Abstract
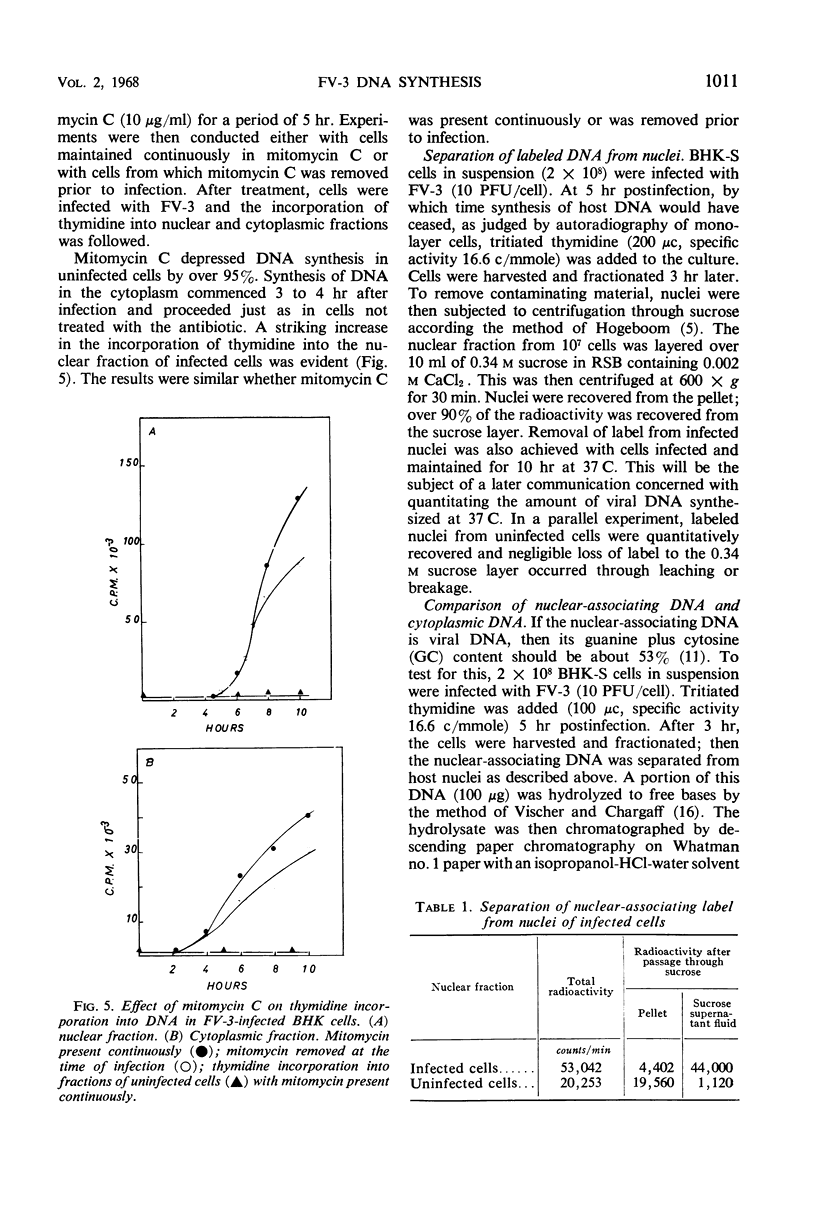
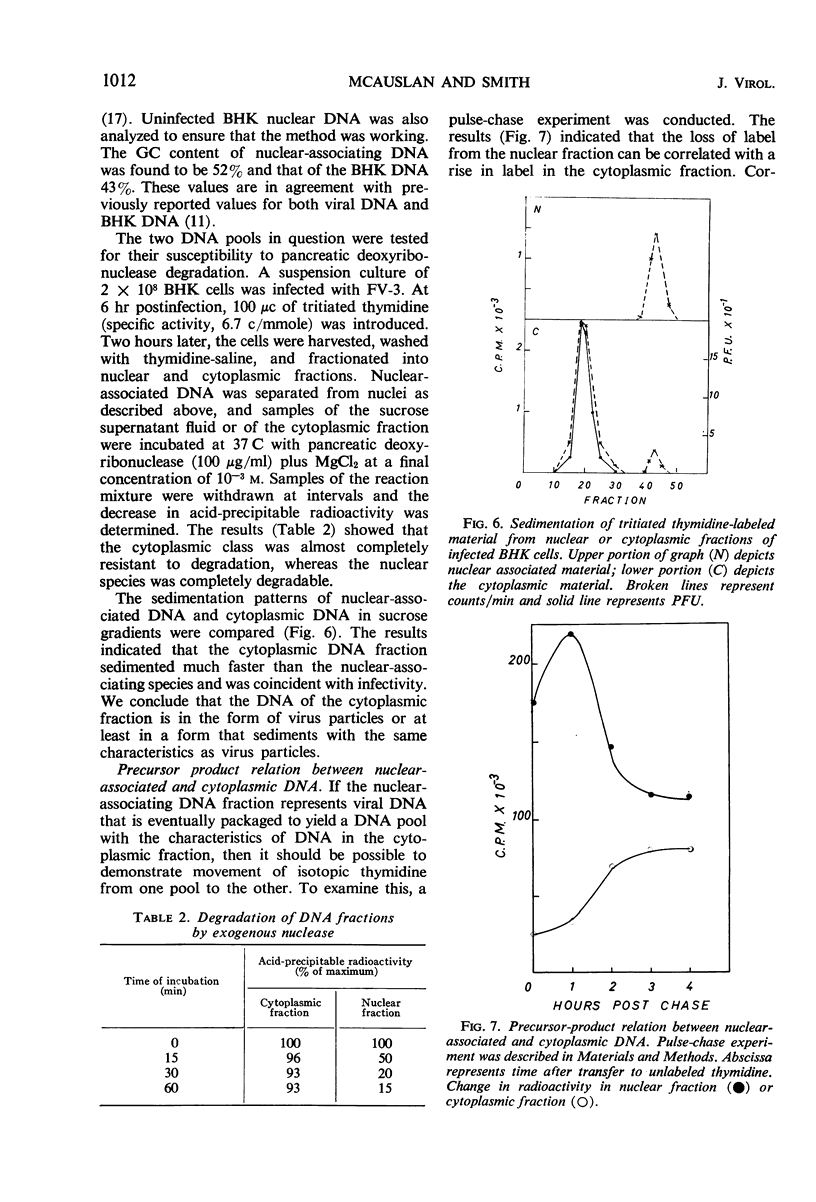
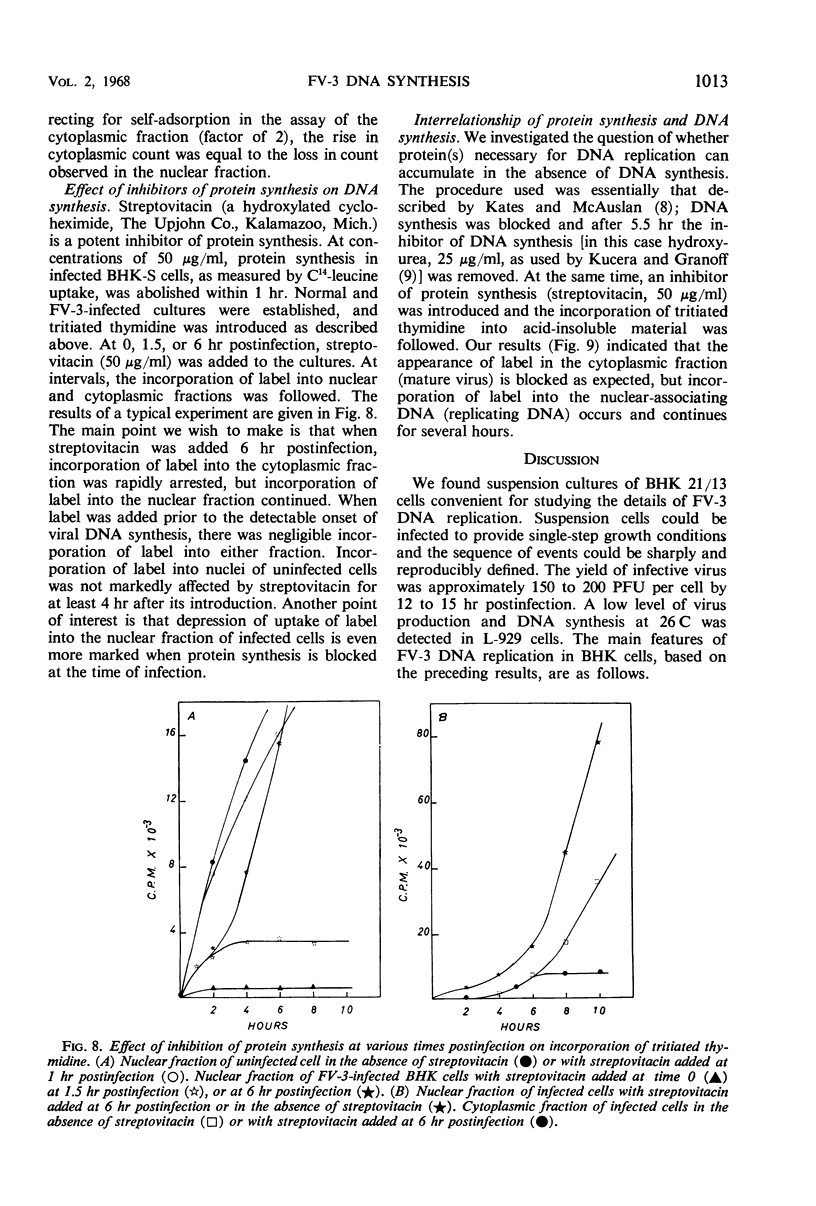
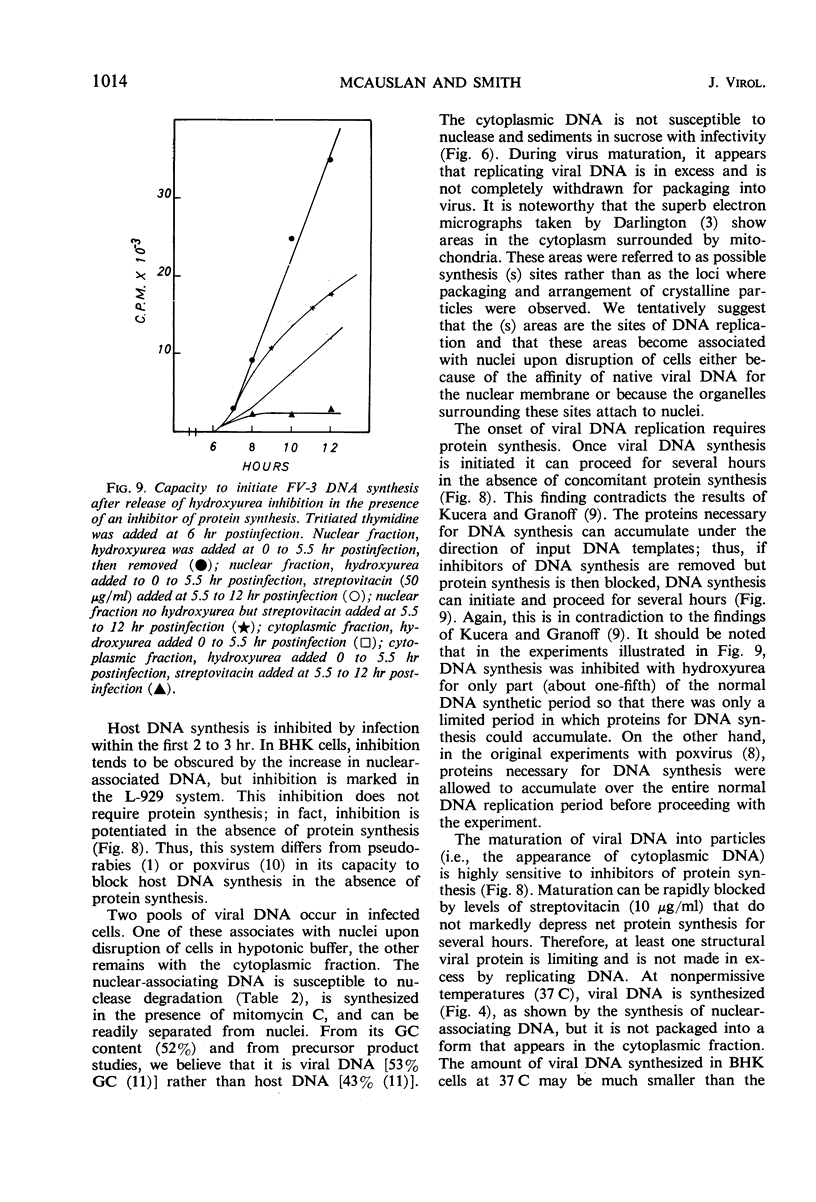
Deoxyribonucleic acid (DNA) synthesis and virus growth in frog virus 3 (FV-3)-infected mammalian cells in suspension were examined. The kinetics of thymidine incorporation into DNA was followed by fractionating infected cells. The cell fractionation procedure separated replicating viral DNA from matured virus. Incorporation of isotope into the nuclear fraction was depressed 2 to 3 hr postinfection; this inhibition did not require protein synthesis. About 3 to 4 hr postinfection, there was an increase in thymidine incorporation into both nuclear and cytoplasmic fractions. The nuclear-associating DNA had a guanine plus cytosine (GC) content of 52%; unlike host DNA it was synthesized in the presence of mitomycin C, it could be removed from nuclei by centrifugation through sucrose, and it was susceptible to nuclease digestion. This nuclear-associating DNA appeared to be a precursor of cytoplasmic DNA of infected cells. The formation of the latter DNA class could be selectively inhibited by conditions (infection at 37 C or inhibition of protein synthesis) that permit continued incorporation of thymidine into nuclear-associating DNA. The cytoplasmic DNA class also had a GC content of 52%, was resistant to nuclease degradation, and its sedimentation profile in sucrose gradients corresponded to that of infective virus. Contrary to previous reports, we found that (i) viral DNA synthesis can continue in the absence of concomitant protein synthesis, and (ii) viral DNA synthesis is not abolished at 37 C. The temperature lesion in FV-3 replication appeared to be in the packaging of DNA into the form that appears in the cytoplasmic fraction of disrupted cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEN-PORAT T., KAPLAN A. S. MECHANISM OF INHIBITION OF CELLULAR DNA SYNTHESIS BY PSEUDORABIES VIRUS. Virology. 1965 Jan;25:22–29. doi: 10.1016/0042-6822(65)90247-3. [DOI] [PubMed] [Google Scholar]
- Darlington R. W., Granoff A., Breeze D. C. Viruses and renal carcinoma of Rana pipiens. II. Ultrastructural studies and sequential development of virus isolated from normal and tumor tissue. Virology. 1966 May;29(1):149–156. doi: 10.1016/0042-6822(66)90204-2. [DOI] [PubMed] [Google Scholar]
- Granoff A., Came P. E., Breeze D. C. Viruses and renal carcinoma of Rana pipiens. I. The isolation and properties of virus from normal and tumor tissue. Virology. 1966 May;29(1):133–148. doi: 10.1016/0042-6822(66)90203-0. [DOI] [PubMed] [Google Scholar]
- HOGEBOOM G. H., SCHNEIDER W. C., STRIEBICH M. J. Cytochemical studies. V. On the isolation and biochemical properties of liver cell nuclei. J Biol Chem. 1952 May;196(1):111–120. [PubMed] [Google Scholar]
- JOKLIK W. K., BECKER Y. THE REPLICATION AND COATING OF VACCINIA DNA. J Mol Biol. 1964 Dec;10:452–474. doi: 10.1016/s0022-2836(64)80066-8. [DOI] [PubMed] [Google Scholar]
- Jungwirth C., Dawid I. B. Vaccinia DNA: separation of viral from host cell DNA. Arch Gesamte Virusforsch. 1967;20(4):464–468. doi: 10.1007/BF01275228. [DOI] [PubMed] [Google Scholar]
- Kates J. R., McAuslan B. R. Relationship between protein synthesis and viral deoxyribonucleic acid synthesis. J Virol. 1967 Feb;1(1):110–114. doi: 10.1128/jvi.1.1.110-114.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucera L. S., Granoff A. Viruses and renal carcinoma of Rana pipiens. VI. Interrelationships of macromolecular synthesis and infectious virus production in frog virus 3-infected BHK 21/13 cells. Virology. 1968 Feb;34(2):240–249. doi: 10.1016/0042-6822(68)90233-x. [DOI] [PubMed] [Google Scholar]
- LOH P. C., PAYNE F. E. EFFECT OF P-FLUOROPHENYLALANINE ON THE SYNTHESIS OF VACCINIA VIRUS. Virology. 1965 Apr;25:560–574. doi: 10.1016/0042-6822(65)90084-x. [DOI] [PubMed] [Google Scholar]
- Maes R., Granoff A. Viruses and renal carcinoma of Rana pipiens. IV. Nucleic acid synthesis in frog virus 3-infected BHK 21/13 cells. Virology. 1967 Nov;33(3):491–502. doi: 10.1016/0042-6822(67)90125-0. [DOI] [PubMed] [Google Scholar]
- Penman S. RNA metabolism in the HeLa cell nucleus. J Mol Biol. 1966 May;17(1):117–130. doi: 10.1016/s0022-2836(66)80098-0. [DOI] [PubMed] [Google Scholar]
- Penman S., Scherrer K., Becker Y., Darnell J. E. POLYRIBOSOMES IN NORMAL AND POLIOVIRUS-INFECTED HELA CELLS AND THEIR RELATIONSHIP TO MESSENGER-RNA. Proc Natl Acad Sci U S A. 1963 May;49(5):654–662. doi: 10.1073/pnas.49.5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEKIGUCHI M., TAKAGI Y. Effect of mitomycin C on the synthesis of bacterial and viral deoxyribonucleic acid. Biochim Biophys Acta. 1960 Jul 15;41:434–443. doi: 10.1016/0006-3002(60)90040-8. [DOI] [PubMed] [Google Scholar]
- SHIBA S., TERAWAKI A., TAGUCHI T., KAWAMATA J. Selective inhibition of formation of deoxyribonucleic acid in Escherichia coli by mitomycin C. Nature. 1959 Apr 11;183(4667):1056–1057. doi: 10.1038/1831056a0. [DOI] [PubMed] [Google Scholar]
- WYATT G. R. The purine and pyrimidine composition of deoxypentose nucleic acids. Biochem J. 1951 May;48(5):584–590. doi: 10.1042/bj0480584. [DOI] [PMC free article] [PubMed] [Google Scholar]