Abstract
Pressure and volume overload results in concentric and eccentric hypertrophy of cardiac ventricular chambers with, respectively, parallel and series replication of sarcomeres. These divergent patterns of hypertrophy were related 40 years ago to disparate wall stresses in both conditions, with systolic wall stress eliciting parallel replication of sarcomeres and diastolic wall stress, series replication. These observations are relevant to clinical practice, as they relate to the excessive hypertrophy and contractile dysfunction regularly observed in patients with aortic stenosis. Stress-sensing mechanisms in cardiomyocytes and activation of cardiomyocyte death by elevated wall stress continue to intrigue cardiovascular scientists.
Pattern recognition in left ventricular hypertrophy
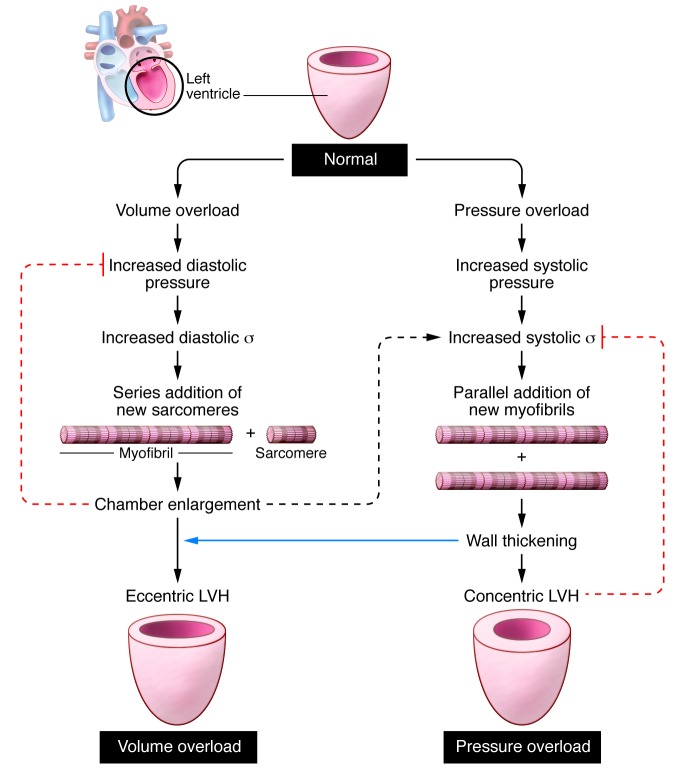
Pressure and volume overload result in divergent patterns of LV hypertrophy. The presence of LV hypertrophy implies a higher-than-normal myocardial mass. Pressure overload usually elicits concentric hypertrophy, with a high ratio of LV wall thickness to radius (h/R). In contrast, volume overload triggers eccentric hypertrophy with a normal h/R ratio (Figure 1). Nearly 40 years ago, an article in these pages related the divergent patterns of LV hypertrophy to disparate LV wall stresses in both conditions (1). In patients with aortic stenosis, both systolic and diastolic LV wall stresses were normal because concentric hypertrophy had succeeded in nicely counterbalancing the effect on LV wall stress of elevated systolic and diastolic LV pressures. Concentric hypertrophy was therefore labeled an adaptive mechanism, which avoids afterload excess to hinder myocardial shortening. In contrast, eccentric hypertrophy, as observed in patients with mitral or aortic regurgitation, corrected systolic LV wall stress but failed to normalize diastolic LV wall stress. Although eccentric hypertrophy resembled physiological growth, its effect on diastolic wall stress suggested that maladaptive hypertrophy develops insidiously during volume overload.
Figure 1. Schematic overview of the development of concentric hypertrophy with the parallel addition of sarcomeres in pressure overload and of eccentric hypertrophy with a series addition of sarcomeres in volume overload.
Chamber enlargement increases systolic wall stress in volume overload (dashed black arrow indicates positive feedback). Wall thickening induces concentric hypertrophy in pressure overload and contributes to eccentric hypertrophy in volume overload (blue arrow). Concentric hypertrophy reduces systolic wall stress in pressure overload, and eccentric hypertrophy reduces diastolic wall stress in volume overload (dashed red lines indicate negative feedback).
Based on the development of concentric hypertrophy in pressure overload and eccentric hypertrophy in volume overload, peak systolic wall stress was proposed as a stimulus for parallel replication of sarcomeres in concentric hypertrophy and end-diastolic wall stress as a stimulus for series replication of cardiomyocytes in eccentric hypertrophy (1). A reflection of LV remodeling in cardiomyocyte remodeling was confirmed more than two decades later by detailed histomorphometric measurements in concentric and eccentric human or animal LV hypertrophy (2). In concentric LV hypertrophy, cardiomyocytes only grow in a transverse direction while keeping cell length constant, whereas in eccentric LV hypertrophy, cardiomyocytes grow proportionally in both longitudinal and transverse directions. The difference in cardiomyocyte remodeling is also reflected in distinct patterns of peptide growth factor induction in concentric and eccentric LV hypertrophy (3).
This 1975 assessment of cardiac remodeling continues to stand out because of its unique methodology: LV remodeling of the human heart was assessed using an integrated, multimodality approach with simultaneous measurement of LV dimension, septal and posterior wall thickness by echocardiography, and LV pressures by high-fidelity micromanometer-tipped catheters (1). This allowed matching of instantaneous LV pressure, radius, and wall thickness throughout the cardiac cycle, something not possible using only imaging and measured blood pressure (4, 5). The ability to match these measurements turns out to be important because of the wide variation in the LV pressure contour, in which the rate of rise, the duration of peak (spiky or broad), and the rate of fall have enormous influence on calculated stress or the presumed force at the level of individual myocytes.
Cardiac remodeling and disease
In concentric LV remodeling, the contribution of LV hypertrophy to the prevailing LV wall stress remains clinically relevant. It is still debated whether asymptomatic patients with extensive LV hypertrophy should undergo aortic valve replacement to prevent progression of LV hypertrophy or whether they should be managed conservatively until symptoms develop (6, 7). Knowledge of LV wall stress helps to solve this clinical dilemma. When LV wall stress is lower than normal, LV hypertrophy is excessive. This probably results from comorbidities such as diabetes mellitus (8) or from a genetic predisposition such as the DD genotype of the ACE gene (9). In both conditions, excessive LV hypertrophy is accompanied by prominent LV fibrosis (10), which predisposes patients to ventricular arrhythmias or sudden death. Under these circumstances, early aortic valve replacement, even in the absence of symptoms, could be the more favorable option.
The appropriateness of LV hypertrophy to LV wall stress is also relevant in the reverse situation in which patients with aortic stenosis present with higher-than-normal LV wall stress (11, 12). Excessive LV wall stress results from insufficient LV hypertrophy with a low h/R ratio and is accompanied by depressed LV contractile performance (11). In the majority of these patients, the depressed LV contractile performance merely reflects afterload mismatch and does not jeopardize postoperative outcome (12). Occasionally, however, the depressed LV contractile performance is worse than predicted by the high LV wall stress, and in these patients it heralds a poor postoperative outcome (12, 13).
A histopathological study of patients with aortic stenosis and varying degrees of LV systolic dysfunction nicely confirmed the concept of insufficient LV hypertrophy underlying depressed LV contractile performance (13). In this study, markers of cardiomyocyte hypertrophy, such as an elevated cardiomyocyte cross-sectional area and increased nuclear DNA content, were present in all patients, irrespective of their LV ejection fraction (EF). In patients with a reduced LVEF (LVEF <50%), cardiomyocyte hypertrophy was paralleled by cardiomyocyte degeneration, which was evident from the disappearance of myofilaments. Cardiomyocyte degeneration resulted from ubiquitin-related autophagy and ischemic cell death or oncosis, i.e., swelling of cardiomyocytes. In patients with a greatly reduced LVEF (<30%), myocardial degeneration was 32 times more frequent than in control myocardium, and all of these patients failed to improve postoperatively. Myocardial fibrosis increased concordantly with cardiomyocyte degeneration and resulted in replacement fibrosis being superimposed on reactive interstitial fibrosis, with a greater likelihood of reentry arrhythmias and poor outcome.
The cause of cardiomyocyte degeneration in aortic stenosis patients with a low EF is unclear. The severity of aortic stenosis does not seem to be involved because the aortic valve orifice area was similar in patients with a normal or low EF (13). A potential explanation could be the rate of progression of aortic valve narrowing, which determines the speed at which a higher wall stress is imposed on the myocardium. RV remodeling as a result of monocrotaline-induced pulmonary hypertension supports this explanation (14). When rats were given low-dose monocrotaline, they developed slow-onset pulmonary hypertension and adaptive RV hypertrophy, but when rats were given high-dose monocrotaline, they developed rapid-onset pulmonary hypertension accompanied by RV failure and premature death. Fourteen days after monocrotaline administration, the extent of RV hypertrophy was still identical in both groups, but microarray analysis of RV myocardium revealed that 63 of the 3,010 cardiac genes screened were differentially expressed between both groups. The differentially expressed genes included those responsible for the activation of proapoptotic pathways. This study implies that the initial rate of rise of the pressure overload stimulus predestines the myocardium to the development of an adaptive or maladaptive phenotype. This conclusion was reinforced by a study in which both groups were subjected to an exercise training program (15). Low-dose monocrotaline rats fared better with regular exercise because of a higher RV myocardial capillary density, but high-dose monocrotaline rats fared worse because of myocardial leukocyte infiltration. The latter probably resulted from excessive elevation of RV wall stress related to repetitive episodes of exercise at a higher pulmonary artery (PA) pressure and was reactive to the activation of cardiomyocyte death pathways. A similar leukocyte infiltration was also observed in aortic stenosis patients with a reduced LVEF (13).
Conclusions
The 1975 study by Grossman et al. (1) continues to raise more questions than it answers. We still do not know the precise mechanisms whereby individual myocytes sense the force patterns imposed by LV pressure or volume overload and transduce the force into sarcomerogenesis that results in cell thickening, lengthening, or both. Also, when does elevated myocardial wall stress activate cardiomyocyte death pathways, which may well be the critical factor underlying the distinction between adaptive and maladaptive hypertrophy? Hopefully, the next 40 years will answer these questions.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2013;123(9):3701–3703. doi:10.1172/JCI69830.
References
- 1.Grossman W, Jones D, McLaurin LP. Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest. 1975;56(1):56–64. doi: 10.1172/JCI108079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerdes AM. Cardiac myocyte remodeling in hypertrophy and progression to failure. J Card Fail. 2002;8(6 suppl):S264–S268. doi: 10.1054/jcaf.2002.129280. [DOI] [PubMed] [Google Scholar]
- 3.Calderone A, Takahashi N, Izzo NJ, Thaik CM, Colucci WS. Pressure- and volume-induced left ventricular hypertrophies are associated with distinct myocyte phenotypes and differential induction of peptide growth factor mRNAs. Circulation. 1995;92(9):2385–2390. doi: 10.1161/01.CIR.92.9.2385. [DOI] [PubMed] [Google Scholar]
- 4.Ganau A, et al. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol. 1992;19(7):1550–1558. doi: 10.1016/0735-1097(92)90617-V. [DOI] [PubMed] [Google Scholar]
- 5.Khouri MG, Peshock RM, Ayers CR, de Lemos JA, Drazner MH. A 4-tiered classification of left ventricular hypertrophy based on left ventricular geometry: the Dallas heart study. Circ Cardiovasc Imaging. 2010;3(2):164–171. doi: 10.1161/CIRCIMAGING.109.883652. [DOI] [PubMed] [Google Scholar]
- 6.Carabello BA. Should severe aortic stenosis be operated on before symptom onset? Aortic valve replacement should be operated on before symptom onset. Circulation. 2012;126(1):112–117. doi: 10.1161/CIRCULATIONAHA.111.079350. [DOI] [PubMed] [Google Scholar]
- 7.Shah PK. Should severe aortic stenosis be operated on before symptom onset? Severe aortic stenosis should not be operated on before symptom onset. Circulation. 2012;126(1):118–125. doi: 10.1161/CIRCULATIONAHA.111.079368. [DOI] [PubMed] [Google Scholar]
- 8.Falcão-Pires I, et al. Diabetes mellitus worsens diastolic left ventricular dysfunction in aortic stenosis through altered myocardial structure and cardiomyocyte stiffness. Circulation. 2011;124(10):1151–1159. doi: 10.1161/CIRCULATIONAHA.111.025270. [DOI] [PubMed] [Google Scholar]
- 9.Dellgren G, Eriksson MJ, Blange I, Brodin LA, Rådegran K, Sylvén C. Angiotensin-converting enzyme gene polymorphism influences degree of left ventricular hypertrophy and its regression in patients undergoing operation for aortic stenosis. Am J Cardiol. 1999;84(8):909–913. doi: 10.1016/S0002-9149(99)00464-6. [DOI] [PubMed] [Google Scholar]
- 10.Fielitz J, et al. Activation of the cardiac renin-angiotensin system and increased myocardial collagen expression in human aortic valve disease. J Am Coll Cardiol. 2001;37(5):1443–1449. doi: 10.1016/S0735-1097(01)01170-6. [DOI] [PubMed] [Google Scholar]
- 11.Gunther S, Grossman W. Determinants of ventricular function in pressure-overload hypertrophy in man. Circulation. 1979;59(4):679–688. doi: 10.1161/01.CIR.59.4.679. [DOI] [PubMed] [Google Scholar]
- 12.Carabello BA, Green LH, Grossman W, Cohn LH, Koster JK, Collins JJ., Jr Hemodynamic determinants of prognosis of aortic valve replacement in critical aortic stenosis and advanced congestive heart failure. Circulation. 1980;62(1):42–48. doi: 10.1161/01.CIR.62.1.42. [DOI] [PubMed] [Google Scholar]
- 13.Hein S, et al. Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: structural deterioration and compensatory mechanisms. Circulation. 2003;107(7):984–991. doi: 10.1161/01.CIR.0000051865.66123.B7. [DOI] [PubMed] [Google Scholar]
- 14.Buermans HP, et al. Microarray analysis reveals pivotal divergent mRNA expression profiles early in the development of either compensated ventricular hypertrophy or heart failure. Physiol Genomics. 2005;21(3):314–323. doi: 10.1152/physiolgenomics.00185.2004. [DOI] [PubMed] [Google Scholar]
- 15.Handoko ML, et al. Opposite effects of training in rats with stable and progressive pulmonary hypertension. Circulation. 2009;120(1):42–49. doi: 10.1161/CIRCULATIONAHA.108.829713. [DOI] [PubMed] [Google Scholar]