Abstract
Streptococcus agalactiae (also known as group B Streptococcus [GBS]) and Streptococcus suis are encapsulated streptococci causing severe septicemia and meningitis. Bacterial capsular polysaccharides (CPSs) are poorly immunogenic, but anti-CPS antibodies are essential to the host defense against encapsulated bacteria. The mechanisms underlying anti-CPS antibody responses are not fully elucidated, but the biochemistry of CPSs, particularly the presence of sialic acid, may have an immunosuppressive effect. We investigated the ability of highly purified S. suis and GBS native (sialylated) CPSs to activate dendritic cells (DCs), which are crucial actors in the initiation of humoral immunity. The influence of CPS biochemistry was studied using CPSs extracted from different serotypes within these two streptococcal species, as well as desialylated CPSs. No interleukin-1β (IL-1β), IL-6, IL-12p70, tumor necrosis factor alpha (TNF-α), or IL-10 production was observed in S. suis or GBS CPS-stimulated DCs. Moreover, these CPSs exerted immunosuppressive effects on DC activation, as a diminution of gamma interferon (IFN-γ)-induced B cell-activating factor of the tumor necrosis factor family (BAFF) expression was observed in CPS-pretreated cells. However, S. suis and GBS CPSs induced significant production of CCL3, via partially Toll-like receptor 2 (TLR2)- and myeloid differentiation factor 88 (MyD88)-dependent pathways, and CCL2, via TLR-independent mechanisms. No major influence of CPS biochemistry was observed on the capacity to induce chemokine production by DCs, indicating that DCs respond to these CPSs in a patterned way rather than a structure-dedicated manner.
INTRODUCTION
Streptococcus agalactiae (also known as group B Streptococcus [GBS]) is a major cause of life-threatening invasive bacterial infections in pregnant women and neonates as well as in the elderly and immunocompromised individuals (1, 2). Clinical manifestations are mainly pneumonia, septicemia, and meningitis. Among 10 GBS serotypes identified, type III is one of the major serotypes associated with invasive neonatal infection and is the most common type in GBS meningitis (2). In addition, GBS type V is emerging as a leading cause of invasive disease in adults (3). Streptococcus suis is an important swine pathogen and an emerging zoonotic pathogen in humans that is able to induce septicemia with sudden death, meningitis, endocarditis, pneumonia, and arthritis (4, 5). Of the 35 serotypes, type 2 is the most virulent and is frequently isolated from both swine and humans (6), and type 14 is also emerging as a zoonotic threat (7). For both pathogens, the capsular polysaccharide (CPS), which defines the serotype, is considered the major virulence factor (8, 9). The structures of type III and V GBS CPSs are formed by different arrangements of the monosaccharides glucose, galactose, and N-acetylglucosamine into unique repeating units that contain a side chain terminated by sialic acid (N-acetylneuraminic acid [Neu5Ac]). The structures of type 2 and 14 S. suis CPSs are composed of the monosaccharides glucose, galactose, N-acetylglucosamine, and rhamnose (for type 2 only) arranged into a unique repeating unit that also contains a side chain terminated by sialic acid. In fact, these streptococci are the sole Gram-positive bacteria possessing sialic acid in their capsules (8, 10, 11). However, despite similarities in the compositions of the CPSs of these two bacterial species, each CPS is composed of a unique arrangement of these sugars conferring a distinct antigenicity. Moreover, sialic acid forms an α-2,6 linkage with the adjacent galactose in S. suis, in contrast to the α-2,3 linkage in GBS. Interestingly, the interplay of CPSs with components of the immune system, including antigen-presenting cells (APCs), seems to differ radically. Experiments using nonencapsulated mutants have shown that S. suis type 2 CPS has a strong antiphagocytic effect, in contrast to GBS type III CPS, and severely interferes with the release of most of the cytokines produced by S. suis-infected APCs. In the case of GBS type III, cytokine production is only partially modified or unaltered by the presence of its CPS (12–15).
Sialic acid of bacterial polysaccharides has been suggested to be involved in immune evasion via several mechanisms. For example, sialic acid of GBS type III CPS interferes with the immune response by molecular mimicry (8) and inhibition of complement activation (16). Some receptors expressed on the surfaces of leukocytes have a distinct preference for specific types of linkage of sialic acid to subterminal sugars. As these binding preferences are likely related to their biological functions, differences in sialic acid linkage in S. suis versus GBS might differentially modulate host immune responses (17). However, knowledge on the specific contributions of sialic acid to the interactions of these two pathogens with the immune system is restricted by the fact that deletion of genes involved in sialic acid synthesis results in considerable or complete loss of CPS expression at the bacterial surface (18, 19).
Dendritic cells (DCs), the most powerful APCs, express a wide variety of pattern recognition receptors (PRRs) that enable them to detect the presence of several pathogens through the recognition of pathogen-associated molecular patterns. Among these PRRs, Toll-like receptors (TLRs) are important for the initiation of the immune response as well as the shaping of adaptive immunity (20). The interactions between DCs and pathogens can strongly influence the magnitude and phenotype of the ensuing cellular and humoral adaptive immune responses, notably via the release of cytokines (21). Purified bacterial CPSs are classically reported to be T cell-independent (TI) antigens which are consequently weak stimulators of the host immune response (22, 23). However, several in vitro studies have demonstrated the ability of bacterial CPSs to interact with APCs, resulting in the production of cytokines and chemokines (24–28). The adaptor molecule myeloid differentiation factor 88 (MyD88), which is involved in intracellular events downstream of TLR signaling, and TLR2 have been suspected to be involved in the interactions of bacterial CPSs with DCs and/or macrophages (25, 27). Nevertheless, the potential role of pure carbohydrates as ligands for TLRs, and more globally for PRRs, remains largely uninvestigated.
Recent reports have shown that DCs play an important role in TI responses and, more precisely, in the development of the humoral response, via the release of B cell-activating factor of the tumor necrosis factor family (BAFF). BAFF is able to enhance B cell proliferation, immunoglobulin (Ig) class switching, and Ig secretion (29, 30). These critical signals are particularly interesting in the context of infection by encapsulated bacteria, where antibodies (Abs) against the CPS have been proven to be essential to the host defense (23). As such, we hypothesized that intra- and interspecies structural differences in CPS might differently modulate DC release of cytokines and chemokines. The goal of this study was thus to evaluate and compare the effects of highly purified CPS preparations from S. suis types 2 and 14 as well as GBS types III and V on DC activation and, more specifically, on the capacity to induce cytokines essential for the development of an effective humoral immune response. The influence of sialic acid was analyzed using chemically desialylated CPS preparations.
MATERIALS AND METHODS
Native CPS purification.
The reference strains of S. suis serotype 2 (S735; ATCC 43765), isolated from a pig with meningitis (31), and S. suis serotype 14 (DAN13730), isolated from a human with meningitis (32), were grown in 150 ml of Todd-Hewitt broth (THB) (Oxoid, Thermo Fisher Scientific) at 37°C for 16 h, diluted to 6 liters in fresh THB, and grown to an optical density at 540 nm (OD540) of 0.8. The cells were pelleted by centrifugation at 10,000 × g for 40 min, suspended by repeated pipetting in 33 mM phosphate-buffered saline (PBS), pH 8.0, and chilled. The CPSs were then purified as previously described (10, 11).
GBS serotype III strain COH-1, isolated from an infant with bacteremia (33), and GBS serotype V strain CJB111 (ATCC BAA-23), isolated from a neonate with septicemia, were used in this study. GBS CPSs were prepared as previously reported (34), with some modifications. Briefly, bacteria were grown in 200 ml THB at 37°C for 16 h, diluted to 8 liters in fresh THB, and grown to an OD540 of 0.8. The cells were pelleted by centrifugation at 10,000 × g for 40 min, washed in PBS, pH 7.3, and treated with 1 N NaOH at 37°C overnight. After neutralization and dialysis, proteins were digested by treatment with 1 mg/ml pronase (Sigma-Aldrich) at 37°C overnight, followed by dialysis. The CPSs were then subjected to re-N-acetylation with 0.8 M acetic anhydride (Sigma) in 5 N NaOH and finally purified by gel filtration on Sephacryl S-300 (GE Healthcare), using 50 mM NH4HCO3 as the eluent.
CPS desialylation.
Highly purified native CPSs were desialylated by mild acid hydrolysis as described previously (10). Briefly, CPS (8 mg) was heated in 1 ml of 70 mM HCl at 60°C for 250 min, neutralized with 2 M NH4OH, dialyzed against deionized water for 48 h at 4°C with a Spectra/Por membrane (molecular size cutoff of 3,500 Da; Spectrum Laboratories), and freeze-dried.
CPS quality controls.
Each purified CPS was subjected to rigorous quality control tests as previously described (10). Nucleic acids were quantified using an ND 1000 spectrometer (Nanodrop). The absorbance was measured at 230 and 260 nm. Calculations were done with Nanodrop software. According to the manufacturer, results are reproducible between 2 and 100 ng/μl. Proteins were quantified by use of a modified Lowry protein assay kit from Pierce on 1-mg/ml CPS samples, using a standard curve prepared with diluted albumin standards from 1 to 1,000 μg/ml. The calculated limit of detection (P ≤ 0.05) was 0.7 to 1.3 μg/ml. Each CPS was analyzed by nuclear magnetic resonance (NMR) as described below. The monosaccharide composition of polysaccharides was confirmed by methanolysis followed by acetylation and analysis by gas chromatography (GC), either with flame ionization detection or coupled to mass spectrometry as previously described (10). The weight-average molecular weight (Mw) of each CPS was determined by size-exclusion chromatography coupled with multiangle light scattering (SEC-MALS) as described below. The presence (native CPS) or absence (desialylated CPS) of sialic acid was verified by NMR and by an enzyme-linked lectin assay (ELLA) as described below.
Nuclear magnetic resonance assay.
S. suis native CPSs were exchanged in phosphate buffer (p2H 8.0) in 2H2O (99.9 atom% 2H), freeze-dried, and dissolved in 2H2O (99.96 atom% 2H) to a final phosphate concentration of 33 mM. The other CPSs were exchanged in 2H2O (99.9 atom% 2H), freeze-dried, and dissolved in 2H2O (99.96 atom% 2H). NMR spectra were acquired on CPS samples at concentrations of ca. 1% to 2%. Conventional 1H spectra were acquired at 14 T on Bruker Avance spectrometers equipped with either a 5-mm TCI CryoProbe at 50°C or a 5-mm PABBO BB inverse gradient probe at 75°C or at 11.75 T on a Bruker Avance 500 spectrometer equipped with a 5-mm triple-resonance TBI probe at 60 to 80°C, using standard Bruker pulse sequences.
Mw characterization of native and desialylated CPSs.
The Mw of CPSs were characterized by SEC-MALS. Chromatographic separation was performed with two 8-mm by 300-mm Shodex OHpak gel filtration columns connected in series (SB 806 and SB 804), preceded by an SB 807G guard column (Showa Denko). Elution was done with a Waters 510 pump (Waters), using a 0.1 M NaNO3 mobile phase filtered through a 0.02-μm membrane (Whatman), at a flow rate of 0.5 ml/min. Samples were dissolved in the SEC eluent at concentrations of 0.7 to 1.0 mg/ml for native CPSs and 2.0 to 3.5 mg/ml for desialylated CPSs and then were injected with a 100- or 200-μl sample loop. Molecular masses were determined with a Dawn EOS MALS detector (Wyatt). A model RI 410 differential refractometer (Waters) was used as a concentration detector. A refractive index increment (dn/dc) of 0.137 ml/g was calculated for 690 nm, using data for xanthan at 436 and 546 nm (35), and the second virial coefficient (A2) was taken as zero. Calculations were performed with ASTRA software, version 6.0.0.108 (Wyatt).
ELLA.
In order to verify the presence or absence of sialic acid in the purified native and desialylated CPSs, ELLA was carried out based on a previously described technique (36), which was adapted to CPSs. Briefly, 200 ng of sample (native or desialylated CPS) was added to wells of an enzyme-linked immunosorbent assay (ELISA) plate (Nunc-Immuno Polysorp). After overnight coating at 4°C, the wells were washed and blocked by the addition of 1× Carbo-Free solution (Vector Laboratories). After washings, the wells were incubated for 1 h with biotinylated Sambucus nigra agglutinin (SNA-I) (Vector Laboratories), which specifically recognizes sialic acid as Neu5Acα-2,6-Galp/GalpNAc (37), or biotinylated Maackia amurensis leukoagglutinin (MAL-I) (Vector Laboratories), which recognizes sialic acid as Neu5Acα-2,3-Galβ-1,4-GlcNAc (38). Horseradish peroxidase (HRP)-labeled avidin D (Vector Laboratories) and 3,3′,5,5′-tetramethylbenzidine were then added. In some experiments, HRP-conjugated Limax flavus agglutinin (LFA) (Vector Laboratories), which recognizes Neu5Ac (39), was used. The enzyme reaction was stopped by the addition of 0.5 M H2SO4, and the absorbance was read at 450 nm with an ELISA plate reader.
Dot-ELISA.
Ten microliters of purified native or desialylated CPS (each at 1 mg/ml) or 10 μl of heat-killed whole bacteria was blotted on a polyvinylidene difluoride (PVDF) Western blot membrane (Roche). Heat-killed bacteria were obtained after incubating bacteria at 60°C for 45 min and were adjusted to 109 CFU/ml. The membrane was blocked for 1 h with a solution of Tris-buffered saline (TBS) containing 2% casein, followed by 2 h of incubation with either the mouse monoclonal Ab (MAb) Z3, which specifically recognizes the sialic acid moiety of S. suis type 2 CPS (40); monospecific polyclonal rabbit sera against S. suis type 2 (41) or S. suis type 14 (32); or commercial rabbit sera against GBS type III or GBS type V CPS (Denka Seiken). The membrane was washed, and the appropriate anti-rabbit or anti-mouse HRP-conjugated Ab (Jackson) was added for 1 h. The membrane was washed 3 times with TBS and revealed with a 4-chloro-1-naphthol solution (Sigma).
Mouse strains and generation of bone marrow-derived dendritic cells.
Six- to 8-week-old mice originating from Jackson Laboratory, including wild-type (WT) C57BL/6, MyD88−/− (B6.129P2-Myd88tm1Defr/J), and TLR2−/− (B6.129-Tlr2tmlKir/J) mice, were used. All experiments involving mice were conducted in accordance with the guidelines and policies of the Canadian Council on Animal Care and the principles set forth in the Guide for the Care and Use of Laboratory Animals by the Animal Welfare Committee of the Université de Montréal (42). Bone marrow-derived DCs were produced according to a previously described technique (13, 43) and cultured in complete medium consisting of RPMI 1640 supplemented with 5% heat-inactivated fetal bovine serum, 10 mM HEPES, 2 mM l-glutamine, 50 μM 2-mercaptoethanol, 100 U/ml penicillin-streptomycin, and 20 μg/ml gentamicin. All reagents were from Gibco (Invitrogen). Cell purity was routinely ≥86 to 90% CD11c+high F4/80−/dim cells as determined by fluorescence-activated cell sorter (FACS) analysis, in agreement with values reported in other studies (44–46).
In vitro DC stimulation assay.
DCs were resuspended at 106 cells/ml in complete medium and stimulated with native or desialylated CPS (5, 50, 100, or 200 μg/ml). At 6 and 24 h, supernatants were collected for cytokine quantification by ELISA, and cells were harvested for analysis of BAFF expression by reverse transcriptase quantitative PCR (RT-qPCR). Cells stimulated with 1 μg/ml ultrapurified Escherichia coli O55:B5 lipopolysaccharide (LPS) (Apotech Corporation) and 10 ng/ml recombinant mouse gamma interferon (IFN-γ) (R&D Systems) served as positive controls for cytokine production and BAFF expression, respectively. Nonstimulated cells served as a negative control. In some experiments, DCs were prestimulated with CPS (at 200 μg/ml) for 6 h prior to incubation with 10 ng/ml IFN-γ for 24 h. Cells were then harvested for BAFF expression analysis. DCs preincubated in complete medium before addition of IFN-γ served as a control. All solutions and CPSs were tested for the absence of endotoxin by use of a Limulus amebocyte lysate gel-clotting test (Pyrotell) with a sensitivity limit of 0.03 endotoxin unit (EU)/ml. Otherwise, the absence of endotoxin contamination during cell stimulation was controlled by parallel assays with polymyxin B sulfate (Sigma) at 20 μg/ml.
Cytokine quantification by ELISA.
Levels of interleukin-1β (IL-1β), IL-6, IL-10, IL-12p70, tumor necrosis factor alpha (TNF-α), CCL2 (MCP-1), and CCL3 (MIP-1α) in cell culture supernatants were measured by sandwich ELISAs using pair-matched Abs from R&D Systems, performed according to the manufacturer's recommendations. Twofold dilutions of recombinant mouse cytokines were used to generate standard curves. Sample dilutions giving OD readings in the linear portion of the appropriate standard curve were used to quantify the levels of each cytokine.
Analysis of BAFF gene expression by real-time RT-qPCR.
Total RNA was isolated from 106 DCs by use of TRIzol reagent (Invitrogen). After elimination of genomic DNA, 800 ng of total RNA was reverse transcribed with a QuantiTect reverse transcription kit (Qiagen). The cDNA was amplified using an SsoFast EvaGreen Supermix kit (Bio-Rad). The PCR amplification program for all cDNAs consisted of an enzyme activation step of 3 min at 98°C followed by 40 cycles of a denaturing step of 2 s at 98°C and an annealing/extension step of 5 s at 56°C. The ATP synthase subunit beta (ATP5B) and cytochrome c1 (CYC1) genes were used as normalizing genes to compensate for potential differences in amounts of cDNA. The primers used for amplification of the different target cDNAs are listed in Table S1 in the supplemental material and were all tested to achieve amplification efficiencies between 98.5% and 100%. The primer sequences were all designed from the NCBI GenBank mRNA sequence, using the Web-based software primerquest from Integrated DNA Technologies. A Bio-Rad CFX-96 sequence detector was used for amplification of cDNA, and quantitation of differences between the different groups was calculated using the 2−ΔΔCT method (47). Nonstimulated DCs were used as the calibrator reference in the analysis.
Statistical analysis.
All data are expressed as means ± standard errors of the means (SEM). Data were analyzed for significance using analysis of variance (ANOVA). Significance is denoted in the figures as follows: *, P < 0.05; and **, P < 0.001. All experiments were repeated at least three times.
RESULTS
S. suis and GBS CPS purification, desialylation, and quality control tests.
On average, from a 6-liter S. suis type 2 or S. suis type 14 culture, 150 mg of crude capsule was obtained, to afford around 30 mg of purified CPS after gel filtration (average yield of 5 mg CPS/liter of culture). From 8 liters of GBS type III or type V culture, 360 mg or 600 mg initial crude capsule was obtained, respectively, to afford around 30 mg of purified CPS after gel filtration (average yield of 3.75 mg CPS/liter of culture). GC and NMR analyses of purified CPSs gave sugar compositions and structures in accord with previous findings (8, 10, 11) (Table 1; see Fig. S1 and S2 in the supplemental material). Each repeating unit is composed of the same four sugars (with an additional rhamnose in S. suis type 2 CPS), with sialic acid located at the terminal side chain. The ratios of the four common sugars are similar in S. suis type 2 and 14 CPSs, whereas they are different between GBS type III and V CPSs. In comparison with S. suis CPSs, the repeating unit of GBS type III and V CPSs has one less galactose, and GBS type V CPS has two additional glucose residues. Indeed, the glucose:galactose:N-acetylglucosamine:N-acetylneuraminic acid:rhamnose sugar ratios for each repeating unit of S. suis type 2, S. suis type 14, GBS type III, and GBS type V CPSs are 1:3:1:1:1, 1:3:1:1:0, 1:2:1:1:0, and 3:2:1:1:0, respectively (Table 1; see Fig. S1 and S2). One of the problems encountered in purifying GBS CPSs is contamination with group B antigen, as the CPS is covalently linked to the cell wall in this bacterial species (48). The absence of rhamnose (a sugar present in group B antigen but not in GBS CPSs) in purified GBS CPS preparations confirmed the absence of contamination with this cell wall antigen. No protein was found above the limit of detection, indicating that there was less than 1.3% (wt/wt) protein in all purified CPSs, and DNA and RNA contamination was less than 1% (wt/wt) (Table 1). SEC-MALS showed that native S. suis type 2 and 14 CPSs had similar Mw, which were 480 and 500 kg/mol, respectively. Native GBS type III and V CPSs had comparable Mw, which were almost 4-fold lower than that of native S. suis CPSs. Desialylation induced a larger Mw decrease in S. suis CPSs than in GBS CPSs, with the highest diminution for desialylated S. suis type 2 CPS (96% of the initial Mw) (Table 1). This could be explained by the presence of rhamnose in the backbone of S. suis type 2 CPS, which forms a linkage more susceptible to acid hydrolysis with the adjacent sugar.
Table 1.
Quality control tests of purified S. suis and GBS CPSsf
| CPSa | Sugar compositionb | Mwc | % (wt/wt) nucleic acidsd | % (wt/wt) proteine |
|---|---|---|---|---|
| S. suis type 2 (n) | 1:2.6:0.9:1.0:0.9 | 480 | 0.8 | NS |
| S. suis type 2 (dS) | 1:2.6:0.9:1.0:0.9 | 21 | 0.8 | NS |
| S. suis type 14 (n) | 1:2.5:0.8:1.0:0.0 | 500 | 0.7 | NS |
| S. suis type 14 (dS) | 1:2.5:0.8:1.0:0.0 | 176 | 0.7 | NS |
| GBS type III (n) | 1:1.5:1.0:0.9:0.0 | 108 | 0.8 | NS |
| GBS type III (dS) | 1:1.5:1.0:0.9:0.0 | 59 | 0.8 | NS |
| GBS type V (n) | 3:1.5:0.8:0.8:0.0 | 128 | 0.9 | NS |
| GBS type V (dS) | 3:1.5:0.8:0.8:0.0 | 91 | 0.9 | NS |
n, native; dS, desialylated.
Determined by GC after methanolysis and acetylation and shown in the order glucose:galactose:N-acetylglucosamine:N-acetylneuraminic acid:rhamnose.
Determined by SEC-MALS (expressed in kg/mol).
Determined by spectrophotometry at 230 and 260 nm.
Determined by spectrophotometry at 750 nm. NS, nonsignificant (under the limit of detection).
See Materials and Methods for more details.
S. suis and GBS CPS recognition by specific sera and sialic acid-binding lectins.
Dot-ELISA experiments on native CPSs showed that recognition of the CPS epitope of each CPS preparation was conserved after purification (Fig. 1). Whereas the native S. suis type 2 CPS was well recognized by MAb Z3, which is specific for the sialic acid part of the capsule, the negative reaction of the desialylated S. suis type 2 CPS attested the absence of sialic acid in the latter (Fig. 1A, left panel). Desialylation of S. suis type 2 CPS resulted in reduced recognition by specific polyclonal Abs (Fig. 1A, right panel), whereas recognition of desialylated preparations of S. suis type 14 CPS was unaltered (Fig. 1B). The capacity of specific polyclonal Abs to react with the desialylated GBS type III CPS was almost completely lost compared to that with native CPS (Fig. 1C). In contrast, native and desialylated preparations of GBS type V CPS were similarly recognized by specific polyclonal Abs (Fig. 1D). These data suggest that there are intra- and interspecies variations in the immunogenic properties exerted by the sialic acid moiety (49–51).
Fig 1.
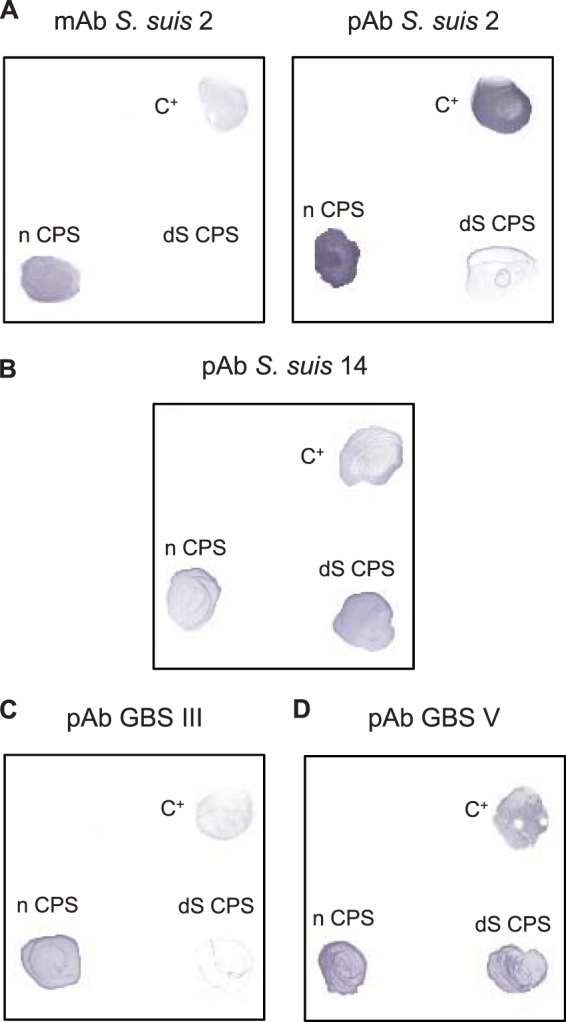
Recognition of S. suis and GBS CPSs by dot-ELISA. S. suis type 2 (A), S. suis type 14 (B), GBS type III (C), or GBS type V (D) whole bacteria (107 CFU) or their respective purified native (n) or desialylated (dS) CPSs (10 μg) were incubated with a MAb directed against the sialic acid moiety of S. suis type 2 CPS (A, left) or with monospecific polyclonal sera (pAb) against S. suis type 2 CPS (A, right), S. suis type 14 CPS (B), GBS type III CPS (C), or GBS type V CPS (D). C+, positive control.
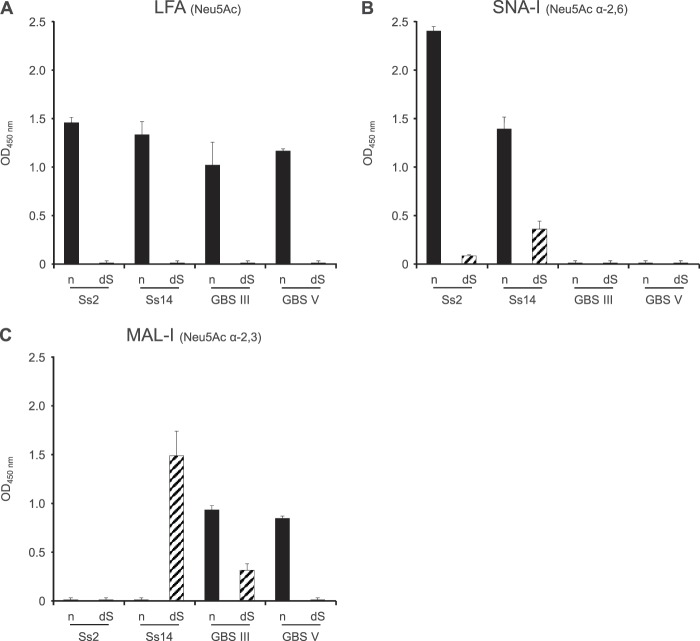
To further confirm the presence or absence of sialic acid in native and desialylated CPSs, an ELLA test was performed. Recognition of native S. suis and GBS CPSs by LFA, which is specific for Neu5Ac, confirmed the integrity of sialic acid in these preparations, whereas the absence of reaction with desialylated preparations demonstrated the absence of this sugar after desialylation by mild acid hydrolysis (Fig. 2A). Recognition of native S. suis CPSs by SNA-I and of native GBS CPSs by MAL-I validated that sialic acid forms α-2,6 and α-2,3 links, respectively, with the adjacent galactose (Fig. 2B and C). In the case of desialylated S. suis type 14 CPS, a positive reaction was observed with both SNA-I and MAL-I. Similarly, a positive reaction was observed for desialylated GBS type III CPS with MAL-I (Fig. 2B and C). Because negative reactions with LFA and NMR analysis (Fig. 2A; see Fig. S1 and S2 in the supplemental material) clearly demonstrated the absence of sialic acid in these desialylated preparations, the positive reaction with SNA-I can be explained by the nonspecific binding of the lectin to d-galactose (37). Recognition of the Galβ-1,4-GlcNAc epitope of desialylated S. suis type 14 and GBS type III CPSs by MAL-I can explain the unspecific reaction of this lectin with these two preparations (52).
Fig 2.
Recognition of S. suis and GBS CPSs by ELLA. Native (n) or desialylated (dS) S. suis type 2 (Ss2), S. suis type 14 (Ss14), GBS type III, or GBS type V CPS (each at 2 μg/ml) was incubated with Limax flavus agglutinin specific for Neu5Ac (LFA) (A), Sambucus nigra agglutinin specific for Neu5Ac α-2,6 links (SNA-I) (B), or Maackia amurensis leukoagglutinin specific for Neu5Ac α-2,3 links (MAL-I) (C). Data are expressed as mean OD450 with SEM for at least three experiments with at least three technical replicates and are corrected for reaction of the dilution buffer with the corresponding lectin.
S. suis and GBS CPSs induce the release of chemokines by DCs.
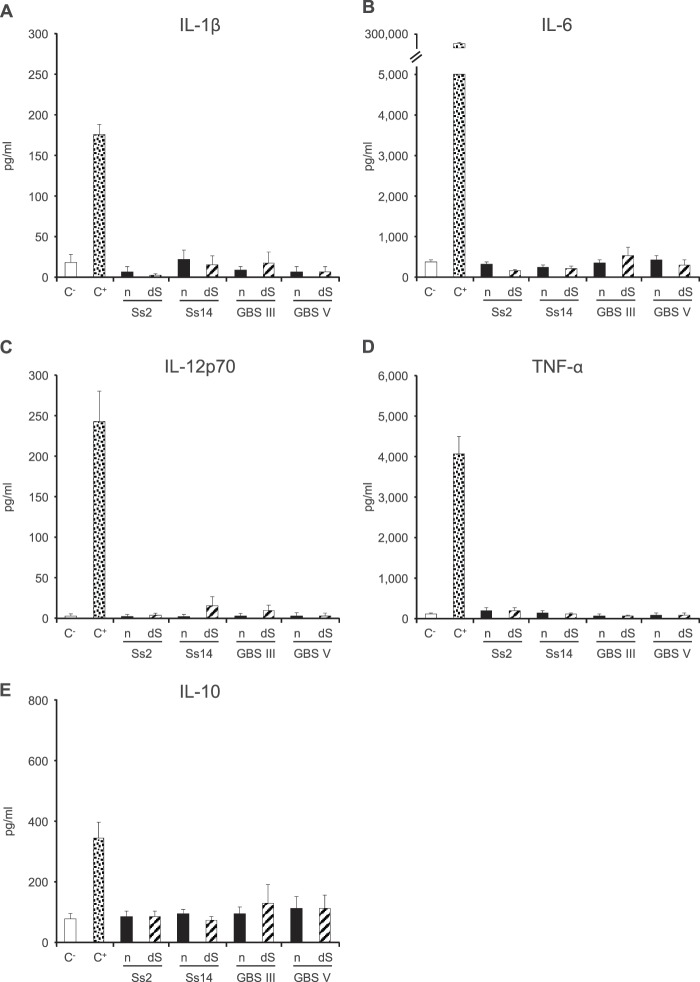
The levels of several cytokines and chemokines in the supernatants of DCs incubated with S. suis type 2 or 14 or GBS type III or V CPS (each at 200 μg/ml) were measured 24 h after stimulation. No significant difference in pro- or anti-inflammatory cytokine production was observed between DCs incubated with the different CPSs and those incubated with medium alone (Fig. 3). Similar results were obtained 6 h after stimulation (data not shown). The presence of sialic acid did not influence the release of these cytokines, as no significant difference was observed between native and desialylated CPSs (Fig. 3).
Fig 3.
Pro- and anti-inflammatory cytokine production by DCs in response to stimulation by S. suis or GBS CPSs for 24 h. Native (n) or desialylated (dS) S. suis type 2 (Ss2), S. suis type 14 (Ss14), GBS type III, or GBS type V CPS (each at 200 μg/ml) was incubated with DCs (106 cells/ml). After 24 h, supernatants were collected, and IL-1β (A), IL-6 (B), IL-12p70 (C), TNF-α (D), and IL-10 (E) levels were quantified by ELISA. Cells stimulated with medium alone and with LPS (1 μg/ml) served as negative (C−) and positive (C+) controls, respectively. Data are expressed as means with SEM (pg/ml) for at least three experiments with at least three technical replicates.
In contrast, S. suis and GBS CPSs induced significant release of the chemokines CCL2 and CCL3 at 24 h (Fig. 4). CCL3 production was induced similarly by all CPS preparations (Fig. 4A). On the other hand, CCL2 production was significantly higher when DCs were activated with S. suis CPSs (P < 0.001) (Fig. 4B). In the case of S. suis, more CCL2 production was observed for DCs stimulated with S. suis type 14 CPS than for those stimulated with S. suis type 2 CPS (P < 0.001). Sialic acid plays a partial inhibitory role in the production of CCL2 for S. suis type 2 CPS only (P < 0.001). To explore more precisely the characteristics of CCL2 and CCL3 production, dose- and time-response analyses were performed. CCL2 release induced by S. suis type 2 CPS was shown to be directly proportional to the CPS concentration and the time of incubation, with maximum release obtained at 24 h with a concentration of 200 μg/ml CPS (Fig. 5). Similar results were obtained with S. suis type 14 and GBS type III and V CPSs, for both CCL2 and CCL3 production (data not shown).
Fig 4.
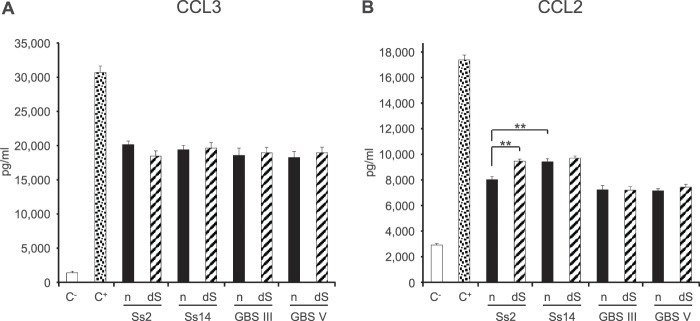
Chemokine production by DCs in response to stimulation by S. suis or GBS CPSs for 24 h. Native (n) or desialylated (dS) S. suis type 2 (Ss2), S. suis type 14 (Ss14), GBS type III, or GBS type V CPS (each at 200 μg/ml) was incubated with DCs (106 cells/ml). After 24 h, supernatants were collected, and CCL3 (A) and CCL2 (B) levels were quantified by ELISA. Cells stimulated with medium alone and with LPS (1 μg/ml) served as negative (C−) and positive (C+) controls, respectively. Data are expressed as means with SEM (pg/ml) for at least three experiments with at least three technical replicates. **, P < 0.001.
Fig 5.
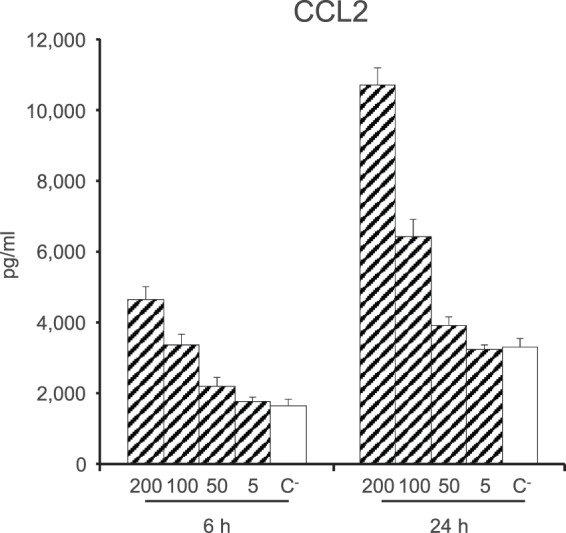
CCL2 production by DCs in response to stimulation by S. suis type 2 CPS is dose and time dependent. Native S. suis type 2 CPS (200, 100, 50, or 5 μg/ml) was incubated with DCs (106 cells/ml). After 6 or 24 h, supernatants were collected and CCL2 levels were quantified by ELISA. Cells stimulated with medium alone served as a negative control (C−). Data are expressed as means with SEM (pg/ml) for at least three experiments with at least three technical replicates.
Involvement of PRRs in chemokine release by DCs stimulated with S. suis and GBS CPSs.
Previous in vitro studies have demonstrated that well-encapsulated S. suis type 2 induces TLR2 mRNA expression by human monocytes (53) and porcine DCs (15) and stimulates cytokine and chemokine production by murine macrophages and DCs in a TLR2- and MyD88-dependent manner (53, 54). Moreover, the release of cytokines by S. suis-stimulated human monocytes was significantly reduced by Ab-mediated blocking of TLR2 but not TLR4 (53). In the case of GBS type III, the killed bacterium induces TLR2 mRNA expression and TLR2-dependent expression of certain cytokines and chemokines by murine macrophages (55). Killed GBS type III stimulates TNF-α release by those cells, in a TLR2-independent but MyD88-dependent way (56). As earlier studies have shown that PRRs such as TLRs are involved in cytokine production by APCs stimulated with purified CPSs from several different bacteria (25, 27, 57), we aimed to evaluate the implication of these receptors in the production of chemokines by DCs activated with our CPSs. Therefore, levels of CCL2 and CCL3 release were compared between WT and TLR2−/− or MyD88−/− DCs incubated with S. suis or GBS CPSs (at 200 μg/ml) for 24 h. As shown in Fig. 6A and 7A, no significant difference in CCL2 production was observed between WT and TLR2−/− DCs or between WT and MyD88−/− DCs for either S. suis or GBS CPSs. On the other hand, TLR2−/− DCs activated with either GBS type III or type V CPS showed a partial reduction of CCL3 production compared to their WT DC counterparts (P < 0.05). In the case of S. suis, a significant effect of TLR2 on CCL3 production was observed only for native S. suis type 2 CPS, indicating the involvement of other receptors (Fig. 6B). Indeed, a significant impairment of CCL3 production by MyD88−/− DCs incubated with all four S. suis or GBS CPSs was observed, with decreases varying between 40 and 50% (P < 0.001) (Fig. 7B). In general, the presence of sialic acid did not seem to significantly modulate the interactions between either GBS or S. suis CPSs and the TLR/MyD88 pathway (Fig. 6 and 7).
Fig 6.
Role of TLR2 in chemokine production by DCs in response to stimulation by S. suis or GBS CPSs for 24 h. Native (n) or desialylated (dS) S. suis type 2 (Ss2), S. suis type 14 (Ss14), GBS type III, or GBS type V CPS (each at 200 μg/ml) was incubated with WT (black bars) or TLR2−/− (white bars) DCs (106 cells/ml). After 24 h, supernatants were collected and CCL2 (A) and CCL3 (B) levels were quantified by ELISA. Cells stimulated with medium alone and with LPS (1 μg/ml) served as negative (C−) and positive (C+) controls, respectively. Data are expressed as means with SEM (pg/ml) for at least three experiments with at least three technical replicates. *, P < 0.05.
Fig 7.
Role of MyD88 in chemokine production by DCs in response to stimulation by S. suis or GBS CPSs for 24 h. Native (n) or desialylated (dS) S. suis type 2 (Ss2), S. suis type 14 (Ss14), GBS type III, or GBS type V CPS (each at 200 μg/ml) was incubated with WT (black bars) or MyD88−/− (white bars) DCs (106 cells/ml). After 24 h, supernatants were collected and CCL2 (A) and CCL3 (B) levels were quantified by ELISA. Cells stimulated with medium alone and with LPS (1 μg/ml) served as negative (C−) and positive (C+) controls, respectively. Data are expressed as means with SEM (pg/ml) for at least three experiments with at least three technical replicates. **, P < 0.001.
Modulation of BAFF gene expression by DCs incubated with S. suis and GBS CPSs.
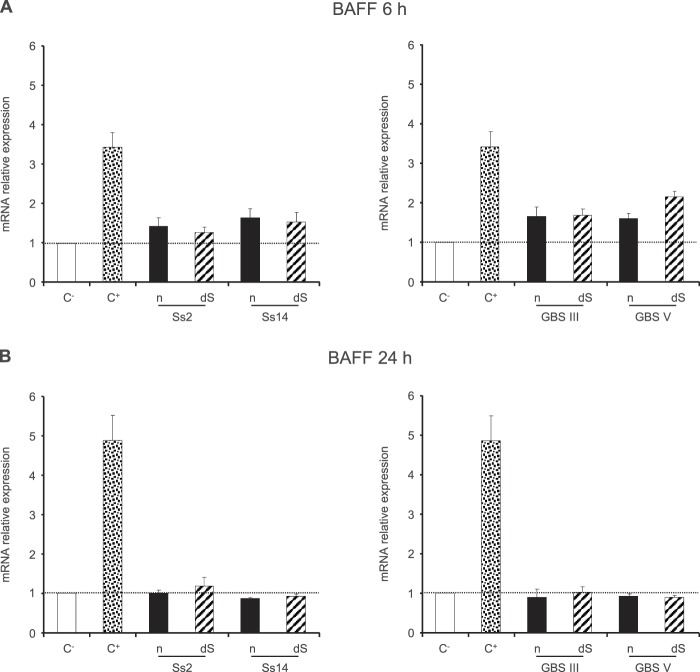
In contrast to the numerous studies that have focused on the effects of BAFF on B cell physiology, there is a relative paucity of evidence concerning BAFF production by DCs. A recent study has shown that the prototype TI antigen NP-Ficoll is able to induce the release of BAFF by murine DCs, which is essential for the development of Ab responses (58). To analyze whether S. suis and GBS CPSs modulate BAFF mRNA expression levels, DCs were stimulated with native CPSs at 6 and 24 h. IFN-γ (10 ng/ml), which is known to stimulate BAFF synthesis by monocytes, macrophages, and DCs (29, 59, 60), was used as a positive control. As shown in Fig. 8, no significant difference in BAFF expression levels was observed between CPS-activated DCs and control nonstimulated cells at either 6 or 24 h. Only a slight upregulation (<2-fold increase) of BAFF mRNA expression was observed at 6 h for all native CPS preparations. As sialic acid has been shown to inhibit B cell activation (61), we wanted to know if this sugar could be involved in the inhibition of BAFF expression as well. Sialic acid did not significantly modulate BAFF expression by DCs (Fig. 8).
Fig 8.
Relative expression of BAFF mRNA by DCs in response to stimulation by S. suis or GBS CPSs for 24 h. Native (n) or desialylated (dS) S. suis type 2 (Ss2), S. suis type 14 (Ss14), GBS type III, or GBS type V CPS (each at 200 μg/ml) was incubated with DCs (106 cells/ml). After 6 h (A) or 24 h (B) of incubation, cells were collected and BAFF mRNA expression was determined by RT-qPCR. Cells stimulated with IFN-γ (10 ng/ml) served as a positive control (C+). Data are expressed as means with SEM for at least three experiments and are relative to the level for DCs stimulated with medium alone (C−), which was arbitrarily fixed to 1.
To further evaluate the capacity of GBS and S. suis CPSs to inhibit BAFF expression by DCs, we evaluated the impact of DC preincubation with CPS on IFN-γ-induced BAFF. To this end, DCs were precultured with the different CPSs for 6 h prior to stimulation with IFN-γ for 24 h. Diminutions of BAFF mRNA expression ranging from 20 to 40% were observed when DCs were preincubated with either S. suis or GBS CPSs in comparison with nonpretreated cells, confirming the inhibitory effect of these bacterial CPSs on BAFF expression (Fig. 9).
Fig 9.
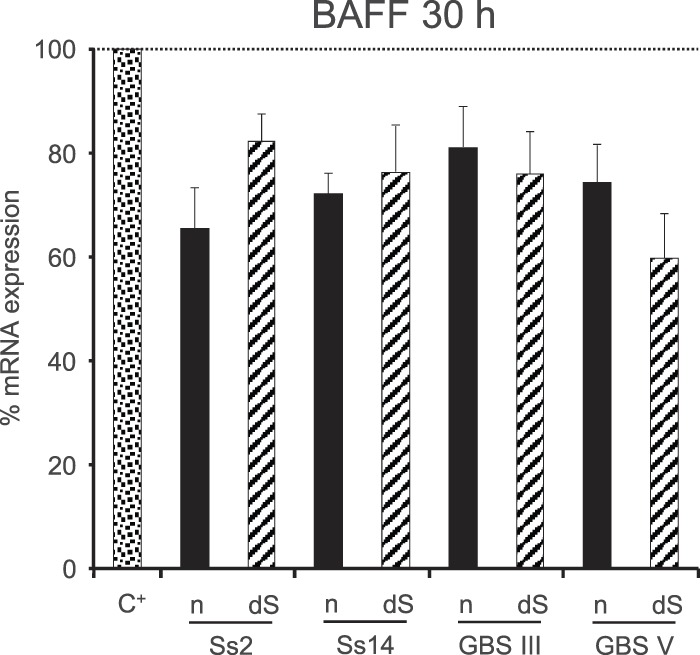
Effect of preincubation of DCs with S. suis or GBS CPSs on IFN-γ-induced expression of BAFF mRNA. DCs (106 cells/ml) were prestimulated with native (n) or desialylated (dS) S. suis type 2 (Ss2), S. suis type 14 (Ss14), GBS type III, or GBS type V CPS (each at 200 μg/ml) for 6 h prior to incubation with IFN-γ (10 ng/ml) for 24 h. Cells were then collected, and BAFF mRNA expression was determined by RT-qPCR. Cells prestimulated with medium alone before addition of IFN-γ served as a positive control (C+). Data are expressed as means with SEM for at least three experiments and are relative to the C+ level, which was arbitrarily fixed to 100%.
DISCUSSION
CPS is a crucial component for both S. suis and GBS. In addition to forming the basis for serotype designation and being the major virulence factor, it has, as an immunogen, a high protective potential in the fight against infections by these two streptococci. Indeed, as shown with other encapsulated bacteria, such as Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae, the specific anti-CPS humoral response could play a decisive role in host survival of S. suis or GBS infections. Previous studies have demonstrated that mouse or pig anti-S. suis type 2 CPS Abs have a protective role in homologous opsonophagocytosis in vitro assays (40, 62, 63) or during in vivo challenge (40, 64). Finally, Abs raised against several GBS CPS serotypes, including serotypes Ia, Ib, II, III, and V, are protective against neonatal infections in both animals and humans and exhibit opsonophagocytosis ability in vitro (65).
However, S. suis and GBS CPSs are poorly immunogenic molecules because they cannot recruit T cell help for B cell functions. Mothers of neonates developing GBS type III disease have low concentrations of anti-CPS Abs in sera at delivery (66), and very few anti-CPS Abs can be detected in pigs infected with S. suis type 2 (62, 64). To counter the low immunogenicity of CPSs, researchers have developed a conjugate vaccine composed of CPS linked to a carrier protein, as is the case for GBS. However, the efficacy of in-trial GBS conjugate vaccines depends on the CPS serotype included in the preparation. Indeed, CPSs of types Ia, Ib, II, and III induce strong protective IgG responses, whereas GBS type V CPS promotes higher concentrations of specific IgM than IgG (65). This suggests that the composition and/or structure of CPS could influence its immunogenicity. Certain structural features of CPSs, such as variations in repeating unit composition or glycosidic linkage positions, are susceptible to producing different immune responses (67–69).
Despite these observations, very few studies have been dedicated to the characterization of CPS activity on APCs and to the corresponding signaling mechanisms. Some bacterial CPS motifs, including sialic acid, are very similar to molecules expressed by human cells or tissues, which could result in immune evasion through molecular mimicry (69). Therefore, chemical alteration of sialic acid of N. meningitidis type B CPS by de-N-acetylation or deletion of this sugar in GBS type V CPS improved the immunogenicity of these two CPSs (50, 70). However, the consequences of capsular sialic acid manipulation on the capacity of CPS to activate the immune system have been evaluated only in the context of Ab production. The mechanisms underlying this effect, such as the modulation of the function of APCs, are poorly known.
In this article, we analyzed the ability of S. suis and GBS CPSs to activate DCs as determined by production of cytokines and chemokines susceptible to being involved in TI responses. Mouse-origin DCs were used because mouse models are well described for both pathogens and because in vitro interactions of GBS or S. suis with mouse-, human-, and/or swine-origin cells show similar results (12, 14, 15, 53, 54, 71, 72). We used native and desialylated CPSs isolated from two of the most virulent and frequently isolated serotypes in humans for each bacterial species in order to examine the role of CPS composition in modulation of DC activation. The capsular preparations underwent a series of rigorous physicochemical and immunologic quality control tests, which attested the high purity of the CPSs, the preservation of epitope recognition, and the absence of sialic acid in the desialylated preparations. Our results demonstrated that native S. suis type 2 and 14 and GBS type III and V CPSs do not induce the release of key proinflammatory cytokines, which confirms the poor immunogenic nature of these molecules. This is in accordance with previous experiments where S. suis type 2 CPS did not induce the production of either IL-1β, IL-6, or TNF-α by human monocytes or murine macrophages (53, 73). However, our results are in contradiction with earlier studies which showed that GBS type III CPS stimulated the production of TNF-α and IL-6 by human cord blood monocytes (74, 75). This difference could be explained by the use of different cells or by variations in the purification method. Nevertheless, the authors of those studies indicated that GBS type III CPS was a poor stimulator compared to other bacterial cell wall components (74, 75). Other studies using CPSs purified from other bacterial species have concluded likewise, that such a molecule is a poor activator of the immune system. S. pneumoniae CPS incubated with human monocyte-derived DCs produced no or very small amounts of IL-12 or IL-10 (76). Similarly, N. meningitidis type C CPS was unable to induce production of IL-6 and TNF-α by human monocytes or macrophages (77). By using desialylated CPSs, we demonstrated for the first time that sialic acid does not seem to play an inhibitory role in the release of proinflammatory cytokines by DCs. The presence of sialic acid has been linked to production of the regulatory cytokine IL-10 (78, 79). However, sialic acid in either GBS CPSs or S. suis CPSs did not result in increased IL-10 production by DCs. Thus, an imbalance between pro- and anti-inflammatory cytokine profiles cannot explain the poorly DC-stimulatory properties of either GBS or S. suis CPSs.
Interestingly, S. suis and GBS CPSs remarkably stimulated DC production of two members of the CC family of chemokines, i.e., CCL2 and CCL3. These two chemokines are known to play a major role in the selective recruitment of monocytes, macrophages, DCs, and lymphocytes to sites of inflammation (80, 81). A high level of CCL2 in the central nervous system is a characteristic of patients with bacterial meningitis (82). Systemic production of this chemokine, as well as its expression in the brain, is a feature of S. suis type 2-infected mice (83), and CCL2 has been associated with clinical signs of GBS sepsis in neonates (84). Recently, whole S. suis type 2 was shown to induce CCL2 production by murine DCs (12) and CCL3 production by total mouse splenocytes (unpublished data). Similarly, studies have shown that whole GBS type III stimulates CCL2 and CCL3 secretion by murine macrophages and/or DCs (85, 86). Our observations with purified CPSs allow a better interpretation of previous data showing different patterns of chemokine production obtained with total leukocytes, monocytes, and/or DCs cultured in the presence of whole S. suis type 2 or GBS types III and V and their respective nonencapsulated mutants. Indeed, in these studies, production of CCL2 and/or CCL3 was significantly diminished with bacteria lacking CPS (53, 72, 86), suggesting an important role of CPS in contributing to the production of these chemokines. Our observations are analogous to those of antecedent studies where S. suis type 2 CPS was able to induce the expression of CCL2 mRNA in a porcine whole-blood culture system and the release of this chemokine by human monocytes and murine macrophages (53, 72). Other studies have reported that purified CPSs from N. meningitidis, Porphyromonas gingivalis, and Bacteroides fragilis stimulate liberation of chemokines by murine macrophages or DCs (25–27). Surprisingly, differences in composition or structure between S. suis and GBS CPSs or the presence of sialic acid did not deeply influence the release of chemokine by DCs.
It is well known that PRRs, including TLRs, are involved in the activation of immune cells by encapsulated bacteria and/or their purified CPSs (27, 57, 87). Our results indicate that the production of CCL2 by native or desialylated S. suis or GBS CPSs is independent of TLR2- or MyD88-related pathways. Similarly, production of CCL2 by murine macrophages incubated with S. suis type 2 CPS has previously been shown to be TLR2 and MyD88 independent (53). Macrophage expression of CCL2 induced by whole GBS type III has also been reported to be TLR2 independent (55).
While CCL2 and CCL3 are two members of the CC family of chemokines presenting similarities in the regulation of their synthesis as well as in their biological functions, CCL3 production induced by S. suis or GBS CPSs was significantly diminished with MyD88−/− DCs and partially affected with TLR2−/− DCs. Interestingly, and in contrast to the case for CCL2, macrophage expression of CCL3 induced by whole GBS type III has been reported to be TLR2 dependent (55). While differential expression of these two chemokines has been reported in other systems (88), the underlying regulatory mechanisms are unknown. Nevertheless, the partial inhibition of CCL3 production in TLR2−/− or MyD88−/− DCs suggests that other TLRs as well as MyD88-independent pathways may be implicated in chemokine release by S. suis or GBS CPSs. The concept of direct interaction of CPSs with TLRs is still controversial. Although we used highly purified CPS material undergoing strict quality controls, the possibility of undetected contamination by traces of lipoproteins cannot be excluded. Cells deficient in TLR2 might also fail to express adequate levels of a receptor that may be relevant for CPS recognition. Members of the large family of lectin receptors are other possible receptor candidates (78, 89) and warrant further investigations.
CPS antigens, with few exceptions, are considered TI antigens. It is well known that the TNF family member BAFF plays a crucial role in the immune response against these antigens. Whereas several studies have shown that the TLR ligand LPS or the prototype TI antigen NP-Ficoll stimulates expression or production of BAFF by total splenocytes, macrophages, or DCs (29, 58, 60, 90), there is a relative paucity of evidence concerning BAFF induction by CPSs. In our study, we observed that S. suis and GBS CPSs were unable to induce a significant expression of BAFF by DCs in comparison with that in unstimulated cells. This is in accordance with a previous study where N. meningitidis type C and GBS type V CPSs did not promote the release of BAFF by murine DCs (58), and it confirms the poor immunogenicity of CPSs. We observed that CPSs presented a suppressive effect on the capacity of IFN-γ to induce BAFF expression, and some CPSs were able to provoke an inhibition of up to 40%. Similarly, N. meningitidis type C and GBS type V CPSs have been shown to inhibit intracellular and extracellular levels of IFN-γ-induced BAFF in murine DCs (58). We evaluated for the first time the influence of sialic acid on this effect. However, sialylation did not play a major role in modulation of BAFF expression. The negative regulatory function of CPSs is not observed only on BAFF mRNA synthesis. The presence of CPS impairs cytokine release by DCs and macrophages activated by S. suis (12, 15, 53, 71). The inhibitory effect of CPS is not specific to cytokine expression but affects other cell functions, such as the expression of costimulatory molecules and major histocompatibility complex class II by DCs (15). S. suis type 2 CPS has been shown to downmodulate phagocytosis by destabilizing lipid microdomains and inhibiting activation of signaling pathways involved in phagocytosis (91, 92), whereas GBS type III CPS impairs bactericidal functions of neutrophils (78).
In conclusion, we found that highly purified CPSs isolated from two distinct serotypes of two different Gram-positive streptococci, S. suis and GBS, were principally poorly immunogenic antigens. However, they were able to specifically induce production of CCL2 and CCL3 by DCs. TLR2 and other MyD88-dependent pathways are partially involved in recognition of these CPSs, which might also implicate a more complex cross talk with other receptors. Interestingly, the effect of CPS composition (including sialic acid) and structure on DC function was less marked than that previously reported for B cell activation (68, 69) or than the observed variations in CPS recognition by specific sera in our dot-ELISA analysis. Thus, DCs seem to recognize and respond to these CPSs in a “patterned” way rather than a structure-dictated manner, which is in agreement with the role of the innate immune system. Further studies on the impact of DC activation status on B cell responses to these TI antigens are guaranteed.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported mainly by the Natural Sciences and Engineering Research Council of Canada (NSERC) through a grant to M.S. (grant 342150-07) and in part by a Canadian Institutes of Health Research grant and by the Fonds de Recherche du Québec–Nature et Technologies (FRQNT)–New Initiatives program to M.S. and M.-R.V.C.
Footnotes
Published ahead of print 17 June 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00113-13.
REFERENCES
- 1.Edwards MS, Baker CJ. 2005. Group B streptococcal infections in elderly adults. Clin. Infect. Dis. 41:839–847 [DOI] [PubMed] [Google Scholar]
- 2.Koenig JM, Keenan WJ. 2009. Group B Streptococcus and early-onset sepsis in the era of maternal prophylaxis. Pediatr. Clin. North Am. 56:689–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blumberg HM, Stephens DS, Modansky M, Erwin M, Elliot J, Facklam RR, Schuchat A, Baughman W, Farley MM. 1996. Invasive group B streptococcal disease: the emergence of serotype V. J. Infect. Dis. 173:365–373 [DOI] [PubMed] [Google Scholar]
- 4.Gottschalk M, Xu J, Calzas C, Segura M. 2010. Streptococcus suis: a new emerging or an old neglected zoonotic pathogen? Future Microbiol. 5:371–391 [DOI] [PubMed] [Google Scholar]
- 5.Segura M. 2009. Streptococcus suis: an emerging human threat. J. Infect. Dis. 199:4–6 [DOI] [PubMed] [Google Scholar]
- 6.Gottschalk M, Segura M, Xu J. 2007. Streptococcus suis infections in humans: the Chinese experience and the situation in North America. Anim. Health Res. Rev. 8:29–45 [DOI] [PubMed] [Google Scholar]
- 7.Kerdsin A, Oishi K, Sripakdee S, Boonkerd N, Polwichai P, Nakamura S, Uchida R, Sawanpanyalert P, Dejsirilert S. 2009. Clonal dissemination of human isolates of Streptococcus suis serotype 14 in Thailand. J. Med. Microbiol. 58:1508–1513 [DOI] [PubMed] [Google Scholar]
- 8.Cieslewicz MJ, Chaffin D, Glusman G, Kasper D, Madan A, Rodrigues S, Fahey J, Wessels MR, Rubens CE. 2005. Structural and genetic diversity of group B Streptococcus capsular polysaccharides. Infect. Immun. 73:3096–3103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fittipaldi N, Segura M, Grenier D, Gottschalk M. 2012. Virulence factors involved in the pathogenesis of the infection caused by the swine pathogen and zoonotic agent Streptococcus suis. Future Microbiol. 7:259–279 [DOI] [PubMed] [Google Scholar]
- 10.Van Calsteren MR, Gagnon F, Lacouture S, Fittipaldi N, Gottschalk M. 2010. Structure determination of Streptococcus suis serotype 2 capsular polysaccharide. Biochem. Cell Biol. 88:513–525 [DOI] [PubMed] [Google Scholar]
- 11.Van Calsteren MR, Calzas C, Goyette-Desjardins G, Okura M, Takamatsu D, Gottschalk M, Segura M. 2013. Structure determination of Streptococcus suis serotype 14 capsular polysaccharide. Biochem. Cell Biol. 91:49–58 [DOI] [PubMed] [Google Scholar]
- 12.Lecours MP, Gottschalk M, Houde M, Lemire P, Fittipaldi N, Segura M. 2011. Critical role for Streptococcus suis cell wall modifications and suilysin in resistance to complement-dependent killing by dendritic cells. J. Infect. Dis. 204:919–929 [DOI] [PubMed] [Google Scholar]
- 13.Lemire P, Houde M, Lecours MP, Fittipaldi N, Segura M. 2012. Role of capsular polysaccharide in group B Streptococcus interactions with dendritic cells. Microbes Infect. 14:1064–1076 [DOI] [PubMed] [Google Scholar]
- 14.Segura MA, Cleroux P, Gottschalk M. 1998. Streptococcus suis and group B Streptococcus differ in their interactions with murine macrophages. FEMS Immunol. Med. Microbiol. 21:189–195 [DOI] [PubMed] [Google Scholar]
- 15.Lecours MP, Segura M, Lachance C, Mussa T, Surprenant C, Montoya M, Gottschalk M. 2011. Characterization of porcine dendritic cell response to Streptococcus suis. Vet. Res. 42:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi S, Aoyagi Y, Adderson EE, Okuwaki Y, Bohnsack JF. 1999. Capsular sialic acid limits C5a production on type III group B streptococci. Infect. Immun. 67:1866–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crocker PR, Paulson JC, Varki A. 2007. Siglecs and their roles in the immune system. Nat. Rev. Immunol. 7:255–266 [DOI] [PubMed] [Google Scholar]
- 18.Chaffin DO, Mentele LM, Rubens CE. 2005. Sialylation of group B streptococcal capsular polysaccharide is mediated by cpsK and is required for optimal capsule polymerization and expression. J. Bacteriol. 187:4615–4626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lecours MP, Fittipaldi N, Takamatsu D, Okura M, Segura M, Goyette-Desjardins G, Van Calsteren MR, Gottschalk M. 2012. Sialylation of Streptococcus suis serotype 2 is essential for capsule expression but is not responsible for the main capsular epitope. Microbes Infect. 14:941–950 [DOI] [PubMed] [Google Scholar]
- 20.Takeuchi O, Akira S. 2010. Pattern recognition receptors and inflammation. Cell 140:805–820 [DOI] [PubMed] [Google Scholar]
- 21.Steinman RM. 1991. The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 9:271–296 [DOI] [PubMed] [Google Scholar]
- 22.Mond JJ, Vos Q, Lees A, Snapper CM. 1995. T cell independent antigens. Curr. Opin. Immunol. 7:349–354 [DOI] [PubMed] [Google Scholar]
- 23.Weintraub A. 2003. Immunology of bacterial polysaccharide antigens. Carbohydr. Res. 338:2539–2547 [DOI] [PubMed] [Google Scholar]
- 24.Simpson SQ, Singh R, Bice DE. 1994. Heat-killed pneumococci and pneumococcal capsular polysaccharides stimulate tumor necrosis factor-α production by murine macrophages. Am. J. Respir. Cell Mol. Biol. 10:284–289 [DOI] [PubMed] [Google Scholar]
- 25.Wang Q, McLoughlin RM, Cobb BA, Charrel-Dennis M, Zaleski KJ, Golenbock D, Tzianabos AO, Kasper DL. 2006. A bacterial carbohydrate links innate and adaptive responses through Toll-like receptor 2. J. Exp. Med. 203:2853–2863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.d'Empaire G, Baer MT, Gibson FC., 3rd 2006. The K1 serotype capsular polysaccharide of Porphyromonas gingivalis elicits chemokine production from murine macrophages that facilitates cell migration. Infect. Immun. 74:6236–6243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zughaier SM. 2011. Neisseria meningitidis capsular polysaccharides induce inflammatory responses via TLR2 and TLR4-MD-2. J. Leukoc. Biol. 89:469–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soell M, Diab M, Haan-Archipoff G, Beretz A, Herbelin C, Poutrel B, Klein JP. 1995. Capsular polysaccharide types 5 and 8 of Staphylococcus aureus bind specifically to human epithelial (KB) cells, endothelial cells, and monocytes and induce release of cytokines. Infect. Immun. 63:1380–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, Cerutti A. 2002. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat. Immunol. 3:822–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Craxton A, Magaletti D, Ryan EJ, Clark EA. 2003. Macrophage- and dendritic cell-dependent regulation of human B-cell proliferation requires the TNF family ligand BAFF. Blood 101:4464–4471 [DOI] [PubMed] [Google Scholar]
- 31.Jacques M, Gottschalk M, Foiry B, Higgins R. 1990. Ultrastructural study of surface components of Streptococcus suis. J. Bacteriol. 172:2833–2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gottschalk M, Higgins R, Jacques M, Mittal KR, Henrichsen J. 1989. Description of 14 new capsular types of Streptococcus suis. J. Clin. Microbiol. 27:2633–2636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuypers JM, Heggen LM, Rubens CE. 1989. Molecular analysis of a region of the group B Streptococcus chromosome involved in type III capsule expression. Infect. Immun. 57:3058–3065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Michon F. June 2001. Procedures for the extraction and isolation of bacterial capsular polysaccharides for use as vaccines or linked to proteins as conjugate vaccines. US patent 6,248,570 B1
- 35.Brandrup J, Immergut EH, Grulke EA. 2005. Polymer handbook, 4th ed. John Wiley & Sons, New York, NY [Google Scholar]
- 36.Gornik O, Lauc G. 2007. Enzyme linked lectin assay (ELLA) for direct analysis of transferrin sialylation in serum samples. Clin. Biochem. 40:718–723 [DOI] [PubMed] [Google Scholar]
- 37.Shibuya N, Goldstein IJ, Broekaert WF, Nsimba-Lubaki M, Peeters B, Peumans WJ. 1987. The elderberry (Sambucus nigra L.) bark lectin recognizes the Neu5Ac(α2–6)Gal/GalNAc sequence. J. Biol. Chem. 262:1596–1601 [PubMed] [Google Scholar]
- 38.Geisler C, Jarvis DL. 2011. Effective glycoanalysis with Maackia amurensis lectins requires a clear understanding of their binding specificities. Glycobiology 21:988–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller RL, Collawn JF, Jr, Fish WW. 1982. Purification and macromolecular properties of a sialic acid-specific lectin from the slug Limax flavus. J. Biol. Chem. 257:7574–7580 [PubMed] [Google Scholar]
- 40.Charland N, Jacques M, Lacouture S, Gottschalk M. 1997. Characterization and protective activity of a monoclonal antibody against a capsular epitope shared by Streptococcus suis serotypes 1, 2 and 1/2. Microbiology 143:3607–3614 [DOI] [PubMed] [Google Scholar]
- 41.Higgins R, Gottschalk M. 1990. An update on Streptococcus suis identification. J. Vet. Diagn. Invest. 2:249–252 [DOI] [PubMed] [Google Scholar]
- 42.Animal Welfare Committee of the Université de Montréal 2012. Guide for the care and use of laboratory animals. Animal Welfare Committee of the Université de Montréal, Montréal, Canada [Google Scholar]
- 43.Segura M, Su Z, Piccirillo C, Stevenson MM. 2007. Impairment of dendritic cell function by excretory-secretory products: a potential mechanism for nematode-induced immunosuppression. Eur. J. Immunol. 37:1887–1904 [DOI] [PubMed] [Google Scholar]
- 44.van den Berg TK, Kraal G. 2005. A function for the macrophage F4/80 molecule in tolerance induction. Trends Immunol. 26:506–509 [DOI] [PubMed] [Google Scholar]
- 45.MacDonald AS, Straw AD, Bauman B, Pearce EJ. 2001. CD8-dendritic cell activation status plays an integral role in influencing Th2 response development. J. Immunol. 167:1982–1988 [DOI] [PubMed] [Google Scholar]
- 46.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. 1999. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 223:77–92 [DOI] [PubMed] [Google Scholar]
- 47.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 48.Deng L, Kasper DL, Krick TP, Wessels MR. 2000. Characterization of the linkage between the type III capsular polysaccharide and the bacterial cell wall of group B Streptococcus. J. Biol. Chem. 275:7497–7504 [DOI] [PubMed] [Google Scholar]
- 49.Gonzalez-Outeirino J, Kadirvelraj R, Woods RJ. 2005. Structural elucidation of type III group B Streptococcus capsular polysaccharide using molecular dynamics simulations: the role of sialic acid. Carbohydr. Res. 340:1007–1018 [DOI] [PubMed] [Google Scholar]
- 50.Guttormsen HK, Paoletti LC, Mansfield KG, Jachymek W, Jennings HJ, Kasper DL. 2008. Rational chemical design of the carbohydrate in a glycoconjugate vaccine enhances IgM-to-IgG switching. Proc. Natl. Acad. Sci. U. S. A. 105:5903–5908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jennings HJ, Lugowski C, Kasper DL. 1981. Conformational aspects critical to the immunospecificity of the type III group B streptococcal polysaccharide. Biochemistry 20:4511–4518 [DOI] [PubMed] [Google Scholar]
- 52.Knibbs RN, Goldstein IJ, Ratcliffe RM, Shibuya N. 1991. Characterization of the carbohydrate binding specificity of the leukoagglutinating lectin from Maackia amurensis. Comparison with other sialic acid-specific lectins. J. Biol. Chem. 266:83–88 [PubMed] [Google Scholar]
- 53.Graveline R, Segura M, Radzioch D, Gottschalk M. 2007. TLR2-dependent recognition of Streptococcus suis is modulated by the presence of capsular polysaccharide which modifies macrophage responsiveness. Int. Immunol. 19:375–389 [DOI] [PubMed] [Google Scholar]
- 54.Lecours MP, Segura M, Fittipaldi N, Rivest S, Gottschalk M. 2012. Immune receptors involved in Streptococcus suis recognition by dendritic cells. PLoS One 7:e44746. 10.1371/journal.pone.0044746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Draper DW, Bethea HN, He YW. 2006. Toll-like receptor 2-dependent and -independent activation of macrophages by group B streptococci. Immunol. Lett. 102:202–214 [DOI] [PubMed] [Google Scholar]
- 56.Henneke P, Takeuchi O, van Strijp JA, Guttormsen HK, Smith JA, Schromm AB, Espevik TA, Akira S, Nizet V, Kasper DL, Golenbock DT. 2001. Novel engagement of CD14 and multiple Toll-like receptors by group B streptococci. J. Immunol. 167:7069–7076 [DOI] [PubMed] [Google Scholar]
- 57.Um SH, Rhee DK, Pyo S. 2002. Involvement of protein kinase C and tyrosine kinase in tumoricidal activation of macrophage induced by Streptococcus pneumoniae type II capsular polysaccharide. Int. Immunopharmacol. 2:129–137 [DOI] [PubMed] [Google Scholar]
- 58.Kanswal S, Katsenelson N, Allman W, Uslu K, Blake MS, Akkoyunlu M. 2011. Suppressive effect of bacterial polysaccharides on BAFF system is responsible for their poor immunogenicity. J. Immunol. 186:2430–2443 [DOI] [PubMed] [Google Scholar]
- 59.Kim HA, Jeon SH, Seo GY, Park JB, Kim PH. 2008. TGF-β1 and IFN-γ stimulate mouse macrophages to express BAFF via different signaling pathways. J. Leukoc. Biol. 83:1431–1439 [DOI] [PubMed] [Google Scholar]
- 60.Nardelli B, Belvedere O, Roschke V, Moore PA, Olsen HS, Migone TS, Sosnovtseva S, Carrell JA, Feng P, Giri JG, Hilbert DM. 2001. Synthesis and release of B-lymphocyte stimulator from myeloid cells. Blood 97:198–204 [DOI] [PubMed] [Google Scholar]
- 61.Courtney AH, Puffer EB, Pontrello JK, Yang ZQ, Kiessling LL. 2009. Sialylated multivalent antigens engage CD22 in trans and inhibit B cell activation. Proc. Natl. Acad. Sci. U. S. A. 106:2500–2505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elliott SD, Clifton-Hadley F, Tai J. 1980. Streptococcal infection in young pigs. V. An immunogenic polysaccharide from Streptococcus suis type 2 with particular reference to vaccination against streptococcal meningitis in pigs. J. Hyg. (Lond.) 85:275–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baums CG, Kock C, Beineke A, Bennecke K, Goethe R, Schroder C, Waldmann KH, Valentin-Weigand P. 2009. Streptococcus suis bacterin and subunit vaccine immunogenicities and protective efficacies against serotypes 2 and 9. Clin. Vaccine Immunol. 16:200–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.del Campo Sepulveda EM, Altman E, Kobisch M, D'Allaire S, Gottschalk M. 1996. Detection of antibodies against Streptococcus suis capsular type 2 using a purified capsular polysaccharide antigen-based indirect ELISA. Vet. Microbiol. 52:113–125 [DOI] [PubMed] [Google Scholar]
- 65.Heath PT. 2011. An update on vaccination against group B Streptococcus. Expert Rev. Vaccines 10:685–694 [DOI] [PubMed] [Google Scholar]
- 66.Baker CJ, Kasper DL. 1976. Correlation of maternal antibody deficiency with susceptibility to neonatal group B streptococcal infection. N. Engl. J. Med. 294:753–756 [DOI] [PubMed] [Google Scholar]
- 67.Avci FY, Kasper DL. 2010. How bacterial carbohydrates influence the adaptive immune system. Annu. Rev. Immunol. 28:107–130 [DOI] [PubMed] [Google Scholar]
- 68.Wessels MR, Paoletti LC, Rodewald AK, Michon F, DiFabio J, Jennings HJ, Kasper DL. 1993. Stimulation of protective antibodies against type Ia and Ib group B streptococci by a type Ia polysaccharide-tetanus toxoid conjugate vaccine. Infect. Immun. 61:4760–4766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Finne J, Leinonen M, Makela PH. 1983. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet ii:355–357 [DOI] [PubMed] [Google Scholar]
- 70.Moe GR, Bhandari TS, Flitter BA. 2009. Vaccines containing de-N-acetyl sialic acid elicit antibodies protective against Neisseria meningitidis groups B and C. J. Immunol. 182:6610–6617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meijerink M, Ferrando ML, Lammers G, Taverne N, Smith HE, Wells JM. 2012. Immunomodulatory effects of Streptococcus suis capsule type on human dendritic cell responses, phagocytosis and intracellular survival. PLoS One 7:e35849. 10.1371/journal.pone.0035849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Segura M, Vanier G, Al-Numani D, Lacouture S, Olivier M, Gottschalk M. 2006. Proinflammatory cytokine and chemokine modulation by Streptococcus suis in a whole-blood culture system. FEMS Immunol. Med. Microbiol. 47:92–106 [DOI] [PubMed] [Google Scholar]
- 73.Segura M, Stankova J, Gottschalk M. 1999. Heat-killed Streptococcus suis capsular type 2 strains stimulate tumor necrosis factor alpha and interleukin-6 production by murine macrophages. Infect. Immun. 67:4646–4654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vallejo JG, Baker CJ, Edwards MS. 1996. Roles of the bacterial cell wall and capsule in induction of tumor necrosis factor alpha by type III group B streptococci. Infect. Immun. 64:5042–5046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vallejo JG, Baker CJ, Edwards MS. 1996. Interleukin-6 production by human neonatal monocytes stimulated by type III group B streptococci. J. Infect. Dis. 174:332–337 [DOI] [PubMed] [Google Scholar]
- 76.Meltzer U, Goldblatt D. 2006. Pneumococcal polysaccharides interact with human dendritic cells. Infect. Immun. 74:1890–1895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kocabas C, Katsenelson N, Kanswal S, Kennedy MN, Cui X, Blake MS, Segal DM, Akkoyunlu M. 2007. Neisseria meningitidis type C capsular polysaccharide inhibits lipooligosaccharide-induced cell activation by binding to CD14. Cell. Microbiol. 9:1297–1310 [DOI] [PubMed] [Google Scholar]
- 78.Carlin AF, Uchiyama S, Chang YC, Lewis AL, Nizet V, Varki A. 2009. Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil Siglec-9 and dampen the innate immune response. Blood 113:3333–3336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ando M, Tu W, Nishijima K, Iijima S. 2008. Siglec-9 enhances IL-10 production in macrophages via tyrosine-based motifs. Biochem. Biophys. Res. Commun. 369:878–883 [DOI] [PubMed] [Google Scholar]
- 80.Deshmane SL, Kremlev S, Amini S, Sawaya BE. 2009. Monocyte chemoattractant protein-1 (MCP-1): an overview. J. Interferon Cytokine Res. 29:313–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Maurer M, von Stebut E. 2004. Macrophage inflammatory protein-1. Int. J. Biochem. Cell Biol. 36:1882–1886 [DOI] [PubMed] [Google Scholar]
- 82.Lahrtz F, Piali L, Spanaus KS, Seebach J, Fontana A. 1998. Chemokines and chemotaxis of leukocytes in infectious meningitis. J. Neuroimmunol. 85:33–43 [DOI] [PubMed] [Google Scholar]
- 83.Dominguez-Punaro MC, Segura M, Plante MM, Lacouture S, Rivest S, Gottschalk M. 2007. Streptococcus suis serotype 2, an important swine and human pathogen, induces strong systemic and cerebral inflammatory responses in a mouse model of infection. J. Immunol. 179:1842–1854 [DOI] [PubMed] [Google Scholar]
- 84.Okazaki K, Kondo M, Kato M, Nishida A, Takahashi H, Noda M, Kimura H. 2008. Temporal alterations in concentrations of sera cytokines/chemokines in sepsis due to group B Streptococcus infection in a neonate. Jpn. J. Infect. Dis. 61:382–385 [PubMed] [Google Scholar]
- 85.Fan H, Williams DL, Zingarelli B, Breuel KF, Teti G, Tempel GE, Spicher K, Boulay G, Birnbaumer L, Halushka PV, Cook JA. 2007. Differential regulation of lipopolysaccharide and Gram-positive bacteria induced cytokine and chemokine production in macrophages by Gαi proteins. Immunology 122:116–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lemire P, Houde M, Segura M. 2012. Encapsulated group B Streptococcus modulates dendritic cell functions via lipid rafts and clathrin-mediated endocytosis. Cell. Microbiol. 14:1707–1719 [DOI] [PubMed] [Google Scholar]
- 87.Mogensen TH, Paludan SR, Kilian M, Ostergaard L. 2006. Live Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis activate the inflammatory response through Toll-like receptors 2, 4, and 9 in species-specific patterns. J. Leukoc. Biol. 80:267–277 [DOI] [PubMed] [Google Scholar]
- 88.Mitchell D, Olive C. 2010. Regulation of Toll-like receptor-induced chemokine production in murine dendritic cells by mitogen-activated protein kinases. Mol. Immunol. 47:2065–2073 [DOI] [PubMed] [Google Scholar]
- 89.Zamze S, Martinez-Pomares L, Jones H, Taylor PR, Stillion RJ, Gordon S, Wong SY. 2002. Recognition of bacterial capsular polysaccharides and lipopolysaccharides by the macrophage mannose receptor. J. Biol. Chem. 277:41613–41623 [DOI] [PubMed] [Google Scholar]
- 90.Moon EY, Lee JH, Oh SY, Ryu SK, Kim HM, Kwak HS, Yoon WK. 2006. Reactive oxygen species augment B-cell-activating factor expression. Free Radic. Biol. Med. 40:2103–2111 [DOI] [PubMed] [Google Scholar]
- 91.Houde M, Gottschalk M, Gagnon F, Van Calsteren MR, Segura M. 2012. Streptococcus suis capsular polysaccharide inhibits phagocytosis through destabilization of lipid microdomains and prevents lactosylceramide-dependent recognition. Infect. Immun. 80:506–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Segura M, Gottschalk M, Olivier M. 2004. Encapsulated Streptococcus suis inhibits activation of signaling pathways involved in phagocytosis. Infect. Immun. 72:5322–5330 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.