Abstract
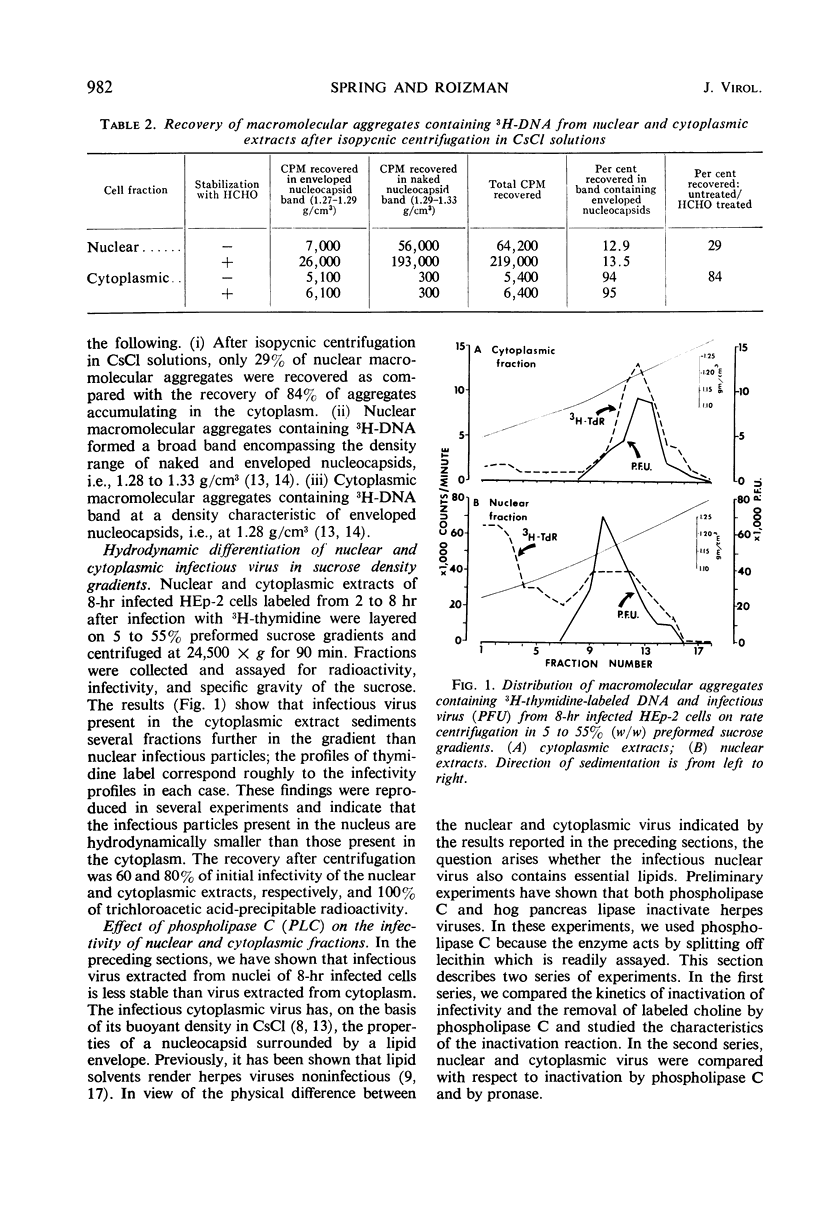
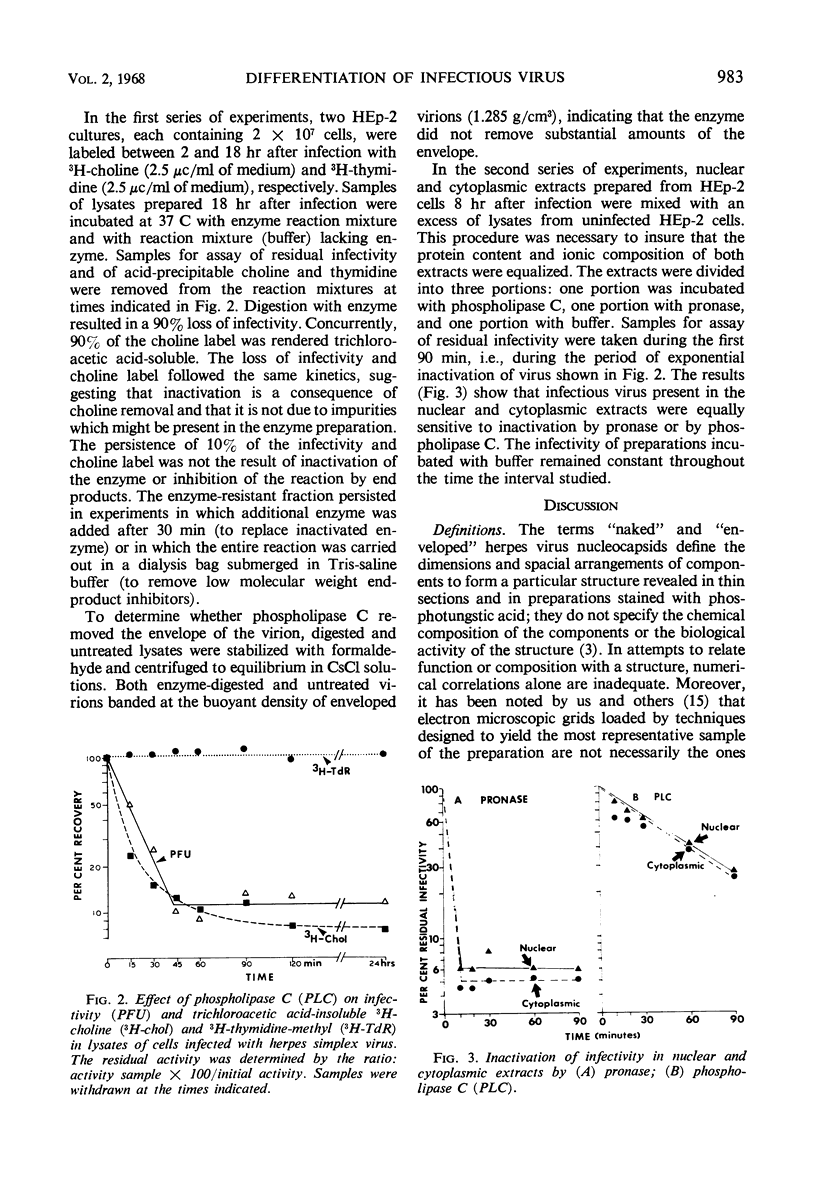
Approximately 67% of infectivity is associated with the nucleus 8 hr after productive infection of HEp-2 cells with herpes simplex virus. Comparison of nuclear and cytoplasmic infectious virus and macromolecular aggregates labeled with 3H-thymidine or with 3H-choline revealed the following. (i) Cytoplasmic infectious virus and macromolecular aggregates banded in CsCl at a density corresponding to enveloped nucleocapsids. The virus was relatively stable; there was only 50% loss of infectivity and only 16% of the virions became disaggregated. (ii) Nuclear macromolecular aggregates banded in CsCl solution at a density corresponding to unenveloped nucleocapsids and, moreover, both the infectious virus and aggregates were highly unstable. (iii) In sucrose density gradients, the nuclear macromolecular aggregates and infectivity sedimented as a single band and migrated more slowly than the corresponding cytoplasmic material. (iv) The infectivity of nuclear and cytoplasmic virus is readily inactivated by digestion with phospholipase C and with pronase. We conclude the following. (i) Cytoplasmic virus consists of enveloped nucleocapsids. (ii) Nuclear virus consists of nucleocapsids covered with lipid or partially enveloped. (iii) The molecular integrity of viral lipids is essential for infectivity. (iv) The envelope protects the nucleocapsid and accelerates adsorption to cells; it is not, however, inherently essential for infectivity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- HOLMES I. H., WATSON D. H. AN ELECTRON MICROSCOPE STUDY OF THE ATTACHMENT AND PENETRATION OF HERPES VIRUS IN BHK21 CELLS. Virology. 1963 Sep;21:112–123. doi: 10.1016/0042-6822(63)90309-x. [DOI] [PubMed] [Google Scholar]
- LWOFF A., HORNE R., TOURNIER P. A system of viruses. Cold Spring Harb Symp Quant Biol. 1962;27:51–55. doi: 10.1101/sqb.1962.027.001.008. [DOI] [PubMed] [Google Scholar]
- MORGAN C., ROSE H. M., HOLDEN M., JONES E. P. Electron microscopic observations on the development of herpes simplex virus. J Exp Med. 1959 Oct 1;110:643–656. doi: 10.1084/jem.110.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REISSIG M., MELNICK J. L. The cellular changes produced in tissue cultures by herpes B virus correlated with the concurrent multiplication of the virus. J Exp Med. 1955 Mar 1;101(3):341–352. doi: 10.1084/jem.101.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROIZMAN B., AURELIAN L. ABORTIVE INFECTION OF CANINE CELLS BY HERPES SIMPLEX VIRUS. I. CHARACTERIZATION OF VIRAL PROGENY FROM CO-OPERATIVE INFECTION WITH MUTANTS DIFFERING IN CAPACITY TO MULTIPLY IN CANINE CELLS. J Mol Biol. 1965 Mar;11:528–538. doi: 10.1016/s0022-2836(65)80008-0. [DOI] [PubMed] [Google Scholar]
- ROIZMAN B., AURELIAN L., ROANE P. R., Jr THE MULTIPLICATION OF HERPES SIMPLEX VIRUS. I. THE PROGRAMMING OF VIRAL DNA DUPLICATION IN HEP-2 CELLS. Virology. 1963 Nov;21:482–498. doi: 10.1016/0042-6822(63)90209-5. [DOI] [PubMed] [Google Scholar]
- ROIZMAN B., ROANE P. R., Jr A physical difference between two strains of herpes simplex virus apparent on sedimentation in cesium chloride. Virology. 1961 Sep;15:75–79. doi: 10.1016/0042-6822(61)90079-4. [DOI] [PubMed] [Google Scholar]
- ROIZMAN B., ROANE P. R., Jr Demonstration of a surface difference between virions of two strains of herpes simplex virus. Virology. 1963 Feb;19:198–204. doi: 10.1016/0042-6822(63)90009-6. [DOI] [PubMed] [Google Scholar]
- Rouhandeh H., Brinkman M. L., Sells L. L. Reactivation of ether-inactivated infectious bovine rhinotracheitis virus. J Infect Dis. 1967 Jun;117(3):237–241. doi: 10.1093/infdis/117.3.237. [DOI] [PubMed] [Google Scholar]
- SMITH K. O. RELATIONSHIP BETWEEN THE ENVELOPE AND THE INFECTIVITY OF HERPES SIMPLEX VIRUS. Proc Soc Exp Biol Med. 1964 Mar;115:814–816. doi: 10.3181/00379727-115-29045. [DOI] [PubMed] [Google Scholar]
- Siegert R., Falke D. Elektronenmikroskopische Untersuchungen über die Entwicklung des Herpesvirus hominis in Kulturzellen. Arch Gesamte Virusforsch. 1966;19(2):230–249. [PubMed] [Google Scholar]
- Spring S. B., Roizman B. Herpes simplex virus products in productive and abortive infection. I. Stabilization with formaldehyde and preliminary analyses by isopycnic centrifugation in CsCl. J Virol. 1967 Apr;1(2):294–301. doi: 10.1128/jvi.1.2.294-301.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring S. B., Roizman B., Schwartz J. Herpes simplex virus products in productive and abortive infection. II. Electron microscopic and immunological evidence for failure of virus envelopment as a cause of abortive infection. J Virol. 1968 Apr;2(4):384–392. doi: 10.1128/jvi.2.4.384-392.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILDY P., RUSSELL W. C., HORNE R. W. The morphology of herpes virus. Virology. 1960 Oct;12:204–222. doi: 10.1016/0042-6822(60)90195-1. [DOI] [PubMed] [Google Scholar]



