Abstract
Antigen-specific Abs are able to enhance or suppress immune responses depending on the receptors that they bind on immune cells. Recent studies have shown that pro- or antiinflammatory effector functions of IgG Abs are also regulated through their Fc N-linked glycosylation patterns. IgG Abs that are agalactosylated (non-galactosylated) and asialylated are proinflammatory and induced by the combination of T cell–dependent (TD) protein antigens and proinflammatory costimulation. Sialylated IgG Abs, which are immunosuppressive, and Tregs are produced in the presence of TD antigens under tolerance conditions. T cell–independent (TI) B cell activation via B cell receptor (BCR) crosslinking through polysaccharides or via BCR and TLR costimulation also induces IgG Abs, but the Fc glycosylation state of these Abs is unknown. We found in mouse experiments that TI immune responses induced suppressive sialylated IgGs, in contrast to TD proinflammatory Th1 and Th17 immune responses, which induced agalactosylated and asialylated IgGs. Transfer of low amounts of antigen-specific sialylated IgG Abs was sufficient to inhibit B cell activation and pathogenic immune reactions. These findings suggest an immune regulatory function for TI immune responses through the generation of immunosuppressive sialylated IgGs and may provide insight on the role of TI immune responses during infection, vaccination, and autoimmunity.
Introduction
Abs regulate the production of new Abs specific for the same antigen via positive or negative feedback mechanisms (1–5). For example, IgG Abs form immune complexes (ICs) with the antigen and generate negative feedback regulation through crosslinking the B cell receptor (BCR) with the IgG inhibitory receptor FcγRIIB (encoded by Fcgr2b), which inhibits BCR signaling and thereby B cell activation and IgG Ab production (2, 3). In addition, FcγRIIB-independent negative feedback effects of IgG Abs have been described (4, 5). For example, T cell–independent type-2 (TI-2) antigen–induced IgG Abs suppress the B cell activation that results from a second challenge with the same TI-2 antigen, independent of FcγRIIB (5).
Recent studies have indicated that the activating or inhibitory functions of IgG Abs are also regulated through the pattern of the Fc glycan linked to Asn297 (6–16). The biantennary core glycan structure, which is composed of 2 N-acetyl-glucosamines (GlcNAc) and 3 mannoses, can be further modified with fucose, bisecting GlcNAc and terminal GlcNAc, galactose, and sialic acid.
The disease severity of RA has been associated with the appearance of proinflammatory asialylated (non-sialylated) and agalactosylated (G0) serum IgG auto-Abs (6, 11, 17–34). Furthermore, the anti-gp120 IgG Abs of HIV patients are less galactosylated and sialylated in long-term nonprogessors, who are infected but show no disease symptoms, compared with infected patients with disease symptoms (15). In contrast, IgG Abs that are both galactosylated and sialylated possess inhibitory properties that underlie the antiinflammatory effect of i.v. IgG (IVIG; pooled serum IgG from healthy donors), which is used in high doses (2 g/kg) to treat autoimmune patients (7–10, 12).
Regarding the physiological development and function of differentially glycosylated IgG Abs, it has recently been shown that tolerance induction with T cell–dependent (TD) protein antigen without a proinflammatory costimulus induces not only Tregs, but also immunosuppressive sialylated IgGs. Small doses (2.5 mg/kg) of sialylated antigen-specific IgGs, in the form of ICs, inhibit the maturation of DCs and proinflammatory immune responses in an antigen-specific manner (13). In contrast, the combination of TD antigens and TLR costimulation induces proinflammatory T and B cell immune responses, including the production of proinflammatory asialylated IgGs (13). For example, TD B cell activation via TLR/MyD88 costimulation plays a major role in the development of pathogenic IgG auto-Abs and autoimmunity, as demonstrated in different mouse models of lupus (35–41).
Recent evidence indicates that B cell activation via BCR and TLR costimulation without the help of T cells can also induce IgG Abs (40, 42–44), although the pro- or antiinflammatory Fc glycosylation pattern of TI-1 and TI-2 IgGs is unknown. In the present study, we compared TI-1 and TI-2 IgGs with respect to TD B cell activation and found that TI B cell activation was associated with the production of immunosuppressive sialylated serum IgGs, which inhibited B cell activation and immune reactions, independent of FcγRIIB.
Results
TI-1 and TI-2 B cell activation induces the production of IgM and IgG Abs and suppresses subsequent antigen-induced immune responses.
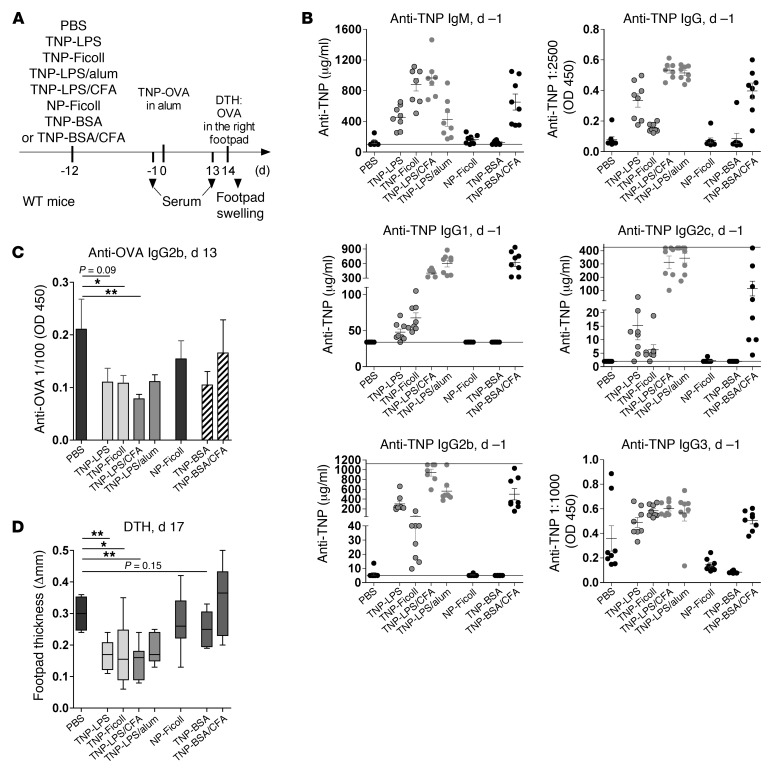
To investigate whether TI B cell activation via BCR and TLR costimulation (TI-1) or via BCR crosslinking (TI-2) induces IgGs and influences subsequent inflammatory immune responses, we studied the effect of TI-1 and TI-2 antigen immunization on the development of disease symptoms resulting from a subsequent antigen-induced T cell–mediated delayed-type hypersensitivity (DTH) response (Figure 1A). Immunization of 8-week-old C57BL/6 WT mice i.p. with the TI-1 antigen 2,4,6-trinitrophenyl–coupled LPS (TNP-LPS) predominantly induced anti-TNP IgM, IgG2c (also known as IgG2ab), and IgG2b Abs (refs. 44–47, Figure 1B, and Supplemental Figures 1 and 2; supplemental material available online with this article; doi: 10.1172/JCI65938DS1). In contrast, immunization with the TI-2 antigen TNP-Ficoll predominantly generated anti-TNP IgM, IgG1, and IgG3 Abs (Figure 1B and Supplemental Figure 2). Both TI antigens hardly induced anti-TNP IgA Abs (Supplemental Figure 2).
Figure 1. TI antigen–specific B cell activation induces IgM and IgG Abs and reduces a subsequent antigen-induced DTH response.
(A) Experimental approach. WT mice were injected i.p. with PBS or 50 μg of TNP-Ficoll, TNP-LPS, TNP-LPS in CFA, TNP-LPS in alum, NP-Ficoll, TNP-BSA, or TNP-BSA in CFA on day –12. DTH was induced by i.p. injection of TNP-OVA in alum on day 0 and OVA in the right footpad on day 14. (B) Anti-TNP IgM, total IgG, and IgG1, IgG2c, IgG2b, and IgG3 subclass serum Ab levels on day –1, determined via ELISA. Anti-TNP IgM, IgG1, IgG2c, and IgG2b serum concentrations were determined using anti-TNP IgM, IgG1, IgG2c, and IgG2b standard Abs (Supplemental Figure 2). Symbols represent data from individual animals. Horizontal lines and error bars represent mean + SEM. (C) Anti-OVA IgG2b serum Ab levels on day 13 were determined via ELISA. Bars represent mean + SEM. (D) Difference in footpad thickness between the right and left footpad, determined 3 days after local DTH induction (i.e., day 17). A box-and-whisker diagram with median and sample minimum and maximum is shown. 1 representative of 3 independent experiments is shown. *P < 0.05, **P < 0.01.
However, immunization with both TI antigens reduced OVA-specific IgG2b Ab responses and footpad swelling after DTH induction with TNP-coupled OVA (TNP-OVA) in alum and subsequent local challenge with OVA (Figure 1, C and D). Moreover, this TI antigen–mediated inhibition was independent of FcγRIIB (Supplemental Figure 3), and immunization with antigen-nonspecific 4-hydroxy-3-nitrophenylacetyl–Ficoll (NP-Ficoll) failed to reduce TNP-OVA–induced DTH reactions (Figure 1 and Supplemental Figure 2).
TD, CD154 (CD40L)-dependent immunization with TNP-BSA in CFA induced IgM, IgG, and IgA anti-TNP serum Abs, but had no inhibitory effect on footpad swelling (Figure 1 and Supplemental Figures 1 and 2). Equal amounts of TNP-BSA without costimulation induced no detectable serum IgGs and showed only weak effects on DTH responses (Figure 1 and Supplemental Figures 1 and 2).
To determine whether the suppressive effect of TI, CD154-independent antigen immunization could be overcome using adjuvant, we administered TNP-LPS in the presence of CFA or alum (Figure 1 and Supplemental Figures 1 and 2). However, neither CFA nor alum was sufficient to overcome the suppressive effect of TI TNP-LPS immunization on footpad swelling (Figure 1 and Supplemental Figure 2).
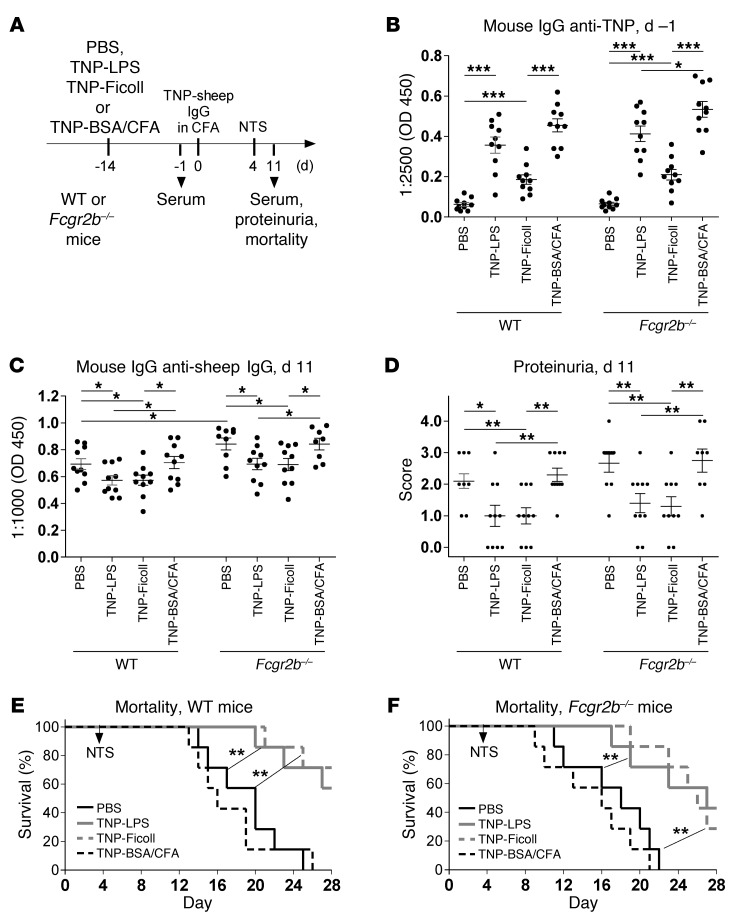
To confirm that TI antigen immunization suppresses subsequent antigen-induced pathogenic TD immune reactions, we studied the effect of TNP-LPS and TNP-Ficoll immunizations on Ab-mediated nephritis induced with TD TNP-coupled sheep IgG (TNP–sheep IgG) in CFA and subsequent transfer of sheep anti–glomerular basement membrane (anti-GBM) nephrotoxic serum (NTS) (Figure 2 and ref. 48). Immunization with TI antigen alone, but not TD TNP-BSA in CFA, reduced the accumulation of mouse IgG anti–sheep IgG serum Abs and nephritis-mediated mortality in both WT and Fcgr2b–/– mice (Figure 2).
Figure 2. TI antigen–specific B cell activation suppresses a subsequent antigen-induced nephritis, independent of FcγRIIB.
(A) Experimental approach. WT and Fcgr2b–/– mice were simultaneously injected i.p. with either PBS or 50 μg of TNP-Ficoll, TNP-LPS, or TNP-BSA in CFA on day –14. Nephritis was induced by i.p. injection of TNP–sheep IgG in CFA on day 0 and NTS on day 4. (B) Anti-TNP mouse IgG serum levels on day –1 were determined via ELISA. Symbols represent data from individual animals. Horizontal lines and error bars represent mean + SEM. (C) Mouse IgG anti–sheep IgG serum levels on day 11 were determined via ELISA. (D) Proteinuria was determined on day 11. (E and F) Kaplan-Meier survival curves of WT (E) and Fcgr2b–/– (F) mice. 1 representative of 2 independent experiments is shown. *P < 0.05, **P < 0.01, ***P < 0.001.
In summary, our results showed that TI antigen–specific B cell activation can induce both IgM and IgG Abs and suppress subsequent pathogenic immune responses driven by T cells and/or pathogenic IgGs in an antigen-specific manner, independent of FcγRIIB. Furthermore, neither CFA nor alum was sufficient to overcome the suppression induced by TI immunization. Immunosuppressive effects have been previously described for both IgM and IgG Abs (1, 4–6, 13, 14, 41, 49–53); in this study, we chose to focus on the suppressive function of TI IgGs.
TI immune responses induce sialylated antigen-specific IgG Abs.
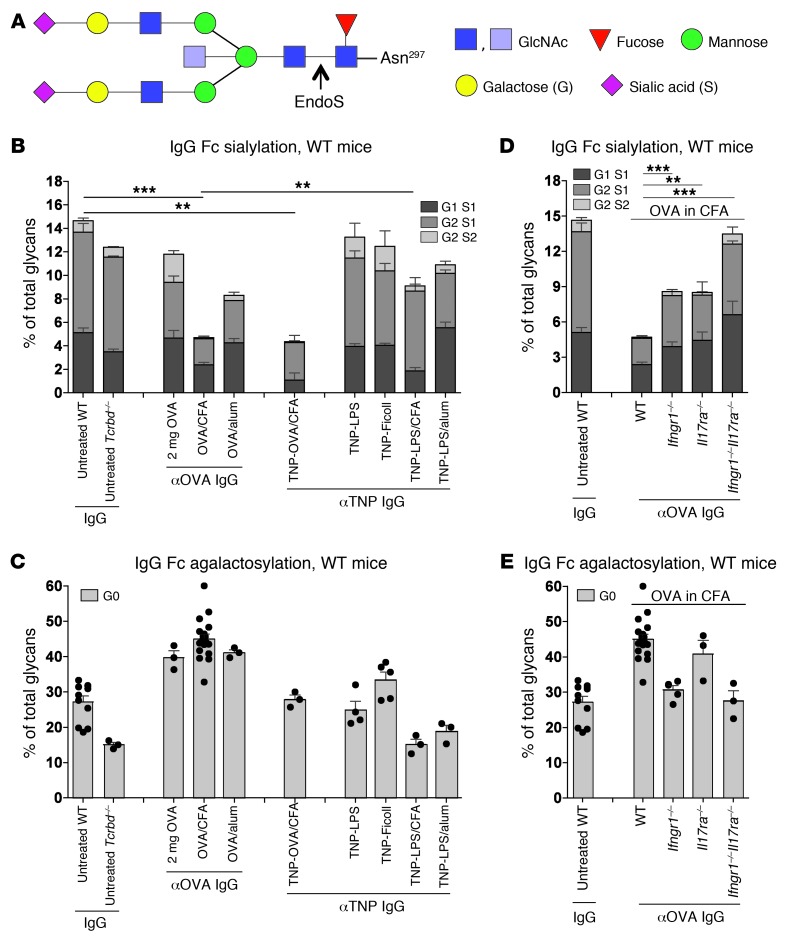
The pro- or antiinflammatory effects of IgG Abs have been shown to correlate with their Fc glycan pattern (Figure 3A). Increasing percentages of G0 serum IgG auto-Abs have been associated with disease severity in RA (6, 11, 17–34), whereas ICs containing sialylated IgG Abs are known to inhibit DC maturation and proinflammatory immune responses in an antigen-specific manner (13). To determine whether IgG Abs induced by TI or TD immunization differ in Fc sialylation and galactosylation, we analyzed the Fc sialic acid content and G0 structures of purified TI or TD antigen–reactive serum IgGs at 14 days after different immunizations in WT mice (Figure 3, B and C, and Supplemental Figure 4).
Figure 3. TI immunization induces sialylated IgG Abs.
(A) The largest IgG Fc glycan coupled to Asn297. EndoS cleavage was used to perform MALDI-TOF MS, exclusive of the Fc glycans coupled to purified total or antigen-reactive IgGs (Supplemental Figure 4). (B and C) Fc sialic acid (B) and G0 (C) contents of total IgG from the pooled sera of 3 10-week-old untreated WT (independent experiments, n = 11) or Tcrbd–/– (n = 3) mice or purified OVA- or TNP-reactive IgGs from the pooled sera of 6–10 WT mice i.p. injected 14 days before with 2 mg pure OVA (n = 3); 100 μg OVA in CFA (n = 18) or alum (n = 3); TNP-OVA in CFA (n = 3); or 50 μg TNP-LPS (n = 4), TNP-Ficoll (n = 5), or TNP-LPS in CFA (n = 3) or alum (n = 3) was analyzed. Bars represent mean + SEM for all independent experiments for each group. Symbols represent data from individual animals. (D and E) Fc sialic acid (D) and G0 (E) contents of purified total IgG from the pooled sera of 3 10-week-old untreated WT mice (n = 11) or purified OVA-reactive IgGs from the pooled sera of 6 10-week-old WT (n = 18), Ifngr1–/– (n = 5), Il17ra–/– (n = 3), or Ifngr1–/–Il17ra–/– (n = 3) mice i.p. injected 14 days before with 100 μg OVA in CFA. Data for total IgG from untreated WT mice and for OVA-reactive IgG from WT mice treated with 2 mg OVA or with 100 μg OVA in CFA were used from our recent studies (13). **P < 0.01, ***P < 0.001.
The sialic acid content of TNP-reactive IgGs induced with 50 μg TI TNP-LPS or TNP-Ficoll was similar to the steady-state level measured in purified total serum IgGs from nonimmunized WT and TCRβ/δ-deficient (Tcrbd–/–) mice as well as the level of TD OVA-reactive IgGs after tolerance induction with 2 mg OVA without costimulation (Figure 3B, Supplemental Figure 4, and ref. 13). The G0 content of the TI TNP-reactive IgGs was comparable to the levels in purified total serum IgGs from nonimmunized WT mice (Figure 3C). Similar results were obtained after TI antigen immunization in Fcgr2b–/– mice (Supplemental Figure 4, D–F).
In contrast, TNP- and OVA-reactive IgG Abs induced by TNP-OVA in CFA or by OVA in CFA showed significantly lower sialic acid contents than IgGs from untreated WT mice (13) or TI antigen–reactive IgG Abs. Only partial desialylation of TD anti-OVA IgGs was observed after Th2-mediated OVA with alum immunization (Figure 3B and Supplemental Figure 4). The G0 content after TD stimulation with OVA was higher than the total IgG content from untreated mice and that of TI TNP-reactive IgGs (Figure 3C). Non–antigen-reactive serum IgGs at the same time point after immunization showed galactosylation and sialylation levels comparable to the total serum IgG obtained from untreated mice (data not shown).
However, TI TNP-LPS immunization with CFA costimulation was not sufficient to induce low-sialylated and low-galactosylated anti-TNP IgGs (Figure 3, B and C). These results indicated that T cell help is important for the induction of asialylated and agalactosylated antigen-reactive IgGs under proinflammatory conditions. Accordingly, low sialylation levels of OVA-reactive serum IgGs induced by OVA in CFA were dependent on the synergistic effects of the Th1 cytokine IFN-γ and the Th17 cytokine IL-17, as demonstrated by the partial lack of proinflammatory low-sialylated anti-OVA IgGs in Ifngr1–/– and Il17ra–/– mice and their complete absence in double-deficient Ifngr1–/–Il17ra–/– mice (Figure 3D and Supplemental Figure 5). The increased G0 content of OVA-reactive IgGs after OVA in CFA immunization was dependent on IFN-γRI signaling (Figure 3E).
In summary, these findings showed that TD protein antigens in the context of a proinflammatory Th1 and Th17 cell–inducing costimulus induced proinflammatory agalactosylated and asialylated IgG Abs, whereas even this costimulus was not sufficient to induce agalactosylated and asialylated IgG Abs after TI immunization. Thus, TI IgG Abs are galactosylated and sialylated.
Antigen-specific sialylated, but not desialylated, IgG Abs suppress pathogenic immune reactions and B cell activation, independent of FcγRIIB.
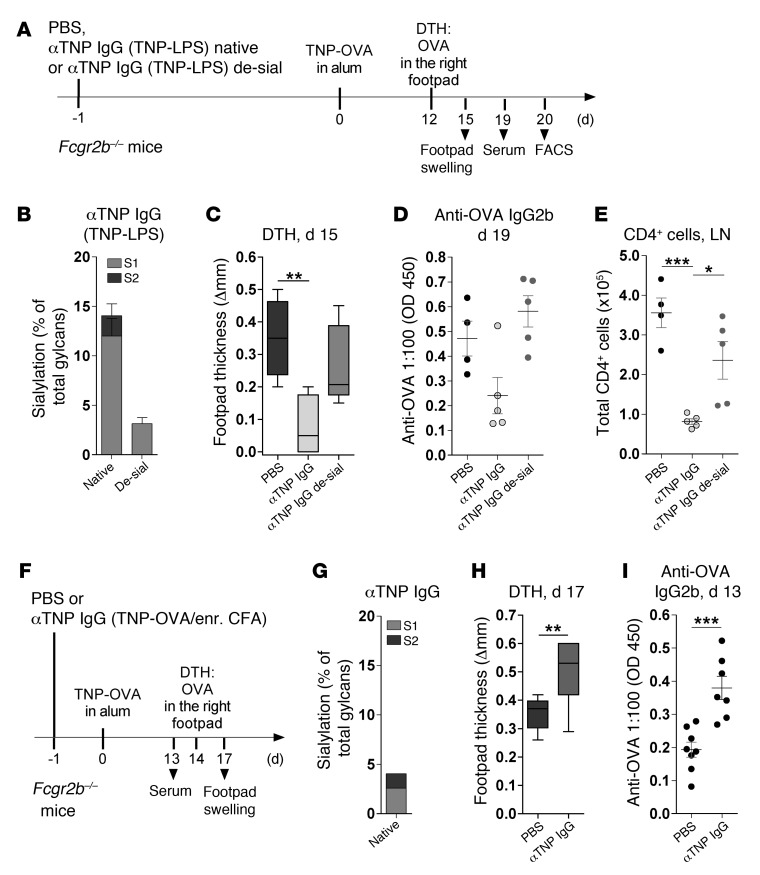
To determine whether the suppressive function of TI IgGs is directly associated with their sialylation level, we transferred native sialylated or sialidase-treated desialylated TNP-reactive serum IgGs (purified from TNP-LPS–immunized mice) into recipient mice 1 day before inducing DTH with TNP-OVA in alum (Figure 4, A and B). In addition, to investigate whether the suppressive function of sialylated IgGs is independent of FcγRIIB, we used Fcgr2b–/– mice as recipients. Transfer of 200 μg of native sialylated, but not sialidase-treated desialylated, TI TNP-specific serum IgGs was sufficient to reduce the pathogenic DTH response, as measured by footpad swelling, anti-OVA IgG2b serum Ab levels, and CD4+ T cell accumulation in local LNs (Figure 4, C–E).
Figure 4. Transfer of antigen-specific sialylated, but not desialylated, TI IgGs suppresses a subsequent antigen-induced DTH response.
(A) Experimental approach for B–E. Fcgr2b–/– mice were injected i.v. on day –1 with PBS (n = 4) or 200 μg native (n = 5) or sialidase-treated desialylated (de-sial; n = 5) anti-TNP IgG Abs purified from the pooled sera of TNP-LPS–immunized WT mice. DTH was induced as in Figure 1. (B) Frequency of Fc sialic acid modifications on native or sialidase-treated TNP-reactive IgGs, determined by EndoS treatment and MALDI-TOF MS (Supplemental Figure 4). Bars represent mean + SEM. (C) Difference in footpad thickness between the right and left footpad at day 15. (D) Anti-OVA IgG2b Ab levels, determined via ELISA. (E) Mice were sacrificed, and draining LNs were analyzed for total CD4+ cell numbers. 1 representative of 2 independent experiments is shown. (F) Experimental approach for G–I. Fcgr2b–/– mice were injected i.v. with PBS (n = 8) or 200 μg anti-TNP IgGs purified from the pooled sera of TNP-OVA in enriched CFA–immunized WT mice (n = 7). DTH was induced as in Figure 1. (G) Frequency of Fc sialic acid modifications on native TNP-specific IgGs, (H) differences in footpad thickness, and (I) anti-OVA IgG2b levels, determined as described above. 1 representative of 2 independent experiments is shown. (C and H) Box-and-whisker diagrams show median and sample minimum and maximum. (D, E, and I) Symbols represent data from individual animals. Horizontal lines represent mean + SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
In contrast, transfer of TNP-reactive low-sialylated IgG serum Abs, induced by immunization with TD TNP-OVA in enriched CFA, enhanced the DTH response and anti-OVA IgG2b serum Ab levels (Figure 4, F–I), potentially through a positive feedback mechanism via activating FcγRs (4, 54).
In summary, sialidase treatment of sialylated TI IgG Abs was sufficient to suspend the immunosuppressive effect of these Abs in Fcgr2b–/– mice. The suppressive effect of sialylated TI IgG Abs was consistent with recent data showing that transfer of sialylated TD IgG Abs induced via protein antigen without costimulation or in vitro sialylated monoclonal murine IgG1 Abs could reduce proinflammatory immune responses in an antigen-specific manner (13). The inhibition of DC maturation by ICs containing sialylated IgGs potentially contributes to this suppressive FcγRIIB-independent effect (13).
We further found that just 100 μg of 15% sialylated anti-TNP murine IgG1 (clone H5) was sufficient to reduce a DTH response in both WT and Fcgr2b–/– mice in an antigen-specific manner (Supplemental Figure 6). Sialylation of clone H5 IgG1 Abs did not influence TNP binding (Supplemental Figure 6 and ref. 13).
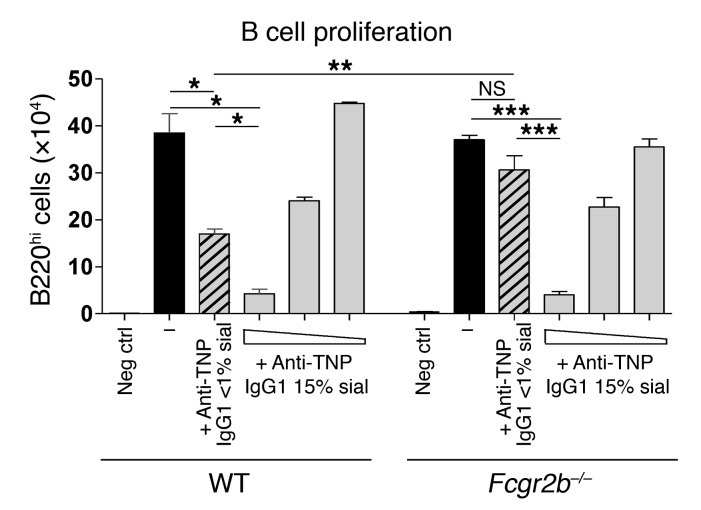
TI-2 antigens have been implicated in the induction of suppressive IgGs, which inhibit a secondary B cell challenge with the same TI-2 antigen, independent of FcγRIIB (5). Furthermore, it has been shown that IVIG treatment can inhibit B cell activation, independent of FcγRIIB (55, 56). To further examine the influence of sialylated IgGs on B cell activation, B cells from WT and Fcgr2b–/– mice were stimulated in vitro with LPS and treated with native asialylated (<1% sialylation) or in vitro sialylated (15% sialylation) monoclonal anti-TNP murine IgG1 hybridoma Abs (clone H5; Figure 5, Supplemental Figure 6, and ref. 13). Asialylated monoclonal murine IgG1 Abs reduced the proliferation of WT B cells, but not Fcgr2b–/– B cells (Figure 5), obviously through an FcγRIIB-dependent mechanism (4, 54). However, sialylated IgG1 Abs inhibited the proliferation of both WT and Fcgr2b–/– B cells (Figure 5), through an FcγRIIB-independent mechanism.
Figure 5. Sialylated, but not asialylated, monoclonal murine IgG1 inhibits LPS-induced B cell proliferation.
LPS-stimulated total splenocytes from WT and Fcgr2b–/– mice were treated with 1 mg/ml native asialylated (<1% sialylated) or 1, 0.1, or 0.01 mg/ml 15% sialylated anti-TNP IgG1 (clone H5; Supplemental Figure 6). No LPS served as a negative control. Live B220hi B cell numbers were calculated on day 3. 1 representative of 2 independent experiments is shown. *P < 0.05, **P < 0.01, ***P < 0.001.
With respect to the results shown in Figures 1 and 2, IgM Abs can also have immunoregulatory properties (1, 41, 49–53). To analyze the feedback effect of differentially sialylated IgM Abs on TI antigen–induced B cell activation, a native sialylated or sialidase-treated desialylated monoclonal anti-TNP IgM Ab was complexed with TNP-Ficoll and injected i.p. into WT mice (Supplemental Figures 7 and 8). The desialylated, but not sialylated, monoclonal IgM Ab enhanced the TNP-Ficoll–induced anti-TNP IgG response. Although the sialylated monoclonal IgM Ab failed to reduce the TNP-Ficoll–induced anti-TNP IgG response in this experiment, these data demonstrated that the sialylation of IgM Abs influences their feedback effect.
In summary, our data showed that TI B cell activation leads to the development of immunosuppressive sialylated IgG Abs, which can inhibit immune responses, such as B cell activation, independent of FcγRIIB.
Discussion
The induction of either a proinflammatory immune response or tolerance to an antigen is a major challenge for the immune system. TD protein antigens in the context of proinflammatory costimuli induce proinflammatory T cells and IgG Abs with low Fc sialylation, whereas TD immune responses without costimulation induce peripheral T cell tolerance (57) and immunosuppressive sialylated IgG Abs (13). Our present results showed that TI-1 and TI-2 B cell activation also induced immunosuppressive sialylated IgG Abs, even in the presence of proinflammatory costimuli. Thus, the help of T cells (especially Th1 and Th17 cells), together with proinflammatory costimuli, are important for the induction of proinflammatory IgG immune responses. In contrast, TD protein antigens under tolerogenic conditions and TI antigens induced sialylated immunosuppressive IgG Abs.
Strategies to improve the success of vaccines have shown that TI antigens, such as polysaccharides, should be coupled to a foreign protein to recruit T cell help (58). The present observation that TI immunizations alone induced suppressive sialylated IgGs may explain this positive protein effect in vaccines. Furthermore, IgG Fc asialylation was more dependent on Th1 and Th17 than on Th2 responses, which may also be important for adjuvant selection in vaccine development.
Different murine models for autoimmune lupus have demonstrated that Th1 and Th17 cells and TD B cell activation via TLR/MyD88 costimulation play a major role in the development of highly mutated, high-affinity pathogenic IgG auto-Abs and autoimmune disease. Recent studies have also shown that TI-1 B cell activation via BCR and TLR costimulation is sufficient to induce IgG auto-Abs (35–45). However, whether the presence of IgG auto-Abs generated in response to TI-1 B cell activation via TLR7 or TLR9 costimulation reflects inflammatory lupus disease or protection must be investigated, as well as the Fc glycosylation pattern of the TI IgG auto-Abs.
The data obtained in the present study showed that TI B cell activation leads to the development of suppressive sialylated IgGs similar to the sialylation level of total serum IgG under steady-state conditions in nonimmunized mice. Steady-state IgG contains both autoreactive and polyreactive IgG Abs, which may play an important role in maintaining self-tolerance (59, 60). TI B cell activation also induces the production of IgM Abs, which may also have suppressive feedback effects, as was previously suggested for IgM Abs in a different context (1, 41, 49–53). In the present study, a desialylated monoclonal anti-TNP IgM Ab enhanced TNP-Ficoll–induced B cell activation in vivo. However, the sialylated anti-TNP IgM Ab failed to inhibit the TI response. The anti-TNP IgM Ab used in this study was a monoclonal Ab produced in HEK293 cells. Sialylated polyclonal serum IgM Abs might have other characteristics that mediate suppressive effects, including different levels of high-mannose and hybrid structures (Supplemental Figure 8) and the presence of the J-chain with unclear functions. However, our data showed that sialylation at least inhibited the positive feedback effects of IgM Abs on TI antigen–induced B cell activation.
The inhibitory properties of sialylated IgGs have been previously described and underlie the antiinflammatory effect of IVIG (7, 8, 10, 12). The sialylated subfraction of IVIG actively modulates marginal zone macrophages via the C-type lectin receptor specific ICAM-3 grabbing nonintegrin–related 1 (SIGN-R1), which is primarily independent of FcγRIIB (8, 12). Sialylated IgGs have a 10-fold lower affinity for activating and inhibiting FcγRs than do asialylated IgGs (7), which further suggests that mechanisms and/or receptors other than FcγRIIB mediate the suppressive effect of sialylated IgGs. Accordingly, small amounts of ICs containing sialylated IgGs were shown to inhibit the maturation of DCs and proinflammatory immune responses in an antigen-specific manner, independent of FcγRIIB (13).
Furthermore, the present data showed that sialylated IgGs inhibited B cell activation, independent of FcγRIIB. Together, these results suggest that sialylated IgG Abs exhibit suppressive effects on different immune cell types, including the inhibition of B cell activation, independent of FcγRIIB.
In summary, our findings identified a novel immune regulatory function for TI immune responses through the generation of antigen-specific immunosuppressive sialylated IgG Abs, which further indicates that the presence of antigen-specific IgG serum Abs is not sufficient to predict the inflammatory quality of an overall immune response. Furthermore, these data may have important implications for understanding TI immune responses to self-antigens and pathogens and for the development of novel vaccination strategies using TI antigens.
Methods
Mice.
C57BL/6 WT mice were purchased from Charles River Laboratories. Fcgr2b–/– mice as well as Il17ra–/– mice (provided by J. Tocker, Amgen, Seattle, Washington, USA) have been previously described (41, 45, 61). Tcrb–/– (no. 002118), Tcrbd–/– (no. 002122), and Ifngr1–/– (no. 003288) mice were purchased from Jackson Laboratories. Il17ra–/– mice were crossed with Ifngr1–/– mice to produce double-deficient Ifngr1–/–Il17ra–/– mice. All mice were backcrossed for at least 8 generations to the C57BL/6 background. Exclusively 8- to 12-week-old female mice were analyzed in the experiments.
Reagents.
TNP-LPS, TNP-Ficoll, NP-Ficoll, NP6-BSA, and TNP9-BSA were purchased from Biosearch Technologies, and OVA was obtained from Calbiochem. TNP3-OVA and TNP13-sheep IgG were prepared in our laboratory using TNP-e-aminocaproyl-OSu (Biosearch Technologies; no. T-1030). CFA (1 mg Mycobacterium tuberculosis/ml) and incomplete Freund adjuvant (IFA) were purchased from Sigma-Aldrich. Enriched CFA (62) was prepared by adding heat-killed M. tuberculosis H37 RA (DIFCO Laboratories) to IFA (5 mg M. tuberculosis/ml).
Cloning and production of anti-TNP murine IgG1, IgG2c, IgG2b, and IgM standard Abs.
The variable VDJ heavy chain (NCBI X65772) and complete κ light chain (NCBI X65774) genes from murine anti-TNP IgE hybridoma IgELa2 cells (ATCC-TIB142) were amplified using PCR from cDNA (see Supplemental Table 1 for primers). To synthesize murine anti-TNP IgG1, IgG2c, IgG2b, and IgM Abs, the variable heavy chain region (AgeI-NheI) in combination with an amplified murine C57BL/6 constant IgG1, IgG2c, IgG2b, or IgM heavy chain region (NheI-BsiWI) (see Supplemental Table 1 for primers) and the complete κ light chain gene (AgeI-HindIII) were cloned into recently described expression vectors (60). The leader sequences of the described expression vectors were used. Mouse anti-TNP Abs were produced by polyethylenimine-mediated cotransfection of HEK293 cells in serum-free medium containing 0.03% Primatone RL/UF (Sheffield BioScience). Anti-TNP IgG subclass Abs were purified using protein G–sepharose (GE Healthcare), and IgM Abs were purified using 2-mercaptopyrimidine-sepharose (GE Healthcare). Ab integrity was verified by SDS-PAGE, and anti-TNP reactivity was tested using ELISA (Supplemental Figures 2 and 7 and data not shown).
Immunization and anti-OVA and anti-TNP IgG Ab purification.
8-week-old mice were immunized i.p. as indicated in Figure 3 and Supplemental Figures 4 and 5. Serum samples were collected on day 14, and pooled serum IgGs were purified with protein G–sepharose. OVA- or TNP-reactive IgGs were additionally purified using OVA or TNP-BSA coupled to a CNBr-activated sepharose 4B column (GE Healthcare) prepared in our laboratory. Antigen-reactive IgG reached up to 5% of total serum IgG, depending on the stimulus (data not shown). Enrichment of OVA- or TNP-reactive IgGs was verified via ELISA. IgG Fc glycan structures were analyzed by MALDI-TOF mass spectrometry (MS).
In vitro sialylation and desialylation of IgG and IgM Abs.
Murine anti-TNP IgG1 hybridoma cells (clone H5) (63) and murine anti-Thy1.1 IgG1 hybridoma cells (clone MRC OX-7) were grown for Ab production in 0.03% Primatone RL/UF. IgG Abs were purified from cell culture media with protein G–sepharose. In vitro sialylation of purified IgG Abs was performed in a 2-step procedure as described previously (7, 13). Briefly, Abs were galactosylated with human β1,4-galactosyltransferase and UDP-galactose and subsequently sialylated with human α2,6-sialyltransferase and CMP–sialic acid (all from Calbiochem). Desialylation of TNP-reactive IgG Abs purified from the pooled sera of TNP-LPS–immunized mice or recombinant monoclonal anti-TNP IgM Abs was performed using a Prozyme Sialidase kit (no. GK80040).
Glycan analysis.
Total or antigen-specific IgG samples were digested with recombinantly expressed endoglycosidase S (EndoS) from Streptococcus pyogenes (64). EndoS specifically cleaves the N-linked glycan at the Fc fragment between the first and second GlcNAc (Figure 3A, Supplemental Figure 4, and ref. 64). Monoclonal anti-TNP IgM Abs were digested with PNGaseF (Supplemental Figure 8 and ref. 65). The resulting N-glycans were purified by solid-phase extraction using reversed-phase C18 and graphitized carbon columns (Alltech). The samples were permethylated according to standard protocols (66) and further investigated by MALDI-TOF MS in duplicate. The spectra were recorded on an Ultraflex III mass spectrometer (Bruker Daltonics) equipped with a Smartbeam laser. Calibration was performed on a glucose ladder, and 2,5-dihydroxybenzoic acid was used as matrix. The spectra were recorded in reflector positive ionization mode, and mass spectra from 3,000 laser shots were accumulated.
DTH responses.
DTH responses were induced by immunization with 100 μg TNP-OVA in alum on day 0, followed by injection with 37.5 μg OVA in montanide ISA 50V (Seppic) into the right footpad on the indicated day. PBS in montanide was injected into the left footpad as a negative control. The difference in footpad thickness between the right and left footpad was determined using an Oditest micrometer gauge (Kroeplin) in a blinded manner.
Nephrotoxic nephritis model.
Nephritis was induced by injection of 100 μg TNP–sheep IgG in CFA on day 0, followed by i.v. injection of 80 μl sheep anti-GBM NTS 4 days later (48). Proteinuria was measured using Multistix 10 Visual stripes (Bayer) and scored as follows: 0, negative; 1, ≤75 mg/dl; 2, ≤125 mg/dl; 3, >125 mg/dl; 4, dead.
ELISA.
OVA-, TNP-, NP-, and sheep IgG–reactive murine Abs and murine IgG1 Abs were measured via ELISA. OVA-, TNP-BSA–, TNP-Ficoll–, NP-BSA–, sheep IgG–, or polyclonal goat anti-mouse IgG1–coated (Bethyl Laboratories) plates were blocked and subsequently incubated with 1:100 (or 1:1,000 as indicated) diluted serum or with purified Abs. Bound Abs were detected with respective horseradish peroxidase–coupled polyclonal goat anti-mouse IgM, IgG, IgG1, IgG2c, IgG2b, IgG3, or IgA secondary Abs (Bethyl Laboratories). Anti-TNP serum IgG1, IgG2c, IgG2b, and IgM Ab concentrations were calculated using standard anti-TNP Abs (described above).
FACS analysis.
Fluorochrome-conjugated anti-mouse CD4 (clone RM4-5) was purchased from BD Biosciences.
In vitro B cell proliferation.
A total of 4 × 105 spleen cells was cultured in RPMI medium containing 10% FCS without IgG (prepared in our laboratory) and treated with 10 μg/ml LPS (Sigma-Aldrich) in 96-well plates. In addition, the cells were incubated with native <1% or 15% sialylated anti-TNP murine IgG1 (clone H5; Supplemental Figure 6). On day 3, total cell numbers were counted and analyzed by FACS after staining for B220 and DAPI to calculate the total numbers of live B cells.
In vivo B cell activation with ICs containing differentially glycosylated IgM.
WT mice were injected i.p. with ICs containing 100 μg TNP-Ficoll and 50 μg differentially glycosylated monoclonal anti-TNP IgMs. Cloning and production were as described above.
Statistics.
Statistical analyses were performed using 2-tailed Student’s t test or the log-rank test for survival curves. A P value less than 0.05 was considered significant.
Study approval.
Mice were bred, maintained, and used in experiments in compliance with approved local institutional animal care and use regulations and with permission of the regional authorities in Berlin, Germany.
Supplementary Material
Acknowledgments
We thank Andreas Radbruch and Fritz Melchers for discussion; Angelina Jahn, Heidi Hecker-Kia, Heidi Schliemann, Tuula Geske, Toralf Kaiser, Katharina Raba, and Detlef Grunow for technical assistance; Mattias Collin for support with EndoS; Birgitta Heyman for support with the anti-TNP IgG1 H5 hybridoma; and J. Tocker for providing Il17ra–/– mice. M. Ehlers was a fellow of the Claussen-Simon-Foundation and was supported by the DFG (EH221-4, EH221-5, RTG 1727, IRTG 1911, SFB/TR 654, and the Excellence Cluster ‘‘Inflammation at Interfaces”) and by the Max Planck Institute for Infection Biology. D. Petzold was supported by the RTG 1727 “Modulation of Autoimmunity” (DFG). S. Eiglmeier was supported by the International Max Planck Research School for Infectious Diseases and Immunology. V. Blanchard and M. Berger were supported by the German Ministry of Research and Education (03IP511) and the Sonnefeld Foundation.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2013;123(9):3788–3796. doi:10.1172/JCI65938.
Constanze Hess’s present address is: Novo Nordisk AIS, Bagsvaerd, Denmark.
Alexandra K. Lorenz and Vivien Holecska’s present address is: Charité-University Medine Berlin, Berlin, Germany.
Anna-Lena Schoen’s present address is: University of Muenster, Muenster, Germany.
Tim Schommartz’s and Maria M.M. Mertes’s present address is: Kailuweit & Uhlemann, Dresden, Germany.
Carolin T. Schoen’s present address is: UCB Pharma GmbH, Smyrna, Georgia, USA.
References
- 1.Brodeur PH, Wortis HH. Regulation of thymus-independent responses: unresponsiveness to a second challenge of TNP-Ficoll is mediated by hapten-specific antibodies. J Immunol. 1980;125(4):1499–1505. [PubMed] [Google Scholar]
- 2.Muta T, Kurosaki T, Misulovin Z, Sanchez M, Nussenzweig MC, Ravetch JV. A 13-amino-acid motif in the cytoplasmic domain of Fc-γ RIIB modulates B-cell receptor signalling. Nature. 1994;368(6466):70–73. doi: 10.1038/368070a0. [DOI] [PubMed] [Google Scholar]
- 3.Takai T, Ono M, Hikida M, Ohmori H, Ravetch JV. Augmented humoral and anaphylactic responses in Fc-γ RII-deficient mice. Nature. 1996;379(6563):346–349. doi: 10.1038/379346a0. [DOI] [PubMed] [Google Scholar]
- 4.Heyman B. Feedback regulation by IgG antibodies. Immunol Lett. 2003;88(2):157–161. doi: 10.1016/S0165-2478(03)00078-6. [DOI] [PubMed] [Google Scholar]
- 5.Obukhanych TV, Nussenzweig MC. T-independent type II immune responses generate memory B cells. J Exp Med. 2006;203(2):305–310. doi: 10.1084/jem.20052036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rademacher TW, Williams P, Dwek RA. Agalactosyl glycoforms of IgG autoantibodies are pathogenic. Proc Natl Acad Sci U S A. 1994;91(13):6123–6127. doi: 10.1073/pnas.91.13.6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313(5787):670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 8.Anthony RM, Wermeling F, Karlsson MC, Ravetch JV. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc Natl Acad Sci U S A. 2008;105(50):19571–19578. doi: 10.1073/pnas.0810163105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anthony RM, Nimmerjahn F, Ashline DJ, Reinhold VN, Paulson JC, Ravetch JV. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science. 2008;320(5874):373–376. doi: 10.1126/science.1154315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nimmerjahn F, Ravetch JV. Anti-inflammatory actions of intravenous immunoglobulin. Annu Rev Immunol. 2008;26:513–533. doi: 10.1146/annurev.immunol.26.021607.090232. [DOI] [PubMed] [Google Scholar]
- 11.Scherer HU, et al. Glycan profiling of anti-citrullinated protein antibodies isolated from human serum and synovial fluid. Arthritis Rheum. 2010;62(6):1620–1629. doi: 10.1002/art.27414. [DOI] [PubMed] [Google Scholar]
- 12.Anthony RM, Kobayashi T, Wermeling F, Ravetch JV. Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway. Nature. 2011;475(7354):110–113. doi: 10.1038/nature10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oefner CM, et al. Tolerance induction with T cell-dependent protein antigens induces regulatory sialylated IgGs. J Allergy Clin Immunol. 2012;129(6):1647–1655. doi: 10.1016/j.jaci.2012.02.037. [DOI] [PubMed] [Google Scholar]
- 14.Karsten CM, et al. Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of Fc gamma RIIB and dectin-1. Nat Med. 2012;18(9):1401–1406. doi: 10.1038/nm.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ackerman ME, et al. Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. J Clin Invest. 2013;123(5):2183–2192. doi: 10.1172/JCI65708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collin M, Ehlers M. The carbohydrate switch between pathogenic and immunosuppressive antigen-specific antibodies. Exp Dermatol. 2013;22(8):511–514. doi: 10.1111/exd.12171. [DOI] [PubMed] [Google Scholar]
- 17.Parekh RB, et al. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 1985;316(6027):452–457. doi: 10.1038/316452a0. [DOI] [PubMed] [Google Scholar]
- 18.Parekh RB, Roitt IM, Isenberg DA, Dwek RA, Ansell BM, Rademacher TW. Galactosylation of IgG associated oligosaccharides: reduction in patients with adult and juvenile onset rheumatoid arthritis and relation to disease activity. Lancet. 1988;1(8592):966–969. doi: 10.1016/s0140-6736(88)91781-3. [DOI] [PubMed] [Google Scholar]
- 19.Parekh R, Isenberg D, Rook G, Roitt I, Dwek R, Rademacher T. A comparative analysis of disease-associated changes in the galactosylation of serum IgG. J Autoimmun. 1989;2(2):101–114. doi: 10.1016/0896-8411(89)90148-0. [DOI] [PubMed] [Google Scholar]
- 20.Rook GA, et al. Changes in IgG glycoform levels are associated with remission of arthritis during pregnancy. J Autoimmun. 1991;4(5):779–794. doi: 10.1016/0896-8411(91)90173-a. [DOI] [PubMed] [Google Scholar]
- 21.Bodman KB, et al. Lymphocytes from patients with rheumatoid arthritis produce agalactosylated IgG in vitro. Clin Exp Immunol. 1992;88(3):420–423. doi: 10.1111/j.1365-2249.1992.tb06465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Zeben D, et al. Early agalactosylation of IgG is associated with a more progressive disease course in patients with rheumatoid arthritis: results of a follow-up study. Br J Rheumatol. 1994;33(1):36–43. doi: 10.1093/rheumatology/33.1.36. [DOI] [PubMed] [Google Scholar]
- 23.Malhotra R, Wormald MR, Rudd PM, Fischer PB, Dwek RA, Sim RB. Glycosylation changes of IgG associated with rheumatoid arthritis can activate complement via the mannose-binding protein. Nat Med. 1995;1(3):237–243. doi: 10.1038/nm0395-237. [DOI] [PubMed] [Google Scholar]
- 24.Pilkington C, Yeung E, Isenberg D, Lefvert AK, Rook GA. Agalactosyl IgG and antibody specificity in rheumatoid arthritis, tuberculosis, systemic lupus erythematosus and myasthenia gravis. Autoimmunity. 1995;22(2):107–111. doi: 10.3109/08916939508995306. [DOI] [PubMed] [Google Scholar]
- 25.Williams PJ, Rademacher TW. Analysis of murine IgG isotype galactosylation in collagen-induced arthritis. Scand J Immunol. 1996;44(4):381–387. doi: 10.1046/j.1365-3083.1996.d01-323.x. [DOI] [PubMed] [Google Scholar]
- 26.Dong X, Storkus WJ, Salter RD. Binding and uptake of agalactosyl IgG by mannose receptor on macrophages and dendritic cells. J Immunol. 1999;163(10):5427–5434. [PubMed] [Google Scholar]
- 27.Kuroda Y, Nakata M, Nose M, Kojima N, Mizuochi T. Abnormal IgG galactosylation and arthritis in MRL-Fas(lpr) or MRL-FasL(gld) mice are under the control of the MRL genetic background. FEBS Lett. 2001;507(2):210–214. doi: 10.1016/S0014-5793(01)02974-X. [DOI] [PubMed] [Google Scholar]
- 28.Axford JS, Cunnane G, Fitzgerald O, Bland JM, Bresnihan B, Frears ER. Rheumatic disease differentiation using immunoglobulin G sugar printing by high density electrophoresis. J Rheumatol. 2003;30(12):2540–2546. [PubMed] [Google Scholar]
- 29.Pasek M, et al. Galactosylation of IgG from rheumatoid arthritis (RA) patients — changes during therapy. Glycoconj J. 2006;23(7–8):463–471. doi: 10.1007/s10719-006-5409-0. [DOI] [PubMed] [Google Scholar]
- 30.Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu Rev Immunol. 2007;25:21–50. doi: 10.1146/annurev.immunol.25.022106.141702. [DOI] [PubMed] [Google Scholar]
- 31.Nimmerjahn F, Anthony RM, Ravetch JV. Agalactosylated IgG antibodies depend on cellular Fc receptors for in vivo activity. Proc Natl Acad Sci U S A. 2007;104(20):8433–8437. doi: 10.1073/pnas.0702936104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van de Geijn FE, et al. Immunoglobulin G galactosylation and sialylation are associated with pregnancy-induced improvement of rheumatoid arthritis and the postpartum flare: results from a large prospective cohort study. Arthritis Res Ther. 2009;11(6):R193. doi: 10.1186/ar2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ercan A, et al. Aberrant IgG galactosylation precedes disease onset, correlates with disease activity, and is prevalent in autoantibodies in rheumatoid arthritis. Arthritis Rheum. 2010;62(8):2239–2248. doi: 10.1002/art.27533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Troelsen LN, et al. IgG glycosylation changes and MBL2 polymorphisms: associations with markers of systemic inflammation and joint destruction in rheumatoid arthritis. J Rheumatol. 2012;39(3):463–469. doi: 10.3899/jrheum.110584. [DOI] [PubMed] [Google Scholar]
- 35.Peng SL, et al. Murine lupus in the absence of alpha beta T cells. J Immunol. 1996;156(10):4041–4049. [PubMed] [Google Scholar]
- 36.Berland R, et al. Toll-like receptor 7-dependent loss of B cell tolerance in pathogenic autoantibody knockin mice. Immunity. 2006;25(3):429–440. doi: 10.1016/j.immuni.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 37.Christensen SR, Shupe J, Nickerson K, Kashgarian M, Flavell RA, Shlomchik MJ. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25(3):417–428. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 38.Christensen SR, Shlomchik MJ. Regulation of lupus-related autoantibody production and clinical disease by Toll-like receptors. Semin Immunol. 2007;19(1):11–23. doi: 10.1016/j.smim.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ehlers M, Ravetch J. Opposing effects of Toll-like receptor stimulation induce autoimmunity or tolerance. Trends Immunol. 2007;28(2):74–79. doi: 10.1016/j.it.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 40.Lanzavecchia A, Sallusto F. Toll-like receptors and innate immunity in B-cell activation and antibody responses. Curr Opin Immunol. 2007;19(3):268–274. doi: 10.1016/j.coi.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 41.Stoehr AD, et al. TLR9 in peritoneal B-1b cells is essential for production of protective self-reactive IgM to control Th17 cells and severe autoimmunity. J Immunol. 2011;187(6):2953–2965. doi: 10.4049/jimmunol.1003340. [DOI] [PubMed] [Google Scholar]
- 42.Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416(6881):603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 43.Herlands RA, Christensen SR, Sweet RA, Hershberg U, Shlomchik MJ. T cell-independent and toll-like receptor-dependent antigen-driven activation of autoreactive B cells. Immunity. 2008;29(2):249–260. doi: 10.1016/j.immuni.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsao PY, Jiao J, Ji MQ, Cohen PL, Eisenberg RA. T cell-independent spontaneous loss of tolerance by anti-double-stranded DNA B cells in C57BL/6 mice. J Immunol. 2008;181(11):7770–7777. doi: 10.4049/jimmunol.181.11.7770. [DOI] [PubMed] [Google Scholar]
- 45.Ehlers M, Fukuyama H, McGaha T, Aderem A, Ravetch J. TLR9/MyD88 signaling is required for class switching to pathogenic IgG2a and 2b autoantibodies in SLE. J Exp Med. 2006;203(3):553–561. doi: 10.1084/jem.20052438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ueda Y, Liao D, Yang K, Patel A, Kelsoe G. T-independent activation-induced cytidine deaminase expression, class-switch recombination, and antibody production by immature/transitional 1 B cells. J Immunol. 2007;178(6):3593–3601. doi: 10.4049/jimmunol.178.6.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kobayashi T, et al. TRAF6 is required for generation of the B-1a B cell compartment as well as T cell-dependent and -independent humoral immune responses. PLoS One. 2009;4(3):e4736. doi: 10.1371/journal.pone.0004736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Madaio MP, Salant DJ, Adler S, Darby C, Couser WG. Effect of antibody charge and concentration on deposition of antibody to glomerular basement membrane. Kidney Int. 1984;26(4):397–403. doi: 10.1038/ki.1984.188. [DOI] [PubMed] [Google Scholar]
- 49.Werwitzke S, et al. Inhibition of lupus disease by anti-double-stranded DNA antibodies of the IgM isotype in the (NZB x NZW)F1 mouse. Arthritis Rheum. 2005;52(11):3629–3638. doi: 10.1002/art.21379. [DOI] [PubMed] [Google Scholar]
- 50.Vas J, Grönwall C, Marshak-Rothstein A, Silverman GJ. Natural antibody to apoptotic cell membranes inhibits the proinflammatory properties of lupus autoantibody immune complexes. Arthritis Rheum. 2012;64(10):3388–3398. doi: 10.1002/art.34537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adachi T, et al. CD22 serves as a receptor for soluble IgM. Eur J Immunol. 2012;42(1):241–247. doi: 10.1002/eji.201141899. [DOI] [PubMed] [Google Scholar]
- 52.Vas J, Grönwall C, Silverman GJ. Fundamental roles of the innate-like repertoire of natural antibodies in immune homeostasis. Front Immunol. 2013;4:4. doi: 10.3389/fimmu.2013.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Silverman GJ, Vas J, Grönwall C. Protective autoantibodies in the rheumatic diseases: lessons for therapy. Nat Rev Rheumatol. 2013;9(5):291–300. doi: 10.1038/nrrheum.2013.30. [DOI] [PubMed] [Google Scholar]
- 54.Getahun A, Heyman B. Studies on the mechanism by which antigen-specific IgG suppresses primary antibody responses: evidence for epitope masking and decreased localization of antigen in the spleen. Scand J Immunol. 2009;70(3):277–287. doi: 10.1111/j.1365-3083.2009.02298.x. [DOI] [PubMed] [Google Scholar]
- 55.Zhuang Q, Bisotto S, Fixman ED, Mazer B. Suppression of IL-4- and CD40-induced B-lymphocyte activation by intravenous immunoglobulin is not mediated through the inhibitory IgG receptor FcgammaRIIb. J Allergy Clin Immunol. 2002;110(3):480–483. doi: 10.1067/mai.2002.127284. [DOI] [PubMed] [Google Scholar]
- 56.Séité JF, Guerrier T, Cornec D, Jamin C, Youinou P, Hillion S. TLR9 responses of B cells are repressed by intravenous immunoglobulin through the recruitment of phosphatase. J Autoimmun. 2011;37(3):190–197. doi: 10.1016/j.jaut.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 57.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 58.Pitsiou GG, Kioumis IP. Pneumococcal vaccination in adults: does it really work? Respir Med. 2011;105(12):1776–1783. doi: 10.1016/j.rmed.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 59.Egner W. The use of laboratory tests in the diagnosis of SLE. J Clin Pathol. 2000;53(6):424–432. doi: 10.1136/jcp.53.6.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tiller T, et al. Development of self-reactive germinal center B cells and plasma cells in autoimmune Fc gamma RIIB-deficient mice. J Exp Med. 2010;207(12):2767–2778. doi: 10.1084/jem.20100171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Müller U, et al. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264(5167):1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 62.Marty I, Péclat V, Kirdaite G, Salvi R, So A, Busso N. Amelioration of collagen-induced arthritis by thrombin inhibition. J Clin Invest. 2001;107(5):631–640. doi: 10.1172/JCI11064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wernersson S, Karlsson MC, Dahlström J, Mattsson R, Verbeek JS, Heyman B. IgG-mediated enhancement of antibody responses is low in Fc receptor gamma chain-deficient mice and increased in Fc gamma RII-deficient mice. J Immunol. 1999;163(2):618–622. [PubMed] [Google Scholar]
- 64.Collin M, Olsén A. Effect of SpeB and EndoS from Streptococcus pyogenes on human immunoglobulins. Infect Immun. 2001;69(11):7187–7189. doi: 10.1128/IAI.69.11.7187-7189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arnold JN, et al. Human serum IgM glycosylation: identification of glycoforms that can bind to mannan-binding lectin. J Biol Chem. 2005;280(32):29080–29087. doi: 10.1074/jbc.M504528200. [DOI] [PubMed] [Google Scholar]
- 66.Wedepohl S, et al. N-Glycan analysis of recombinant L-Selectin reveals sulfated GalNAc and GalNAc-GalNAc Motifs. J Proteome Res. 2010;9(7):3403–3411. doi: 10.1021/pr100170c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.