2011. The role of interleukin-17 in the Helicobacter pylori induced infection and immunity. Helicobacter 16:1–8 [DOI] [PubMed] [Google Scholar]
- 34.Sjostedt A, Conlan JW, North RJ. 1994. Neutrophils are critical for host defense against primary infection with the facultative intracellular bacterium Francisella tularensis in mice and participate in defense against reinfection. Infect. Immun. 62:2779–2783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conlan JW, KuoLee R, Shen H, Webb A. 2002. Different host defences are required to protect mice from primary systemic versus pulmonary infection with the facultative intracellular bacterial pathogen, Francisella tularensis LVS. Microb. Pathog. 32:127–134 [DOI] [PubMed] [Google Scholar]
- 36.KuoLee R, Harris G, Conlan JW, Chen W. 2011. Role of neutrophils and NADPH phagocyte oxidase in host defense against respiratory infection with virulent Francisella tularensis in mice. Microbes Infect. 13:447–456 [DOI] [PubMed] [Google Scholar]
- 37.Rathinam VA, Jiang Z, Waggoner SN, Sharma S, Cole LE, Waggoner L, Vanaja SK, Monks BG, Ganesan S, Latz E, Hornung V, Vogel SN, Szomolanyi-Tsuda E, Fitzgerald KA. 2010. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat. Immunol. 11:395–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mariathasan S, Weiss DS, Dixit VM, Monack DM. 2005. Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J. Exp. Med. 202:1043–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elkins KL, Cooper A, Columbini SM, Cowley SC, Kieffer TL. 2002. In vivo clearance of an intracellular bacterium, Francisella tularensis LVS, is dependent on the p40 subunit of interleukin 12 (IL-12) but not on IL-12 p70. Infect. Immun. 70:1936–1948 [DOI] [PMC free article] [PubMed] [Google Scholar]