Abstract
The transferrin-binding proteins TbpA and TbpB enable Neisseria gonorrhoeae to obtain iron from human transferrin. The lipoprotein TbpB facilitates, but is not strictly required for, TbpA-mediated iron acquisition. The goal of the current study was to determine the contribution of two conserved regions within TbpB to the function of this protein. Using site-directed mutagenesis, the first mutation we constructed replaced the lipobox (LSAC) of TbpB with a signal I peptidase cleavage site (LAAA), while the second mutation deleted a conserved stretch of glycine residues immediately downstream of the lipobox. We then evaluated the resulting mutants for effects on TbpB expression, surface exposure, and transferrin iron utilization. Western blot analysis and palmitate labeling indicated that the lipobox, but not the glycine-rich motif, is required for lipidation of TbpB and tethering to the outer membrane. TbpB was released into the supernatant by the mutant that produces TbpB LSAC. Neither mutation disrupted the transport of TbpB across the bacterial cell envelope. When these mutant TbpB proteins were produced in a strain expressing a form of TbpA that requires TbpB for iron acquisition, growth on transferrin was either abrogated or dramatically diminished. We conclude that surface tethering of TbpB is required for optimal performance of the transferrin iron acquisition system, while the presence of the polyglycine stretch near the amino terminus of TbpB contributes significantly to transferrin iron transport function. Overall, these results provide important insights into the functional roles of two conserved motifs of TbpB, enhancing our understanding of this critical iron uptake system.
INTRODUCTION
Neisseria gonorrhoeae is the etiologic agent of the sexually transmitted infection gonorrhea. According to the CDC, over 300,000 cases of gonorrhea were reported in the United States in 2010 (1). The actual number of cases, however, is anticipated to be even greater due to the high prevalence of asymptomatic infection, particularly in women (2, 3). Symptoms of gonococcal infection typically include cervicitis in women and urethritis and epididymitis in men. When left untreated, gonorrhea may lead to serious downstream sequelae, including pelvic inflammatory disease, ectopic pregnancy, infertility, and disseminated gonococcal infection (4–6). In addition, gonococcal infection has been associated with increased HIV transmission rates (7, 8). Over the years, N. gonorrhoeae has exhibited increasing rates of antibiotic resistance, culminating recently in the identification of clinical isolates that are resistant to all currently recommended antibiotics (9, 10). The serious potential sequelae of gonococcal infection combined with the evolution of drug-resistant strains have increased the urgency to understand the pathogenesis of N. gonorrhoeae in the hopes of identifying new therapeutic or vaccine targets.
N. gonorrhoeae requires iron for growth and reproduction. To meet this need within the iron-limited environment of the human host, the organism expresses a series of membrane-bound iron acquisition systems (for a review, see reference 11). The surface receptor components of these systems bind iron-carrying host proteins, such as hemoglobin (Hb), lactoferrin (Lf), and transferrin (Tf). Among these, the Tf receptor is of particular interest. The Tf iron acquisition system is universally produced by all gonococcal strains and is well conserved (12–14). In addition, in a human experimental infection model, a mutant unable to utilize lactoferrin and to produce the transferrin-binding proteins (TBPs) was unable to establish infection (15). For these reasons, the TBPs represent an important virulence factor and potential vaccine target for N. gonorrhoeae.
The gonococcal Tf receptor is a bipartite system comprised of TbpA, an integral outer membrane protein, and TbpB, a surface-exposed lipoprotein (11, 16–18). After Tf binding by the TBPs, iron is extracted from the Tf molecule and transported into the periplasm via a pore formed by TbpA. While TbpA is absolutely required for uptake of iron from Tf, TbpB exhibits an important role in increasing the efficiency of this process, at least in part, by preferentially binding the holotransferrin molecule and excluding apo-Tf (19, 20). While both TbpA and TbpB are capable of binding Tf, the degree to which the TBPs interact with one another during the process of iron acquisition is only partially understood. We are especially interested in how conserved regions of the TbpB protein affect presentation of the TbpB molecule on the bacterial surface and its interaction with TbpA, thereby influencing iron uptake.
One region of note is the conserved LSAC sequence at the amino terminus of TbpB. This 4-residue motif represents a prototypical lipobox, with the cysteine residue serving as the first amino acid in the mature protein and the predicted site of lipidation (21, 22). The LSAC sequence is the site of action for signal II peptidase. After cleavage by signal II peptidase, the Cys residue becomes the mature amino terminus, which is subsequently modified by the addition of a diacyl glycerol. The amino-terminal Cys residue is further modified with a third acyl group added to the free amino terminus of the peptide. We have previously shown that TbpB is lipidated by incorporation of radiolabeled palmitate (16). While we predict that the amino terminal lipid moiety affixed to TbpB is required for anchoring of the protein to the outer membrane, the contribution of lipidation modification to transport of TbpB across the outer membrane and insertion into the outer leaflet has not been evaluated to date.
A second conserved motif of interest is located two amino acids downstream of the LSAC site. This region consists of four glycine residues in tandem. While this conserved polyglycine stretch has the potential for important structural and functional contributions to TbpB, the role of this motif has not yet been determined. The goal of the current study was to characterize the effects of mutagenesis of the LSAC motif and the polyglycine stretch of TbpB, with respect to protein transport, surface exposure, and utilization of transferrin as an iron source.
MATERIALS AND METHODS
Strains, plasmids, and media.
All strains and plasmids used in this study are described in Tables 1 and 2, respectively. Plasmids were routinely propagated in E. coli TOP10 (Invitrogen). E. coli was cultured on Luria-Bertani medium, and when necessary, the antibiotic ampicillin (100 μg/ml), kanamycin (50 μg/ml), or chloramphenicol (34 μg/ml) was included in the culture medium. Gonococci were cultured on GC medium base (Difco) containing Kellogg's supplement and 12 μM Fe(NO3)3 at 37°C in an atmosphere supplemented with 5% CO2 (23). To induce iron stress and maximize production of the transferrin-binding proteins, gonococci were grown in Chelex-treated defined medium (CDM) as previously described (24). To test for growth on solid-phase media, bacteria were plated on CDM agarose plates supplemented with appropriate iron sources.
Table 1.
Strains used in this study
| Strain | Description | Reference or source |
|---|---|---|
| E. coli TOP10 | F− mcrA Δ(mrr-hsdRMS-mcrBC) ϕ80lacZΔM15 ΔlacX74 recA1 deoR araD139 Δ(ara-leu)7697 galU galK rpsL(StrR) endA1 nupG | Invitrogen |
| N. gonorrhoeae | ||
| FA19 | Wild type | 14 |
| FA6747 | TbpA− (tbpA::mTn3Cm) | 17 |
| FA6905 | TbpB− (ΔtbpB) | 20 |
| FA6815 | TbpA− TbpB− (tbpB::Ω) | 16 |
| MCV515 | TbpA L9HA | 27 |
| MCV516 | TbpA L9HA TbpB− (ΔtbpB) | 27 |
| MCV601 | LbpB− (lbpB::Ω) | 28 |
| MCV839 | TbpB LSAC (lbpB::Ω) | This study |
| MCV840 | TbpA− TbpB LSAC (tbpA::mTn3cat lbpB::Ω) | This study |
| MCV843 | TbpB ΔGly (lbpB::Ω) | This study |
| MCV844 | TbpA− TbpB ΔGly (tbpA::mTn3cat, lbpB::Ω) | This study |
| MCV858 | TbpA L9HA TbpB LSAC (lbpB::Ω) | This study |
| MCV860 | TbpA L9HA TbpB ΔGly (lbpB::Ω) | This study |
Table 2.
Plasmids
| Plasmid | Descriptiona | Reference or source |
|---|---|---|
| pCR2.1 TOPO | Kanr Ampr | Invitrogen |
| pHSS6GCU | Vector containing the gonococcal uptake sequence (Kanr) | 26 |
| pUNCH755 | Plasmid containing the downstream region of tbpA with an mTn3Cm insertion between XbaI sites | 16 |
| pVCU521 | pCR2.1 containing the tbpA L9HA gene fragment | 27 |
| pVCU758 | pVCU521 containing the tbpA::mTn3Cm XbaI fragment from pUNCH755 | This study |
| pVCU828 | TOPO pCR2.1 containing the tbpB gene fragment with the sequence encoding the LSAC mutation | This study |
| pVCU829 | pHSS6GCU containing the EcoRI fragment from pVCU828 | This study |
| pVCU832 | TOPO pCR2.1 containing the ΔGly tbpB gene fragment | This study |
| pVCU833 | pHSS6GCU containing the EcoRI fragment from pVCU832 | This study |
Kanr, kanamycin resistant; Ampr, ampicillin resistant.
Gonococcal transformation.
Piliated gonococci were grown on solid GC medium as described above. Bacteria were suspended in GC broth (GCB) containing Kellogg's supplement and 10 mM MgCl2. The bacteria were incubated with chromosomal or linearized plasmid DNA either in liquid suspension or on the surface of an agar plate. For liquid transformations, bacteria were subsequently plated to GC medium plates containing the appropriate antibiotic. For plate transformations, the initial plating was on GC medium plates containing no antibiotics. Colonies were then passed to plates with appropriate antibiotics. In both cases, transformants were selected and then screened for the desired mutations by PCR.
Site-directed mutagenesis.
Mutagenesis was carried out using the method of gene splicing by overlap extension (25). Two primary PCRs were carried out with high-fidelity Taq polymerase (Invitrogen) using gonococcal chromosomal DNA as the template. The primers for each primary PCR included a gene-specific primer that annealed to the tbpB gene, while the other primer also annealed to tbpB but contained the desired nucleotide substitutions for the mutations (Table 3). The primary PCRs resulted in two PCR products, each including the mutated region of interest contiguous with either the upstream or downstream flanking region. Following gel purification, these PCR products were used as the templates for a secondary PCR. The primers for this secondary PCR annealed to tbpB on either side of the mutagenized nucleotides. The final PCR resulted in a tbpB amplicon containing the mutation of interest (Table 2). The final PCR product was gel purified and cloned into the vector TOPO pCR2.1. Plasmid pVCU828 encoded a portion of the TbpB protein in which the lipobox sequence (LSAC) had been replaced with the sequence LAAA. The tbpB gene fragment from pVCU828 was excised using EcoRI and subcloned into the gonococcal transformation vector, pHSS6GCU (26), to create plasmid pVCU829. Similarly, plasmid pVCU832 encoded a portion of the TbpB protein in which amino acids 3 to 6 of the mature, wild-type protein were deleted. The mutated tbpB gene fragment from pVCU832 was excised using EcoRI and subcloned into pHSS6GCU to create plasmid pVCU833.
Table 3.
Oligonucleotides
| Name | Sequence (5′–3′)a |
|---|---|
| oVCU154 | CGGTTGCAGGCGGTTTGAAAAGCAACTTGG |
| oVCU237 | TCCCTGTTTCGTATCGGTTACAAAATGCCACACAC |
| oVCU287 | CCCGTGTTTTTGGCTAGCGCTGCTCTGGGCGGAGGC |
| oVCU288 | GCCTCCGCCCAGAGCAGCGCTAGCCAAAAACACGGG |
| oVCU370 | GCCGTGTTTTTGTTGAGCGCTTGTCTGAGTTTCGATCTTGATTCTGTCGATACCG |
| oVCU371 | CGGTATCGACAGAATCAAGATCGAAACTCAGACAAGCGCTCAAAAAGCGCGGGC |
| oVCU197 | GGCTTGGAAACGGTCAGCGTCGTCGGCAAA |
| GC750 | TTCGCACTCGGATGCCAATTC |
Mutagenic nucleotides are underlined.
Generation of double mutants.
To generate the double mutant MCV858, strain FA19 was used as the recipient strain for transformation with two genetically unlinked markers (encoding TbpA L9HA and TbpB LSAC). Congression was used to provide a selectable marker for gonococcal transformation as previously described (27). Briefly, linearized plasmid DNA encoding the TbpA loop 9 HA epitope insertion and plasmid DNA encoding the TbpB LSAC mutation were added simultaneously as donor DNAs to competent gonococci. Neither mutation included antibiotic resistance markers for selection of transformants that had undergone homologous recombination. Therefore, chromosomal DNA from MCV601 (lbpB::Ω) (28) was also added to the transformation mixture to provide a marker for selection of transformed bacteria. Colonies that survived after selection on streptomycin plates were subsequently screened by PCR and DNA sequencing to confirm acquisition of the other, unlinked tbp-specific mutations.
To generate the double mutant MCV860 (TbpA L9HA, TbpB ΔGly), MCV843 (TbpB ΔGly) was used as the recipient strain. To provide a selectable marker for incorporation of the TbpA L9HA epitope insertion, a new plasmid was constructed. The previously described plasmids pVCU521 and pUNCH755 (16, 27) were digested with XbaI. The pUNCH755 fragment containing am mTn3(Cm) cassette was then ligated to pVCU521. The resulting plasmid, pVCU758, contained a portion of the tbpA gene, including the loop 9 HA epitope insertion mutation. The plasmid also contained a DNA sequence contiguous with tbpA in the chromosome but harboring a mTn3(Cm) cassette for antibiotic selection. This plasmid was linearized and used for transformation of MCV843, selecting for chloramphenicol resistance. DNA sequencing of amplicons generated by PCR amplification of the tbpA and tbpB genes from the resultant transformants confirmed that the two desired mutations were successfully integrated into the gonococcal chromosome.
Palmitate labeling of lipidated proteins.
To detect lipidation of TbpB, the Click-iT labeling system (Invitrogen) was employed. Bacterial cultures were grown in iron-depleted, Chelex-treated, defined medium as described above. After an initial doubling period, azide-labeled palmitate was added to a final concentration of 50 μM. Bacteria were incubated an additional 4 h at 37°C. Bacteria were harvested by centrifugation, washed twice with sterile phosphate-buffered saline, and resuspended in lysis buffer (50 mM Tris, 0.1% SDS [pH 8.0]). Benzonase nuclease (Invitrogen) was added, and the bacteria were incubated at 4°C for 1 h to ensure lysis. Intact bacteria and cellular debris were pelleted by low-speed centrifugation, and the bacterial lysate was then transferred to a clean tube. Solubilized proteins were coupled to biotin alkyne according to the manufacturer's instructions. Samples were loaded onto 7.5% SDS-PAGE gels and subjected to electrophoresis and Western blot analysis as described below.
TCA precipitation.
Bacteria were grown under iron-limiting conditions as described above. After 4 h of iron stress, the bacteria were pelleted by centrifugation. The supernatant was subjected to ultracentrifugation at 140,000 × g to remove any possible membrane blebs. To the resulting supernatant, 100% (wt/vol) trichloroacetic acid (TCA) was added to the culture supernatant to a final concentration of 10% (vol/vol). The supernatant was incubated on ice for 15 min. Precipitated proteins were collected by centrifugation, and the protein pellet was washed three times with PBS. The resulting pellet was then resuspended in Laemmli solubilizing buffer and subjected to electrophoresis (29) and Western blot analysis as described below.
SDS-PAGE and Western blot analysis.
Protein samples or whole-cell pellets were resuspended in Laemmli solubilizing buffer, and except in the case of the palmitate-azide labeled samples, β-mercaptoethanol was added to 5%. Samples were heated prior to being loaded onto a 7.5% acrylamide gel and subjected to electrophoresis. Following separation, proteins were transferred to nitrocellulose.
For detection of TbpB, membranes were blocked with 5% nonfat milk in low-salt Tris-buffered saline (50 mM Tris, 150 mM NaCl [pH 7.5], 0.05% Tween 20). Blocked membranes were probed with polyclonal rabbit antiserum against TbpB (30). Goat anti-rabbit IgG conjugated to horseradish peroxidase (HRP) or alkaline phosphatase (AP) was used as the secondary antibody. HRP conjugates were detected using Opti-4CN (Bio-Rad); AP conjugates were detected using the nitroblue tetrazolium (NBT) and 5-bromo-4-chloro-3-indolylphosphate (BCIP) development system (Sigma). As we have shown previously (19, 20), TbpB can be detected in gonococcal whole-cell lysates using human transferrin conjugated to HRP (hTf-HRP). Therefore, in some assays, blocked membranes were probed with hTf-HRP (Jackson ImmunoResearch). TbpB was detected using the ECL Plus chemiluminescence detection system (Pierce).
For detection of TbpA, membranes were blocked with 5% bovine serum albumin (BSA) in high-salt Tris-buffered saline (20 mM Tris, 500 mM NaCl, 0.02% NaN3 [pH 7.5], 0.05% Tween 20). Blocked membranes were probed with polyclonal rabbit antiserum against TbpA (17). Goat anti-rabbit IgG conjugated to alkaline phosphatase was used as the secondary antibody, and the membranes were developed using NBT and BCIP (Sigma).
For palmitate-labeling studies, membranes were blocked with 1% BSA in high-salt Tris-buffered saline with Tween 20. The membranes were probed with avidin-HRP (Bio-Rad) and developed using the ECL Plus chemiluminescence detection system (Pierce).
All blots were scanned using an Epson V200 scanner, and images were processed using Adobe Photoshop.
Solid-phase transferrin binding assay.
Bacteria were grown under iron-limiting conditions as described above. Liquid culture was applied to nitrocellulose membranes using a Bio-Rad dot blot apparatus. Loading volume was standardized based on final culture densities. Dried membranes were blocked with 5% nonfat milk in low-salt Tris-buffered saline. Membranes were then probed with hTf-HRP, washed, and visualized using Opti-4CN.
Protease accessibility assay.
Surface-exposure of mutated TbpB proteins was assessed by susceptibility to exogenously added trypsin as described previously (20). Bacteria were grown under iron-limiting conditions as described above. After 3 h of iron stress, cultures were removed in aliquots to sterile polypropylene tubes. Trypsin (1 mg/ml in PBS containing 10 mM Ca2+ and 10 mM Mg2+) was added to a final concentration of 2.5 μg/ml, and the samples were incubated at 37°C with 5% CO2. Aliquots were taken at 0, 15, and 30 min after addition of the protease. Whole bacterial cells were harvested by centrifugation and resuspended in Laemmli solubilizing buffer. Samples were then subjected to electrophoresis and Western blotting to probe for TbpB using hTf-HRP, as described above. Only TbpB binds to hTf following SDS-PAGE and electroblotting; no other gonococcal proteins are detected by this assay, including TbpA. Furthermore, only amino-terminal fragments of TbpB are detected by hTf-HRP binding (13), which is advantageous in the protease accessibility assay (20). Polyclonal antiserum against TbpB detects all proteolytic fragments generated after trypsin digest and is thus not the ideal reagent for this assay.
Tbp cross-linking assay.
Gonococci were grown in a modified iron-depleted, Chelex-treated, defined medium to which was added 0.7 mM l-photoleucine and 0.1 mM l-photomethionine (Thermo Scientific) as cross-linking reagents. After 4 h of growth, cultures were harvested, UV irradiated, and then standardized to culture density. Whole-cell lysates were prepared, and the cross-linked samples were subjected to SDS-PAGE and Western blot analysis as described above. TbpA was detected using polyclonal rabbit serum specific for TbpA (17). TbpB was detected on Western blots using a polyclonal anti-TbpB rabbit serum (30) and hTf-HRP (20). Triplicate sets of the hTf-HRP probed blots were scanned and quantitated using NIH ImageJ (31).
RESULTS
Site-directed mutagenesis.
The gonococcal TbpB protein contains a prototypical lipobox with the sequence LSAC, in which the cysteine is the first amino acid in the mature protein and the predicted site of lipidation. To confirm the role of this motif in lipidation and tethering of the mature TbpB protein to the outer membrane, we used alanine substitution mutagenesis to replace the LSAC motif with the sequence LAAA. The effect of this mutation was to replace the lipobox with a consensus signal I peptidase cleavage site, thereby theoretically preventing lipidation of the mature protein but allowing cleavage and export. This mutant therefore allowed us to evaluate the role of the lipidated mature amino terminus of TbpB in transport of the protein across the cytoplasmic membrane, the periplasm, and the outer membrane.
The tbpB gene also encodes four consecutive glycine residues two amino acids downstream of the lipidation site. To evaluate the role of this conserved region, we created a glycine deletion mutant (TbpB ΔGly) in which these residues were absent from the mature protein. This approach avoided the possibility that replacement with alanine residues, which are similar in size and charge to glycine, would mask any potential defects in TbpB function.
Mutagenesis of the LSAC motif decreases the amount of detectable, cell-associated TbpB.
To discern the effects of mutating the lipobox or the nearby polyglycine motif on TbpB expression, bacteria were grown under iron-depleted conditions. Whole-cell lysates were prepared and subjected to SDS-PAGE followed by electroblotting. Both MCV839 (TbpB LSAC) and the glycine deletion mutant (MCV843) produced full-length TbpB, as demonstrated by Western blot analysis (Fig. 1). While MCV843 produced TbpB at levels similar to those in the wild-type strain, detection of cell-associated TbpB was diminished in MCV839. Since tbpA lies downstream of tbpB in a bicistronic operon, we also tested the mutant strains for effects on production of TbpA. By Western blot analysis, we detected full-length TbpA in both MCV839 and MCV843 at levels comparable to wild type, indicating that neither of these mutations affected TbpA expression (Fig. 1). These results indicate that deletion of the glycine residues did not impact expression levels of either of the TBPs. Furthermore, since TbpA levels were unaffected in MCV839, we hypothesized that the reduced TbpB levels observed in MCV839 were due to changes in retention of the protein in the whole-cell pellet, rather than effects on gene expression.
Fig 1.
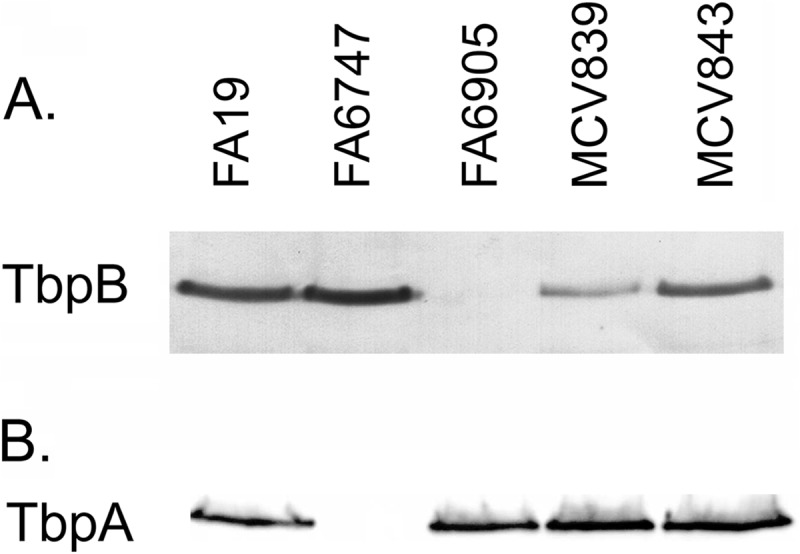
TbpB is produced by mutants expressing TbpB LSAC and TbpB ΔGly. Gonococci were grown under iron-depleted conditions, and whole-cell lysates were standardized for protein content. Proteins were separated by SDS-PAGE and transferred to nitrocellulose. MCV839 (TbpB LSAC) and MCV843 (TbpB ΔGly) were evaluated for expression of transferrin-binding proteins, along with the control strains FA19 (wild type), FA6747 (TbpA−), and FA6905 (TbpB−). (A) Blot probed with anti-TbpB serum; (B) blot probed with anti-TbpA serum.
TbpB is palmitate labeled in MCV843 (TbpB ΔGly) but not in MCV839 (TbpB LSAC).
As the terminal cysteine in the LSAC motif is predicted to be the site of lipidation in TbpB, it was anticipated that replacing the LSAC motif with the sequence LAAA would prevent lipidation. As shown in Fig. 2A, we confirmed this hypothesis utilizing the Click-iT-based technology (Invitrogen). Bacteria were grown under iron-depleted conditions with the addition of azide-labeled palmitate. Biotin alkyne was then conjugated to any proteins containing the azide-labeled palmitate. We then detected palmitoylated proteins using avidin-HRP. By using this approach, TbpB was shown to be lipidated in both the wild-type strain and in MCV843 (TbpB ΔGly; Fig. 2A, arrow). Although full-length TbpB was detected in strain MCV839 by Western blotting (Fig. 2B, arrow), the mutant TbpB protein was not palmitate labeled (Fig. 2A). These observations confirm that the LSAC motif is necessary for lipidation of TbpB and furthermore demonstrate that deleting the neighboring polyglycine motif has no detectable effect on lipidation.
Fig 2.
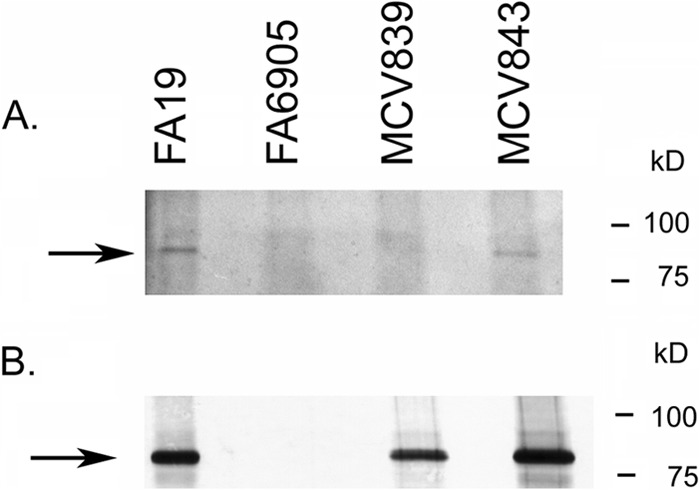
TbpB LSAC produced by MCV839 is not lipidated. (A) Gonococci were grown under iron-depleted conditions in the presence of azide-labeled palmitic acid. Bacteria were lysed, and azide-labeled proteins were conjugated to biotin alkyne (Click-iT labeling system; Invitrogen). Following separation by SDS-PAGE and transfer to nitrocellulose, proteins were probed with avidin-HRP, followed by detection with ECL. MCV839 (TbpB LSAC mutant) and MCV843 (TbpB ΔGly mutant) were tested for lipidation of TbpB, along with the control strains FA19 (wild type) and FA6905 (TbpB−). The arrow marks the location of full-length TbpB. Note that there is a lipidated protein slightly larger than TbpB which is apparent in all lanes. TbpB is produced and lipidated only in FA19 and MCV843. (B) The samples described for panel A were tested for production of TbpB by probing the blot with anti-TbpB serum, followed by goat anti-rabbit IgG secondary antibody conjugated to alkaline phosphatase. Bands were detected with NBT/BCIP. Approximate positions of molecular mass markers are shown on the right.
TbpB is released into the supernatant by MCV839 (TbpB LSAC).
Because our initial analysis of MCV839 (TbpB LSAC) detected significantly reduced levels of cell-associated TbpB compared to those in the wild type (Fig. 1), we speculated that the lack of lipidation resulted in release of TbpB into the culture medium due to its inability to be tethered to the outer membrane. To test this, bacteria were grown in iron-depleted, Chelex-treated, defined medium and cells were pelleted. The supernatant was subjected to ultracentrifugation to eliminate any potential membrane blebs from the culture medium, and then the supernatant was TCA precipitated to recover soluble proteins released from the cell during growth. In the wild-type strain, no TbpB was detectable in the supernatant, and all of the protein remained cell associated in the pellet fraction (Fig. 3A, lanes P). This was in contrast to MCV839 (TbpB LSAC), which released significant amounts of TbpB into the culture supernatant (Fig. 3A, lanes S). As a control, we tested both pellet and supernatant fractions for the presence of TbpA. As shown in Fig. 3B, as expected, no TbpA was detected in the supernatant fractions (S) of either wild-type FA19 or MCV839 (TbpB LSAC). This indicates that mutation of the LSAC motif prevented anchoring of TbpB to the cell surface but did not affect transport of TbpB across the outer membrane.
Fig 3.
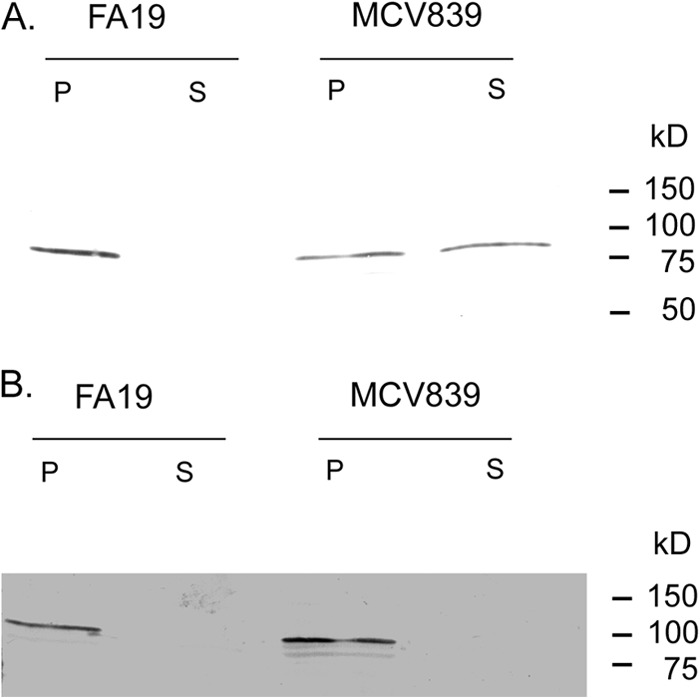
TbpB LSAC produced by MCV839 is released into the culture supernatant. Gonococci were grown under iron-depleted conditions. Whole cells were pelleted and resuspended in Laemmli solubilizing buffer. The supernatant fraction was ultracentrifuged, TCA precipitated, and resuspended in Laemmli solubilizing buffer. The whole-cell (P) and supernatant (S) fractions from gonococcal strains FA19 (wild type) and MCV839 (TbpB LSAC mutant) were separated by SDS-PAGE, transferred to nitrocellulose, and then probed for the presence of TbpB (A) using anti-TbpB serum. As a control, the presence of TbpA was assessed in the same samples by probing with anti-TbpA serum (B).
TbpB surface accessibility in MCV839 (TbpB LSAC) is dependent on TbpA.
While N. gonorrhoeae expresses several lipoproteins, the mechanism by which they are localized to the outer membrane is currently unknown. Based upon the strict conservation of both the LSAC motif and the polyglycine motif, we hypothesized that these elements could be involved in trafficking of TbpB to the cell surface. To evaluate the role of each of these regions in surface exposure of TbpB, we first grew the strains under iron-depleted conditions and analyzed transferrin binding to the cell surface by solid-phase binding assay. Since TbpA also binds transferrin in whole-cell binding assays, we measured transferrin binding to gonococcal TbpB in the background of a tbpA knockout strain. Whole bacteria were applied to nitrocellulose and then probed with hTf-HRP to determine whether mutant TbpB proteins were accessible on the bacterial surface. As shown in Fig. 4A, MCV840 (TbpB LSAC) did not bind transferrin, indicating absence of functional TbpB on the cell surface when TbpA was not expressed. MCV844 (TbpB ΔGly) expressed TbpB on the cell surface (Fig. 4A).
Fig 4.
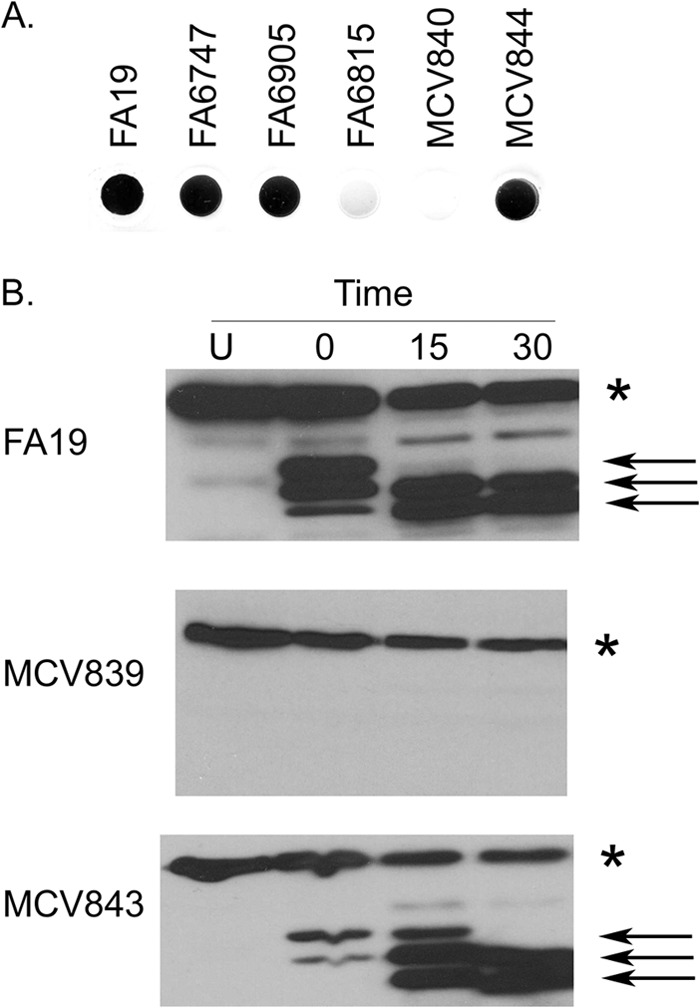
TbpB LSAC is retained on the cell surface only when TbpA is also produced. (A) Bacteria were grown under iron-depleted conditions and spotted onto nitrocellulose. Blots were probed with hTf-HRP. Strains tested included the controls FA19 (wild type), FA6747 (TbpA−), FA6905 (TbpB−), and FA6815 (TbpA− TbpB−). MCV840 produces TbpB LSAC in the background of a strain that does not produce TbpA. MCV844 produces TbpB ΔGly in the background of a strain that does not produce TbpA. (B) Bacteria were grown under iron-depleted conditions prior to exposure to trypsin. Whole cells were exposed to trypsin for 0, 15, or 30 min, after which aprotinin was added to stop digestion. Undigested samples (U) were also included as a control. Whole-cell lysates were generated from trypsin-treated cells and controls. Proteins were separated by SDS-PAGE, transferred to nitrocellulose, and probed with hTf-HRP. Strains tested include MCV839 (produces the TbpB LSAC mutant in the background of a strain that produces TbpA), MCV843 (produces the TbpB ΔGly mutant in the background of a strain that produces TbpA), and the wild-type strain, FA19. The asterisk marks the location of full-length TbpB. TbpB produced by all of the strains is degraded over time of exposure to trypsin, indicating that TbpB is surface exposed in these strains. The arrows mark the positions of lipidated, proteolytically cleaved TbpB fragments, which remain tethered to the cell surface.
We also performed protease accessibility assays utilizing the TbpB mutant strains that also produced a wild-type TbpA (Fig. 4B). Trypsin treatment of the bacterial cell surface resulted in the appearance of smaller, protease-digested fragments that were lipidated and thus remained cell associated in both FA19 (wild type) and MCV843 (TbpB ΔGly) (Fig. 4B, arrows). These results indicate that TbpB was surface exposed in these strains. In contrast, lipidated, proteolytic digestion products that remained cell associated were not detectable when MCV839 (TbpB LSAC) was exposed to trypsin (Fig. 4B). This result is consistent with the conclusion that the TbpB LSAC mutant is not tethered to the outer membrane via the lipid anchor. Interestingly, full-length TbpB was detectable in MCV839 (TbpB LSAC) (Fig. 4B, asterisk), but the amount of TbpB decreased with time of trypsin exposure. This finding indicates that in strain MCV839 (TbpB LSAC), TbpB is surface exposed and therefore accessible to trypsin. This appears to be the case only when TbpA is present, as no TbpB was detected on the cell surface in a tbpA mutant (Fig. 4A). Cumulatively, these data suggest that the TbpB protein produced by MCV839 is not lipidated, is secreted into the supernatant, and furthermore is detectable only on the whole-cell surface when TbpA is also produced.
Impact of TbpB mutagenesis on transferrin iron transporter function.
A previously described gonococcal strain, MCV515, contains an HA epitope tag in outer membrane loop 9 of TbpA (L9HA) (27). While TbpB acts as an accessory protein that enhances the efficiency of transferrin iron uptake when TbpA is wild type, the TbpA L9HA mutant grows on transferrin only when TbpB is also produced. We hypothesize that epitopes of TbpA that are key to iron extraction or iron internalization are disrupted in this mutant, making iron acquisition impossible without a wild-type TbpB. To test whether the mutants expressing TbpB LSAC or TbpB ΔGly were disrupted in TbpB's iron internalization function, we constructed two double mutants expressing TbpA L9HA in addition to either TbpB LSAC (strain MCV858) or TbpB ΔGly (strain MCV860). The addition of the mutation encoding TbpA L9HA resulted in strains that retained the phenotypes described above for both MCV839 and MCV843 (data not shown). To test the ability of TbpB LSAC and TbpB ΔGly proteins to compensate for the TbpA L9HA growth defect, we grew the strains on defined media containing human transferrin as the sole iron source. As transferrin can be variably iron saturated, with an average physiological saturation of 30%, we tested growth across a range of saturation levels, including 5, 10, 25, and 40%. While the parental strain, MCV515, was able to grow using human transferrin as the sole iron source (Fig. 5, region 3 of the plates), regardless of saturation level, growth was completely abrogated in MCV858 (TbpB LSAC, TbpA L9HA), even at 40% saturation (Fig. 5, region 5). This phenotype was identical to that of the tbpB knockout mutant in the TbpA L9HA background (Fig. 5, region 4). While the mutant that produced TbpB ΔGly demonstrated modest transferrin-dependent growth, the growth was significantly reduced (Fig. 5, region 6) compared to that of the parent strain, MCV515 (Fig. 5, region 3). This reduction in growth was most obvious at lower transferrin saturation levels, but was still apparent at 40% saturation. None of the strains tested were impaired for growth on ferric nitrate as the sole iron source (data not shown), indicating that the observed growth defect is specific to utilization of transferrin iron. Cumulatively, these data indicate that lipidation of TbpB is necessary for the function of this protein in transferrin iron utilization. Additionally, the glycine motif is also important for the function of TbpB, although some transferrin iron internalization is accomplished in the absence of this conserved sequence.
Fig 5.
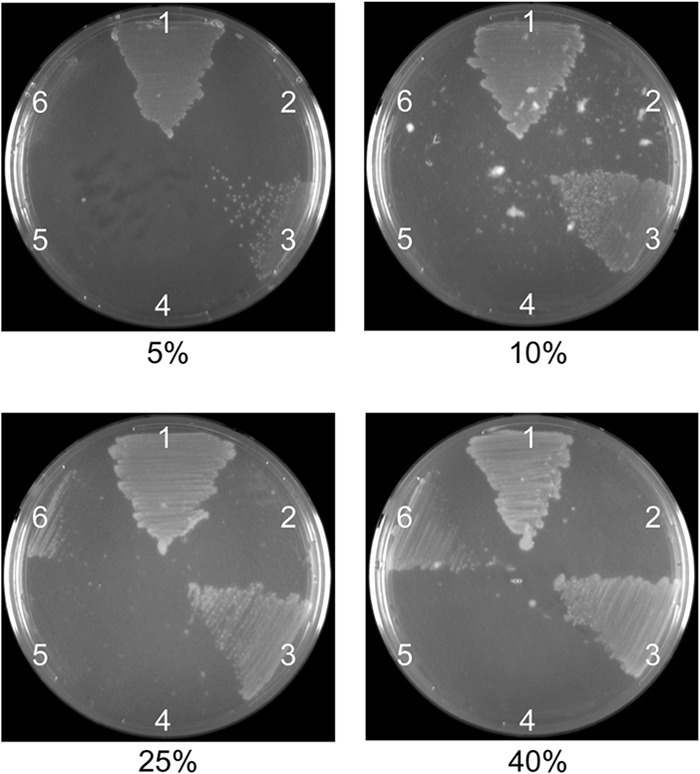
TbpB mutations diminish transferrin iron utilization. Gonococci were plated on CDM agarose plates containing human transferrin at various levels of saturation (5 to 40%, as indicated). Strains tested are labeled as follows: 1, FA19 (wild type); 2, FA6815 (TbpA− TbpB−); 3, MCV515 (TbpA L9HA TbpB+); 4, MCV516 (TbpA L9HA TbpB−); 5, MCV858 (TbpA L9HA TbpB LSAC); 6, MCV860 (TbpA L9HA TbpB ΔGly).
Mutation of the TbpB LSAC and ΔGly motifs does not impair interaction with TbpA.
To determine the effects of the LSAC and ΔGly mutations on physical interaction between TbpA and TbpB, a cross-linking assay was performed. Gonococci were grown with photoactivatable, cross-linking amino acids under iron-depleted conditions. Interacting proteins, including the TBPs, could then be cross-linked by UV exposure. When gonococci were grown with cross-linkers and exposed to UV light, unresolvable, high-molecular-weight protein products were detectable with polyclonal antisera against both TbpA (see Fig. S1A in the supplemental material) and TbpB (see Fig. S1B), indicating cross-linking and formation of very large species comprised of both proteins. A single species of TbpB was detected by ligand-binding blotting, as shown in Fig. 6A. Both full-length and higher-molecular-weight, cross-linked species of TbpB and TbpA were detectable by Western blotting using specific polyclonal antisera, as shown in Fig. 6B and C, respectively. Detection of high-molecular-weight, cross-linked species by Western blotting was apparent, but specific cross-linked species were not resolvable (Fig. 6B and C). As an indication of TbpA-TbpB interaction, we measured loss of full-length TbpB in the hTf-HRP-probed blots (Fig. 6A). In the wild-type strain, FA19, photoactivation resulted in the loss of wild-type-sized TbpB. In the tbpA knockout mutant (FA6747), the wild-type TbpB protein was detectable to the same degree with and without photoactivation, indicating that cross-linking of TbpB was dependent upon the presence of TbpA. We tested whether the mutations in the amino terminus of TbpB affected the interaction with, and therefore cross-linking to, TbpA. As shown in Fig. 6A, both mutant TbpB proteins remained competent for TbpA interaction in this cross-linking assay, suggesting that the defects imposed by the mutations were not due to an inability to interact with TbpA. Interestingly, MCV839 (TbpB LSAC) remained competent for TbpA interaction, despite the inability of the protein to be tethered on the outer membrane without its lipid anchor. These observations are consistent with those of the protease accessibility assay (Fig. 4B), which allowed us to conclude that small amounts of TbpB were accessible to trypsin and therefore surface exposed when TbpA was produced. Cumulatively, these results suggest that TbpB, without a lipid anchor, retains its affinity for TbpA, to which it can be cross-linked. As shown in Fig. 7, we quantitated the loss of full-length TbpB as a function of cross-linking in the wild-type and mutant strains. In this analysis, MCV839 (TbpB LSAC) and MCV843 (TbpB ΔGly) were indistinguishable from the wild-type strain with respect to TbpB cross-linking.
Fig 6.
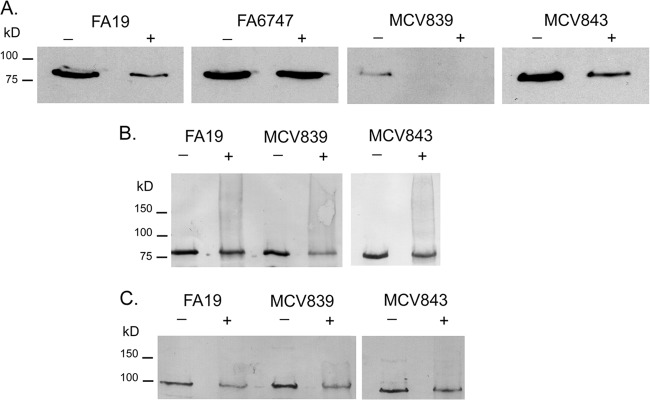
Cross-linking of TbpB and TbpA to form high-molecular-weight complexes depends on production of TbpA. Gonococci were grown under iron-depleted conditions in the presence of photoactivatable cross-linkers. Following UV irradiation (+), whole-cell lysates were prepared. Samples that were not exposed to UV irradiation (−) were included as negative controls. Strains tested include FA19 (wild type), FA6747 (TbpB+ TbpA−), MCV839 (TbpB LSAC TbpA+), and MCV843 (TbpB ΔGly TbpA+). Proteins were separated by SDS-PAGE and transferred to nitrocellulose. Blots were probed with hTf-HRP (A), anti-TbpB serum (B), or anti-TbpA serum (C). Approximate positions of molecular mass markers are indicated on the left.
Fig 7.
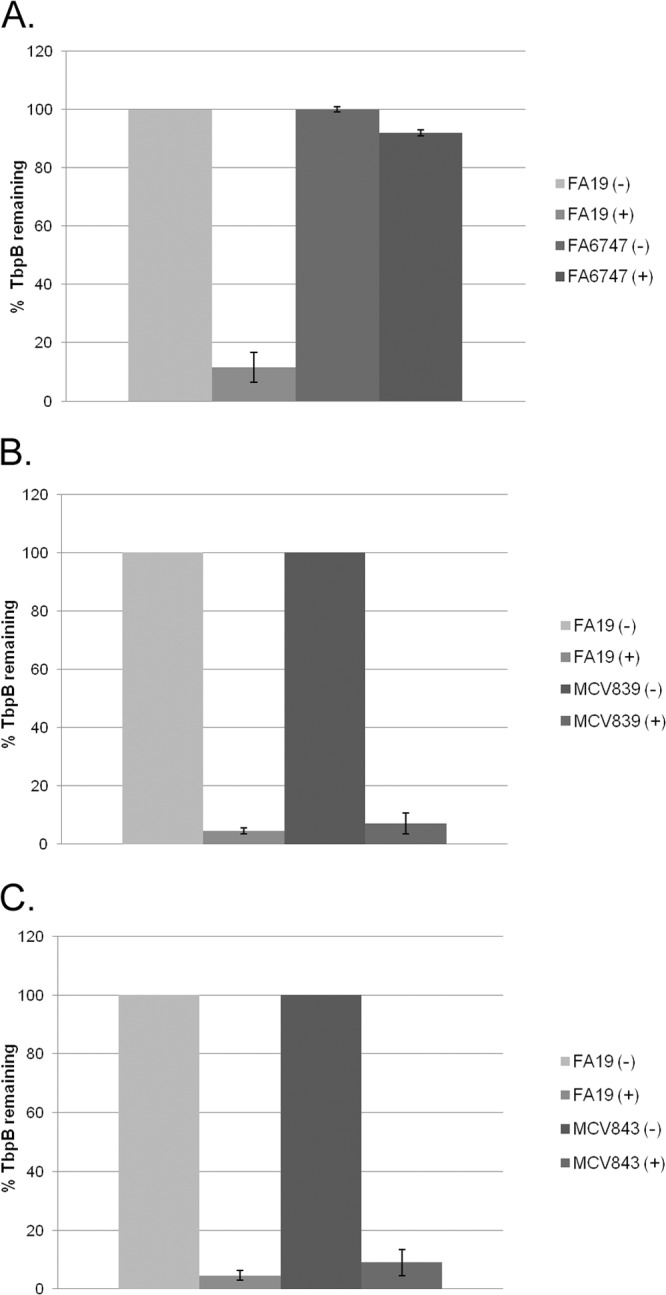
Quantitation of extent of TbpB cross-linking in mutant strains. Triplicate blots of cross-linked samples were probed with hTf-HRP (one representative blot is shown in Fig. 6A), scanned, and quantitated using NIH ImageJ. The percentage of full-length, wild-type TbpB that is detected in the mutant strains with (+) and without (−) cross-linking is shown. (A) Comparison of FA19 (wild type) and FA6747 (TbpA−) before and after cross-linking. (B) Comparison of FA19 (wild type) and MCV839 (TbpB LSAC). (C) Comparison of FA19 (wild type) and MCV843 (TbpB ΔGly).
DISCUSSION
From the results described in the current study, we conclude that while the LSAC motif is required for tethering of TbpB to the bacterial surface, it is not necessary for transport of the protein across the bacterial cell envelope. While it was anticipated that mutation of the lipobox would result in TbpB's being produced without its lipid anchor, the finding that the mutated protein is nevertheless exported to the cell surface is noteworthy and was somewhat unexpected. The lipid anchor is absolutely necessary for the function of TbpB, with respect to transferrin iron utilization in concert with TbpA. In addition, deletion of the conserved polyglycine motif had significant negative effects on growth when transferrin was supplied as the sole iron source, despite the fact that the mutant protein is apparently normally surface exposed. Together, these data significantly enhance our understanding of the posttranslational processing of TbpB and the role of conserved motifs in TbpB on transferrin iron acquisition.
The gonococcal transferrin binding proteins are considered attractive vaccine candidates and potential therapeutic targets (32). This is due to their ubiquitous expression among strains, high degree of conservation, and importance in establishing infection in a human experimental infection model. TbpA is a TonB-dependent outer membrane transporter and is absolutely required for transferrin iron utilization (17). The lipoprotein TbpB, on the other hand, is not required for gonococcal transferrin iron utilization, although its presence makes the process more efficient (16).
Recent studies (33, 34) have yielded new insights into how TbpA and TbpB work together during the process of iron acquisition. According to recently published crystal structures, TbpA and TbpB both bind to the C lobe of hTf, although each of the TBPs interacts with distinct sites on the glycoprotein. TbpB preferentially binds holo-hTf, and data suggest that this binding is specific for Fe carried in the C lobe (34). Likewise, TbpA binds specifically to the C lobe of hTf, although it does not distinguish between apo- and holo-Tf (34). Thus, one of TbpB's functions may be to selectively bind hTf that is usable by the gonococcus prior to passing on the hTf molecule to TbpA for stripping and transport of Fe. In this proposed mechanism, it is apparent that surface exposure of TbpB would be critical for its function. Moreover, the three-part complex comprised of TbpA, TbpB, and Tf demonstrated the presence of a potential iron chamber, enclosed by domains of each of the three proteins (34). Therefore, another function of TbpB may be to enhance iron uptake by virtue of the capacity to capture iron in the iron chamber and prevent its diffusion subsequent to release from transferrin.
TbpB was previously demonstrated to be lipidated (16) and fully surface exposed (35), similar to other neisserial lipoproteins, as well as lipoproteins of several other Gram-negative bacteria, such as Klebsiella, Bordetella, and Borrelia spp. (36–38). The palmitate labeling results presented in the current study indicate that the LSAC motif constituting the lipobox of TbpB is necessary for lipidation. Abrogation of lipidation resulted in less TbpB being retained on the cell surface, indicating that the lipid moiety is essential for tethering of TbpB to the outer leaflet of the outer membrane. Furthermore, when lipidation of TbpB was prevented in the TbpA L9HA background, growth on transferrin was completely abolished, confirming the functional importance of surface-tethered TbpB. Unlipidated TbpB was surface exposed, as detected by protease accessibility, and furthermore, the unmodified TbpB retained the ability to interact with TbpA, suggesting that the lipid moiety is not necessary for TbpA-TbpB interaction.
While we have shown that the LSAC lipobox is critical for lipidation of TbpB, the presence of the lipid moiety is not required for transport of TbpB across the cytoplasmic membrane, periplasm, and outer membrane. In E. coli, lipoprotein transport has been well characterized, with the periplasmic Lol system shuttling lipoproteins destined for the outer membrane to their proper location (39). However, unlike the majority of E. coli lipoproteins, which tend to be oriented toward the periplasm, many lipoproteins produced by the gonococcus and other related species are oriented toward the extracellular milieu (35, 38). In addition, while N. gonorrhoeae possesses homologues of some of the Lol proteins, the chromosome does not encode a LolE homologue. Furthermore, it has yet to be established whether these neisserial Lol proteins exhibit the same functionality as those in E. coli. This raises the question of how N. gonorrhoeae transports bacterial lipoproteins to the outer membrane and “flips” them outward, ultimately leading to tethering of the protein via the lipid moiety to the outer leaflet. One possibility is that specific sequences within the protein act as trafficking signals. The data presented in the current study demonstrate that replacement of the LSAC motif with a signal I peptidase cleavage site does not cause TbpB to accumulate in the periplasm; rather, the unlipidated protein is transported across the outer membrane and released into the extracellular milieu. This indicates that the TbpB lipobox is not required for processing and transport of TbpB across the outer membrane. However, our results indicate that the exported TbpB is released into the supernatant. Some fraction of the released protein is surface localized in a TbpA-dependent manner. Previous studies have demonstrated a physical interaction between TbpA and TbpB (18, 34, 40). Likewise, our cross-linking data confirm a physical interaction between TbpA and TbpB, even in the mutant that expresses TbpB LSAC. We propose that the retention of unlipidated TbpB in MCV839 is due to its affinity for TbpA. This unlipidated TbpB, however, whether by virtue of its not being tethered to the membrane or its diminished presence on the bacterial surface, is still unable to functionally participate in transferrin iron utilization.
Although the LSAC motif does not appear to act as a localization signal, the polyglycine tract just downstream of the lipobox could also potentially play a role in TbpB trafficking. This motif is well conserved not only among different gonococcal strains but also among other gonococcal lipoproteins, such as LbpB and HpuA. This is notable, as the lipoprotein SphB1 of Bordetella pertussis contains a conserved region of 14 glycine residues adjacent to the lipidation site, followed by a long proline-rich region, and these domains have been implicated in proper localization of SphB1 to the outer membrane (36). While much shorter, the gonococcal TbpB polyglycine tract could similarly act as a signal for lipoprotein localization. When we deleted these residues, however, TbpB remained surface exposed and appropriately lipidated. Interestingly, when the glycine deletion mutation was expressed in a TbpA mutant strain that requires TbpB for transferrin iron utilization, growth on transferrin was significantly diminished, despite its wild-type expression on the bacterial surface.
One possible explanation for the growth defects observed in the mutant that produces TbpB ΔGly is that this region provides a critical structural role as opposed to lipoprotein transport. While published structural data have provided important insights into the interaction between TbpA, TbpB, and Tf, the proteins used in these in vitro studies did not include the amino-terminal 22 residues of mature TbpB (33, 34, 41). Our data provide important new insights into the critical role of this region in the functionality of this lipoprotein. In fact, a recent study indicated that the “anchor peptide” of meningococcal TbpB, comprised of amino acids 2 to 40, contributes to the physical interaction between TbpA and TbpB, which is likely necessary for the cooperative activity of the two proteins (42). Further analysis showed that the interaction does not require the first seven amino acids, which contains the homologous polyglycine tract. However, while it was not necessarily implicated in binding to TbpA, it was speculated that the polymeric stretch of small amino acids may provide flexibility as well as length to the anchor peptide (42). It has been suggested that this would allow greater accessibility of TbpB at the surface to interact with transferrin and could also be involved in conformational changes upon hTf binding that would bring TbpB closer to the surface and in closer proximity to TbpA (42). In the context of this model, the results of the current study indicate that deletion of the polyglycine tract potentially impairs TbpB's ability to appropriately interact with TbpA upon hTf binding and/or might prevent subsequent interaction with TbpA to form the iron chamber, which allows for efficient iron internalization through the beta-barrel.
Overall, our results underscore the importance of two conserved TbpB motifs for proper localization and function of the protein, providing new insights regarding this member of the transferrin iron acquisition system. These data give us a more detailed understanding of how TbpB interacts with both TbpA and hTf. This knowledge, in turn, has the potential to aid in development of targeted therapies against gonococcal infection based on this critical iron acquisition system.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by U.S. Public Health Service grants AI065555 and AI084400 from the National Institute of Allergy and Infectious Diseases at the National Institutes of Health. This work was also supported by the SE STI Center grant (U19 AI31496) from the National Institute of Allergy and Infectious Diseases.
Footnotes
Published ahead of print 8 July 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00280-13.
REFERENCES
- 1.Centers for Disease Control and Prevention 2011. Sexually transmitted disease surveillance 2010. U.S. Department of Health and Human Services, Atlanta, GA [Google Scholar]
- 2.Barlow D, Phillips I. 1978. Gonorrhoea in women. Diagnostic, clinical, and laboratory aspects. Lancet i:761–764 [DOI] [PubMed] [Google Scholar]
- 3.McCormack WM, Stumacher RJ, Johnson K, Donner A. 1977. Clinical spectrum of gonococcal infection in women. Lancet i:1182–1185 [DOI] [PubMed] [Google Scholar]
- 4.Holmes KK, Counts GW, Beaty HN. 1971. Disseminated gonococcal infection. Ann. Intern. Med. 74:979–993 [DOI] [PubMed] [Google Scholar]
- 5.Sparling PF. 1990. Biology of Neisseria gonorrhoeae, p 131–138 In Holmes KK, Mardh P-A, Sparling PF, Weisner PJ. (ed), Sexually transmitted diseases, 2nd ed. McGraw Hill, New York, NY [Google Scholar]
- 6.Westrom L. 1980. Incidence, prevalence, and trends of acute pelvic inflammatory disease and its consequences in industrialized countries. Am. J. Obstet. Gynecol. 138:880–892 [DOI] [PubMed] [Google Scholar]
- 7.Cohen MS, Hoffman IF, Royce RA, Kazembe P, Dyer JR, Daly CC, Zimba D, Vernazza PL, Maida M, Fiscus SA, Eron JJ, AIDSCAP Malawi Research Group 1997. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. Lancet 349:1868–1873 [DOI] [PubMed] [Google Scholar]
- 8.McClelland RS, Wang CC, Mandaliya K, Overbaugh J, Reiner MT, Penteleeff DD, Lavreys L, Ndinya-Achola J, Bwayo JJ, Kreiss JK. 2001. Treatment of cervicitis is associated with decreased cervical shedding of HIV-1. AIDS 15:105–110 [DOI] [PubMed] [Google Scholar]
- 9.Ohnishi M, Golparian D, Shimuta K, Saika T, Hoshina S, Iwasaku K, Nakayama S, Kitawaki J, Unemo M. 2011. Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea?: detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob. Agents Chemother. 55:3538–3545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Unemo M, Shafer WM. 2011. Antibiotic resistance in Neisseria gonorrhoeae: origin, evolution, and lessons learned for the future. Ann. N. Y. Acad. Sci. 1230:E19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cornelissen CN, Hollander A. 2011. TonB-dependent transporters expressed by N. gonorrhoeae. Front. Microbiol. 2:117. 10.3389/fmicb.2011.00117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cornelissen CN, Anderson JE, Boulton IC, Sparling PF. 2000. Antigenic and sequence diversity in gonococcal transferrin-binding protein A (TbpA). Infect. Immun. 68:4725–4735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cornelissen CN, Anderson JE, Sparling PF. 1997. Characterization of the diversity and the transferrin-binding domain of gonococcal transferrin-binding protein 2. Infect. Immun. 65:822–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mickelsen PA, Sparling PF. 1981. Ability of Neisseria gonorrhoeae, Neisseria meningitidis, and commensal Neisseria species to obtain iron from transferrin and iron compounds. Infect. Immun. 33:555–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cornelissen CN, Kelley M, Hobbs MM, Anderson JE, Cannon JG, Cohen MS, Sparling PF. 1998. The transferrin receptor expressed by gonococcal strain FA1090 is required for the experimental infection of human male volunteers. Mol. Microbiol. 27:611–616 [DOI] [PubMed] [Google Scholar]
- 16.Anderson JE, Sparling PF, Cornelissen CN. 1994. Gonococcal transferrin-binding protein 2 facilitates but is not essential for transferrin utilization. J. Bacteriol. 176:3162–3170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cornelissen CN, Biswas GD, Tsai J, Paruchuri DK, Thompson SA, Sparling PF. 1992. Gonococcal transferrin-binding protein 1 is required for transferrin utilization and is homologous to TonB-dependent outer membrane receptors. J. Bacteriol. 174:5788–5797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noinaj N, Buchanan SK, Cornelissen CN. 2012. The transferrin-iron import system from pathogenic Neisseria species. Mol. Microbiol. 86:246–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cornelissen CN, Anderson JE, Sparling PF. 1997. Energy-dependent changes in the gonococcal transferrin receptor. Mol. Microbiol. 26:25–35 [DOI] [PubMed] [Google Scholar]
- 20.Cornelissen CN, Sparling PF. 1996. Binding and surface exposure characteristics of the gonococcal transferrin receptor are dependent on both transferrin-binding proteins. J. Bacteriol. 178:1437–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayashi S, Wu HC. 1990. Lipoproteins in bacteria. J. Bioenerg. Biomembr. 22:451–471 [DOI] [PubMed] [Google Scholar]
- 22.Yamaguchi K, Yu F, Inouye M. 1988. A single amino acid determinant of the membrane localization of lipoproteins in E. coli. Cell 53:423–432 [DOI] [PubMed] [Google Scholar]
- 23.Kellogg DS, Jr, Peacock WL, Jr, Deacon WE, Brown L, Pirkle CI. 1963. Neisseria gonorrhoeae. I. Virulence genetically linked to clonal variation. J. Bacteriol. 85:1274–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.West SEH, Sparling PF. 1987. Aerobactin utilization by Neisseria gonorrhoeae and cloning of a genomic DNA fragment that complements Escherichia coli fhuB mutations. J. Bacteriol. 169:3414–3421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horton RM, Cai ZL, Ho SN, Pease LR. 1990. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. Biotechniques 8:528–535 [PubMed] [Google Scholar]
- 26.Elkins C, Thomas CE, Seifert HS, Sparling PF. 1991. Species-specific uptake of DNA by gonococci is mediated by a 10-base-pair sequence. J. Bacteriol. 173:3911–3913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yost-Daljev MK, Cornelissen CN. 2004. Determination of surface-exposed, functional domains of gonococcal transferrin-binding protein A. Infect. Immun. 72:1775–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kenney CD, Cornelissen CN. 2002. Demonstration and characterization of a specific interaction between gonococcal transferrin binding protein A and TonB. J. Bacteriol. 184:6138–6145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- 30.Thomas CE, Zhu W, Dam CNV, Davis NL, Johnson RE, Sparling PF. 2006. Vaccination of mice with gonococcal TbpB expressed in vivo from Venezuelan Equine Encephalitis viral replicon particles. Infect. Immun. 74:1612–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9:671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cornelissen CN. 2008. Identification and characterization of gonococcal iron transport systems as potential vaccine antigens. Future Microbiol. 3:287–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moraes TF, Yu RH, Strynadka NC, Schryvers AB. 2009. Insights into the bacterial transferrin receptor: the structure of transferrin-binding protein B from Actinobacillus pleuropneumoniae. Mol. Cell 35:523–533 [DOI] [PubMed] [Google Scholar]
- 34.Noinaj N, Easley NC, Oke M, Mizuno N, Gumbart J, Boura E, Steere AN, Zak O, Aisen P, Tajkhorshid E, Evans RW, Gorringe AR, Mason AB, Steven AC, Buchanan SK. 2012. Structural basis for iron piracy by pathogenic Neisseria. Nature 483:53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeRocco AJ, Cornelissen CN. 2007. Identification of transferrin-binding domains in TbpB expressed by Neisseria gonorrhoeae. Infect. Immun. 75:3220–3232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coutte L, Willery E, Antoine R, Drobecq H, Locht C, Jacob-Dubuisson F. 2003. Surface anchoring of bacterial subtilisin important for maturation function. Mol. Microbiol. 49:529–539 [DOI] [PubMed] [Google Scholar]
- 37.d'Enfert C, Ryter A, Pugsley AP. 1987. Cloning and expression in Escherichia coli of the Klebsiella pneumoniae genes for production, surface localization and secretion of the lipoprotein pullulanase. EMBO J. 6:3531–3538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haake DA. 2000. Spirochaetal lipoproteins and pathogenesis. Microbiology 146:1491–1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tokuda H, Matsuyama S. 2004. Sorting of lipoproteins to the outer membrane in E. coli. Biochim. Biophys. Acta 1694:5–13 [DOI] [PubMed] [Google Scholar]
- 40.Fuller CA, Yu RH, Irwin SW, Schryvers AB. 1998. Biochemical evidence for a conserved interaction between bacterial transferrin binding protein A and transferrin binding protein B. Microb. Pathog. 24:75–87 [DOI] [PubMed] [Google Scholar]
- 41.Calmettes C, Alcantara J, Yu RH, Schryvers AB, Moraes TF. 2012. The structural basis of transferrin sequestration by transferrin-binding protein B. Nat. Struct. Mol. Biol. 19:358–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang X, Yu RH, Calmettes C, Moraes TF, Schryvers AB. 2011. Anchor peptide of transferrin-binding protein B is required for interaction with transferrin-binding protein A. J. Biol. Chem. 286:45165–45173 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


