Abstract
Enterohemorrhagic Escherichia coli (EHEC) causes hemorrhagic colitis and life-threatening complications. The main reservoirs for EHEC are healthy ruminants. We reported that SdiA senses acyl homoserine lactones (AHLs) in the bovine rumen to activate expression of the glutamate acid resistance (gad) genes priming EHEC's acid resistance before they pass into the acidic abomasum. Conversely, SdiA represses expression of the locus of enterocyte effacement (LEE) genes, whose expression is not required for bacterial survival in the rumen but is necessary for efficient colonization at the rectoanal junction (RAJ) mucosa. Our previous studies show that SdiA-dependent regulation was necessary for efficient EHEC colonization of cattle fed a grain diet. Here, we compared the SdiA role in EHEC colonization of cattle fed a forage hay diet. We detected AHLs in the rumen of cattle fed a hay diet, and these AHLs activated gad gene expression in an SdiA-dependent manner. The rumen fluid and fecal samples from hay-fed cattle were near neutrality, while the same digesta samples from grain-fed animals were acidic. Cattle fed either grain or hay and challenged with EHEC orally carried the bacteria similarly. EHEC was cleared from the rumen within days and from the RAJ mucosa after approximately one month. In competition trials, where animals were challenged with both wild-type and SdiA deletion mutant bacteria, diet did not affect the outcome that the wild-type strain was better able to persist and colonize. However, the wild-type strain had a greater advantage over the SdiA deletion mutant at the RAJ mucosa among cattle fed the grain diet.
INTRODUCTION
Enterohemorrhagic Escherichia coli (EHEC) is responsible for major outbreaks and sporadic cases of hemorrhagic colitis and hemolytic uremic syndrome (HUS) throughout the world. Infection can begin after exposure to as few as 50 CFU. EHEC colonizes the large intestine of humans and forms attaching and effacing (AE) lesions on intestinal epithelial cells (1). The AE lesion is characterized by the destruction of the microvilli and rearrangement of the cytoskeleton to form pedestal-like structures that cup the bacteria (2–4). The genes involved in the formation of the AE lesion are encoded within a chromosomal pathogenicity island named the locus of enterocyte effacement (LEE) (5). The LEE region contains five major operons, LEE1, LEE2, LEE3, LEE5, and LEE4 (6–8), that encode a type III secretion system (TTSS) (9), an adhesin (intimin) (10) and its receptor (Tir) (11), and effector proteins, which together with Tir are translocated into the epithelial cell through the bacterial TTSS (12–16) (Fig. 1). The LEE-encoded TTSS also translocates effector proteins encoded outside the LEE region (NleA, B, C, D, E, and F and EspFu/TccP); these other effectors are also important for virulence and pedestal formation (17–23). Additionally, EHEC also requires the LEE to efficiently colonize the rectoanal junction (RAJ) mucosa of healthy cattle, the bacteria's main reservoir (24, 25).
Fig 1.
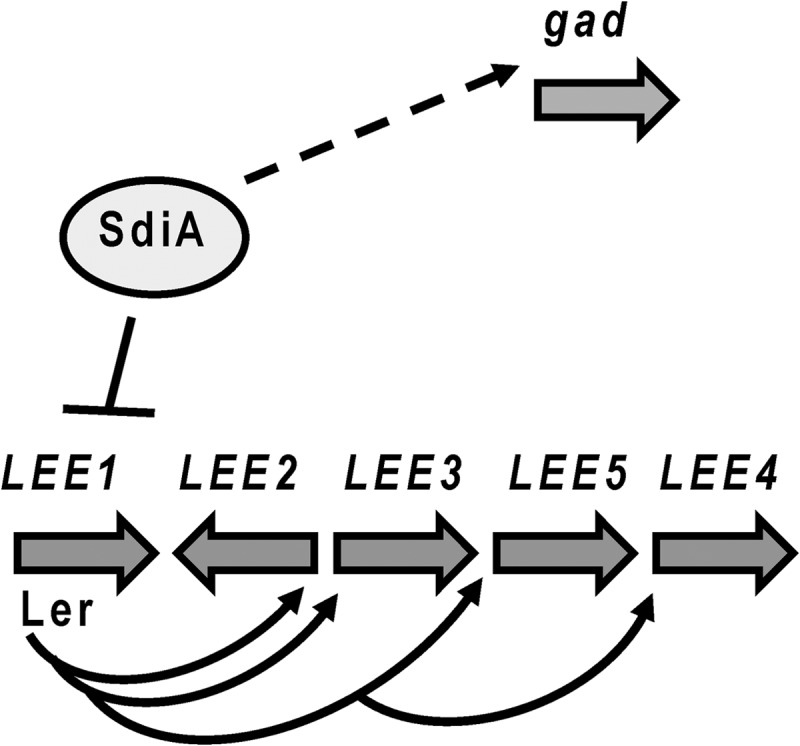
Schematic regulatory model of SdiA virulence gene regulation in EHEC. SdiA directly repressed expression of ler (solid line), encoding the master activator of all LEE genes. SdiA activates gad expression indirectly (dashed line).
Several studies have also shown that other genes beyond the LEE are needed for EHEC to efficiently colonize cattle. The glutamate decarboxylase (gad) acid resistance system is important for EHEC's survival and colonization of the bovine gastrointestinal tract (26). Our previous study shows that duodenal digesta from animals fed either a grain diet or a hay diet was acidic, with pH values ranging from 4.33 to 4.75 (27). Acid resistance is important for bacterial survival in the bovine gastrointestinal tract. We reported that SdiA senses acyl homoserine lactones (AHLs) in the rumen to activate expression of the glutamate acid resistance (gad) genes to induce EHEC's acid resistance before passing into the acidic abomasum stomach. A signature-tagged mutagenesis (STM) study also shows that several genes, including sdiA, play a role in the colonization of the bovine intestine (28). Our previous competition studies show that an EHEC sdiA mutant is defective for survival within the rumen and colonization of the RAJ mucosa among cattle fed a grain diet (29). SdiA is a LuxR-type transcription factor. LuxR transcription factors are part of the LuxR/I-type quorum sensing systems found in several Gram-negative bacteria. In these systems, the signaling molecule is usually an AHL. The LuxR/I system was the first to be described in Vibrio fischeri (30). The luciferase operon in Vibrio fischeri is regulated by two proteins, LuxI, which is responsible for the production of the AHL autoinducer, and LuxR, which is activated by this autoinducer to increase transcription of the luciferase operon (31, 32). Since this initial description, homologues of LuxR-LuxI have been identified in other bacteria and shown to regulate the transcription of several genes involved in a variety of phenotypes, including biofilm formation, regulation of several virulence genes, and production of pigments, among others (33–35). The LuxI-type proteins are the AHL synthases. AHLs have a conserved homoserine lactone ring connected through an amide bond to a variable acyl chain. Acyl chains vary among 4 to 18 carbons, and the third position may or may not be modified (carbonyl group, hydroxyl, or fully reduced). Different acyl chains ensure that different AHLs will be recognized by different LuxR-type proteins. The substrates used by LuxI-type proteins for AHL synthesis are S-adenosyl methionine (SAM) to synthesize the homoserine lactone ring, and the acyl chains are acquired from lipid metabolism, carried by various acyl carrier proteins (ACP) (36–38). The LuxR-type proteins usually recognize a specific AHL. Due to this feature, this signaling system has been associated primarily with intraspecies signaling. However, there are examples of LuxR-type proteins (such as SdiA) that recognize more than one AHL and are involved primarily in interspecies signaling (39–42).
AHLs have been detected in the rumen of cattle (29, 43, 44) but not in the other gastrointestinal (GI) tract compartments of these animals (29). Through SdiA, rumen AHLs repress transcription of the LEE and activate expression of the gad genes (Fig. 1), promoting EHEC colonization in cattle (29). It is assumed that these AHLs are being produced by the rumen microbiota. Our previous studies concerning the role of SdiA in EHEC cattle colonization were performed using animals fed a grain diet (29). It is known that dietary conditions can have profound effects in the composition of the GI microbiota (45). It is important to determine the role of SdiA in bacterial colonization of cattle fed different diets. Here, we showed that cattle fed a forage hay diet also harbor AHLs in their rumen and regulate EHEC SdiA-dependent gene expression in a fashion similar to that of cattle fed grain. In both grain and hay diets, SdiA was important for survival within the rumen and colonization of the RAJ mucosa, although SdiA's role in RAJ mucosa colonization seems to be more pronounced in animals fed a grain diet.
MATERIALS AND METHODS
Strains and plasmids.
EHEC strain 86-24 was isolated from a patient with bloody diarrhea (46). The sdiA mutant strain DH1 and complemented strain DH2 (sdiA gene cloned into pACYC177) have been previously reported (29). E. coli strains were grown aerobically in Luria-Bertani (LB) medium or Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) at 37°C unless otherwise stated. Antibiotics were added at the following concentrations: 100 μg ml−1 ampicillin, 30 μg ml−1 chloramphenicol, and 50 μg ml−1 kanamycin.
Quantitative real-time RT-PCR.
RNA was extracted from strains 86-24, DH1 (86-24 sdiA mutant), and DH2 (mutant complemented with sdiA), grown in DMEM at 37°C to an optical density at 600 nm (OD600) of 1.0 in the absence or presence of rumen-extracted AHLs, where 5 μl of the evaporated rumen extract was added to DMEM. RNA from three biological replicate cultures of each strain was extracted at the late exponential growth phase (OD600 of 1.0) using the RiboPure bacteria RNA isolation kit (Ambion) according to the manufacturer's guidelines. The LerrtF/LerrtR and GadXrtF/GadXrtR primers used in the real-time assays were designed using Primer Express version 1.5 (Applied Biosystems) and have been previously reported (29). Real-time reverse transcription-PCR (RT-PCR) was performed in a one-step reaction using an ABI 7500 sequence detection system (Applied Biosystems). For each 20-μl reaction mixture, 10 μl 2× SYBR master mix, 0.1 μl Multi-Scribe reverse transcriptase (Applied Biosystems), and 0.1 μl RNase inhibitor (Applied Biosystems) were added. Amplification efficiency of each of the primer pairs was verified using standard curves of known RNA concentrations. Melting-curve analysis was used to ensure template specificity by heating products to 95°C for 15 s, followed by cooling to 60°C and heating to 95°C while monitoring fluorescence. Once the amplification efficiency and template specificity were determined for each primer pair, relative quantification analysis was used to analyze the unknown samples using the following conditions for cDNA generation and amplification: 1 cycle at 48°C for 30 min, 1 cycle at 95°C for 10 min, and 40 cycles at 95°C for 15 s and 60°C for 1 min. The RNA polymerase subunit A gene (rpoA) was used as the endogenous control. Real-time RT-PCR primers for the LEE genes and rpoA have been previously described (47).
AHL extraction from rumen and detection of extracted AHL through TLC.
AHL extraction and detection were performed as previously described (48). Briefly, 30 ml of rumen fluid was extracted 2 times with dichloromethane and concentrated to 5 μl using a rotary evaporator. For analytical thin-layer chromatography (TLC), 5 μl of concentrated extract was applied to C18 reverse-phase TLC plates (200-μM layer; Whatman), and the chromatograms were developed with methanol-water (70:30, vol/vol). After development, the dried plates were overlaid with a culture of the Agrobacterium tumefaciens traI::lacZ (49) indicator strain and 80 μg/ml 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) for 16 h at 30°C. For preparative plates, 50 ml of GI content was extracted 2 times with dichloromethane and concentrated to 5 μl and the plates were spotted, but no chromatography was performed before overlay. The positive control, N-hexanoyl-l-homoserine lactone (C6-AHL) (402 pmol), was also included. For the alkali-acid treatment, 5 μl of rumen extract in dichloromethane was evaporated and 100 μl of 0.1 M NH4OH was added. After 3 h, 50 μl of the alkali-treated sample was evaporated, while 50 μl of 1 M HCl was added to the remaining 50 μl of alkali-treated sample. After 3 h, the alkali-acid sample was evaporated. This process was repeated with 15 pmol of C8-oxo-AHL. A total of 5 μl of dichloromethane was added back to all samples, and analytical TLC was performed.
Animals and diet.
All protocols were approved by the Institutional Animal Care and Use and Biosafety Committees at the University of Idaho. Eight 15-month-old Charolais heifers were surgically fitted with ruminal cannulae and identified with ear tags. The animals were housed in an isolation barn in separate containment pens and had free access to salt blocks and water. The experiment was divided into two parts based on different diet regimens. Survival and persistence of the EHEC sdiA mutant (DH1) was tested in the same cattle fed a high-grain diet (previously published [29]) or a hay diet. Cattle were acclimated for 1 month to either a grain diet containing 90% corn and 10% alfalfa hay (29) or, subsequently, after the first experiment was completed and cattle were no longer shedding EHEC, to a forage-based diet containing 90% alfalfa hay and 10% grain prior to bacterial challenge. The forage-based diet is referred to throughout as the hay diet. Rumen fluid and fecal pH values were measured using a Φ45 pH meter (Beckmann Instruments, Fullerton, CA).
Bacterial strains and challenge method.
EHEC strain 86-24 (stx deletion and streptomycin resistance) and its derivative sdiA mutant (streptomycin and chloramphenicol resistance, strain DH1) were used in this trial. There was no difference in the growth rates of the parental and the sdiA mutant strains in LB broth. The parental and mutant bacteria were grown separately for 18 h at 37°C in LB broth with aeration, adjusted to the same OD600, and mixed in a 1:1 ratio prior to cattle challenge. Each animal received a single dose of 1010 CFU containing equal numbers of the parental and sdiA mutant strains placed directly into the rumen.
Sampling and culture.
Rumen fluid was sampled from each heifer prior to bacterial challenge and thereafter as indicated in Results. Approximately 30 ml of rumen fluid was obtained from rumen samples collected aseptically with a sterile gloved hand from each heifer. Rumen fluid was filtered through three layers of cheese cloth into a sterile 50-ml tube and held on ice for transport to the laboratory for culture.
Rectoanal junction mucosal swab (RAMS) samples were obtained as previously described (50, 51) prior to bacterial challenge and on the days indicated. Briefly, a sterile foam swab was inserted into the rectum of each steer and moved 10 times in a circular motion around the RAJ mucosa. Each swab was placed in 3 ml Trypticase soy broth (TSB) and held on ice for transport to the laboratory.
E. coli O157 in the bovine samples were cultured and identified using MacConkey sorbitol agar (Difco, Sparks, MD) plates supplemented with 50 ng/ml cefixime (Sigma, St. Louis, MO), 2.5 μg/ml potassium tellurite (Sigma), 40 μg/ml vancomycin (Sigma), and 0.1 mg/ml 4-methylumbelliferyl-β-d-glucuronide (MUG) (Biosynth, Switzerland) (SMAC-CTVM). TSB was used for enrichment culture, as in our previous publications (50, 51). E. coli O157:H7 colonies were identified as sorbitol negative and MUG negative and confirmed to be of the O157 serotype by latex agglutination. The numerical data obtained by this procedure are noted as CFU/ml from rumen fluid or CFU/swab from RAMS. Samples negative by direct culture were enriched by incubation at 37°C overnight with aeration, and serial dilutions were plated on SMAC-CTVM. Positive enrichment cultures were given a numerical value of <10 CFU.
After isolation on SMAC-CTMV, the ratio of parental wild-type (WT) to mutant CFU in samples was determined by antibiotic resistance using SMAC-streptomycin and SMAC-chloramphenicol agar. At least 20 separate isolates from each sample were characterized. The competitive index for isolates from each heifer at each sampling time was determined by dividing the number of mutant isolates by the number of WT isolates. Output and input ratios were compared using a Mann-Whitney test. A P value of <0.05 was considered significant.
RESULTS
AHLs are present in the rumen of cattle fed a hay diet.
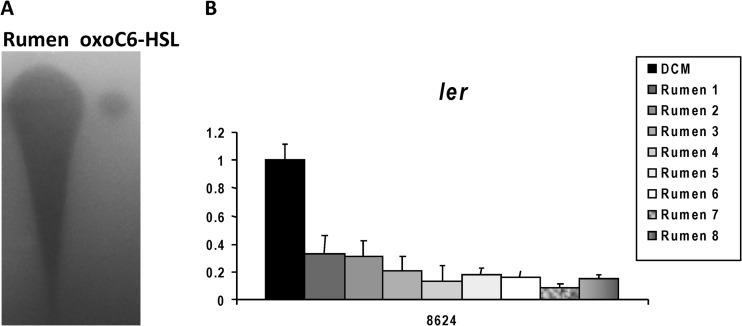
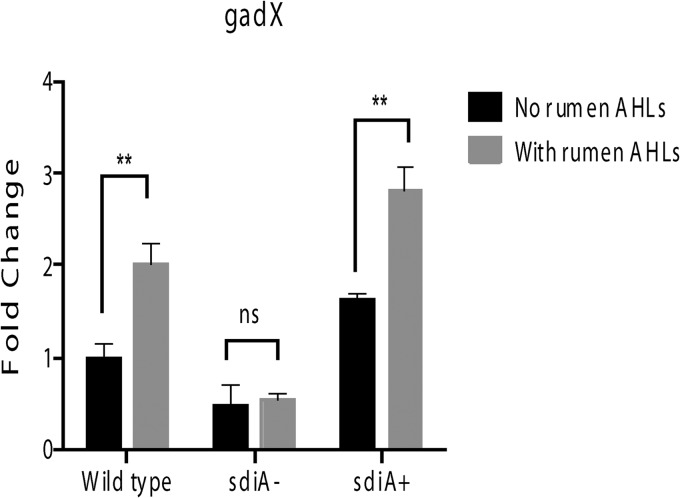
Our previous studies with cattle fed a grain diet show that AHLs are present in rumen fluid and that these AHLs activate transcription of the gad genes in an SdiA-dependent fashion (29). Because changes in diet can profoundly affect the composition of the GI microbiota (45, 52), which we assume is responsible for the AHL produced within the ruminant rumen, we wanted to assess whether AHLs are present in the rumen of the same heifers fed a forage hay diet. Figure 2A shows the presence of AHLs in the rumen, and the AHLs extracted from the eight different rumen samples repressed expression of ler in WT EHEC (Fig. 2B). Rumen-extracted AHLs also activated expression of gadX (the major regulator of the gad genes) in WT EHEC but not in the sdiA mutant. This regulation was rescued with gene complementation (Fig. 3).
Fig 2.
AHLs are also present within the rumen of hay-fed cattle. (A) TLC from AHLs extracted from 30 ml (evaporated to 10 μl, which was added to the TLC) of rumen fluid and the positive control, oxo-C6-HSL. (B) Quantitative RT-PCR (qRT-PCR) of ler in the absence (DCM, dichloromethane) and presence of rumen AHLs.
Fig 3.
Rumen AHLs activate gad expression in an SdiA-dependent manner. qRT-PCR of the gadX gene in the WT, sdiA mutant, and complemented strains in the absence and presence of rumen AHLs.
pH of rumen and feces.
The grain diet, compared to the hay diet, resulted in more acidic conditions in the rumen and feces. The mean pH values of rumen fluid and feces from the heifers fed the grain diet were 5.98 and 6.03 (of note, the data of grain-fed cattle have been previously published [29]), respectively, whereas the mean pH values of rumen fluid and feces from the heifers fed the hay diet were 7.16 and 7.27, respectively (Table 1).
Table 1.
pH values of rumen fluid and feces of cattle fed hay
| Heifer | pH valuea at postdose day: |
|||
|---|---|---|---|---|
| 7 |
28 |
|||
| Rumen fluid | Feces | Rumen fluid | Feces | |
| V1 | 7.22 | 7.15 | 7.12 | 7.28 |
| V2 | 6.95 | 7.03 | 7.24 | 7.37 |
| V3 | 7.17 | 7.28 | 7.09 | 7.14 |
| V4 | 7.29 | 7.19 | 7.33 | 7.43 |
| V5 | 7.07 | 7.43 | 7.07 | 7.11 |
| V6 | 7.36 | 7.73 | 7.34 | 7.25 |
| V7 | 7.09 | 7.07 | 7.25 | 7.41 |
| V8 | 7.03 | 7.23 | 6.98 | 7.33 |
| Mean | 7.15 | 7.26 | 7.18 | 7.29 |
| SE | 0.15 | 0.23 | 0.13 | 0.12 |
pH values of rumen and fecal samples of grain-fed heifer have been previously published.
EHEC persistence in the rumen and at the RAJ mucosa in the hay-fed heifers compared to that in the grain-fed heifers.
All eight heifers were EHEC O157:H7 culture negative before bacterial challenge. Each received 1010 CFU containing equal numbers of the parental and sdiA deletion mutant. Rumen fluid samples from all heifers were culture positive for E. coli O157:H7 at 1 h, 4 h, 1 day, and 2 days postchallenge (Table 2). By day 3, some heifers were rumen fluid culture negative for EHEC. When fed the grain diet, all animals were rumen culture negative by day 8 (of note, the data of grain fed cattle have been previously published [29]). When fed the hay diet, all animals were rumen culture negative by day 11. Among the grain-fed heifers, only one was culture positive by enrichment culture on day 19, and all heifers were negative for EHEC thereafter (29). However, three animals among the eight hay-fed heifers remained culture positive for the bacteria on day 24, and one was culture positive for 31 days (Table 3).
Table 2.
Number of E. coli O157 CFU in rumen fluid of hay-fed heifers after oral dose
| Heifera |
E. coli O157:H7 CFU/ml of rumen fluid after oral doseb |
|||||
|---|---|---|---|---|---|---|
| 1 h | 4 h | Day 1 | Day 2 | Day 3 | Day 7 | |
| V1 | 8.60 × 105 | 5.10 × 103 | 1.55 × 103 | 3.10 × 101 | N | N |
| V2 | 2.47 × 105 | 3.70 × 104 | 7.90 × 102 | 1.00 × 102 | N | N |
| V3 | 1.13 × 106 | 2.70 × 104 | 1.12 × 103 | 5.00 × 101 | 5.00 × 101 | W+M+ |
| V4 | 7.50 × 105 | 2.56 × 104 | 4.00 × 102 | 1.80 × 102 | 1.60 × 102 | W+M+ |
| V5 | 2.43 × 106 | 9.50 × 103 | 2.60 × 102 | 3.00 × 101 | W+M+ | N |
| V6 | 4.40 × 105 | 2.80 × 104 | 1.30 × 103 | 2.00 × 101 | W+M+ | W+M+ |
| V7 | 1.75 × 106 | 4.40 × 103 | 7.10 × 102 | 7.00 × 101 | N | N |
| V8 | 9.40 × 106 | 3.50 × 104 | 2.60 × 102 | 2.00 × 101 | 9.00 × 101 | W+M+ |
Eight 15-month-old Charolais heifers were challenged with an oral dose of 1010 CFU containing equal numbers of the parental strain and the sdiA mutant. Samples were taken (hour or day) postdose.
N, sample was negative by both direct and enrichment culture methods; W+M+, sample was positive only by enrichment culture and contained both the parental and the mutant strains. Bacterial culture data on grain-fed heifer have been previously published.
Table 3.
Number of E. coli O157:H7 CFU recovered from RAMS samples from hay-fed heifers
| Heifera |
E. coli O157:H7 CFU/swab on postdose dayb |
|||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 7 | 11 | 15 | 21 | 24 | |
| V1 | 2.43 × 104 | 9.60 × 103 | 8.10 × 104 | N | N | N | N | N |
| V2 | 2.82 × 104 | 1.20 × 104 | 6.60 × 103 | 7.80 × 102 | 8.10 × 103 | 5.40 × 103 | 2.70 × 103 | 1.08 × 104 |
| V3 | 8.40 × 104 | 7.20 × 103 | 3.00 × 103 | 2.46 × 104 | 8.40 × 103 | 1.80 × 104 | 1.70 × 103 | 3.75 × 104 |
| V4 | 5.40 × 104 | 1.90 × 104 | 2.70 × 104 | 5.40 × 104 | 1.20 × 102 | N | N | N |
| V5 | 2.91 × 103 | 2.40 × 103 | 9.30 × 102 | W+M+ | W+M+ | 6.00 × 101 | N | N |
| V6 | 3.90 × 105 | 7.20 × 103 | 1.56 × 103 | 4.40 × 104 | 9.00 × 101 | 6.00 × 101 | 3.00 × 101 | W+M− |
| V7 | 1.80 × 104 | 5.40 × 103 | 5.40 × 103 | 1.20 × 103 | W−M+ | N | N | N |
| V8 | 1.65 × 105 | 2.04 × 104 | 2.82 × 104 | 3.50 × 103 | 9.00 × 101 | 3.00 × 101 | N | N |
Eight 15-month-old Charolais heifers were challenged with an oral dose of 1010 CFU containing equal numbers of the parental strain and the sdiA mutant. RAMS were taken days postchallenge.
N, sample was negative by both direct and enrichment culture; W+M+, sample was positive only by enrichment culture and contained both the wild type and the mutant; W+M−, sample was positive by enrichment culture only and only the parental strain was isolated; W−M+, sample was positive by enrichment culture only and only the mutant strain was isolated. Grain-fed heifer data have been previously published.
The parental wild-type strain outcompeted the sdiA deletion mutant in the rumen and at the RAJ mucosa among heifers fed either the grain or hay diet.
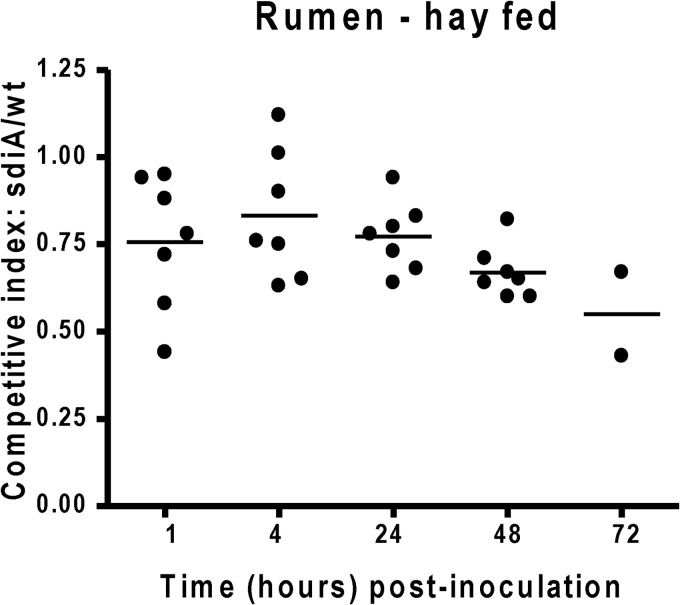
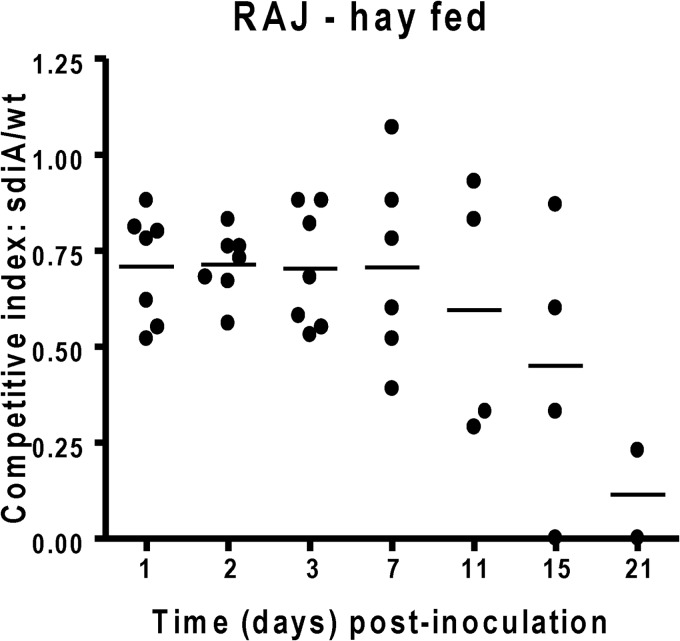
Fewer sdiA mutant CFU than wild-type CFU were recovered from rumen fluid among all heifers beginning 1 h postchallenge (Fig. 4) (of note, the data of grain-fed cattle have been previously published [29]). The advantage of the WT over the sdiA mutant in the rumen was similar in both grain- and hay-fed animals. Similarly, fewer sdiA mutant CFU than wild-type CFU were recovered from the RAMS samples of all heifers, except from one sample on day 3 (Fig. 5). However, in the RAJ, even though the WT had a competitive advantage in animals in both diets, this advantage was more pronounced in cattle on a grain diet (28) (Fig. 4).
Fig 4.
Competition index of the EHEC WT and sdiA mutant in hay-fed cattle. Eight 15-month-old Charolais heifers were fed a grain diet or a hay diet and challenged with equal numbers of the parental wild type and the sdiA mutant placed directly into the rumen. The ratio of mutant to WT isolates was determined in the rumen fluid. Competitive index (CI) values equal to 1 indicate no difference between strains, values of <1 indicate that the wild type survives/colonizes better than the mutant, and values of >1 indicate that the mutant survives/colonizes better than the wild type. The horizontal line indicates the mean CI value.
Fig 5.
Competition index of the EHEC WT and sdiA mutant in hay-fed cattle. Eight 15-month-old Charolais heifers were fed a grain diet or a hay diet and challenged with equal numbers of the parental wild type and the sdiA mutant placed directly into the rumen. The ratio of mutant to WT isolates was determined in RAJ. Competitive index (CI) values equal to 1 indicate no difference between strains, values of <1 indicate that the wild type survives/colonizes better than the mutant, and values of >1 indicate that the mutant survives/colonizes better than the wild type. The horizontal line indicates the mean CI value.
DISCUSSION
Colonization of cattle herds by EHEC is linked to EHEC outbreaks, either directly through contaminated beef products or contact with animals or indirectly by environmental distribution of the pathogen in manure that then contaminates water or other food products. The effect of diet on cattle carriage of EHEC has been controversial and confounded by studies with generic E. coli that try to extrapolate results to include E. coli O157:H7. Many studies show that a grain diet supports higher concentrations of generic E. coli in cattle (27, 53–55). However, in experimental challenge studies that specifically measure E. coli O157:H7, this is not the case. Forage diets show either no differences in concentration and duration of E. coli O157:H7 shedding or higher concentrations and increased durations of E. coli O157:H7 shedding (27, 56–59). This study, using the same eight animals and comparing their carriage of EHEC while fed a grain (29) or hay diet, supported earlier work showing that cattle fed a forage-based diet are colonized by EHEC longer than ruminants fed a grain-based diet (53, 57, 59). Understanding the effect of diet in EHEC colonization of cattle is significant, given that grain diets predominate in feedlots.
An important consideration of the effect of different diets on EHEC cattle colonization has to do with changes in the composition of the GI microbiota (60, 61). Given that EHEC recognizes the AHL chemical signals produced by the rumen microbiota through SdiA to modulate its gene expression toward efficient colonization of cattle (29) and animals fed a hay diet are colonized by EHEC for longer periods of time (Table 3), we wanted to investigate whether SdiA regulation is relevant for EHEC colonization of cattle fed either diets. We previously reported that AHLs are present within the rumen of grain-fed cattle and that such AHLs can decrease expression of the LEE and increase expression of the gad genes. Deletion of sdiA removes the ability to sense AHLs and regulate LEE and gad gene expression, and as a result the sdiA mutant did not grow well in rumen and was defective, compared to the WT, for colonization of the RAJ mucosa of these cattle (29). Here, we report that these same animals, after clearing carriage of EHEC and being acclimated to a hay diet, still harbor AHLs in their rumen, and these AHLs can still repress ler and increase gad gene expression in an SdiA-dependent way (Fig. 2 and 3). The WT EHEC fared better than the sdiA mutant in the rumen and the RAJ mucosa of cattle fed either diet (29) (Fig. 4 and 5). In the rumen, there were no differences in the competition of the WT and the sdiA mutant between the two diets (29) (Fig. 4). However, the WT had a bigger advantage over the sdiA mutant for colonization at the RAJ mucosa of animals fed a grain diet compared to those with forage diets (29) (Fig. 5). These data suggest that a grain diet is more conducive to the SdiA-dependent EHEC colonization of cattle in the RAJ.
In these studies, we are not utilizing different populations of heifers fed different diets but rather the same animals acclimated to two different diets (grain and hay), which diminishes variability. We can suggest from our studies that cattle fed a hay diet are colonized by EHEC for a longer period of time and that the SdiA system, although important for EHEC colonization of cattle fed either diet, had a more pronounced role with the grain-fed cattle. Typical finishing diets used in feedlots are ≥90% grain, suggesting that interference with this quorum sensing signaling system may be a potential strategy to diminish contamination of meat products, as well as cross-contamination of other food products.
ACKNOWLEDGMENTS
This work was supported by NIH grant AI077613. This work was supported, in part, by the Idaho Agriculture Experiment Station (C.J.H.) and by NIH grants P20-RR16454 (NCRR) (C.J.H.) and P20-GM103408 (NIGMS) (C.J.H.).
We thank Lonie Austin for technical assistance and animal handling.
Footnotes
Published ahead of print 8 July 2013
REFERENCES
- 1.Kaper JB, Nataro JP, Mobley HL. 2004. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2:123–140 [DOI] [PubMed] [Google Scholar]
- 2.Moon HW, Whipp SC, Argenzio RA, Levine MM, Giannella RA. 1983. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect. Immun. 41:1340–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knutton S, Baldini MM, Kaper JB, McNeish AS. 1987. Role of plasmid-encoded adherence factors in adhesion of enteropathogenic Escherichia coli to HEp-2 cells. Infect. Immun. 55:78–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tzipori S, Wachsmuth IK, Chapman C, Birden R, Brittingham J, Jackson C, Hogg J. 1986. The pathogenesis of hemorrhagic colitis caused by Escherichia coli O157:H7 in gnotobiotic piglets. J. Infect. Dis. 154:712–716 [DOI] [PubMed] [Google Scholar]
- 5.McDaniel TK, Jarvis KG, Donnenberg MS, Kaper JB. 1995. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc. Natl. Acad. Sci. U. S. A. 92:1664–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elliott SJ, Hutcheson SW, Dubois MS, Mellies JL, Wainwright LA, Batchelor M, Frankel G, Knutton S, Kaper JB. 1999. Identification of CesT, a chaperone for the type III secretion of Tir in enteropathogenic Escherichia coli. Mol. Microbiol. 33:1176–1189 [DOI] [PubMed] [Google Scholar]
- 7.Mellies JL, Elliott SJ, Sperandio V, Donnenberg MS, Kaper JB. 1999. The Per regulon of enteropathogenic Escherichia coli: identification of a regulatory cascade and a novel transcriptional activator, the locus of enterocyte effacement (LEE)-encoded regulator (Ler). Mol. Microbiol. 33:296–306 [DOI] [PubMed] [Google Scholar]
- 8.Elliott SJ, Wainwright LA, McDaniel TK, Jarvis KG, Deng YK, Lai LC, McNamara BP, Donnenberg MS, Kaper JB. 1998. The complete sequence of the locus of enterocyte effacement (LEE) from enteropathogenic Escherichia coli E2348/69. Mol. Microbiol. 28:1–4 [DOI] [PubMed] [Google Scholar]
- 9.Jarvis KG, Giron JA, Jerse AE, McDaniel TK, Donnenberg MS, Kaper JB. 1995. Enteropathogenic Escherichia coli contains a putative type III secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc. Natl. Acad. Sci. U. S. A. 92:7996–8000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jerse AE, Yu J, Tall BD, Kaper JB. 1990. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc. Natl. Acad. Sci. U. S. A. 87:7839–7843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kenny B, DeVinney R, Stein M, Reinscheid DJ, Frey EA, Finlay BB. 1997. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell 91:511–520 [DOI] [PubMed] [Google Scholar]
- 12.McNamara BP, Donnenberg MS. 1998. A novel proline-rich protein, EspF, is secreted from enteropathogenic Escherichia coli via the type III export pathway. FEMS Microbiol. Lett. 166:71–78 [DOI] [PubMed] [Google Scholar]
- 13.Kenny B, Jepson M. 2000. Targeting of an enteropathogenic Escherichia coli (EPEC) effector protein to host mitochondria. Cell Microbiol. 2:579–590 [DOI] [PubMed] [Google Scholar]
- 14.Elliott SJ, Krejany EO, Mellies JL, Robins-Browne RM, Sasakawa C, Kaper JB. 2001. EspG, a novel type III system-secreted protein from enteropathogenic Escherichia coli with similarities to VirA of Shigella flexneri. Infect. Immun. 69:4027–4033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tu X, Nisan I, Yona C, Hanski E, Rosenshine I. 2003. EspH, a new cytoskeleton-modulating effector of enterohaemorrhagic and enteropathogenic Escherichia coli. Mol. Microbiol. 47:595–606 [DOI] [PubMed] [Google Scholar]
- 16.Kanack KJ, Crawford JA, Tatsuno I, Karmali MA, Kaper JB. 2005. SepZ/EspZ is secreted and translocated into HeLa cells by the enteropathogenic Escherichia coli type III secretion system. Infect. Immun. 73:4327–4337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng W, Puente JL, Gruenheid S, Li Y, Vallance BA, Vazquez A, Barba J, Ibarra JA, O'Donnell P, Metalnikov P, Ashman K, Lee S, Goode D, Pawson T, Finlay BB. 2004. Dissecting virulence: systematic and functional analyses of a pathogenicity island. Proc. Natl. Acad. Sci. U. S. A. 101:3597–3602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campellone KG, Robbins D, Leong JM. 2004. EspFU is a translocated EHEC effector that interacts with Tir and N-WASP and promotes Nck-independent actin assembly. Dev. Cell 7:217–228 [DOI] [PubMed] [Google Scholar]
- 19.Gruenheid S, Sekirov I, Thomas NA, Deng W, O'Donnell P, Goode D, Li Y, Frey EA, Brown NF, Metalnikov P, Pawson T, Ashman K, Finlay BB. 2004. Identification and characterization of NleA, a non-LEE-encoded type III translocated virulence factor of enterohaemorrhagic Escherichia coli O157:H7. Mol. Microbiol. 51:1233–1249 [DOI] [PubMed] [Google Scholar]
- 20.Mundy R, Jenkins C, Yu J, Smith H, Frankel G. 2004. Distribution of espI among clinical enterohaemorrhagic and enteropathogenic Escherichia coli isolates. J. Med. Microbiol. 53:1145–1149 [DOI] [PubMed] [Google Scholar]
- 21.Dahan S, Wiles S, La Ragione RM, Best A, Woodward MJ, Stevens MP, Shaw RK, Chong Y, Knutton S, Phillips A, Frankel G. 2005. EspJ is a prophage-carried type III effector protein of attaching and effacing pathogens that modulates infection dynamics. Infect. Immun. 73:679–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garmendia J, Frankel G. 2005. Operon structure and gene expression of the espJ–tccP locus of enterohaemorrhagic Escherichia coli O157:H7. FEMS Microbiol. Lett. 247:137–145 [DOI] [PubMed] [Google Scholar]
- 23.Shaw RK, Smollett K, Cleary J, Garmendia J, Straatman-Iwanowska A, Frankel G, Knutton S. 2005. Enteropathogenic Escherichia coli type III effectors EspG and EspG2 disrupt the microtubule network of intestinal epithelial cells. Infect. Immun. 73:4385–4390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naylor SW, Low JC, Besser TE, Mahajan A, Gunn GJ, Pearce MC, McKendrick IJ, Smith DG, Gally DL. 2003. Lymphoid follicle-dense mucosa at the terminal rectum is the principal site of colonization of enterohemorrhagic Escherichia coli O157:H7 in the bovine host. Infect. Immun. 71:1505–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheng H, Lim JY, Knecht HJ, Li J, Hovde CJ. 2006. Role of Escherichia coli O157:H7 virulence factors in colonization at the bovine terminal rectal mucosa. Infect. Immun. 74:4685–4693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Price SB, Wright JC, DeGraves FJ, Castanie-Cornet MP, Foster JW. 2004. Acid resistance systems required for survival of Escherichia coli O157:H7 in the bovine gastrointestinal tract and in apple cider are different. Appl. Environ. Microbiol. 70:4792–4799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grauke LJ, Wynia SA, Sheng HQ, Yoon JW, Williams CJ, Hunt CW, Hovde CJ. 2003. Acid resistance of Escherichia coli O157:H7 from the gastrointestinal tract of cattle fed hay or grain. Vet. Microbiol. 95:211–225 [DOI] [PubMed] [Google Scholar]
- 28.Dziva F, van Diemen PM, Stevens MP, Smith AJ, Wallis TS. 2004. Identification of Escherichia coli O157: H7 genes influencing colonization of the bovine gastrointestinal tract using signature-tagged mutagenesis. Microbiology 150:3631–3645 [DOI] [PubMed] [Google Scholar]
- 29.Hughes DT, Terekhova DA, Liou L, Hovde CJ, Sahl JW, Patankar AV, Gonzalez JE, Edrington TS, Rasko DA, Sperandio V. 2010. Chemical sensing in mammalian host-bacterial commensal associations. Proc. Natl. Acad. Sci. U. S. A. 107:9831–9836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nealson KH, Platt T, Hastings JW. 1970. Cellular control of the synthesis and activity of the bacterial luminescent system. J. Bacteriol. 104:313–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engebrecht J, Nealson K, Silverman M. 1983. Bacterial bioluminescence: isolation and genetic analysis of functions from Vibrio fischeri. Cell 32:773–781 [DOI] [PubMed] [Google Scholar]
- 32.Engebrecht J, Silverman M. 1984. Identification of genes and gene products necessary for bacterial bioluminescence. Proc. Natl. Acad. Sci. U. S. A. 81:4154–4158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parsek MR, Greenberg EP. 2000. Acyl-homoserine lactone quorum sensing in Gram-negative bacteria: a signaling mechanism involved in associations with higher organisms. Proc. Natl. Acad. Sci. U. S. A. 97:8789–8793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davies DG, Parsek MR, Pearson JP, Iglewski BH, Costerton JW, Greenberg EP. 1998. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280:295–298 [DOI] [PubMed] [Google Scholar]
- 35.de Kievit TR, Iglewski BH. 2000. Bacterial quorum sensing in pathogenic relationships. Infect. Immun. 68:4839–4849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parsek MR, Val DL, Hanzelka BL, Cronan JE, Jr, Greenberg EP. 1999. Acyl homoserine-lactone quorum-sensing signal generation. Proc. Natl. Acad. Sci. U. S. A. 96:4360–4365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalogeraki VS, Winans SC. 1995. The octopine-type Ti plasmid pTiA6 of Agrobacterium tumefaciens contains a gene homologous to the chromosomal virulence gene acvB. J. Bacteriol. 177:892–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giammanco A, Maggio M, Giammanco G, Morelli R, Minelli F, Scheutz F, Caprioli A. 1996. Characteristics of Escherichia coli strains belonging to enteropathogenic E. coli serogroups isolated in Italy from children with diarrhea. J. Clin. Microbiol. 34:689–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dyszel JL, Soares JA, Swearingen MC, Lindsay A, Smith JN, Ahmer BM. E. coli K-12 and EHEC genes regulated by SdiA. PLoS One 5:e8946. 10.1371/journal.pone.0008946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Michael B, Smith JN, Swift S, Heffron F, Ahmer BM. 2001. SdiA of Salmonella enterica is a LuxR homolog that detects mixed microbial communities. J. Bacteriol. 183:5733–5742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dyszel JL, Smith JN, Lucas DE, Soares JA, Swearingen MC, Vross MA, Young GM, Ahmer BM. 2010. Salmonella enterica serovar Typhimurium can detect the acyl homoserine lactone production of Yersinia enterocolitica in mice. J. Bacteriol. 192:29–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noel JT, Joy J, Smith JN, Fatica M, Schneider KR, Ahmer BM, Teplitski M. 2010. Salmonella SdiA recognizes N-acyl homoserine lactone signals from Pectobacterium carotovorum in vitro, but not in a bacterial soft rot. Mol. Plant Microbe Interact. 23:273–282 [DOI] [PubMed] [Google Scholar]
- 43.Edrington TS, Farrow RL, Sperandio V, Hughes DT, Lawrence TE, Callaway TR, Anderson RC, Nisbet DJ. 2008. Acyl-homoserine-lactone autoinducer in the gastrointestinal tract of feedlot cattle and correlation to season, E. coli O157:H7 prevalence, and diet. Curr. Microbiol. 58:227–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Erickson DL, Nsereko VL, Morgavi DP, Selinger LB, Rode LM, Beauchemin KA. 2002. Evidence of quorum sensing in the rumen ecosystem: detection of N-acyl homoserine lactone autoinducers in ruminal contents. Can. J. Microbiol. 48:374–378 [DOI] [PubMed] [Google Scholar]
- 45.Faith JJ, McNulty NP, Rey FE, Gordon JI. 2011. Predicting a human gut microbiota's response to diet in gnotobiotic mice. Science 333:101–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Griffin PM, Ostroff SM, Tauxe RV, Greene KD, Wells JG, Lewis JH, Blake PA. 1988. Illnesses associated with Escherichia coli O157:H7 infections. A broad clinical spectrum. Ann. Intern. Med. 109:705–712 [DOI] [PubMed] [Google Scholar]
- 47.Walters M, Sperandio V. 2006. Autoinducer 3 and epinephrine signaling in the kinetics of locus of enterocyte effacement gene expression in enterohemorrhagic Escherichia coli. Infect. Immun. 74:5445–5455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shaw PD, Ping G, Daly SL, Cha C, Cronan JE, Jr, Rinehart KL, Farrand SK. 1997. Detecting and characterizing N-acyl-homoserine lactone signal molecules by thin-layer chromatography. Proc. Natl. Acad. Sci. U. S. A. 94:6036–6041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fuqua C, Winans SC. 1996. Conserved cis-acting promoter elements are required for density-dependent transcription of Agrobacterium tumefaciens conjugal transfer genes. J. Bacteriol. 178:435–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rice DH, Sheng HQ, Wynia SA, Hovde CJ. 2003. Rectoanal mucosal swab culture is more sensitive than fecal culture and distinguishes Escherichia coli O157:H7-colonized cattle and those transiently shedding the same organism. J. Clin. Microbiol. 41:4924–4929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sheng H, Davis MA, Knecht HJ, Hancock DD, Van Donkersgoed J, Hovde CJ. 2005. Characterization of a Shiga toxin-, intimin-, and enterotoxin hemolysin-producing Escherichia coli ONT:H25 strain commonly isolated from healthy cattle. J. Clin. Microbiol. 43:3213–3220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Callaway TR, Dowd SE, Edrington TS, Anderson RC, Krueger N, Bauer N, Kononoff PJ, Nisbet DJ. 2010. Evaluation of bacterial diversity in the rumen and feces of cattle fed different levels of dried distillers grains plus solubles using bacterial tag-encoded FLX amplicon pyrosequencing. J. Anim. Sci. 88:3977–3983 [DOI] [PubMed] [Google Scholar]
- 53.Krause DO, Smith WJ, Conlan LL, Gough JM, Williamson MA, McSweeney CS. 2003. Diet influences the ecology of lactic acid bacteria and Escherichia coli along the digestive tract of cattle: neural networks and 16S rDNA. Microbiology 149:57–65 [DOI] [PubMed] [Google Scholar]
- 54.Gilbert RA, Tomkins N, Padmanabha J, Gough JM, Krause DO, McSweeney CS. 2005. Effect of finishing diets on Escherichia coli populations and prevalence of enterohaemorrhagic E. coli virulence genes in cattle faeces. J. Appl. Microbiol. 99:885–894 [DOI] [PubMed] [Google Scholar]
- 55.Diez-Gonzalez F, Callaway TR, Kizoulis MG, Russell JB. 1998. Grain feeding and the dissemination of acid-resistant Escherichia coli from cattle. Science 281:1666–1668 [DOI] [PubMed] [Google Scholar]
- 56.Hovde CJ, Austin PR, Cloud KA, Williams CJ, Hunt CW. 1999. Effect of cattle diet on Escherichia coli O157:H7 acid resistance. Appl. Environ. Microbiol. 65:3233–3235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kudva IT, Hunt CW, Williams CJ, Nance UM, Hovde CJ. 1997. Evaluation of dietary influences on Escherichia coli O157:H7 shedding by sheep. Appl. Environ. Microbiol. 63:3878–3886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tkalcic S, Brown CA, Harmon BG, Jain AV, Mueller EP, Parks A, Jacobsen KL, Martin SA, Zhao T, Doyle MP. 2000. Effects of diet on rumen proliferation and fecal shedding of Escherichia coli O157:H7 in calves. J. Food Prot. 63:1630–1636 [DOI] [PubMed] [Google Scholar]
- 59.Van Baale MJ, Sargeant JM, Gnad DP, DeBey BM, Lechtenberg KF, Nagaraja TG. 2004. Effect of forage or grain diets with or without monensin on ruminal persistence and fecal Escherichia coli O157:H7 in cattle. Appl. Environ. Microbiol. 70:5336–5342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muegge BD, Kuczynski J, Knights D, Clemente JC, Gonzalez A, Fontana L, Henrissat B, Knight R, Gordon JI. 2011. Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science 332:970–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. 2011. Human nutrition, the gut microbiome and the immune system. Nature 474:327–336 [DOI] [PMC free article] [PubMed] [Google Scholar]