Abstract
Management of coinfection with malaria and HIV is a major challenge to public health in developing countries, and yet potential drug-drug interactions between antimalarial and antiviral regimens have not been adequately investigated in people with both infections. Each of the constituent components of artemether-lumefantrine, the first-line regimen for malaria treatment in Nigeria, and nevirapine, a major component of highly active antiretroviral therapy, are drugs metabolized by the cytochrome P450 3A4 isoenzyme system, which is also known to be induced by nevirapine. We examined potential interactions between lumefantrine and nevirapine in 68 HIV-positive adults, all of whom were diagnosed with asymptomatic Plasmodium falciparum infections by microscopy. Post hoc PCR analysis confirmed the presence of P. falciparum in only a minority of participants. Day 7 capillary blood levels of lumefantrine were significantly higher in HIV-positive participants than in 99 HIV-negative controls (P = 0.0011). Associations between day 7 levels of lumefantrine and risk of persistent parasitemia could not be evaluated due to inadequate power. Further investigations of the impact of nevirapine on in vivo malaria treatment outcomes in HIV-infected patients are thus needed.
INTRODUCTION
Malaria and HIV are two of the most important health problems facing developing countries and are among the most common infections in sub-Saharan Africa. HIV coinfection is thought to contribute to 3 million additional malaria cases, higher malaria parasite densities in immunosuppressed children, and a 5% greater mortality rate (1, 2). HIV also increases the risk of Plasmodium falciparum infection progressing to clinical malaria in adults, especially in those with advanced immunosuppression, by eroding the efficacy of acquired immunity (3). The choice of antimalarial drug for the treatment of HIV patients therefore is of utmost importance considering the dangers of comorbidity, but sufficient pharmacokinetic and parasitological evidence to make this choice is currently lacking.
Combination therapies in current use for malaria in Africa comprise a derivative of the artemisinin family of drugs combined with at least one nonartemisinin partner drug. The most widely used such combination is artemether plus lumefantrine (coartemether; AL). Artemether is metabolized in the liver by the isoenzyme CYP3A4, to its active metabolite dihydroartemisinin (DHA), with peak plasma concentration being reached around 2 to 3 h after oral administration (4); elimination half-life is estimated at approximately 1 h. There is thus only limited opportunity for DHA to participate in drug-drug interactions. Lumefantrine is partially metabolized to desbutyl-lumefantrine, predominantly through CYP3A4, reaching peak plasma levels approximately 10 h after oral administration, and is then cleared slowly, showing a terminal half-life of 4 to 6 days in P. falciparum malaria cases (5–9). Oral bioavailability of lumefantrine is variable and highly dependent on administration with fatty foods (5, 9, 10).
The antiretroviral drug nevirapine (NVP) is a nonnucleoside reverse transcriptase inhibitor that is well absorbed after oral administration with >90% bioavailability, generally achieved about 4 h after oral dosing, and has a long half-life (11). NVP is extensively metabolized by the same CYP3A4 isoform as artemether and lumefantrine and is also known to upregulate the isoenzyme (12, 13). Thus, NVP autoinduces its own metabolism and potentially that of any other drugs metabolized through this route. This raises the possibility of significant drug-drug interactions of NVP with lumefantrine and other antimalarials (1). Kredo and colleagues (6) initiated a pharmacokinetic study in 18 South African volunteers that were HIV infected and receiving NVP therapy, compared to 18 naive controls, each of whom took a full adult course of AL; none of the study subjects were infected with Plasmodium sp. This study found differences between NVP recipients and controls in several pharmacokinetic parameters for lumefantrine, the most important of which was a significantly higher day 7 lumefantrine concentration in the NVP group. These authors concluded that further studies of drug-drug interactions between NVP and lumefantrine were urgently needed in malaria-infected subjects.
Artemether-lumefantrine, which is currently the recommended therapy for malaria treatment, was introduced in Nigeria in 2005 as the first-line regimen for uncomplicated malaria. Rivers State, in the Niger Delta area of southern Nigeria, has a high prevalence of HIV infection (7.4% of the population), and malaria transmission is hyperendemic. The study was designed to address the lack of data regarding the pharmacokinetics of AL among HIV-positive subjects in this setting, where asymptomatic parasite carriage is common. We hypothesized that following treatment with AL for concomitant P. falciparum infections, day 7 blood concentrations of lumefantrine in HIV-positive individuals on NVP therapy would differ from those in HIV-negative individuals. Any such difference may also have a measurable impact on parasite clearance in treated asymptomatic individuals, as day 7 lumefantrine concentration is known to be an important determinant of antimalarial efficacy in individuals with symptomatic malaria (4).
MATERIALS AND METHODS
Study area.
The study was carried out at the University of Port Harcourt Teaching Hospital and the Braithwaite Memorial Specialist Hospital Port Harcourt, Nigeria, from September 2010 to May 2011. Port Harcourt is the capital of Rivers State in the Niger Delta, rich in the nation's oil resources. The region is dotted with oil and gas activities which attract an international workforce, and commercial sex workers follow the camp (14). These socioeconomic conditions contribute to a high estimated population prevalence of HIV infection of 7.4% (15). Malaria is holoendemic in Nigeria, with transmission all year round, but malaria cases are most common during the rainy season, from April to September, with peaks of rain and intense transmission between May and July. Annual rainfall averages more than 3,550 mm in the region.
Patients and samples.
This paper describes an exploratory pharmacokinetic study with a simple unmatched case-control design, ancillary to a study designed to track molecular markers of drug resistance in HIV-infected individuals, using active detection of P. falciparum infection followed by treatment with AL, which is the regimen recommended by the University of Port Harcourt Teaching Hospital guidelines for uncomplicated malaria in adults. The work was conducted from September 2010 to May 2011. The main endpoints of the current analysis were day 7 peripheral blood lumefantrine levels and parasite carriage at day 3 and day 28 posttreatment. The primary endpoint for which the study was designed and powered was carriage of parasite genetic markers of antimalarial resistance. This analysis is ongoing and will be reported elsewhere.
Participants were recruited if they met the following eligibility criteria: 16 to 65 years of age, willingness to have HIV status confirmed from clinical records or by a point-of-care test, P. falciparum positive by microscopic examination of a blood film, and provision of a signed informed consent form. HIV-positive patients were recruited from the HIV adult clinic of both hospitals. HIV-negative participants were recruited from the hospital communities, including staff and students. HIV-negative patients were screened and confirmed virus negative with the use of the HIV Determine point-of-care test (Alere Medical Co., Ltd., Matshuhidai-shi, Chiba, Japan). Each was then screened for malaria by standard microscopy. Permission for the study was obtained from the Research Ethics Committees of the University of Port Harcourt Teaching Hospital, the Braithwaite Memorial Specialist Hospital, and the London School of Hygiene and Tropical Medicine, London, United Kingdom.
Enrolled patients were treated with AL (Coartem; Novartis Pharma, Nigeria) according to the manufacturer's dosing regimen: 4 tablets twice daily for 3 days for persons with a weight of >35 kg. Patients were advised to eat before taking the tablets. Most of the patients took their first dose at the site, having been preinformed to eat before coming. Patients were followed up till day 28. On day 7, capillary blood samples were taken from a finger prick.
For drug measurements, 100 μl of blood was measured using a pipette and dropped on a filter paper (glass microfiber paper; Fisherbrand FB59431) pretreated with 0.75 M tartaric acid (Fisher Scientific). The papers were allowed to air dry and were then stored in individual pouches with a silica desiccant to absorb moisture. The preserved papers were transferred to the London School of Hygiene and Tropical Medicine. Filter paper-adsorbed blood samples were analyzed for lumefantrine using liquid chromatography mass spectrometry (LCMS; Thermo Finnigan LCQ instrument) by following a modified protocol based on previously published methods (16). Briefly, bloodspots were extracted in methanol-water (4:1; 350 μl), and the extracts were filtered through a cotton wool plug. Each sample (20 μl) was separated on a Dionex Acclaim 120 3-μm C18 column (4.6 by 150 mm, with 120-Å pore size, fitted with a guard column) and eluted with ammonium formate (20 mM, pH 2.7) and methanol (vol/vol; 85:15) isocratically at a flow rate of 500 μl/min. The column temperature was maintained at 35°C. The electrospray ionization (ESI) source was operated in positive mode with the capillary temperature set to 350°C and sheath and auxiliary gas (nitrogen) flow rates of 60 and 20 arbitrary units, respectively. Peak identity was confirmed by using blood spiked with lumefantrine standards (0 to 30 μg/ml), adsorbed onto filter paper, and extracted in the same manner as the patient samples. Quantitation was performed using selective ion monitoring for the transition of m/z 530 to 512. The lower limit of detection was determined to be 0.1 μg/ml, the lower limit of quantification was 1.0 μg/ml, and the upper limit of quantification was 20.0 μg/ml.
Plasmodium falciparum DNA was prepared from dried spots (10 to 20 μl) on Whatman paper as previously described (17), and codons 24 to 201 of the pfmdr1 locus were amplified by nested PCR (18). Relative quantification of parasite DNA was performed by an established quantitative PCR (qPCR) method, as previously described (19).
Data were entered into spreadsheets and analyzed in STATA 11 (Stata Corp., Madison WI). Continuous data were compared between groups using Wilcoxon's rank sum test, while categorical comparisons in 2-by-2 format were performed using the χ2 distribution.
RESULTS
Out of 80 attendees at the two HIV clinics who agreed to have a malaria film read, 68 were identified as positive for P. falciparum and returned for day 7 follow-up (85%). None of these individuals reported concurrent symptoms suggestive of clinical malaria. A total of 126 individuals agreed to have a rapid HIV test performed, of which 99 were found to be negative for HIV-specific antibodies, were identified as infected with P. falciparum, and attended for day 7 follow-up (79%); none of these individuals were symptomatic. These 167 participants were treated with a full adult course of AL and followed up on days 3, 7, and 28 for repeat blood sampling.
To confirm microscopic diagnosis of P. falciparum parasitemia at enrollment, nested PCR amplification of the amino-terminal fragment of the pfmdr1 gene was carried out on DNA extracted from the first blood sample taken from each participant. Nested PCR was also performed on DNA extracted from all day 3 and day 28 filter paper blood samples. Unexpectedly, a high proportion of enrollees (78.1%) were found to be aparasitemic by nested PCR, suggesting poor specificity of the original microscopic diagnosis (Table 1). There was a strong association between PCR positivity at day 0 and day 3 (odds ratio [OR], 5.56; 95% confidence interval [CI], 1.76 to 17.32; P = 0.0004), suggesting good reproducibility of parasite detection for the PCR method, in contrast to results obtained with microscopy.
Table 1.
Parasite carriage by microscopy and PCR in 68 HIV-positive and 99 HIV-negative individualsa
| HIV status | No. of individuals (%) PCR positive for P. falciparum on day: |
||
|---|---|---|---|
| 0 | 3 | 28 | |
| Positive (n = 68b) | 17 (29.9) | 8 | 12 |
| Negative (n = 99) | 20 (17.2) | 12 | 12 |
All 167 individuals were reported as positive for P. falciparum parasites on microscopic examination of thick blood films.
Sixty-seven of these individuals were receiving daily nevirapine antiretroviral therapy, and one received efavirenz. All HIV-positive patients also received the nucleoside reverse transcriptase inhibitors lamivudine and zidovudine.
Using the PCR data as a more reliable test for parasite carriage, we found weak evidence that HIV-positive people were more likely to be parasitemic at day 0 (OR, 2.05; 95% CI, 0.917 to 4.60; P = 0.054), which may reflect slightly higher parasite densities in this group and thus a greater likelihood of parasites being correctly identified by the screening microscopists. HIV-positive subjects were not significantly more likely to be PCR positive for P. falciparum at day 3 and/or day 28 after AL treatment than were HIV-negative individuals (OR, 1.75; 95% CI, 0.776 to 3.95; P = 0.141).
Both HIV status and lumefantrine concentration at day 7 were recorded for all 167 individuals. We examined the distribution of lumefantrine concentration at day 7 in all study participants and found highly significant departure from normality (z score = 7.581, P < 0.0001), which remained after (natural) logarithmic transformation (z = 5.372, P < 0.0001). In an exploratory analysis following the methods of Kredo et al. (6), we removed as outliers 5 samples with extremely low measured lumefantrine concentrations (0, 0, 0.01, 0.08, 0.08 μM, all in the HIV-negative group) and log transformed as described above. Departure from the normal distribution was then marginally nonsignificant (z = 1.594, P = 0.054). After consideration of these findings, we decided to take the conservative approach of using only nonparametric methods for testing statistical significance of comparisons and retained all data in the analysis.
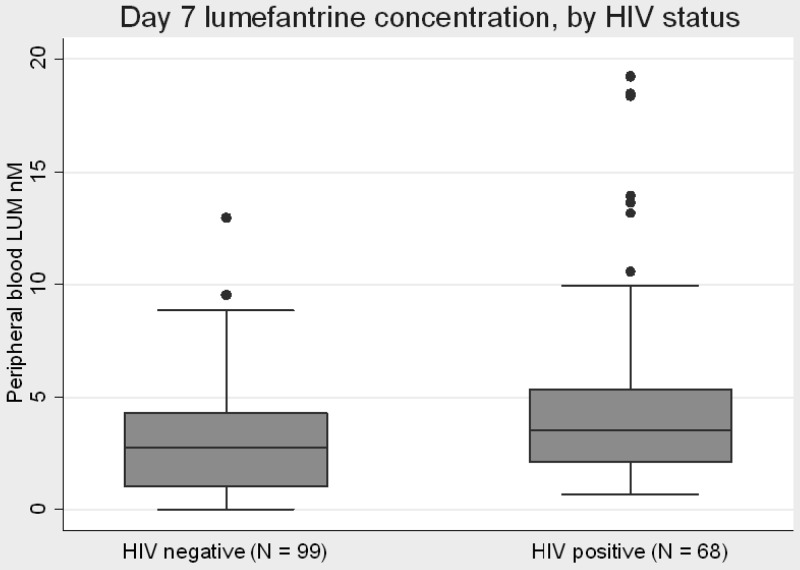
HIV status, and thus nevirapine use, was found to have a significant effect on the concentration of lumefantrine 7 days after treatment (Wilcoxon rank sum test z = −3.270, P = 0.0011), with a median concentration in the HIV-negative group of 2.75 μM (interquartile range [IQR], 1.03 to 4.31) and in the HIV-positive group of 3.55 μM (IQR, 2.07 to 5.37) (Fig. 1). However, the 5 individuals with extremely low lumefantrine readings (identified in the previous paragraph) were all in the HIV-negative group, so to test for possible bias caused by this group, we performed the comparison with these 5 measurements removed. In this exploratory analysis, a significant association remained between HIV status and lumefantrine concentration at 7 days after AL treatment (z = −2.830, P = 0.0046).
Fig 1.
Day 7 lumefantrine concentration in AL-treated participants. Midline of each box plot is the median, with the edges of the box representing the interquartile interval. Whiskers delineate the 5th and 95th percentiles. Lumefantrine concentration was below the normal limits of detection in five individuals, all in the HIV-negative group (see the text).
As many of our subjects were shown to be parasite negative by PCR, we tested for any effect of parasitemia on lumefantrine concentrations at day 7. Overall, in all 166 evaluable individuals, PCR-positive parasitemia at day 0 was not associated with any difference in day 7 lumefantrine concentration in our sample set (37 positive versus 129 negative individuals). There was a weak association between day 3 PCR-detected parasitemia and higher lumefantrine concentrations (z = −2.305, P = 0.021), suggesting that greater lumefantrine bioavailability among NVP recipients was not improving AL treatment outcomes. This effect was not strong enough to confer a statistically significant deficit in parasite clearance for HIV-positive individuals as a group; considering only those participants with follow-up data from both day 3 and day 28 (n = 140), 33.9% of HIV-positive individuals had PCR-detectable parasitemia on either or both day 3 and day 28, compared to 22.7% of HIV-negative individuals (OR, 1.75; 95% CI, 0.776 to 3.95; P = 0.141). Assessment of day 0 parasitemia using qPCR was performed for 8 individuals (including 5 who were HIV positive) who were subsequently PCR positive on day 3, and 15 who had cleared parasites by day 3 (including 9 who were HIV positive). This exploratory analysis did not provide any evidence that higher starting parasitemia increased the likelihood of an individual remaining PCR positive for P. falciparum on day 3 (Wilcoxon rank sum test z = −0.904, P = 0.37).
DISCUSSION
The coformulated combination of artemether, a sesquiterpene lactone derived from the natural compound artemisinin, with the aryl amino-alcohol lumefantrine, as a systemic racemic fluorene mixture, has become the most widely distributed and available antiretroviral chemotherapy throughout Africa. As antiretroviral chemotherapies have also become more widely available for treatment of HIV patients in health systems in Africa, detailed understanding of any interactions between these two chemotherapies is urgently needed. In this study, we show that HIV-positive adults taking regular NVP who were treated with AL for microscopically apparent P. falciparum infection had significantly higher day 7 plasma concentrations of lumefantrine than treated adults who were HIV test negative and not receiving NVP. However, we found no evidence that submicroscopic parasite persistence at day 3 after AL treatment was prevented in individuals with higher day 7 plasma levels of lumefantrine; in fact, HIV-positive individuals were slightly more likely to have PCR-detectable parasitemia on day 3 or day 28 than were HIV-negative participants, although this was not significant.
Our findings are consistent with those of Kredo et al. (6) and confirm that drug-drug interactions between AL and NVP are potentially important. However, NVP stimulation of the CYP3A4 isoenzyme would be expected, a priori, to lower peripheral lumefantrine levels, due to an increase in the amount of lumefantrine metabolized to desbutyl-lumefantrine, a potent derivative that is normally found at a concentration between 0.5% and 5% of that of the parent compound at day 7 in the few studies available (8, 20). Food intake also alters lumefantrine metabolism; we were not able to supervise the food intake of our participants while they were taking AL, but all were informed of the need to accompany their medication with fatty food. The apparently increased bioavailability of lumefantrine in NVP recipients produced no measurable parasitological benefit in our patients; on the contrary, one-third of HIV-positive (and thus NVP-receiving) participants were found to have persisting PCR-detectable P. falciparum parasitemia at day 3 and/or day 28, compared to less than a quarter of the control group. This difference, which suggests that perturbation of the immune system in HIV infection may have some impact on antimalarial effectiveness in these dual-treated patients, was not statistically significant. The case-control design used here may be prone to selection bias, and this could affect parasitological outcomes. However, univariate analysis of posttreatment parasitemia versus age, weight, gender, and educational attainment found no evidence of confounding by any of these parameters (data not shown). The recent observation that coadministration of NVP with AL leads to reduced maximal concentration of both artemether and DHA (21) suggests an alternate explanation for reduced parasite clearance at day 3 in patients receiving both regimens. Nevertheless, further studies of the parasitological impact of antiretroviral-antimalarial drug-drug interactions in adequately powered studies are urgently needed, not least because of the important role of the host immune system in clearing drug-treated malaria parasites (3, 22). In our study, all HIV-infected participants were identified through attendance at a weekly clinic in which all received NVP (except for a single patient on efavirenz; when this patient was excluded from the analysis, the relationship between NVP use and lumefantrine concentration at day 7 remained significant). Compliance with antiretroviral treatment was not evaluated directly. Future studies with HIV patients not receiving NVP may permit discrimination between drug-drug interactions and the impact of retroviral disease per se on lumefantrine bioavailability.
A major weakness of our study was the poor quality of enrollment microscopic diagnosis, such that the majority of participants had in fact failed a major inclusion criterion. This had two main impacts. First, the study was greatly underpowered to evaluate any parasitological outcomes, as so many participants were actually uninfected (with P. falciparum). Second, we were not able to analyze parasite densities with any confidence and thus were left with the binary variable of PCR positivity as the remaining reliable measure of malaria infection. Further, by this method, we cannot rule out the possibility that some of our positive PCRs on posttreatment blood samples were detecting gametocytes of P. falciparum only. These sexual-stage parasite forms are infective to Anopheles sp. mosquitoes but do not contribute to clinical malaria symptoms and cannot divide. Gametocytes are well known to survive in a minority of AL-treated patients after clearance of the actively dividing asexual parasite stages (23, 24). Nevertheless, we have recently described persistence of asexual parasites in asymptomatic Ghanaian school children treated with artemisinin combination therapy, suggesting that subclinical parasitemia may be more difficult to clear than previously thought (25).
In conclusion, this is the second study to find evidence that NVP-recipient HIV patients harbor a significantly higher peripheral blood concentration of lumefantrine than do HIV-negative controls, 7 days after receiving a full treatment course of AL. Our findings corroborate the findings of Kredo et al. (6) in a larger group of AL-treated individuals, some of whom were infected with P. falciparum. Insufficient parasitological data were available to determine whether this difference in lumefantrine concentration provides any parasitological benefit to NVP-treated HIV patients with malaria infections.
ACKNOWLEDGMENTS
This work was supported by a doctoral studentship awarded to Ifeyinwa Chijioke-Nwauche by the Rivers State Sustainable Development Agency and by the MALACTRES Project (EU FP7). C.J.S. is supported by Public Health England.
Footnotes
Published ahead of print 17 June 2013
REFERENCES
- 1.Khoo S, Back D, Winstanley P. 2005. The potential for interactions between antimalarial and antiretroviral drugs. AIDS 19:995–1005 [DOI] [PubMed] [Google Scholar]
- 2.Korenromp EL, Williams BG, de Vlas SJ, Gouws E, Gilks CF, Ghys PD, Nahlen BL. 2005. Malaria attributable to the HIV-1 epidemic, sub-Saharan Africa. Emerg. Infect. Dis. 11:1410–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitworth J, Morgan D, Quigley M, Smith A, Mayanja B, Eotu H, Omoding N, Okongo M, Malamba S, Ojwiya A. 2000. Effect of HIV-1 and increasing immunosuppression on malaria parasitaemia and clinical episodes in adults in rural Uganda: a cohort study. Lancet 356:1051–1056 [DOI] [PubMed] [Google Scholar]
- 4.Ezzet F, Mull R, Karbwang J. 1998. Population pharmacokinetics and therapeutic response of CGP 56697 (artemether + benflumetol) in malaria patients. Br. J. Clin. Pharmacol. 46:553–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Djimdé A, Lefèvre G. 2009. Understanding the pharmacokinetics of Coartem. Malar. J. 8(Suppl 1):S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kredo T, Mauff K, Van der Walt JS, Wiesner L, Maartens G, Cohen K, Smith P, Barnes KI. 2011. Interaction between artemether-lumefantrine and nevirapine-based antiretroviral therapy in HIV-1-infected patients. Antimicrob. Agents Chemother. 55:5616–5623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lefèvre G, Bindschedler M, Ezzet F, Schaeffer N, Meyer I, Thomsen MS. 2000. Pharmacokinetic interaction trial between co-artemether and mefloquine. Eur. J. Pharm. Sci. 10:141–151 [DOI] [PubMed] [Google Scholar]
- 8.Salman S, Page-Sharp M, Griffin S, Kose K, Siba PM, Ilett KF, Mueller I, Davis TM. 2011. Population pharmacokinetics of artemether, lumefantrine, and their respective metabolites in Papua New Guinean children with uncomplicated malaria. Antimicrob. Agents Chemother. 55:5306–5313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Vugt M, Ezzet F, Phaipun L, Nosten F, White NJ. 1998. The relationship between capillary and venous concentrations of the antimalarial drug lumefantrine (benflumetol). Trans. R. Soc. Trop. Med. Hyg. 92:564–565 [DOI] [PubMed] [Google Scholar]
- 10.Ezzet F, van Vugt M, Nosten F, Looareesuwan S, White NJ. 2000. Pharmacokinetics and pharmacodynamics of lumefantrine (benflumetol) in acute falciparum malaria. Antimicrob. Agents Chemother. 44:697–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerber JG. 2000. Using pharmacokinetics to optimize antiretroviral drug-drug interactions in the treatment of human immunodeficiency virus infection. Clin. Infect. Dis. 30(Suppl 2):S123–S129 [DOI] [PubMed] [Google Scholar]
- 12.Back DJ, Khoo SH. 2003. The role of clinical pharmacology in optimizing antiretroviral therapy. Br. J. Clin. Pharmacol. 55:473–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Havlir D, Cheeseman SH, McLaughlin M, Murphy R, Erice A, Spector SA, Greenough TC, Sullivan JL, Hall D, Myers M, Lamson M, Richman DD. 1995. High-dose nevirapine: safety, pharmacokinetics, and antiviral effect in patients with human immunodeficiency virus infection. J. Infect. Dis. 171:537–545 [DOI] [PubMed] [Google Scholar]
- 14.Nwauche CA, Akani CI. 2006. An assessment of high risk sexual behavior and HIV transmission-among migrant oil workers in the Niger Delta area of Nigeria. Niger J. Clin. Pract. 9:48–51 [PubMed] [Google Scholar]
- 15.Federal Ministry of Health Nigeria 2008. Technical report (2008). National HIV sero-prevalence sentinel survey among pregnant women attending antenatal clinics in Nigeria. FMOH, Abuja, Nigeria [Google Scholar]
- 16.Blessborn D, Römsing S, Annerberg A, Sundquist D, Björkman A, Lindegardh N, Bergqvist Y. 2007. Development and validation of an automated solid-phase extraction and liquid chromatographic method for determination of lumefantrine in capillary blood on sampling paper. J. Pharmaceut. Biomed. 45:282–287 [DOI] [PubMed] [Google Scholar]
- 17.Dlamini SV, Beshir K, Sutherland CJ. 2010. Markers of anti-malarial drug resistance in Plasmodium falciparum isolates from Swaziland: identification of pfmdr1-86F in natural parasite isolates. Malaria J. 9:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Humphreys GA, Merinopoulos I, Ahmed J, Whitty CJM, Mutabingwa TK, Sutherland CJ, Hallett RL. 2007. Amodiaquine and artemether-lumefantrine select distinct alleles of the Plasmodium falciparum pfmdr1 gene in Tanzanian children treated for uncomplicated malaria. Antimicrob. Agents Chemother. 51:991–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beshir K, Hallett RL, Eziefula AC, Bailey R, Watson J, Wright SG, Chiodini PL, Polley SD, Sutherland CJ. 2010. Measuring the efficacy of anti-malarial drugs in vivo: quantitative PCR measurement of parasite clearance. Malaria J. 9:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGready R, Stepniewska K, Lindegardh N, Ashley EA, La Y, Singhasivanon P, White NJ, Nosten F. 2006. The pharmacokinetics of artemether and lumefantrine in pregnant women with uncomplicated falciparum malaria. Eur. J. Clin. Pharmacol. 62:1021–1031 [DOI] [PubMed] [Google Scholar]
- 21.Byakika-Kibwika P, Lamorde M, Mayito J, Nabukeera L, Namakula R, Mayanja-Kizza H, Katabira E, Ntale M, Pakker N, Ryan M, Hanpithakpong W, Tarning J, Lindegardh N, de Vries PJ, Khoo S, Back D, Merry C. 2012. Significant pharmacokinetic interactions between artemether/lumefantrine and efavirenz or nevirapine in HIV-infected Ugandan adults. J. Antimicrob. Chemother. 67:2213–2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Djimde AA, Guindo AB, Kayentao K, Diourte Y, Niare-Doumbo S, Coulibaly D, Kone AK, Cissoko Y, Tekete M, Fofana B, Dicko A, Diallo DA, Wellems TE, Kwiatkowski D, Plowe CV. 2003. Clearance of drug-resistant parasites as a model for protective immunity in Plasmodium falciparum malaria. Am. J. Trop. Med. Hyg. 69:558–563 [PubMed] [Google Scholar]
- 23.Bousema JT, Schneider P, Gouagna LC, Drakeley CJ, Tostmann A, Houben R, Githure JI, Ord R, Sutherland CJ, Omar SA, Sauerwein RW. 2006. Moderate effect of artemisinin-based combination therapy on transmission of Plasmodium falciparum. J. Infect. Dis. 193:1151–1159 [DOI] [PubMed] [Google Scholar]
- 24.Sutherland CJ, Ord R, Dunyo S, Jawara M, Drakeley CJ, Alexander N, Coleman R, Pinder M, Walraven G, Targett GAT. 2005. Reduction of malaria transmission to Anopheles mosquitoes with a six-dose regimen of co-artemether. PLoS Med. 2:e92. 10.1371/journal.pmed.0020092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dinko B, Oguike MC, Larbi JB, Bousema JT, Sutherland CJ. 2013. Persistent detection of Plasmodium falciparum, P. malariae, P. ovale curtisi and P. ovale wallikeri after ACT treatment of asymptomatic Ghanaian school-children. Int. J. Parasitol. DDR 3:45–50 [DOI] [PMC free article] [PubMed] [Google Scholar]