Abstract
Neisseria meningitidis (meningococcus) is a symbiont of the human nasopharynx. On occasion, meningococci disseminate from the nasopharynx to cause invasive disease. Previous work showed that purified meningococcal peptidoglycan (PG) stimulates human Nod1, which leads to activation of NF-κB and production of inflammatory cytokines. No studies have determined if meningococci release PG or activate Nod1 during infection. The closely related pathogen Neisseria gonorrhoeae releases PG fragments during normal growth. These fragments induce inflammatory cytokine production and ciliated cell death in human fallopian tubes. We determined that meningococci also release PG fragments during growth, including fragments known to induce inflammation. We found that N. meningitidis recycles PG fragments via the selective permease AmpG and that meningococcal PG recycling is more efficient than gonococcal PG recycling. Comparison of PG fragment release from N. meningitidis and N. gonorrhoeae showed that meningococci release less of the proinflammatory PG monomers than gonococci and degrade PG to smaller fragments. The decreased release of PG monomers by N. meningitidis relative to N. gonorrhoeae is partly due to ampG, since replacement of gonococcal ampG with the meningococcal allele reduced PG monomer release. Released PG fragments in meningococcal supernatants induced significantly less Nod1-dependent NF-κB activity than released fragments in gonococcal supernatants and tended to induce less interleukin-8 (IL-8) secretion in primary human fallopian tube explants. These results support a model in which efficient PG recycling and extensive degradation of PG fragments lessen inflammatory responses and may be advantageous for maintaining meningococcal carriage in the nasopharynx.
INTRODUCTION
Neisseria meningitidis (meningococcus) colonizes the nasopharynx of up to 40% of the healthy human adult population (1). In ∼1,525 cases annually in the United States, N. meningitidis disseminates from the nasopharynx to cause a highly inflammatory meningitis and septicemia (2). Even with treatment, there is a high rate of morbidity and mortality associated with meningococcal disease. Giardin et al. showed that purified meningococcal peptidoglycan (PG) stimulates NF-κB activation via the intracellular pattern recognition receptor Nod1, which could contribute to the inflammatory state observed during invasive disease (3). That study demonstrated that N. meningitidis peptidoglycan is inflammatory, although it remains unclear if meningococci release PG during infection. Even though meningococcal disease is highly inflammatory, meningococci are usually carried asymptomatically, implying that the bacteria are able to control release of inflammatory compounds and/or have differential interactions with host cells.
Neisseria gonorrhoeae (gonococcus) is the etiologic agent of the sexually transmitted disease gonorrhea and is closely related to N. meningitidis. During normal growth, gonococci release fragments of cell-wall-derived PG (4). Nearly 50% of gonococcal PG is broken down per generation, but in the recycling process, most PG fragments are brought back into the cytoplasm and a much lower percentage of PG fragments is released (5). Gonococci release 15% of their PG per generation (6). AmpG, a permease selective for 1,6-anhydro-muramyl residues, is required for PG recycling in gonococci and other Gram-negative bacteria, such as Escherichia coli (6–9). An ampG deletion in gonococci leads to a large increase in PG monomer release (6).
Released PG fragments stimulate inflammation and cell damage in multiple bacterial infections. In Bordetella pertussis infection, release of the 1,6-anhydro-disaccharide tetrapeptide PG monomer (also known as tracheal cytotoxin [TCT]) causes the death and sloughing of ciliated cells of the trachea (10–12). Examination of the mechanism involved in this cell damage, determined using the hamster tracheal ring model, demonstrated that the damage is immune mediated. Nonciliated cells sense TCT and respond with interleukin-1 (IL-1) production (12). Ciliated cells respond to IL-1 with increased expression of inducible nitric oxide synthase (iNOS), and the amount of nitric oxide produced is sufficient to kill the ciliated cells (12, 13). Similar pathology is seen in gonococcal infection of human fallopian tubes (FT), and ciliated cells die and slough from the epithelium (14). The addition of gonococcal PG monomers (a mixture of 1,6-anhydro-disaccharide tetrapeptide and 1,6-anhydro-disaccharide tripeptide) was shown to recapitulate the cell damage (15). During Shigella flexneri infections, release of tripeptide monomer leads to Nod1 stimulation and inflammatory cytokine production (16). Human Nod1 (hNod1) specifically recognizes PG fragments containing diaminopimelic acid (DAP) as the C-terminal amino acid, including the tripeptide monomer (17). In gonococcal infections, it is unclear if it is the tetrapeptide PG monomer (TCT), tripeptide PG monomer, or a combination of both that causes the ciliated cell damage in the fallopian tube.
Since N. meningitidis is most often an asymptomatic colonizer (1), we hypothesized that proinflammatory PG fragment release from N. meningitidis may be lower than that from bacteria that typically cause disease. This limiting of PG fragment release could contribute to the ability of meningococci to exist as a member of the nasopharyngeal microbiota. Therefore, we determined the identity and amounts of PG fragments released by meningococci. We also examined PG recycling in N. meningitidis and found that meningococci are very efficient in uptake of liberated PG fragments, recycling 96% of PG monomers. Quantitative comparison with N. gonorrhoeae revealed that meningococci release less of the proinflammatory monomers than gonococci and differ in the ratio of tripeptide monomer and tetrapeptide monomer. In a direct test of inflammatory potential, supernatants from N. meningitidis were found to stimulate less NF-κB activation via Nod1 than supernatants from N. gonorrhoeae. Meningococcal supernatants also induced less IL-8 production in human fallopian tube tissue than gonococcal supernatants, suggesting that differences in the release and breakdown of PG fragments contribute to differences in the inflammatory response to the pathogenic Neisseria.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All strains used for this study are listed in Table 1. Meningococci and nonpiliated gonococci were both grown in gonococcal base liquid medium (GCBL) with Kellogg's supplements and 0.042% NaHCO3 (cGCBL) with aeration or on GCB agar plates (Difco) in the presence of 5% CO2 (18). E. coli cells were grown in LB broth or on LB agar plates (Difco). Chloramphenicol was used for selection at concentrations of either 25 μg/ml (E. coli) or 5 μg/ml (N. meningitidis). Kanamycin was used at 40 μg/ml (E. coli) and 80 μg/ml (N. meningitidis). Erythromycin was used for selection of meningococci at 10 μg/ml and for E. coli at a concentration of 500 μg/ml. Isopropyl-β-d-thiogalactopyranoside (IPTG) was used at a concentration of 1 mM to induce expression of the ampG complement.
Table 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| Strains | ||
| N. meningitidis | ||
| ATCC 13102 | Clinical isolate, serogroup C | ATCC |
| ATCC 13102 Str | ATCC 13102 rpsL(K43R) | 40 |
| ATCC 13102 cap | ATCC 13102 Str transformed with pHC10 [rpsL(K43R) siaD::cat] | This study |
| KL1074 | ATCC 13102 cap transformed with pKL37 [rpsL(K43R) siaD::cat ΔampG::kan] | This study |
| KL1090 | KL1074 transformed with pKL47 [rpsL(K43R) siaD::cat ΔampG::kan ampG+] | This study |
| N. gonorrhoeae | ||
| MS11 | Wild-type N. gonorrhoeae | 41 |
| DG130 | MS11 transformed with pGC3 (ampG::ermC) | This study |
| DG132 | MS11 ΔampG | 6 |
| EC505 | DG130 transformed with pEC006 (meningococcal ampG) | This study |
| Plasmids | ||
| pKL26 | pDG132 (ΔampG) insert subcloned into pKH6 at HindIII and SpeI | This study |
| pKL37 | aph3 from pKH99 (EcoRV EcoICRI) in ΔampG of pKL26 at blunted BglII | This study |
| pKL47 | ampG coding sequence in pMR33 complementation vector at SacI and XbaI | This study |
| pEC006 | ampG coding region from ATCC 13102 with MS11 flanking DNA in pIDN3 at SacI and BamHI | This study |
| pIDN3 | Cloning plasmid containing Neisseria DNA uptake sequences | 42 |
| pHC10 | siaD::cat construct from serogroup C N. meningitidis | 43 |
| pGC3 | N. gonorrhoeae ampG::ermC in pZero2.1 | W. Goldman |
| pDG132 | In-frame ampG deletion construct | 6 |
| pMR33 | IPTG-inducible Neisseria complementation vector for insertion at iga-trpB | 44 |
| pKH6 | Cloning vector derived from pIDN1 and pGCC6 | 40 |
| pKH99 | aph3 from pHSS6 in BamHI site of pIDN2 | 40 |
Plasmid and strain construction.
All plasmids used in this study are listed in Table 1. A strain with a mutation in ampG was constructed to examine meningococcal PG recycling. The fragment containing an in-frame deletion of ampG and flanking DNA was excised from pDG132 with restriction enzymes HindIII and SpeI and subcloned into the cloning vector pKH6 at the same restriction sites to generate plasmid pKL26. The kanamycin resistance marker was cut from pKH99 with EcoRV and EcoICRI. Plasmid pKL26 was cut with BglII and blunted with T4 DNA polymerase. The kanamycin resistance marker was inserted into the blunted BglII site in the same orientation as ampG to generate pKL37. Strain ATCC 13102 Str was transformed with pHC10 in order to generate a capsule-null variant, ATCC 13102 cap. To generate an ampG deletion in N. meningitidis, ATCC 13102 cap was transformed with pKL37 linearized with NheI. Deletion of ampG was confirmed by PCR. An ampG complementation construct was generated by amplifying the ampG coding region from ATCC 13102 chromosomal DNA with primers SacI ampG F (CGGGAGCTCGCGATATTTGCTACAATAGGC) and XbaI ampG R (GCCCTCTAGACACAATATCAGGTAAACGCTCC). Restriction sites in the primer sequences are underlined. Both the PCR product and plasmid pMR33 were digested with SacI and XbaI and then ligated together to create pKL47. Strain KL1074 was transformed with pKL47 linearized with NcoI to make the ampG complement strain KL1090.
The DNA fragment containing the ampG coding region with 50 bp of flanking DNA was amplified from ATCC 13102 chromosomal DNA using primers ampG-F2 (GGCGATATTTGCTACAATAGGCT) and ampG-1325R (CAATATCAGGTAAACGCTCCAGTTTGA). A fragment containing 600 bp of ampG upstream DNA sequence was amplified from MS11 chromosomal DNA using primers Mc-ampG-SacI-F3 (ATTCAGAGCTCCATCGGCGGCATCATCAAAC) and ampG-5′-flank-R (AGCCTATTGTAGCAAATATCGCC), while the fragment containing around 400 bp of ampG downstream DNA sequence was amplified similarly using primers ampG-3′-flank-F (TCAAACTGGAGCGTTTACCTGATATTG) and ampG-3′flank-R-BamHI (CTCAGGATCCGTTCTTTATATGAGCGGCAGG). The full-length DNA fragment containing N. meningitidis ATCC 13102-derived ampG coding sequence flanked by N. gonorrhoeae MS11-derived ampG upstream and downstream DNA sequence was generated using a PCR-based method of extension overlap using the aforementioned DNA fragments and primers Mc-ampG-SacI-F3 and ampG-3′flank-R-BamHI. The full-length DNA fragment and plasmid pIDN3 were digested with SacI and BamHI and ligated together to generate pEC006. To generate a gonococcal strain expressing meningococcal ampG, strain DG130 (N. gonorrhoeae ampG::ermC) was transformed with plasmid pEC006. The resulting strain, EC505, was screened for loss of erythromycin resistance, and the expected construction was confirmed by PCR and DNA sequencing.
Characterization of released PG fragments.
Labeling and characterization of released fragments were performed according to the methods described by Rosenthal and Dziarski as modified by Cloud and Dillard (19, 20). Quantitative fragment release analysis was performed as described by Garcia and Dillard (6). Meningococci and gonococci were pulse-labeled with 10 μCi/ml [6-3H]glucosamine in GCBL without glucose but containing pyruvate as the carbon source. A 2-h chase period in cGCBL followed. For quantitative release, radioactive counts per minute (cpm) in cell pellets after labeling were determined by liquid scintillation counting. The chase period started with equal numbers of cpm in each strain. After the chase, the cells were removed by centrifugation and supernatants were passed through a 0.22-μm-pore filter. PG fragments in the supernatants were separated by size exclusion chromatography and detected by liquid scintillation counting. Relative amounts of PG fragments were determined by calculating the area under the curve. The monomer fractions were pooled, concentrated, and desalted.
High-performance liquid chromatography (HPLC) analysis was carried out as described by Kohler et al. (21). Desalted PG monomers were separated by reversed-phase HPLC over a 4.6- by 250-mm C18 column using a 4 to 13% acetonitrile gradient over 30 min. PG monomers were detected by liquid scintillation counting.
PG monomers were analyzed by liquid chromatography-mass spectrometry (LC-MS) at the University of Wisconsin—Madison Biotechnology Center Mass Spectrometry facility. Liquid chromatography separation was performed on an Agilent 1200 high-performance liquid chromatography system using an Agilent Zorbax SB-C18 2.1- by 50-mm column with 1.8-μm particles. The following solvents were used: A, 0.1% formic acid in water; B, 0.1% formic acid in acetonitrile. The flow rate was 250 μl/min, and the gradient was as follows: 2% B, hold for 1 min, ramp to 40% B at 48 min, ramp to 60% B at 55 min, ramp to 95% B at 60 min, return to 2% B at 62 min, and hold for 18 min. The column was held at 35°C. The mass spectrometer was an Agilent LC/Mass selective detector time of flight (MSD TOF) model G1969A operated in the positive-ion mode. Data were collected over the m/z range 100 to 3,200 with 10,000 transients collected per scan and 0.89 scan acquired per second. The source was held at 35°C with electrospray voltage of 3,600 V. The drying gas was 8 liters/min, the nebulizer gas was at 40 psi (gauge) (psig), and the fragmentor was at 160 V. Spectra were internally calibrated using a dual-electrospray ionization (ESI) source in which the second electrospray delivered calibrant solution at a flow rate of 20 μl/min.
Peptidoglycan turnover.
Meningococcal PG was metabolically labeled with 10 μCi/ml [6-3H]glucosamine as described above. During a 2-h chase period, 500-μl samples were taken every 30 min. Whole sacculi were purified from each sample as described by Cloud and Dillard (20). Cell pellets were resuspended in 165 μl 50 mM sodium phosphate buffer, pH 5.0. An equivalent amount of boiling 8% SDS was added to the sample. After 30 min of boiling, whole sacculi were collected by centrifugation at 41,000 relative centrifugal force (RCF) for 30 min at 15°C. The supernatant was removed, and the pellet was resuspended in 100 μl sterile water. Radiation in the entire sample was detected by liquid scintillation. The data shown are the average of three independent experiments, with error bars representing the standard deviation. Significance was determined using the Student t test.
NF-κB activation in epithelial cells.
HEK293-Blue hNod1 cells (InvivoGen) were grown in Dulbecco's modified Eagle's medium (DMEM) with 4.5 g/liter glucose, 2 mM l-glutamine, 10% fetal bovine serum (FBS), 100 μg/ml Normocin, 30 μg/ml blasticidin, and 100 μg/ml Zeocin at 37°C with 5% CO2. These cells overexpress human Nod1 and express a soluble alkaline phosphatase NF-κB reporter. Gonococci and meningococci were grown into the log phase in liquid culture. Cultures were centrifuged to remove bacteria, and supernatants were filtered with a 0.22-μm-pore filter. Total protein in the cell pellet was determined by bicinchoninic acid (BCA) assay (Pierce), and supernatants were normalized based on total protein. HEK293-Blue cells were resuspended at a density of 2.8 × 105 cells/ml in DMEM with 4.5 g/liter glucose, 2 mM l-glutamine, and 10% heat-inactivated fetal bovine serum (FBS). Cells (180 μl) were plated in a 96-well plate along with 20 μl of bacterial supernatant, 10 μg/ml Tri-DAP positive control (InvivoGen), 10 μg/ml Tri-Lys negative control (InvivoGen), or medium-only control and incubated at 37°C with 5% CO2 for 24 h. After incubation, 20 μl of supernatant was added to 200 μl of QUANTI-Blue reagent (InvivoGen). The reaction mixture was incubated at 37°C for 2 h. Absorbance was read at 650 nm in a plate reader to determine alkaline phosphatase activity. The values reported are the mean fold increase over medium only from three independent biological replicates performed in duplicate. Significance was determined using the Student t test.
FTOC and cytokine analysis.
Samples of human fallopian tube (FT) were obtained from consenting donors via the National Human Tissue Resource Center at the National Disease Research Interchange in accordance with the University of Wisconsin—Madison Human Studies protocol. This work was approved by the University of Wisconsin—Madison Human Subjects Institutional Review Board (IRB). Upon arrival, FT samples were placed in Eagle's minimal essential medium (MEM) (Cellgro) with HEPES (pH 7.4) and penicillin-streptomycin, where they were trimmed to remove muscle tissue and cut to expose epithelia prior to dissection with a 3-mm biopsy punch. Segments were removed to individual wells of a 24-well plate containing 1 ml of the above medium and cultured overnight at 37°C with 5% CO2. Following overnight incubation, segments were evaluated microscopically to confirm the presence of peripheral ciliated cell activity. Prior to treatment, each punch was washed with sterile phosphate-buffered saline (PBS) and removed to a new 24-well plate containing 900 μl of the above medium without antibiotics. A minimum number of three segments per condition were then treated with either 100 μl of GCBL (mock) or supernatant from strain MS11, DG132, ATCC 13102, or KL1074 grown into log phase in liquid culture as described above. At 24 h postinfection, 250 μl of fallopian tube organ culture (FTOC) medium was removed, filtered by centrifugation through a 0.22-μm-pore filter (Costar), and stored at −80°C. Filtered FTOC supernatants were assayed for the presence of IL-8 by sandwich enzyme-linked immunosorbent assay (ELISA) using the Ready-Set-Go human IL-8 ELISA kit (eBioscience), according to the manufacturer's standard protocol. Significance was determined by the Mann-Whitney test.
RESULTS
Peptidoglycan fragment release by N. meningitidis.
Purified N. meningitidis peptidoglycan (PG) induces an inflammatory response and inflammatory cytokine production (3), but it has not been determined if meningococci release PG fragments or how PG affects meningococcal disease and carriage. We hypothesized that meningococci could be limiting proinflammatory PG fragment release, which may facilitate carriage in the human nasopharynx. To examine PG fragment release from N. meningitidis, we used a pulse-chase, metabolic labeling method that has been used to characterize PG fragment release from N. gonorrhoeae and other bacteria. Growth of N. meningitidis strain ATCC 13102 in the presence of [6-3H]glucosamine with pyruvate as the carbon source was successful in incorporating label into the SDS-insoluble cell wall PG. However, a substantial amount of label was incorporated into other molecules, likely the polysaccharide capsule. Therefore, we constructed an isogenic capsule-null strain of ATCC 13102 by introduction of an siaD mutation. The unencapsulated strain exhibited 1.9-fold-increased labeling efficiency for introduction of [6-3H]glucosamine into the cell wall PG (data not shown). All subsequent labeling with [6-3H]glucosamine was performed on capsule-null strains.
We metabolically labeled ATCC 13102 cap with [6-3H]glucosamine, and after a 2-h chase period, the supernatant was collected for evaluation of released PG fragments. PG fragments in the medium were separated by size exclusion chromatography, and by comparison with known standards, we were able to identify most of the PG fragments released by N. meningitidis. The release profile for strain ATCC 13102 cap shows the presence of PG dimers, PG monomers, free disaccharide, and a monosaccharide fraction containing 1,6-anhydro-N-acetylmuramic acid (anhydro-MurNAc) and possibly other molecules (Fig. 1).
Fig 1.
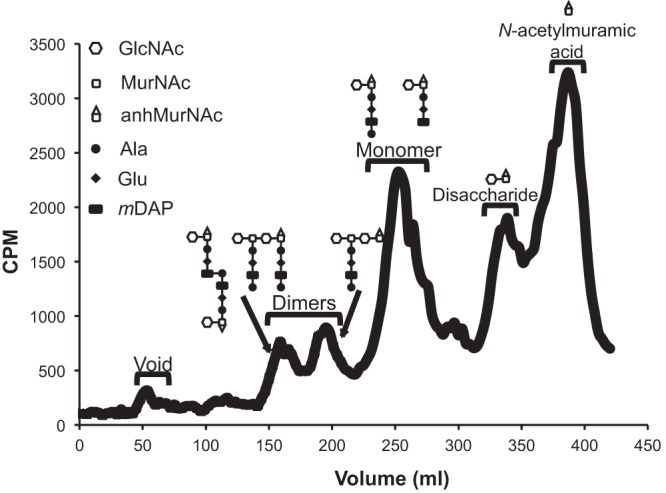
Meningococci release peptidoglycan fragments during normal growth. Meningococci were metabolically labeled with [6-3H]glucosamine. After a chase period, supernatants were collected. Radiolabeled fragments were separated by size exclusion chromatography and detected by liquid scintillation counting. During logarithmic growth, meningococci release PG dimers, monomers, free disaccharide, and anhydro-N-acetylmuramic acid. GlcNAc, N-acetylglucosamine; MurNAc, N-acetylmuramic acid; anhMurNAc, anhydro-N-acetylmuramic acid. Although only one structure is shown, the location of the peptide on the tetrasaccharide-peptide is not known.
As is seen in the release of PG fragments by N. gonorrhoeae, the major PG fragments released by N. meningitidis include PG monomers and free disaccharide (Fig. 1). PG monomers are generated by the action of lytic transglycosylases that break down PG strands into 1,6-anhydro-disaccharide tripeptide and 1,6-anhydro-disaccharide tetrapeptide monomers (11, 22–24). Free disaccharides are generated by the N-acetylmuramyl-l-alanine amidase AmiC, which cleaves peptides from the glycan chains (6, 25). The PG fragment that elutes at the 390-ml point in the N. meningitidis profile (Fig. 1) was only barely detected in N. gonorrhoeae profiles and has not been previously analyzed (22). We performed additional size exclusion chromatography and demonstrated that this molecule released by N. meningitidis is larger than glucosamine and N-acetylglucosamine (data not shown). Mass spectrometry of an unlabeled sample demonstrated the presence of 1,6-anhydro-N-acetylmuramic acid (anhydro-MurNAc) at this elution time in the meningococcal profile. The less abundant PG fragments released by N. meningitidis include PG dimers: peptide-cross-linked PG dimer, glycosidically linked PG dimer, and a tetrasaccharide with a single peptide chain. We also noted that N. meningitidis has an additional PG monomer peak not found in the release profile of N. gonorrhoeae, representing a molecule smaller than the tripeptide monomer. The presence of this smaller monomer suggests that meningococci degrade liberated PG monomers, possibly removing more of the peptide side chain or removing the glucosamine from the PG monomers.
Since PG monomers induce an inflammatory response in multiple bacterial infections (12, 15, 26) and purified N. meningitidis peptidoglycan stimulates Nod1, we examined what types of PG monomers are released by N. meningitidis. PG monomers released by unencapsulated ATCC 13102 were purified by size exclusion chromatography and analyzed by LC/MS (Fig. 2). The mass spectrometry analysis identified mass-to-charge ratios consistent with 1,6-anhydro-disaccharide tetrapeptide monomer (Fig. 2A), 1,6-anhydro-disaccharide tripeptide monomer (Fig. 2B), reducing disaccharide tetrapeptide monomer, and reducing disaccharide tripeptide monomer (data not shown). Together, the size exclusion analysis and the LC/MS data show that meningococci release known proinflammatory PG fragments into the extracellular milieu.
Fig 2.
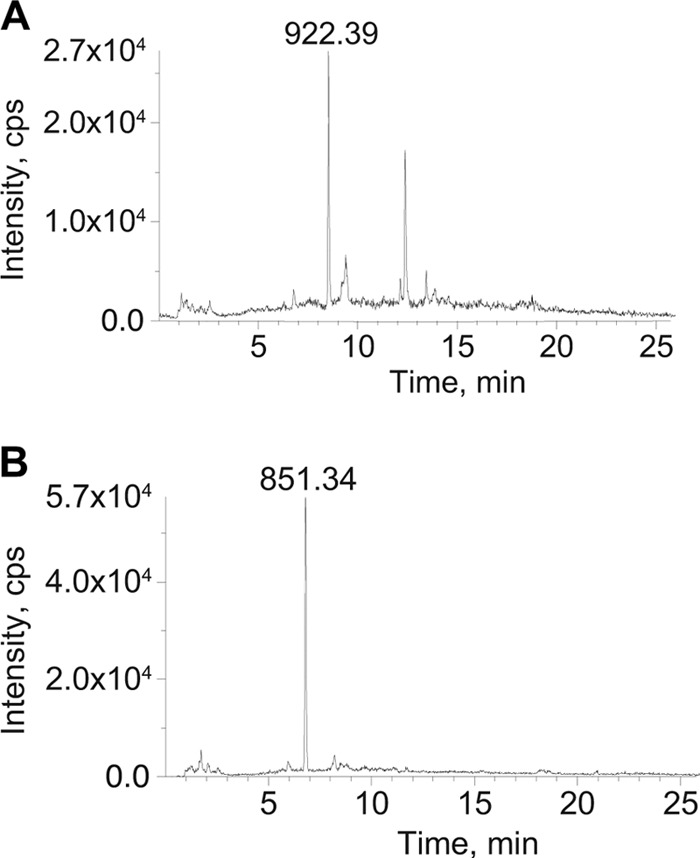
Meningococci release anhydro-disaccharide tripeptide monomer and anhydro-disaccharide tetrapeptide monomer. PG fragments in supernatant from unlabeled ATCC 13102 were separated by size exclusion chromatography. The monomer fractions were pooled, desalted, and concentrated. LC/MS analysis showed species with mass-to-charge ratios consistent with 1,6-anhydro-disaccharide tetrapeptide monomer (A) and 1,6-anhydro-disaccharide tripeptide monomer (B).
Meningococci efficiently recycle peptidoglycan.
Examination of the N. meningitidis genome sequences shows that meningococci have a homologue of the anhydromuramyl-specific permease, AmpG, which is involved in uptake of PG fragments for recycling in N. gonorrhoeae, Citrobacter freundii, and other Gram-negative bacterial species (6, 7, 9). In order to characterize meningococcal PG recycling, we made a deletion of ampG in N. meningitidis and a complemented ampG mutant and examined PG turnover during growth. We metabolically labeled capsule-null N. meningitidis strain ATCC 13102, the isogenic ampG deletion mutant, and the complemented strain and measured the radioactivity remaining in purified sacculi over a 2-h chase period. We found that the ampG mutant released significantly more PG throughout the chase period than both the wild-type (WT) and complemented strains (Fig. 3), suggesting that meningococci recycle significant amounts of PG fragments generated during cell wall growth and use AmpG in this process.
Fig 3.
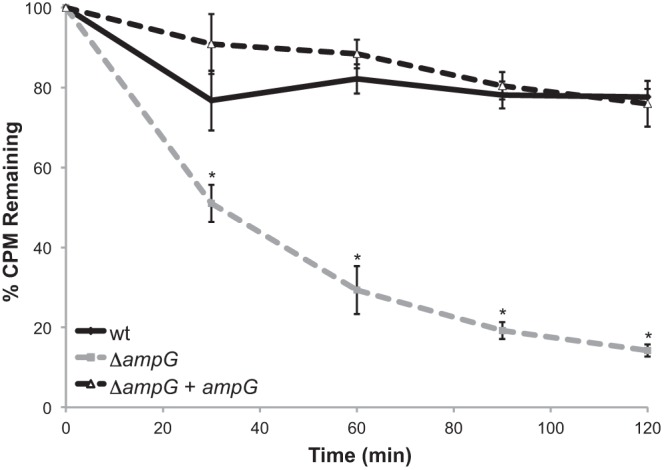
Turnover of peptidoglycan is significantly increased in an ampG mutant over the wild type. Meningococcal strains were labeled with [6-3H]glucosamine. During the 120-min chase period, samples were taken every 30 min. Whole sacculi were purified, and total cpm were determined by liquid scintillation counting. The ampG deletion mutant KL1074 showed significantly more PG turnover than its parent, ATCC 13102 cap, and the complemented strain, KL1090. *, P < 0.05 by ANOVA. Error bars represent the standard deviation. n = 3.
To determine the effects of an ampG deletion on fragment release, the wild-type and ampG mutant strains were radiolabeled as described above; however, at the beginning of the chase period the cultures were diluted to give equal amounts of radioactivity in the wild-type and ampG mutant cultures to allow for a quantitative comparison of PG fragment release. The ampG deletion strain showed increased release of PG monomers and free disaccharide relative to the wild-type parent strain (Fig. 4). The amount of monomer released by the ampG mutant was 29 times greater than that released by the wild type, indicating that wild-type meningococci recycle 96% of monomers. Similarly, meningococci were found to recycle 90% of disaccharide fragments. Previous work with N. gonorrhoeae showed that gonococci recycle 85% of PG monomer (6). Therefore, meningococci release 4% of their PG monomer and gonococci release 15%, indicating that 3.7-fold more of this proinflammatory molecule is released by N. gonorrhoeae. Our results suggest that N. meningitidis recycles PG fragments via AmpG more efficiently than does N. gonorrhoeae.
Fig 4.
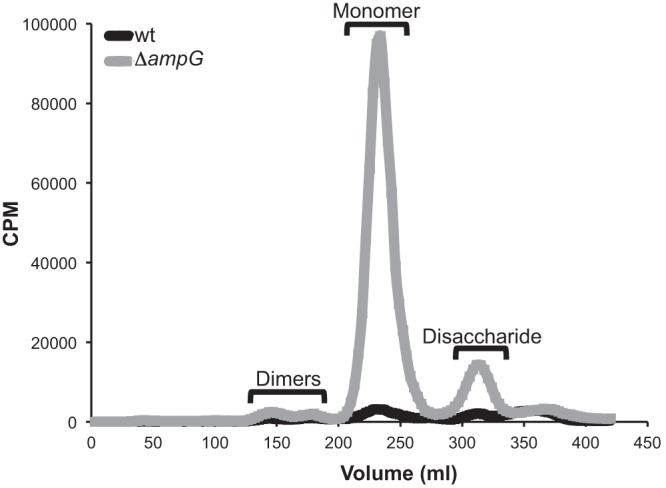
Deletion of ampG results in increased monomer and free disaccharide release over the level in the wild type. An ampG deletion mutant (KL1074) and its parent (ATCC 13102 cap) were labeled with [6-3H]glucosamine. Following a 2-h chase, supernatants were collected, and fragments were separated by size exclusion chromatography and detected by liquid scintillation counting. Based on differences in monomer peak area, meningococci recycle 96% of PG monomers.
We wondered whether the differences in PG fragment release between meningococci and gonococci could be directly attributed to AmpG. Alignment of AmpG from N. meningitidis and N. gonorrhoeae showed that AmpG is 97% identical at the amino acid level, with the differences spread throughout the amino acid sequence. We constructed a gonococcal strain, EC505, that expresses meningococcal ampG in place of the native gonococcal ampG. Quantitative comparison of EC505 to the parental gonococcal strain MS11 showed that EC505 releases 50.7% as much PG monomer as MS11 (Fig. 5). Release of PG dimers and free disaccharide was also reduced, although release of anhydro-MurNAc was unchanged. This result indicates that AmpG itself is partially responsible for the lower PG monomer release from N. meningitidis compared to N. gonorrhoeae.
Fig 5.
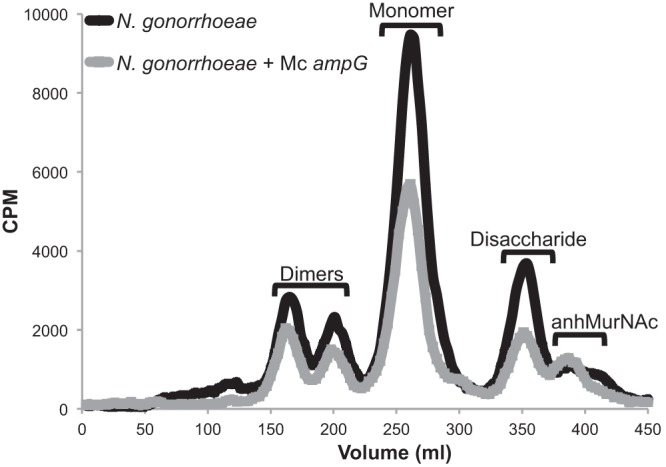
Expression of meningococcal ampG increases recycling efficiency in N. gonorrhoeae. Gonococcal strains expressing either gonococcal ampG or meningococcal ampG were equivalently labeled with [6-3H]glucosamine, and fragment release was analyzed. Gonococci expressing meningococcal ampG show a 1.97-fold decrease in peptidoglycan monomer release compared to wild-type gonococci.
Meningococci release less proinflammatory PG monomers than gonococci.
To examine differences in PG fragment release between N. meningitidis and N. gonorrhoeae, we labeled meningococci and gonococci with [6-3H]glucosamine and used a quantitative method to determine differences in PG fragment release between the two species (Fig. 6). We also determined that meningococci and gonococci incorporate [6-3H]glucosamine into PG at nearly identical labeling frequencies—56.06% and 56.58%, respectively. We found that N. meningitidis releases 2.8 times less PG monomer than N. gonorrhoeae (Fig. 6). The differences in monomer release between the pathogenic neisseriae are likely partially due to differences in ampG (Fig. 5). The levels of free disaccharide release were very similar between meningococci and gonococci, but dimer release appeared slightly reduced in the meningococcal profile. As noted above, the meningococcal profile includes a PG monomer peak for a molecule smaller than the tripeptide monomer and a large peak for anhydro-N-acetylmuramic acid. The latter is also seen to a smaller extent in the N. gonorrhoeae profile of released fragments. The appearance of these smaller PG fragments suggests that N. meningitidis is degrading multiple PG fragments to smaller products.
Fig 6.
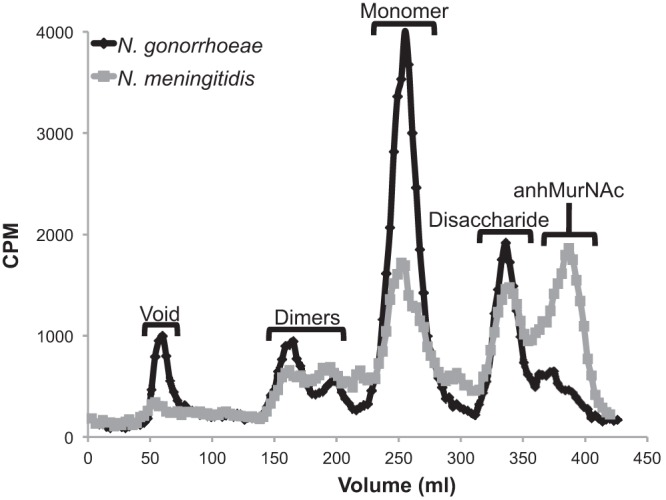
N. meningitidis releases less cytotoxic monomer than N. gonorrhoeae. Meningococci and gonococci were quantitatively labeled with [6-3H]glucosamine. After a chase period, supernatants were collected, and fragments were separated by size exclusion chromatography and detected by liquid scintillation counting. N. meningitidis releases less monomer than N. gonorrhoeae and has altered PG multimer release.
The PG monomer fractions from N. meningitidis and N. gonorrhoeae were further analyzed by reversed-phase HPLC (Fig. 7). The N. meningitidis monomer fraction contained both tripeptide monomer (human Nod1 agonist) and tetrapeptide monomer (TCT), consistent with our LC/MS analysis described above. The amount of tripeptide monomer released by N. meningitidis was 1.8-fold as much as tetrapeptide monomer. In addition, we detected three peaks with retention times that are consistent with those of reducing monomers, i.e., monomers lacking the 1,6-anhydro bond on the N-acetylmuramic acid. The PG monomers released by N. gonorrhoeae included tripeptide monomer and tetrapeptide monomer. The gonococcal strain released 2.6 times more tripeptide monomer than tetrapeptide monomer, consistent with previous results (21). The decrease in the ratio of tripeptide monomer to tetrapeptide monomer in N. meningitidis could alter PG sensing by Nod1 as human Nod1 only recognizes tripeptide monomer and not tetrapeptide monomer (17). In total, these data indicate that meningococci are breaking down PG more extensively or to different products than those found in gonococci.
Fig 7.
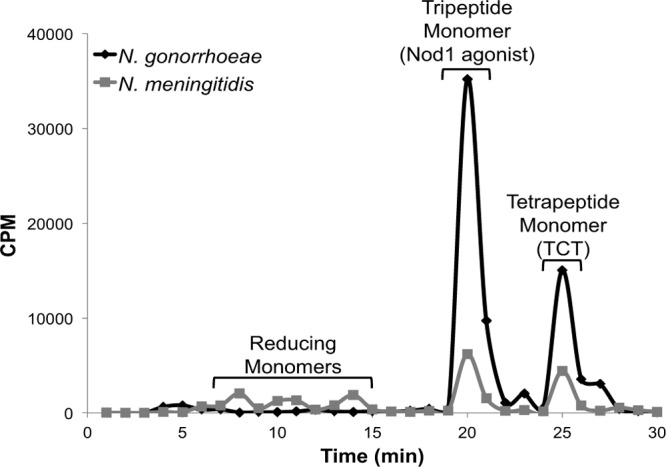
Meningococci release PG monomers in different proportions than gonococci. Radiolabeled PG monomer was pooled and analyzed by reversed-phase HPLC over a C18 column. Meningococci release 1.8 times more tripeptide monomer than tetrapeptide monomer. N. gonorrhoeae releases 2.6 times more tripeptide monomer than tetrapeptide monomer, suggesting that meningococci degrade PG fragments differently than gonococci.
Meningococci stimulate less Nod1-dependent activation of NF-κB than gonococci.
To determine if differences in PG fragment release and recycling between N. meningitidis and N. gonorrhoeae may result in altered host responses, we measured Nod1-dependent NF-κB activation in response to supernatants from meningococcal and gonococcal cultures. We exposed HEK293 cells overexpressing human Nod1 (hNod1) along with an alkaline phosphatase NF-κB reporter to meningococcal or gonococcal supernatants or a medium-only control. Supernatants were from bacteria grown to the log phase and were normalized based on total protein in the cell pellet. After 24 h of exposure to bacterial supernatants, alkaline phosphatase activity in the medium was measured and compared to the activity in the medium-only control (Table 2). NF-κB activity was significantly higher in cells expressing hNod1 than the parental null line, indicating that we were detecting hNod1-dependent NF-κB activity (data not shown). As expected, HEK293 cells overexpressing hNod1 showed NF-κB activity in response to tripeptide containing DAP, but not to tripeptide containing lysine. We found that gonococcal supernatants stimulated significantly more hNod1-dependent NF-κB activity than meningococcal supernatants, suggesting that differences in the amounts and types of PG fragments released by these species will affect NF-κB activity and inflammatory cytokine production during infection (Table 2).
Table 2.
Meningococci stimulate less hNod1-dependent NF-κB activity than gonococci
| Sample | Nod1-dependent PhoA activity (fold change)a |
|---|---|
| N. gonorrhoeae | 6.4 ± 0.5 |
| N. meningitidis | 3.9 ± 0.9b |
| Tri-DAP | 12.0 ± 4.1 |
| Tri-Lys | 1.0 ± 0.04 |
Fold increase over medium-only control.
Significantly less than the fold change stimulated by N. gonorrhoeae by t test (P = 0.011).
Cytokine induction in human fallopian tube explants.
While N. gonorrhoeae and N. meningitidis are naturally human-restricted pathogens, and few relevant in vivo models of human disease are available, a human fallopian tube organ culture (FTOC) model has been established to study the pathogenicity of the gonococcus (27). In this model, PG fragments from gonococci have been shown to cause ciliated cell death in fallopian tube mucosa (15) and infection with gonococci has been shown to induce the production of proinflammatory cytokines as well as cause ciliated cell death (28–30). Since PG fragments are known to stimulate the secretion of proinflammatory cytokines and chemokines, the human FTOC model could be used to evaluate the in vivo relevance of proportional differences in bacterial PG fragment release. Fallopian tube tissue was obtained from two donors and was treated with supernatants from N. meningitidis, N. gonorrhoeae, or ampG mutants of these species. After 24 h, tissue culture medium was removed, filtered, and assayed for levels of IL-8. In Fig. 8, each point represents the IL-8 produced from a single fallopian tube tissue segment. N. gonorrhoeae supernatants induced an average of 131 and 145 ng/ml IL-8 for the WT and ampG mutant, respectively. The average for IL-8 induced by the wild-type N. meningitidis supernatant appeared considerably lower at 85 ng/ml, but the ampG mutant gave an average IL-8 concentration of 136 ng/ml. There is a high degree in variability in response between the tissue sections, which may partly be due to differences in the human donors. The differences between the treatment groups do not reach statistical significance but show a trend toward lower IL-8 levels for the wild-type N. meningitidis-treated tissue and increased levels when ampG is mutated. These trends are in agreement with the results seen in hNod1-reporter cells (Table 2) and with the amounts of PG monomers released by these strains (Fig. 4 and 6).
Fig 8.
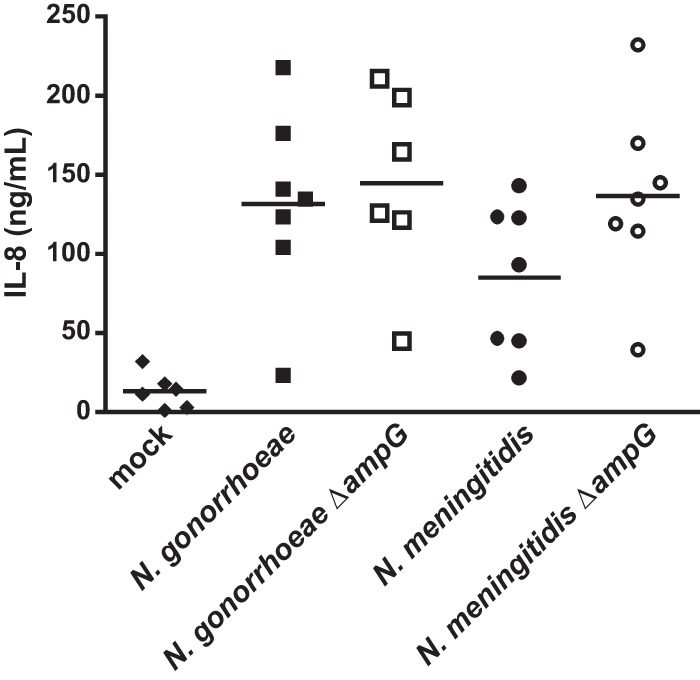
Fallopian tube organ culture explants secrete the proinflammatory cytokine IL-8 in response to exposure to meningococcal and gonococcal supernatants. Explants from two patients were exposed to supernatants from meningococci, gonococci, and isogenic ampG deletion mutants. After 24 h of exposure, IL-8 secretion was measured by ELISA. Data points are representative of IL-8 secretion from two independent experiments with ≥ 3 replicates per condition per experiment. Bars indicate the mean. There was no statistically significant difference between samples as determined by the Mann-Whitney test.
DISCUSSION
It appears that all bacteria that produce PG release some portion of what they produce into the environment in the course of normal growth. PG fragments are generated as the cell grows—PG strands in the cell wall are degraded to make a space for insertion of additional strands, and PG is broken down in the process of septum formation and splitting (31). It is how the PG is broken down, into what form, that affects its potential to elicit an immune response. Also, the amounts of PG fragments taken back into the cytoplasm for recycling affect the amounts available for release into the environment and thus possible detection by the immune system (32). In this study, we found that N. meningitidis has high-efficiency recycling and increased degradation of PG fragments, resulting in reduced release of proinflammatory PG fragments relative to N. gonorrhoeae.
We found that N. meningitidis releases soluble PG fragments, including the PG monomers that are known to induce inflammatory responses and cell death in various types of infections (11, 15, 16, 33). These PG fragments may contribute to the large inflammatory response that occurs during meningococcal sepsis or meningitis. Meningococci also release other proinflammatory molecules, including lipooligosaccharide (endotoxin) and Toll-like receptor 2 (TLR2) agonist PorB (34, 35). It is unclear how an organism that makes such potent inflammatory molecules can normally be found as an asymptomatic colonizer of humans, but we speculate that limiting PG fragment release may reduce inflammatory responses during nasopharyngeal colonization.
By comparing the amounts of PG fragments released by the wild-type strain and an ampG mutant, we determined that N. meningitidis normally recycles 96% of the PG monomers liberated from the cell wall. This rate of recycling is very similar to that seen with E. coli but is substantially higher than the rate determined for N. gonorrhoeae (36). Gonococci recycle 85% of PG monomers (6). The higher rate of recycling in N. meningitidis is partly due to differences in ampG. Replacement of gonococcal ampG with the allele from N. meningitidis resulted in an almost 2-fold reduction in PG monomer release (Fig. 5). Thus, although ampG does not account for all of the 2.8- to 3.7-fold reduction in amounts of PG monomer released by N. meningitidis, it does explain a substantial part of this difference (Fig. 4 and 6) (6). The meningococcal and gonococcal AmpG sequences are 97% identical at the amino acid level. It is possible that these minor differences may affect AmpG stability, activity, or interaction with other proteins involved in PG fragment recycling or release. Also, expression of ampG may be higher in meningococci than in gonococci. It is clear that there are differences between meningococci and gonococci in PG fragment recycling in addition to the amounts of PG monomers recycled. In N. gonorrhoeae, an ampG mutant is not affected in release of free disaccharide, and an ampD (recycling-defective) mutant shows increased uptake of free disaccharide (6). These data suggest that gonococci sense and respond to levels of PG in the cytoplasm. In N. meningitidis, mutation of ampG leads to an increase in free disaccharide and PG monomer release over that in the wild type, thus demonstrating that AmpG is responsible for free disaccharide uptake in meningococci and that meningococcal uptake of free disaccharide differs from gonococcal free disaccharide uptake.
A comparison of the PG fragments released by N. meningitidis and N. gonorrhoeae shows that N. meningitidis releases smaller amounts of PG monomers and releases additional PG fragments not released by N. gonorrhoeae. These additional PG fragments include smaller PG dimers, smaller PG monomers, and 1,6-anhydro-N-acetylmuramic acid. The presence of these PG fragments indicates that meningococci are breaking down the liberated PG fragments more extensively than gonococci. Furthermore, the HPLC analysis of meningococcal PG monomers shows that this fraction contains not just 1,6-anhydro-disaccharide tetrapeptide monomer and 1,6-anhydro-disaccharide tripeptide monomer but also contains reducing monomers. The net effect of the highly efficient monomer recycling and additional PG fragment degradation contributes to detoxification of released PG fragments, as evidenced by meningococcal supernatants inducing less Nod-1-dependent NF-κB activation than gonococcal supernatants. (Table 2). Also the tendency of gonococcal supernatants to induce more IL-8 production in fallopian tube tissue than was found using meningococcal supernatants is consistent with this idea (Fig. 8). E. coli also degrades PG fragments prior to their release. In E. coli, liberated PG monomers are degraded by the N-acetylmuramyl-l-alanine amidase AmiD (37). AmiD converts PG monomers into free peptides and free disaccharides. The free peptides could still participate in inducing inflammation through the Nod1 pathway since free tetrapeptides or free tripeptides would be Nod1 agonists in mice or humans, respectively (38). However, in the B. pertussis model, peptides have to be blocked at the N terminus to have cell-damaging activity (39), and thus AmiD activity would detoxify PG monomers.
This is the first characterization of PG fragments released by N. meningitidis during normal growth. We found that meningococci release multiple PG fragments, including proinflammatory PG monomers. Meningococcal PG monomer release is lower than that of N. gonorrhoeae, and this difference is partly due to differences in ampG. Meningococci also release additional PG fragments not present in the gonococcal PG fragment release profile, indicating that meningococci are breaking down PG further than gonococci. Reduction of proinflammatory monomer release and additional PG degradation may be advantageous for meningococci in maintaining an asymptomatic colonization of the human nasopharynx.
ACKNOWLEDGMENTS
We thank Sanjay Ram for the generous gift of plasmid pHC10.
This work was supported by NIH grants R01AI097157 and R21AI072605 to J.P.D.
Footnotes
Published ahead of print 8 July 2013
REFERENCES
- 1.Stephens DS, Greenwood B, Brandtzaeg P. 2007. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet 369:2196–2210 [DOI] [PubMed] [Google Scholar]
- 2.Cohn AC, MacNeil JR, Harrison LH, Hatcher C, Theodore J, Schmidt M, Pondo T, Arnold KE, Baumbach J, Bennett N, Craig AS, Farley M, Gershman K, Petit S, Lynfield R, Reingold A, Schaffner W, Shutt KA, Zell ER, Mayer LW, Clark T, Stephens D, Messonnier NE. 2010. Changes in Neisseria meningitidis disease epidemiology in the United States, 1998–2007: implications for prevention of meningococcal disease. Clin. Infect. Dis. 50:184–191 [DOI] [PubMed] [Google Scholar]
- 3.Girardin SE, Boneca IG, Carneiro LA, Antignac A, Jehanno M, Viala J, Tedin K, Taha MK, Labigne A, Zahringer U, Coyle AJ, DiStefano PS, Bertin J, Sansonetti PJ, Philpott DJ. 2003. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science 300:1584–1587 [DOI] [PubMed] [Google Scholar]
- 4.Rosenthal RS. 1979. Release of soluble peptidoglycan from growing gonococci: hexaminidase and amidase activities. Infect. Immun. 24:869–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hebeler BH, Young FE. 1976. Chemical composition and turnover of peptidoglycan in Neisseria gonorrhoeae. J. Bacteriol. 126:1180–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia DL, Dillard JP. 2008. Mutations in ampG or ampD affect peptidoglycan fragment release from Neisseria gonorrhoeae. J. Bacteriol. 190:3799–3807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobs C, Huang LJ, Bartowsky E, Normark S, Park JT. 1994. Bacterial cell wall recycling provides cytosolic muropeptides as effectors for beta-lactamase induction. EMBO J. 13:4684–4694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindquist S, Weston-Hafer K, Schmidt H, Pul C, Korfmann G, Erickson J, Sanders C, Martin HH, Normark S. 1993. AmpG, a signal transducer in chromosomal beta-lactamase induction. Mol. Microbiol. 9:703–715 [DOI] [PubMed] [Google Scholar]
- 9.Johnson JW, Fisher JF, Mobashery S. 2013. Bacterial cell-wall recycling. Ann. N. Y. Acad. Sci. 1277:54–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldman WE, Klapper DG, Baseman JB. 1982. Detection, isolation, and analysis of a released Bordetella pertussis product toxic to cultured tracheal cells. Infect. Immun. 36:782–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenthal RS, Nogami W, Cookson BT, Goldman WE, Folkening WJ. 1987. Major fragment of soluble peptidoglycan released from growing Bordetella pertussis is tracheal cytotoxin. Infect. Immun. 55:2117–2120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heiss LN, Moser SA, Unanue ER, Goldman WE. 1993. Interleukin-1 is linked to the respiratory epithelial cytopathology of pertussis. Infect. Immun. 61:3123–3128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flak TA, Goldman WE. 1999. Signalling and cellular specificity of airway nitric oxide production in pertussis. Cell. Microbiol. 1:51–60 [DOI] [PubMed] [Google Scholar]
- 14.McGee ZA, Johnson AP, Taylor-Robinson D. 1981. Pathogenic mechanisms of Neisseria gonorrhoeae: observations on damage to human fallopian tubes in organ culture by gonococci of colony type 1 or type 4. J. Infect. Dis. 143:413–422 [DOI] [PubMed] [Google Scholar]
- 15.Melly MA, McGee ZA, Rosenthal RS. 1984. Ability of monomeric peptidoglycan fragments from Neisseria gonorrhoeae to damage human fallopian-tube mucosa. J. Infect. Dis. 149:378–386 [DOI] [PubMed] [Google Scholar]
- 16.Nigro G, Fazio LL, Martino MC, Rossi G, Tattoli I, Liparoti V, De Castro C, Molinaro A, Philpott DJ, Bernardini ML. 2008. Muramylpeptide shedding modulates cell sensing of Shigella flexneri. Cell. Microbiol. 10:682–695 [DOI] [PubMed] [Google Scholar]
- 17.Girardin SE, Jehanno M, Mengin-Lecreulx D, Sansonetti PJ, Alzari PM, Philpott DJ. 2005. Identification of the critical residues involved in peptidoglycan detection by Nod1. J. Biol. Chem. 280:38648–38656 [DOI] [PubMed] [Google Scholar]
- 18.Kellogg DS, Jr, Peacock WL, Jr, Deacon WE, Brown L, Pirkle CL. 1963. Neisseria gonorrhoeae. I. Virulence genetically linked to clonal variation. J. Bacteriol. 85:1274–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenthal RS, Dziarski R. 1994. Isolation of peptidoglycan and soluble peptidoglycan fragments. Methods Enzymol. 235:235–285 [DOI] [PubMed] [Google Scholar]
- 20.Cloud KA, Dillard JP. 2002. A lytic transglycosylase of Neisseria gonorrhoeae is involved in peptidoglycan-derived cytotoxin production. Infect. Immun. 70:2752–2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kohler PL, Hamilton HL, Cloud-Hansen K, Dillard JP. 2007. AtlA functions as a peptidoglycan lytic transglycosylase in the Neisseria gonorrhoeae type IV secretion system. J. Bacteriol. 189:5421–5428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cloud-Hansen KA, Hackett KT, Garcia DL, Dillard JP. 2008. Neisseria gonorrhoeae uses two lytic transglycosylases to produce cytotoxic peptidoglycan monomers. J. Bacteriol. 190:5989–5994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sinha RK, Rosenthal RS. 1980. Release of soluble peptidoglycan from growing gonococci: demonstration of anhydro-muramyl-containing fragments. Infect. Immun. 29:914–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee M, Hesek D, Llarrull LI, Lastochkin E, Pi H, Boggess B, Mobashery S. 2013. Reactions of all Escherichia coli lytic transglycosylases with bacterial cell wall. J. Am. Chem. Soc. 135:3311–3314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heidrich C, Templin MF, Ursinus A, Merdanovic M, Berger J, Schwarz H, de Pedro MA, Höltje JV. 2001. Involvement of N-acetylmuramyl-L-alanine amidases in cell separation and antibiotic-induced autolysis of Escherichia coli. Mol. Microbiol. 41:167–178 [DOI] [PubMed] [Google Scholar]
- 26.Girardin SE, Tournebize R, Mavris M, Page AL, Li X, Stark GR, Bertin J, DiStefano PS, Yaniv M, Sansonetti PJ, Philpott DJ. 2001. CARD4/Nod1 mediates NF-kappaB and JNK activation by invasive Shigella flexneri. EMBO Rep. 2:736–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melly MA, Gregg CR, McGee ZA. 1981. Studies of toxicity of Neisseria gonorrhoeae for human fallopian tube mucosa. J. Infect. Dis. 143:423–431 [DOI] [PubMed] [Google Scholar]
- 28.Maisey K, Nardocci G, Imarai M, Cardenas H, Rios M, Croxatto HB, Heckels JE, Christodoulides M, Velasquez LA. 2003. Expression of proinflammatory cytokines and receptors by human fallopian tubes in organ culture following challenge with Neisseria gonorrhoeae. Infect. Immun. 71:527–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGee ZA, Clemens CM, Jensen RL, Klein JJ, Barley LR, Gorby GL. 1992. Local induction of tumor necrosis factor as a molecular mechanism of mucosal damage by gonococci. Microb. Pathog. 12:333–341 [DOI] [PubMed] [Google Scholar]
- 30.Velasquez L, Garcia K, Morales F, Heckels JE, Orihuela P, Rodas PI, Christodoulides M, Cardenas H. 2012. Neisseria gonorrhoeae pilus attenuates cytokine response of human fallopian tube explants. J. Biomed. Biotechnol. 2012:491298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Typas A, Banzhaf M, Gross CA, Vollmer W. 2012. From the regulation of peptidoglycan synthesis to bacterial growth and morphology. Nat. Rev. Microbiol. 10:123–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boudreau MA, Fisher JF, Mobashery S. 2012. Messenger functions of the bacterial cell wall-derived muropeptides. Biochemistry 51:2974–2990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koropatnick TA, Engle JT, Apicella MA, Stabb EV, Goldman WE, McFall-Ngai MJ. 2004. Microbial factor-mediated development in a host-bacterial mutualism. Science 306:1186–1188 [DOI] [PubMed] [Google Scholar]
- 34.Zughaier SM, Tzeng YL, Zimmer SM, Datta A, Carlson RW, Stephens DS. 2004. Neisseria meningitidis lipooligosaccharide structure-dependent activation of the macrophage CD14/Toll-like receptor 4 pathway. Infect. Immun. 72:371–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Massari P, Visintin A, Gunawardana J, Halmen KA, King CA, Golenbock DT, Wetzler LM. 2006. Meningococcal porin PorB binds to TLR2 and requires TLR1 for signaling. J. Immunol. 176:2373–2380 [DOI] [PubMed] [Google Scholar]
- 36.Greenway DL, Perkins HR. 1985. Turnover of the cell wall peptidoglycan during growth of Neisseria gonorrhoeae and Escherichia coli. Relative stability of newly synthesized material. J. Gen. Microbiol. 131:253–263 [DOI] [PubMed] [Google Scholar]
- 37.Uehara T, Park JT. 2007. An anhydro-N-acetylmuramyl-L-alanine amidase with broad specificity tethered to the outer membrane of Escherichia coli. J. Bacteriol. 189:5634–5641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Magalhaes JG, Philpott DJ, Nahori MA, Jehanno M, Fritz J, Le Bourhis L, Viala J, Hugot JP, Giovannini M, Bertin J, Lepoivre M, Mengin-Lecreulx D, Sansonetti PJ, Girardin SE. 2005. Murine Nod1 but not its human orthologue mediates innate immune detection of tracheal cytotoxin. EMBO Rep. 6:1201–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luker K, Tyler A, Marshall G, Goldman W. 1995. Tracheal cytotoxin structural requirements for respiratory epithelial damage in pertussis. Mol. Microbiol. 16:733–743 [DOI] [PubMed] [Google Scholar]
- 40.Woodhams KL, Benet ZL, Blonsky SE, Hackett KT, Dillard JP. 2012. Prevalence and detailed mapping of the gonococcal genetic island in Neisseria meningitidis. J. Bacteriol. 194:2275–2285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swanson J. 1972. Studies on gonococcus infection. II. Freeze-fracture, freeze-etch studies on gonococci. J. Exp. Med. 136:1258–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamilton HL, Schwartz KJ, Dillard JP. 2001. Insertion-duplication mutagenesis of Neisseria: use in characterization of DNA transfer genes in the gonococcal genetic island. J. Bacteriol. 183:4718–4726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ram S, Cox AD, Wright JC, Vogel U, Getzlaff S, Boden R, Li J, Plested JS, Meri S, Gulati S, Stein DC, Richards JC, Moxon ER, Rice PA. 2003. Neisserial lipooligosaccharide is a target for complement component C4b. Inner core phosphoethanolamine residues define C4b linkage specificity. J. Biol. Chem. 278:50853–50862 [DOI] [PubMed] [Google Scholar]
- 44.Ramsey ME, Hackett KT, Kotha C, Dillard JP. 2012. New complementation constructs for inducible and constitutive gene expression in Neisseria gonorrhoeae and Neisseria meningitidis. Appl. Environ. Microbiol. 78:3068–3078 [DOI] [PMC free article] [PubMed] [Google Scholar]


