Abstract
The incidence of superficial dermatophytoses is high in developed countries, and there remains a need for effective topical antifungals. In this study, we evaluated the in vitro antifungal activity of naftifine hydrochloride, the active ingredient in naftifine hydrochloride cream and gel 1% and 2%, against dermatophytes. The MICs and minimum fungicidal concentrations (MFCs) of naftifine hydrochloride against 350 clinical strains, including Trichophyton rubrum, T. mentagrophytes, T. tonsurans, Epidermophyton floccosum, and Microsporum canis, were determined using the CLSI methodology. Subsets from this test panel were subsequently tested in a time-kill assay at 0.125×, 0.25×, 0.5×, and 1× the MFC for each isolate. CFU counts were performed over a period of 48 h of incubation. Additionally, in order to determine the potential for resistance development, six strains were subjected to 15 serial passages in concentrations higher than the MIC for each strain. MICs were determined following each passage. The MIC range against the dermatophyte isolates tested was 0.015 to 1.0 μg/ml, with naftifine hydrochloride being fungicidal against 85% of the Trichophyton species. The time-kill assay showed dose-dependent activity, with the greatest reduction in the numbers of CFU corresponding to the highest drug concentration. There was no increase in MIC for any strains following repeated exposure to naftifine hydrochloride. Naftifine hydrochloride demonstrated potent activity against all dermatophytes tested, and none of the isolates within this test panel demonstrated the potential for the development of resistance. Thus, future clinical studies of naftifine hydrochloride against dermatophytes may be warranted for the treatment of superficial dermatophytoses.
INTRODUCTION
The prevalence of superficial fungal infections has been estimated to be as high as 25% among the worldwide population, though the distribution of clinical manifestations and the causative agents vary by geographical location (1). Superficial mycoses are most often caused by dermatophytes, which belong to one of three genera, Trichophyton, Microsporum, and Epidermophyton (2). After onychomycosis and tinea capitis, the most prevalent superficial mycosis in the United States and Canada is tinea pedis (affecting approximately 40% of the population), followed by tinea corporis and tinea cruris (3, 4). The most common dermatophyte isolated in this region is Trichophyton rubrum, followed by T. mentagrophytes and Epidermophyton floccosum (1). The clinical manifestations and species distribution in the countries of Western Europe, Asia, and Australia mimic those in the developed countries of North America, with tinea pedis being the most prevalent in these areas (1, 5–7).
With the incidence of superficial dermatophytoses remaining so high in developed countries, there remains a need for topical antifungals that are safe and effective. Naftifine hydrochloride, the original member of the allylamine class, has shown potent in vitro antifungal activity against dermatophytes (8, 9) and subsequently has been shown to be an effective topical agent for the treatment of tinea cruris, tinea corporis, and tinea pedis (10).
However, with the increased use of antifungal agents, including azoles, for the treatment of superficial dermatophytoses, the development of resistance remains a possibility and needs to be monitored. The development of fluconazole resistance among Candida spp. is well documented, and there has been evidence of development of resistance to itraconazole by filamentous fungi (11, 12). Further, Gupta and Kohli have reported increased MICs in T. rubrum following treatment with ketoconazole (13). Though to our knowledge there are no published reports of the development of resistance in dermatophytes following repeated exposure to terbinafine or other allylamines, it is important to determine whether such resistance to members of this antifungal class is likely to occur.
Further, as new antifungal agents are developed, there is a need to understand the fungicidal properties and pharmacodynamic characteristics of these agents. Data collected from time-kill studies provide information regarding the rate and extent of fungicidal activity as the relationship between concentration and effect. In the study described in this report, we assessed the in vitro activity of naftifine hydrochloride, the active ingredient in naftifine hydrochloride cream and gel 1% and 2%, against the dermatophyte strains shown to be the most common causative agents of tinea pedis and other superficial dermatophytic infections by determination of the MICs and minimum fungicidal concentration (MFCs), time-kill assays, and development-of-resistance studies.
MATERIALS AND METHODS
MICs and MFCs.
The MICs and MFCs of naftifine hydrochloride against 350 clinical dermatophyte strains taken from the culture collection of the Center for Medical Mycology, Case Western Reserve University, Cleveland, OH, were determined. These included 75 strains each of T. rubrum, T. mentagrophytes, Epidermophyton floccosum, and Microsporum canis and 50 strains of T. tonsurans.
MIC testing was performed according to the CLSI M38-A2 standard method for the susceptibility testing of dermatophytes developed at the Center for Medical Mycology (14, 15). Naftifine hydrochloride was dissolved in dimethyl sulfoxide (Sigma-Aldrich, St. Louis, MO), and serial dilutions were prepared in RPMI 1640 (Hardy Diagnostics, Santa Maria, CA) and added to wells of a microdilution plate. Inocula were prepared in RPMI 1640 to a concentration of 1 × 103 to 3 × 103 CFU/ml and added to the drug dilutions, and the mixtures were incubated at 35°C for 4 days. The MIC inhibition endpoint was defined as the lowest concentration of antifungal exhibiting an 80% reduction in growth compared to that for the growth control.
MFC determinations were performed according to the modifications suggested by Canton et al. (16) and Ghannoum and Isham (17). Specifically, the total contents of each clear well from the MIC assay were subcultured onto potato dextrose agar (PDA). To avoid antifungal carryover, the aliquots were allowed to soak into the agar overnight and streaked for isolation once they were dry, thus removing the cells from the drug source. Fungicidal activity was defined as a >99.9% reduction in the number of CFU/ml from the starting inoculum count, and fungistatic activity was defined as a ≤99.9% reduction.
Time-kill assay.
For the time-kill assay, four strains each of T. rubrum, T. mentagrophytes, and E. floccosum from the MIC-MFC study were selected. The assay was conducted using a modification of the method described by Klepser et al. (18) Suspensions of test organisms (inoculum, 3 × 103 conidia/ml in Sabouraud dextrose broth) were added to concentrations of naftifine hydrochloride of 0.125×, 0.25×, 0.5×, and 1× the MFC for each isolate. (The MFC values for all Trichophyton strains tested were 0.125 to 0.25 μg/ml, while the MFC values for the four E. floccosum strains were 0.5, 1.0, 2.0, and 8 μg/ml.) At predetermined time points (0, 2, 4, 8, 12, 24, and 48 h), a 0.1-ml sample was removed and diluted with saline, and a 30-μl aliquot from each dilution was plated in duplicate onto a PDA plate. Colony counts were determined following 4 days of incubation at 30°C. A time-kill curve was plotted for each isolate (log10 number of conidia/ml versus time).
Development of resistance.
Three strains each of T. mentagrophytes and T. tonsurans, taken from the set of isolates from the MIC-MFC study, were selected to determine whether repeated exposure to naftifine hydrochloride would cause development of resistance to the drug.
A repeat MIC assay was set up for each strain, in which the contents of the well at 0.5× MIC was subcultured to a PDA plate. An inoculum from this subculture (0.5 ml of a 107-CFU/ml suspension) was added to 10 ml of naftifine hydrochloride in RPMI 1640 at 0.5× MIC, 1× MIC, 2× MIC, and 4× MIC for 4 days. Tubes were then centrifuged at 3,000 rpm for 10 min, and the sediment (cell suspension) was subcultured to PDA. This subculture was subsequently used to repeat the MIC procedure as described above to determine whether an increase in MIC occurred. The process was performed in duplicate and repeated for a total of 15 passages. An indication of the development of resistance to naftifine hydrochloride was defined as an increase in the MIC of greater than 3 dilutions.
RESULTS
MICs and MFCs.
As can be seen in Table 1, naftifine hydrochloride demonstrated potent antifungal activity against all of the dermatophyte isolates tested. The naftifine hydrochloride MIC range, MIC50, and MIC90 were 0.015 to 1.0 μg/ml, 0.06 μg/ml, and 0.25 μg/ml, respectively, for all dermatophyte strains tested.
Table 1.
MIC and MFC values of naftifine hydrochloride against dermatophytes
| Species (no. of isolates tested) | MIC (μg/ml) |
MFC (μg/ml) |
||||
|---|---|---|---|---|---|---|
| Range | 50% | 90% | Range | 50% | 90% | |
| E. floccosum (75) | 0.12–0.25 | 0.12 | 0.12 | 0.5–>64 | 8.0 | >32 |
| M. canis (75) | 0.03–0.5 | 0.12 | 0.25 | 0.25–>64 | >32 | >64 |
| T. mentagrophytes (75) | 0.03–1.0 | 0.06 | 0.5 | 0.12–>32 | 0.25 | 8.0 |
| T. rubrum (75) | 0.015–0.25 | 0.06 | 0.06 | 0.06–>0.5 | 0.06 | 0.25 |
| T. tonsurans (50) | 0.03–0.12 | 0.06 | 0.06 | 0.06–>0.5 | 0.25 | >0.5 |
| All dermatophytes (350) | 0.015–1.0 | 0.06 | 0.25 | 0.06–>64 | 0.5 | >32 |
Naftifine hydrochloride MFC values were lower for Trichophyton species than for the E. floccosum and M. canis strains tested (Table 1). Importantly, naftifine hydrochloride demonstrated fungicidal activity against 85% of the Trichophyton species tested (81% of the T. mentagrophytes, 74% of the T. tonsurans, and 96% of the T. rubrum strains), with fungicidality being defined as an MFC within 3 dilutions of the MIC. In contrast, naftifine hydrochloride demonstrated fungistatic activity against 72% of the E. floccosum and 83% of the M. canis strains tested.
Development of resistance.
As can be seen in Table 2, the MICs of all isolates undergoing repeated exposure to naftifine hydrochloride remained constant throughout the serial passages (i.e., no increase in MIC values was noted). The initial naftifine hydrochloride MIC range for the six test strains was 0.03 to 0.12 μg/ml, and the MIC for each strain following 15 serial passages was 0.06 μg/ml. This demonstrates that naftifine hydrochloride did not have the potential for inducing resistance following serial passage of the dermatophytes tested.
Table 2.
MICs following repeated exposure to naftifine hydrochloride
| Isolate | MIC (μg/ml) after the indicated exposure |
||||
|---|---|---|---|---|---|
| Initial | Passage 15 |
||||
| 0.5× | 1× | 2× MIC | 4× MIC | ||
| T. mentagrophytes MRL 10799 | 0.03 | 0.06 | 0.06 | 0.06 | 0.06 |
| T. mentagrophytes MRL 10759 | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 |
| T. mentagrophytes MRL 10840 | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 |
| T. tonsurans MRL 10152 | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 |
| T. tonsurans MRL 10260 | 0.12 | 0.06 | 0.06 | 0.06 | 0.06 |
| T. tonsurans MRL 10326 | 0.12 | 0.06 | 0.06 | 0.06 | 0.06 |
Time-kill assay.
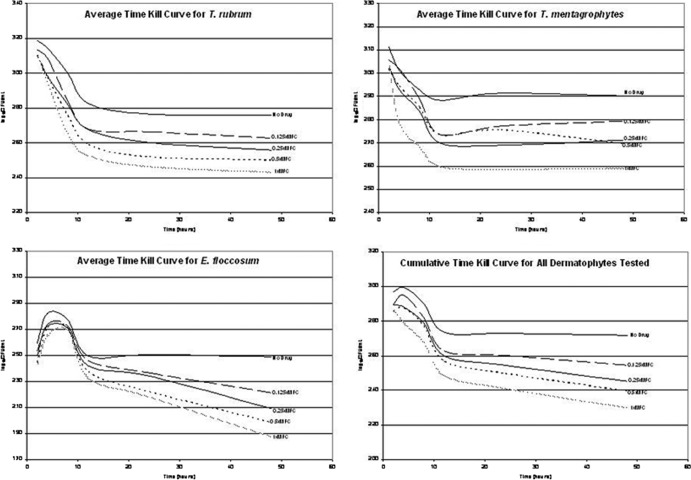
The time-kill curves for all dermatophytes tested (Fig. 1) showed that naftifine hydrochloride inhibited all strains in a dose-dependent manner. As expected, the greatest reduction in the numbers of CFU corresponded to the highest drug concentration in each case.
Fig 1.
Time-kill curves for naftifine hydrochloride against dermatophyte strains.
DISCUSSION
In this study, the antifungal activity of naftifine hydrochloride was evaluated. When testing a new antimicrobial agent or formulation, it is important to demonstrate antifungal efficacy, including both growth inhibition and fungicidal properties. Our data showed that naftifine hydrochloride possesses potent antifungal activity against dermatophytes, as measured by MIC, MFC, and time-kill assays. In this regard, our data were in agreement with previously reported in vitro results of the activity of naftifine hydrochloride (MIC range, 0.001 to 0.5 μg/ml) against dermatophyte strains (8, 19).
In addition, our fungicidality testing showed that naftifine hydrochloride is fungicidal against the majority of dermatophyte strains. This fungicidality is due to the mechanism of action of naftifine hydrochloride and the allylamine class of antifungals, which exert their fungicidal activity through the inhibition of squalene epoxidase (20). This inhibition results in the accumulation of squalene, which is known to be toxic to fungi.
Further, with the use of time-kill assays, we showed that naftifine hydrochloride activity is dose dependent, with the greatest reduction in the numbers of CFU corresponding to the highest drug concentration.
Most importantly, we demonstrated that repeated exposure to naftifine hydrochloride did not result in the development of resistance within the tested dermatophyte strains. The underlying reason for this lack of ability to induce resistance could be due to its fungicidal activity. This reason may be comparable to that for amphotericin B, another fungicidal agent, to which resistance has not been reported in over 50 years of use. Similarly, Bradley et al. did not identify any development of resistance to terbinafine, another member of the allylamine class, in the large phase III trial that brought terbinafine to the market (21). Though a small subset of subjects from this trial failed terbinafine therapy, data indicated that this failure was not associated with resistance development but rather was associated with host-related factors.
Taken together, our data suggest that naftifine hydrochloride may be an excellent candidate antifungal for the treatment of superficial dermatophytoses; future clinical studies are warranted to support this premise.
Footnotes
Published ahead of print 1 July 2013
REFERENCES
- 1.Havlickova A, Czaika VA, Friedrich M. 2008. Epidemiological trends in skin mycoses worldwide. Mycoses 51(Suppl 4):2–15 [DOI] [PubMed] [Google Scholar]
- 2.Aly R. 1994. Ecology and epidemiology of dermatophyte infection. J. Am. Acad. Dermatol. 31(3 Pt 2):S21–S25 [DOI] [PubMed] [Google Scholar]
- 3.Legge BS, Grady JF, Lacey AM. 2008. The incidence of tinea pedis in diabetic versus nondiabetic patients with interdigital macerations: a prospective study. J. Am. Podiatr. Med. Assoc. 98:353–356 [DOI] [PubMed] [Google Scholar]
- 4.Panackal AA, Halpern EF, Watson AJ. 2009. Cutaneous fungal infections in the United States: analysis of the National Ambulatory Medical Care Survey (NAMCS) and National Hospital Ambulatory Medical Care Survey (NHAMCS), 1995-2004. Int. J. Dermatol. 46:704–712 [DOI] [PubMed] [Google Scholar]
- 5.Stock I. 2008. Antimycotic therapy of tinea pedis and other foot mycoses. Med. Monatsschr. Pharm. 31:247–256 [PubMed] [Google Scholar]
- 6.Delgado Florencio V, Abad Romero-Baimas J. 1999. On the epidemiology of dermatophytoses in Granada (Andalousia, Spain). Rev. Iberoam. Micol. 16:53–56 (In Spanish.) [PubMed] [Google Scholar]
- 7.Seebacher C, Bouchara JP, Mignon B. 2008. Updates on the epidemiology of dermatophyte infections. Mycopathologia 166:335–352 [DOI] [PubMed] [Google Scholar]
- 8.Venugopal PV, Venugopal TV. 1994. Antidermatophytic activity of allylamine derivatives. Indian J. Pathol. Microbiol. 37:381–388 [PubMed] [Google Scholar]
- 9.Birnbaum JE. 1990. Pharmacology of the allylamines. J. Am. Acad. Dermatol. 23(4 Pt 2):782–785 [DOI] [PubMed] [Google Scholar]
- 10.Monk JP, Brogden RN. 1991. Naftifine. A review of its antimicrobial activity and therapeutic use in superficial dermatomycoses. Drugs 42:659–672 [DOI] [PubMed] [Google Scholar]
- 11.Maertens JA. 2004. History of the development of azole derivatives. Clin. Microbiol. Infect. 10(Suppl 1):1–10 [DOI] [PubMed] [Google Scholar]
- 12.Andrade TS, Castro LG, Nunes RS, Gimenes VM, Cury AE. 2004. Susceptibility of sequential Fonsecaea pedrosoi isolates from chromoblastomycosis patients to antifungal agents. Mycoses 47:216–221 [DOI] [PubMed] [Google Scholar]
- 13.Gupta AK, Kohli Y. 2003. Evaluation of in vitro resistance in patients with onychomycosis who fail antifungal therapy. Dermatology 207:375–380 [DOI] [PubMed] [Google Scholar]
- 14.Ghannoum MA, Chaturvedi V, Espinel-Ingroff A, Pfaller MA, Rinaldi MG, Lee-Yang W, Warnock DW. 2004. Intra- and interlaboratory study of a method for testing the antifungal susceptibilities of dermatophytes. J. Clin. Microbiol. 42:2977–2979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CLSI 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard, 2nd ed. CLSI document M38-A2 CLSI, Wayne, PA [Google Scholar]
- 16.Canton E, Peman J, Viudes A, Quindos G, Gobernado M, Espinel-Ingroff A. 2003. Minimum fungicidal concentrations of amphotericin B for bloodstream Candida species. Diagn. Microbiol. Infect. Dis. 45:203–206 [DOI] [PubMed] [Google Scholar]
- 17.Ghannoum MA, Isham N. 2007. Voriconazole and caspofungin cidality against non-albicans Candida species. Infect. Dis. Clin. Pract. 15:250–253 [Google Scholar]
- 18.Klepser ME, Ernst EJ, Lewis RE, Ernst ME, Pfaller MA. 1998. Influence of test conditions on antifungal time-kill curve results: proposal for standardized methods. Antimicrob. Agents Chemother. 42:1207–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Georgopoulos A, Petranyi G, Mieth H, Drews J. 1981. In vitro activity of naftifine, a new antifungal agent. Antimicrob. Agents Chemother. 19:386–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petranyi G, Ryder NS, Stütz A. 1984. Allylamine derivatives: new class of synthetic antifungal agents inhibiting fungal squalene epoxidase. Science 224:1239–1241 [DOI] [PubMed] [Google Scholar]
- 21.Bradley MC, Leidich S, Isham N, Elewski BE, Ghannoum MA. 1999. Antifungal susceptibilities and genetic relatedness of serial Trichophyton rubrum isolates from patients with onychomycosis of the toenail. Mycoses 42(Suppl 2):105–110 [PubMed] [Google Scholar]