Abstract
The fitness and virulence costs associated with the clinical acquisition of colistin resistance by Acinetobacter baumannii were evaluated. The growth of strain CR17 (colistin resistant) was less than that of strain CS01 (colistin susceptible) when the strains were grown in competition (72-h competition index, 0.008). In a murine sepsis model, CS01 and CR17 reached spleen concentrations when coinfecting of 9.31 and 6.97 log10 CFU/g, respectively, with an in vivo competition index of 0.016. Moreover, CS01 was more virulent than CR17 with respect to mortality and time to death.
TEXT
Acinetobacter baumannii is increasingly clinically relevant due to the rising number of nosocomial infections that it causes and its ability to develop resistance to all antimicrobials, including colistin (CST) (1). Although the rate of outbreaks of CST-resistant strains remains low, their incidence is increasing due to the rise in the use of this antibiotic (2).
CST resistance in A. baumannii may occur via mutations in the PmrAB two-component system (3), which leads to the addition of phosphoethanolamine to the lipid A molecule (4, 5), or by the loss of the bacterial lipopolysaccharide due to mutation or insertional inactivation of the genes responsible for lipid A biosynthesis (lpxA, lpxC, and lpxD) (6, 7). In previous reports, the in vitro acquisition of nonstable CST resistance in the A. baumannii ATCC 19606 strain selected by growth under increasing pressure of the antibiotic, associated with mutations in the PmrAB system, was associated with changes in the expression of numerous proteins (8). This phenotype was associated with decreased fitness and virulence compared to its parental susceptible strain (9). This decrease in virulence could explain the low prevalence of CST resistance in clinical settings. To illustrate, a report from Rolain et al. described the colonizing nature of a strain that acquired CST resistance after the clinical administration of CST (10), which was also associated with a mutation in the PmrAB system (11).
We have previously reported the acquisition of CST resistance in a strain from a CST-treated patient that maintained its ability to cause infection (12). The objective of the present work was to study the cost in terms of fitness and virulence of the CST resistance in this clinical A. baumannii strain.
(This work was presented in part at the 22nd European Congress of Clinical Microbiology and Infectious Diseases [ECCMID], London, United Kingdom, 2012.)
Two previously described clinical A. baumannii isolates were used, the CST-susceptible CS01 strain (CST MIC, <0.03 mg/liter), isolated from the cerebrospinal fluid (CSF) of a patient with meningitis prior to CST treatment, and its CST-resistant derivative (CR17; CST MIC, >16 mg/liter), which was isolated from CSF 9 days after initiation of treatment with CST (12). The MIC of CST for the CR17 strain was maintained after 10 serial passages on plates in the absence of CST, and spontaneous reversion of CR17 to the susceptible phenotype was not seen during the course of the experiment, indicating that the CST-resistant phenotype was stable. In order to characterize mutations in the PmrAB system, genomic DNA from CS01 and CR17 strains was extracted by resuspending a single colony in 25 μl of water and then lysing the cells by incubation at 100°C for 10 min. After centrifugation, the genomic DNA in the supernatant was used to amplify the pmrA and pmrB genes with specific primers (9), and the amplified sequences were cloned into the pGEM-T Easy vector (Promega Biotech Ibérica SL, Madrid, Spain) and sequenced using standard methods. In the resistant CR17 strain, no mutations were found in pmrB, the sensor kinase element. In the response regulator element pmrA, a Met-to-Lys alteration was found at position 12.
For in vitro growth, bacterial duplication time, and competition index (CI) experiments, growth curves were performed for both strains separately and growing together. Briefly, bacteria at a concentration of 5 × 105 CFU/ml were grown in 20 ml of Mueller-Hinton broth (MH; Becton, Dickinson Microbiology Systems, Cockeysville, MD). At 2, 4, 8, 24, 48, and 72 h, 100-μl aliquots were taken and susceptible and resistant CFU were determined by plating serial log10 dilutions on MH agar or MH agar plus 8 mg/liter of CST (Sigma Chemical Co., St. Louis, MO, USA). The CI was defined as the number of CR17 CFU recovered/number of CS01 CFU recovered, divided by the number of CR17 CFU inoculated/number of CS01 CFU inoculated. Duplication times were 43 min for CS01 and 40.7 min for CR17. Significant differences in in vitro growth between strains were not observed when they were grown separately. However, CR17 growth was reduced compared to that of CS01 when the two strains were grown in competition (Fig. 1A), with a CI at 24 h of 0.097 and a CI at 72 h of 0.008. These results are similar to those obtained with a CST-resistant strain derived in vitro by growth of the A. baumannii ATCC 19606 strain in the presence of CST, but with unstable resistance to CST (9).
Fig 1.
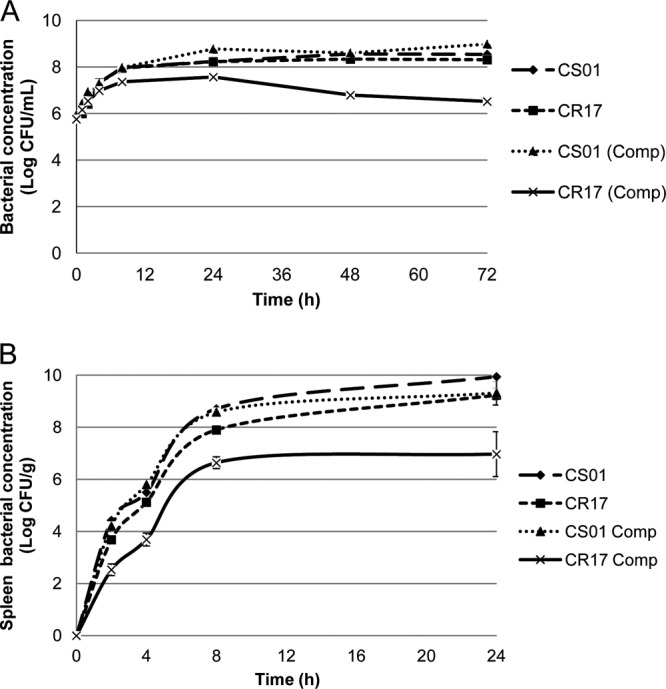
In vitro (A) and in vivo (B) growth of A. baumannii CS01 and CR17, separately and in competition.
For in vivo bacterial growth and CI experiments in an animal model of peritoneal sepsis, three groups of 19 C57BL/6 female mice (University of Seville, Seville, Spain) were inoculated intraperitoneally with 0.5 ml containing 5 log10 CFU/ml (100% lethal dose [LD100]; see below) of each strain, CS01 and CR17, separately and with a mixed inoculum (50% of each strain). Subgroups of three mice were sacrificed at 2, 4, and 8 h, and 10 mice were sacrificed at 24 h (for calculation of the CI). Spleens were removed aseptically and homogenized (Stomacher 80; Tekmar Co., Cincinnati, OH). CFU were determined after plating in MH agar with or without CST, and the CI was calculated as described above. For the duration of this experiment (24 h), the in vivo growth of CS01 reached a maximal concentration in the spleen of 10 log10 CFU/g, whereas CR17 reached a maximal concentration of 9.17 log10 CFU/g (Fig. 1B). When the strains were grown in competition, the maximum concentration of CS01 decreased to 9.31 log10 CFU/g (0.69 log10 decrease), while that of CR17 decreased to 6.97 log10 CFU/g (2.2 log10 decrease). The in vivo CI at 24 h was 0.016. These results show a lower fitness of the CR17 strain in vivo, suggesting a lower infecting ability than that of its CST-susceptible parental strain. These results are also in concordance with the experiments performed previously with an in vitro CST-resistant induced strain (9). The in vivo studies were approved by the Ethics and Clinical Research Committee of the University Hospital Virgen del Rocío, Seville, Spain.
The virulence of both strains was assessed in a murine peritoneal sepsis model by measuring mortality and lethal doses according to the Reed and Muench method (13), as well as by measuring survival time of infected mice. Briefly, for each strain, groups of 10 animals were infected intraperitoneally (i.p.) with an inoculum of 0.5 ml starting at 8 log10 CFU/ml and serially 10-fold diluted until 100% survival was reached. Bacteria were mixed with porcine mucin (Sigma-Aldrich, Madrid, Spain) at 5% (wt/vol) prior to inoculation. The higher mortality of CS01 than of CR17 in the peritoneal sepsis model is shown in Table 1, and LD100, LD50, and LD0 are shown in Table 2. In animals inoculated with the minimal lethal dose that produced 100% mortality for both strains (5 log10 CFU/ml), the survival time for CS01 was shorter than that for CR17 (mean ± standard deviation, 23.6 ± 3.29 h versus 28.4 ± 2.19 h; P < 0.026, Student t test) (Fig. 2). These results show a loss in virulence of the clinical strain associated with the acquisition of CST resistance produced by clinical treatment with CST and are consistent with the lower virulence seen in strains that acquired CST resistance in vitro (9). However, it should be noted that a large difference was seen between the virulence of the A. baumannii ATCC 19606 strain (LD50, 6.4 log10 CFU) (9) and the clinical strain CS01 (LD50, 3.29 log10 CFU), highlighting the strain-dependent virulence in A. baumannii that has been described previously (14).
Table 1.
Mortality in a murine model of peritoneal sepsis after infection with clinical strain Acinetobacter baumannii CS01 and its colistin-resistant mutant CR17
| Strain | Mortality (%) at inoculum (log10 CFU/ml)a: |
||||||
|---|---|---|---|---|---|---|---|
| 8 | 7 | 6 | 5 | 4 | 3 | 2 | |
| CS01 | 100 | 100 | 100 | 100 | 100 | 20 | 0 |
| CR17 | 100 | 100 | 100 | 100 | 10 | 0 | 0 |
Inoculum volume, 500 μl.
Table 2.
Lethal doses of clinical strain Acinetobacter baumannii CS01 and its colistin-resistant mutant CR17 in a murine model of peritoneal sepsis
| Strain | LD (log10 CFU/ml) |
||
|---|---|---|---|
| LD100 | LD50 | LD0 | |
| CS01 | 4 | 3.29 | 2 |
| CR17 | 5 | 4.39 | 3 |
Fig 2.
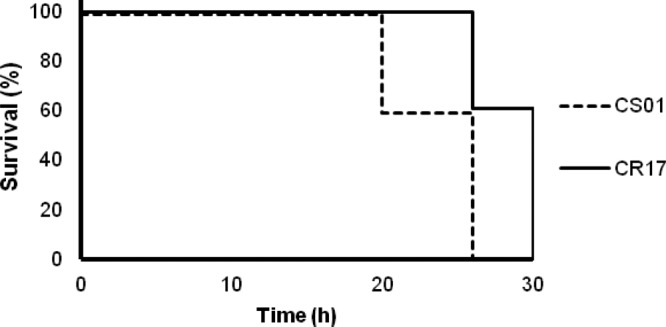
Time of survival with the minimal lethal dose (5 log10 CFU/ml) of A. baumannii CS01 and CR17 that produced 100% mortality (survival was assessed every 2 h). *, P = 0.029, log rank test.
In summary, we have shown decreased fitness and lower virulence associated with the acquisition of CST resistance due to antibiotic pressure during clinical administration of CST. However, although these results could in part explain the limited spread of CST resistance in clinical settings, there are many more factors (1) that may influence the infective ability of clinical strains (14), leading to the emergence of CST-resistant strains able to produce severe infections (12), causing major clinical concern.
Nucleotide sequence accession number.
The nucleotide sequence of the A. baumannii CR17 pmrA gene was submitted to the EMBL database (GenBank accession number KC776915).
ACKNOWLEDGMENTS
This work was supported in part by the Consejería de Salud of the Junta de Andalucía (PI-0044-2011) and by the Ministerio de Economía y Competitividad, Instituto de Salud Carlos III, cofinanced by European Development Regional Fund “A way to achieve Europe” (ERDF), Spanish Network for the Research in Infectious Diseases (REIPI RD12/0015). M.J.M. is supported by the Subprograma Miguel Servet from the Ministerio de Economía y Competitividad of Spain (CP11/00314).
Footnotes
Published ahead of print 8 July 2013
REFERENCES
- 1.McConnell MJ, Actis L, Pachon J. 2013. Acinetobacter baumannii: human infections, factors contributing to pathogenesis and animal models. FEMS Microbiol. Rev. 37:130–155 [DOI] [PubMed] [Google Scholar]
- 2.Vila J, Pachon J. 2012. Therapeutic options for Acinetobacter baumannii infections: an update. Expert Opin. Pharmacother. 13:2319–2336 [DOI] [PubMed] [Google Scholar]
- 3.Adams MD, Nickel GC, Bajaksouzian S, Lavender H, Murthy AR, Jacobs MR, Bonomo RA. 2009. Resistance to colistin in Acinetobacter baumannii associated with mutations in the PmrAB two-component system. Antimicrob. Agents Chemother. 53:3628–3634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beceiro A, Llobet E, Aranda J, Bengoechea JA, Doumith M, Hornsey M, Dhanji H, Chart H, Bou G, Livermore DM, Woodford N. 2011. Phosphoethanolamine modification of lipid A in colistin-resistant variants of Acinetobacter baumannii mediated by the pmrAB two-component regulatory system. Antimicrob. Agents Chemother. 55:3370–3379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arroyo LA, Herrera CM, Fernandez L, Hankins JV, Trent MS, Hancock RE. 2011. The pmrCAB operon mediates polymyxin resistance in Acinetobacter baumannii ATCC 17978 and clinical isolates through phosphoethanolamine modification of lipid A. Antimicrob. Agents Chemother. 55:3743–3751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moffatt JH, Harper M, Adler B, Nation RL, Li J, Boyce JD. 2011. Insertion sequence ISAba11 is involved in colistin resistance and loss of lipopolysaccharide in Acinetobacter baumannii. Antimicrob. Agents Chemother. 55:3022–3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moffatt JH, Harper M, Harrison P, Hale JD, Vinogradov E, Seemann T, Henry R, Crane B, St Michael F, Cox Adler B, Nation RL, Li J, Boyce JD. 2010. Colistin resistance in Acinetobacter baumannii is mediated by complete loss of lipopolysaccharide production. Antimicrob. Agents Chemother. 54:4971–4977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez-Reyes M, Rodriguez-Falcon M, Chiva C, Pachon J, Andreu D, Rivas L. 2009. The cost of resistance to colistin in Acinetobacter baumannii: a proteomic perspective. Proteomics 9:1632–1645 [DOI] [PubMed] [Google Scholar]
- 9.Lopez-Rojas R, Dominguez-Herrera J, McConnell MJ, Docobo-Perez F, Smani Y, Fernandez-Reyes M, Rivas L, Pachon J. 2011. Impaired virulence and in vivo fitness of colistin-resistant Acinetobacter baumannii. J. Infect. Dis. 203:545–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rolain JM, Roch A, Castanier M, Papazian L, Raoult D. 2011. Acinetobacter baumannii resistant to colistin with impaired virulence: a case report from France. J. Infect. Dis. 204:1146–1147 [DOI] [PubMed] [Google Scholar]
- 11.Rolain JM, Diene SM, Kempf M, Gimenez G, Robert C, Raoult D. 2013. Real-time sequencing to decipher the molecular mechanism of resistance of a clinical pan-drug-resistant Acinetobacter baumannii isolate from Marseille, France. Antimicrob. Agents Chemother. 57:592–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopez-Rojas R, Jimenez-Mejias ME, Lepe JA, Pachon J. 2011. Acinetobacter baumannii resistant to colistin alters its antibiotic resistance profile: a case report from Spain. J. Infect. Dis. 204:1147–1148 [DOI] [PubMed] [Google Scholar]
- 13.O'Reilly T, Cleeland R, Squires EL. 1996. Evaluation of antimicrobials in experimental animal infections, p 604–765 In Lorian V. (ed), Antibiotics in laboratory medicine, 4th ed. Williams & Wilkins, Baltimore, MD [Google Scholar]
- 14.Eveillard M, Soltner C, Kempf M, Saint-Andre JP, Lemarie C, Randrianarivelo C, Seifert H, Wolff M, Joly-Guillou ML. 2010. The virulence variability of different Acinetobacter baumannii strains in experimental pneumonia. J. Infect. 60:154–161 [DOI] [PubMed] [Google Scholar]


