Abstract
The aim of this study was to use in vitro and in vivo models to assess the impact of lipopolysaccharide (LPS) from two different bacterial species on blood-brain barrier (BBB) integrity and brain uptake of colistin. Following repeated administration of LPS from Pseudomonas aeruginosa, the brain-to-plasma ratio of [14C]sucrose in Swiss outbred mice was not significantly increased. Furthermore, while the brain uptake of colistin in mice increased 3-fold following administration of LPS from Salmonella enterica, LPS from P. aeruginosa had no significant effect on colistin brain uptake. This apparent species-dependent effect did not appear to correlate with differences in plasma cytokine levels, as the concentrations of tumor necrosis factor alpha and interleukin-6 following administration of each LPS were not different (P > 0.05). To clarify whether this species-specific effect of LPS was due to direct effects on the BBB, human brain capillary endothelial (hCMEC/D3) cells were treated with LPS from P. aeruginosa or S. enterica and claudin-5 expression was measured by Western blotting. S. enterica LPS significantly (P < 0.05) reduced claudin-5 expression at a concentration of 7.5 μg/ml. In contrast, P. aeruginosa LPS decreased (P < 0.05) claudin-5 expression only at the highest concentration tested (i.e., 30 μg/ml). Coadministration of therapeutic concentrations of colistin ameliorated the S. enterica LPS-induced reduction in claudin-5 expression in hCMEC/D3 cells and the perturbation in BBB function in mice. This study demonstrates that BBB disruption induced by LPS is species dependent, at least between P. aeruginosa and S. enterica, and can be ameliorated by colistin.
INTRODUCTION
The blood-brain barrier (BBB) is formed by specialized endothelial cells of cerebral microvessels. Unlike endothelial cells in other organs, the layer of endothelial cells lining the cerebral microvessels constitutes a physical barrier between blood and brain tissue by a complex network of tight junctions (TJs) (1). The TJs consist of transmembrane proteins spanning the intercellular cleft; these proteins include occludin, claudins (particularly claudin-5) (2), and several cytoplasmic proteins, such as zonula occludens (3). Under normal conditions, these TJ proteins seal the paracellular route of the BBB, thus preventing the movement of relatively small hydrophilic molecules from the blood into the brain, ensuring homeostatic regulation of the central nervous system (CNS) (4).
Alterations to TJ proteins and increases in BBB permeability are observed during various disease states, including peripheral hyperalgesia, bacterial meningitis, systemic inflammation, and sepsis (5–8). Indeed, we have demonstrated that systemic administration to mice of lipopolysaccharide (LPS), a key component of the outer membrane of Gram-negative bacteria, from Salmonella enterica (as a model of bacterium-induced inflammation) leads to BBB dysfunction (9). One proposed mechanism for the decreased paracellular integrity of the BBB in response to LPS involves elevated plasma concentrations of cytokines, especially tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6). Studies have demonstrated that direct administration of cytokines significantly enhances BBB permeability both in animals and in brain endothelial cell monolayers (10–14). However, in our previous studies involving inoculation of Pseudomonas aeruginosa to mice, despite significant increases in the plasma concentrations of the three above-mentioned cytokines, the BBB paracellular integrity was not compromised (15). These studies suggested a lack of correlation between the release of these three cytokines and BBB disruption. This lack of correlation was further strengthened by the observations that LPS from S. enterica, which induced a lower proinflammatory response than P. aeruginosa bacterial infection, caused greater BBB disruption (15). This suggests that there may be a direct TJ-disrupting effect of LPS via activation of LPS receptors expressed at the brain endothelial cells. It has been shown that LPS can cause increased permeability when applied directly to the BBB in animals and decreased paracellular integrity in isolated brain microvascular endothelial cell monolayers (8, 16–20). However, whether there is a species-dependent effect of LPS between P. aeruginosa and S. enterica when applied directly to the BBB remains to be clarified.
In the present study, the effects of LPS from both S. enterica and P. aeruginosa (as species inducing systemic infection) on (i) the BBB paracellular route in mice, (ii) the brain uptake of the polymyxin antibiotic colistin, and (iii) the plasma cytokine concentrations were compared. To further assess whether any species-dependent BBB disruption was due to specific effects on the BBB, the impact of each LPS on the expression of claudin-5 was measured in an immortalized human brain capillary endothelial (hCMEC/D3) cell line (21, 22). Given the potentially neurotoxic consequences of BBB disruption resulting from LPS-induced inflammation, we were interested to determine whether the clinically utilized antibiotic colistin could ameliorate the BBB-disrupting effects of S. enterica LPS. Colistin is a polypeptide antibiotic belonging to the polymyxin family, and it is now increasingly being used to treat Gram-negative bacterial infections as last-line salvage therapy. In addition to its bactericidal effect, colistin has also been shown to bind to LPS and prevent the pathophysiologic effects of the endotoxin in the circulation (23). On the basis of this knowledge, the hypotheses of this study are (i) that LPSs from S. enterica and P. aeruginosa exhibit species-dependent effects on BBB dynamics and (ii) that coadministration of colistin prevents LPS-induced BBB disruption through sequestration of free LPS in the systemic circulation.
MATERIALS AND METHODS
Chemicals and reagents.
Colistin sulfate was supplied by Zhejiang Shenghua Biok Biology Co., Ltd. (EP5 grade; Zhejiang, China). [14C]sucrose was provided by American Radiolabeled Chemicals (St. Louis, MO). LPSs from both Salmonella enterica serotype Typhimurium and Pseudomonas aeruginosa and Trizma were purchased from Sigma-Aldrich (Castle Hill, New South Wales, Australia). Solid-phase extraction cartridges (C18 Sep-Pak, 100 mg) were obtained from Waters (Milford, MA), and Tween 20 was obtained from Aldrich Chemical Company (Milwaukee, WI). Endothelial basal medium 2 (EBM-2) and an endothelial growth medium SingleQuot kit were purchased from Lonza (Walkersville, MD). Fetal bovine serum (FBS) and Dulbecco's phosphate-buffered saline were purchased from Invitrogen (Auckland, New Zealand), and rat tail collagen type I was purchased from BD Biosciences (Bedford, MA). The primary rabbit anti-claudin-5 and mouse anti-β-actin antibodies were obtained from Abcam (Cambridge, MA), and the secondary goat anti-mouse and donkey anti-rabbit antibodies were purchased from LI-COR Biosciences (Lincoln, NE). All other reagents were of analytical and/or high-pressure liquid chromatography (HPLC) grade, and water was prepared from a Millipore purification system (Millipore Corporation, Billerica, MA).
Effect of P. aeruginosa LPS on BBB integrity and brain uptake of colistin.
Animal experiments were approved by the Monash Institute of Pharmaceutical Sciences Animal Ethics Committee and were performed in accordance with the Australian National Health and Medical Research Council (NHMRC) guidelines for the care and use of animals for scientific purposes. Male Swiss outbred mice (age, 6 to 8 weeks; weight, 25 to 30 g) were used in all studies. Mice had free access to food and water during all experimental periods.
Mice were administered intraperitoneally (i.p.) 200 μl of 0.9% (wt/vol) saline (control) or LPS (P. aeruginosa, 3 mg/kg of body weight in saline) at 0, 6, and 24 h. At 4 h after the last administration, mice (n = 6) were intravenously administered a 50-μl dose of [14C]sucrose (2 μCi in saline), plasma and brain samples were collected at 5 min postdose, and radioactivity in plasma and brain was determined using liquid scintillation counting (Tri-Carb 2800 TR; PerkinElmer, Boston, MA) (9). The brain-to-plasma (B:P) ratio of [14C]sucrose at 5 min was calculated using the following formula: (number of disintegrations per minute [dpm] per gram of brain tissue)/(number of dpm per milliliter of plasma). To determine the brain uptake of colistin in mice treated with LPS from P. aeruginosa, a 200 μl colistin sulfate solution was administered subcutaneously (s.c.) to mice (40 mg/kg) at 4 h after the third LPS or saline dose. Plasma and brain samples (n = 4) were harvested 0.5 h later, and the concentrations of colistin in brain homogenate and plasma were determined by HPLC to obtain B:P ratios (24). These B:P ratios were compared to those obtained following administration of LPS from S. enterica under the same experimental conditions (9).
Effect of P. aeruginosa LPS on plasma cytokine concentrations.
Plasma concentrations of proinflammatory cytokines (TNF-α, IL-1β, and IL-6) at 4 h after the last injection of saline or P. aeruginosa LPS were measured by mouse cytokine kits (Ready-SET-Go!; eBioscience, San Diego, CA). According to the company's instructions, a 50-μl plasma sample was added to a 96-well plate, and the absorbance at 450 nm was recorded with a Fluostar Optima microplate reader (BMG Labtech, Mount Eliza, Victoria, Australia). Standard curves were established using cytokines of known concentrations present in the kits with a quantification range of between 8 and 1,000 pg/ml. No absorbance was detected with untreated plasma, suggesting that the assay was not affected by the endogenous components within plasma. These plasma cytokine concentrations were compared to those obtained following administration of LPS from S. enterica under the same experimental conditions (15).
Impact of LPS on claudin-5 expression in vitro.
The hCMEC/D3 cells (passages 36 and 37) were kindly provided by Pierre-Olivier Couraud (INSERM, France). The cells were seeded on rat tail collagen-coated 6-well plates (density, 50,000 cells/cm2) and cultured in EBM-2 supplemented with vascular endothelial growth factor, insulin-like growth factor 1, epidermal growth factor, basic fibroblast growth factor, hydrocortisone, ascorbate, and penicillin-streptomycin from the endothelial growth medium SingleQuot kit and 2.5% (vol/vol) FBS, as recommended by the manufacturer. The cells were maintained at 37°C in an atmosphere of 5% CO2–95% O2. Culture medium was changed every 1 to 2 days until cells reached a confluent monolayer.
Once they were confluent (at 4 to 5 days postseeding), the hCMEC/D3 cells were treated with serum-free medium or LPS (from S. enterica or P. aeruginosa in serum-free medium) at concentrations of 3.75, 7.5, 15, and 30 μg/ml (n = 3 replicates/concentration). These concentrations of LPS were predicted on the basis of a bioavailability of approximately 10% from the i.p. cavity (25) with the doses which were administered to mice (i.e., 3 mg/kg). To match the time period of exposure to the in vivo studies, the cells were exposed to serum-free medium or LPS for 28 h. The cells were then lysed using ice-cold radioimmunoprecipitation assay (RIPA) buffer (150 mM sodium chloride, 1.0% [vol/vol] Triton X-100, 0.5% [wt/vol] sodium deoxycholate, 0.1% [wt/vol] sodium dodecyl sulfate, 50 mM Tris base, pH 8.0) supplemented with 4% of Complete protease inhibitor cocktail (Roche Pharmaceuticals, Basel, Switzerland) and 1 mM phenylmethylsulfonyl fluoride. Total protein levels from each sample lysate were quantified with a bicinchoninic acid protein assay kit (Pierce, Rockford, IL) using bovine serum albumin (BSA) as a standard, and these samples were then loaded at 7.5 μg/well and run on a 12% Tris-glycine polyacrylamide gel. Proteins were separated by electrophoresis at 120 V for 1.5 h in a minigel apparatus (Bio-Rad Laboratories, Hercules, CA). After separation, proteins were transferred onto a nitrocellulose membrane (pore size, 0.22 μm; Bio-Rad Laboratories GmbH, Munich, Germany), the membranes were washed in Tris-buffered saline (1 mM Tris-HCl, 150 mM sodium chloride, pH 7.6) supplemented with 0.05% (vol/vol) Tween 20 (TBS-T) and blocked with 5% (wt/vol) BSA dissolved in TBS-T. After 2 h, the 5% BSA solution was removed and the membrane was incubated overnight with the primary antibodies diluted in TBS-T at 4°C. The membranes were rinsed again with TBS-T and then incubated with the secondary antibodies diluted in TBS-T for 2 h at room temperature. Bands were visualized by exposure to a LI-COR Odyssey scanner (Lincoln, NE), and the optical density was quantified using ImageJ analysis software (National Institutes of Health). The expression of claudin-5 was measured relative to the levels of the housekeeping protein β-actin.
Effect of colistin on LPS-mediated BBB disruption.
Studies investigating whether colistin can ameliorate the BBB-disrupting effect of LPS from S. enterica were performed in hCMEC/D3 cells, and the results were confirmed in vivo. hCMEC/D3 cells were grown to confluence and treated with either (i) serum-free medium, (ii) LPS from S. enterica (15 μg/ml in serum-free medium), (iii) colistin sulfate (30 μg/ml in serum-free medium), or (iv) LPS from S. enterica (15 μg/ml in serum-free medium) and colistin sulfate (30 μg/ml in serum-free medium) (n = 3 per treatment). The concentration of colistin chosen was based on the plasma concentration obtained after subcutaneous administration of a 40-mg/kg dose to mice (9). At 28 h after treatment, cells were lysed and the expression of claudin-5 was quantified, as described above.
To confirm whether any ameliorative effect of colistin observed in vitro was reflective of the in vivo setting, male Swiss outbred mice (n = 4 per treatment) were randomly divided into four groups, and mice in each group were administered an i.p. dose of S. enterica LPS (3 mg/kg in saline) or saline with either a subcutaneous dose of colistin (40 mg/kg in saline) or saline at 0, 6, and 24 h. At 4 h after the last dose, mice were intravenously administered a 50-μl solution of [14C]sucrose (2 μCi in saline) as a BBB integrity marker, and plasma and brain samples were collected at 5 min postdose. The radioactivity in plasma and brain homogenate was determined using liquid scintillation counting, as described above, and B:P ratios of [14C]sucrose were determined for each treatment group. In addition, plasma cytokine concentrations (TNF-α, IL-1β, and IL-6) in each mouse were measured as described above.
Data analysis.
All data are presented as the mean ± standard deviation (SD), unless otherwise stated. For comparisons of the B:P ratios ([14C]sucrose or colistin) or cytokine concentrations between saline-treated and P. aeruginosa LPS-treated animals, Student's t test was employed. When comparing the hCMEC/D3 cell β-actin-normalized expression of claudin-5 between different LPS concentrations, a one-way analysis of variance followed by a Newman-Keuls multiple-comparisons test (PASW Statistics for Windows, version 17.0; Chicago, IL) was used. The one-way analysis of variance was also used when comparing the claudin-5 expression or B:P ratios of [14C]sucrose between saline-, LPS (S. enterica)-, and colistin-treated hCMEC/D3 cells or mice. A difference with a P value of <0.05 was considered significant.
RESULTS
Effect of LPS on BBB integrity and brain uptake of colistin.
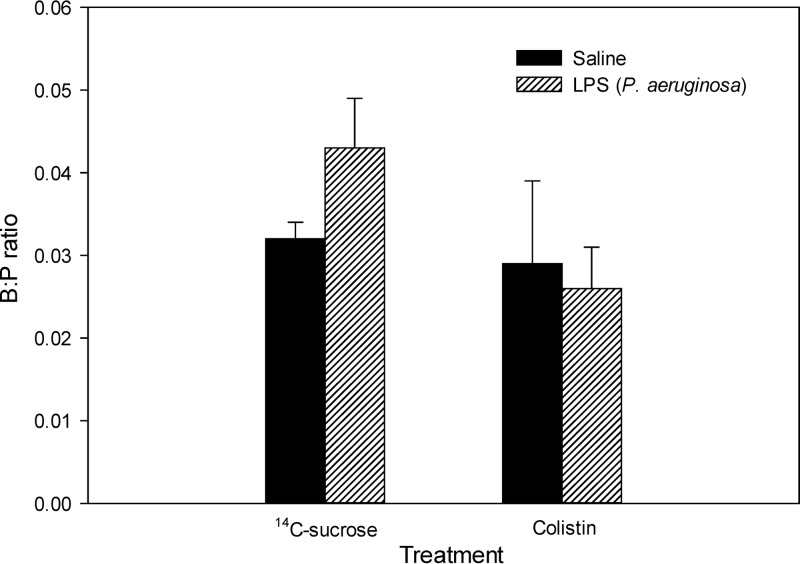
The B:P ratios of [14C]sucrose and colistin in saline- and P. aeruginosa LPS-treated mice are shown in Fig. 1. No significant difference in the B:P ratio of [14C]sucrose was detected between saline- and P. aeruginosa LPS-treated mice. The B:P ratios of [14C]sucrose were 0.032 ± 0.004 and 0.043 ± 0.015, respectively. This inability of P. aeruginosa LPS to increase the B:P ratio of [14C]sucrose was in contrast to what we had previously observed with S. enterica LPS (9). Consistent with this species-dependent effect on BBB integrity, the B:P ratios of colistin in saline- or P. aeruginosa LPS-treated mice were very low, with values of 0.029 ± 0.010 and 0.026 ± 0.005, respectively. These B:P ratios were not significantly different between saline- and LPS-treated mice (Fig. 1b), again inconsistent with our previous findings with S. enterica LPS (9).
Fig 1.
B:P ratios of [14C]sucrose (n = 6) and colistin (n = 4) in Swiss outbred mice at 4 h after the last dose of saline or P. aeruginosa LPS (3 mg/kg at 0, 6, and 24 h). Data are presented as the mean ± SEM.
Plasma cytokine concentrations following LPS treatment.
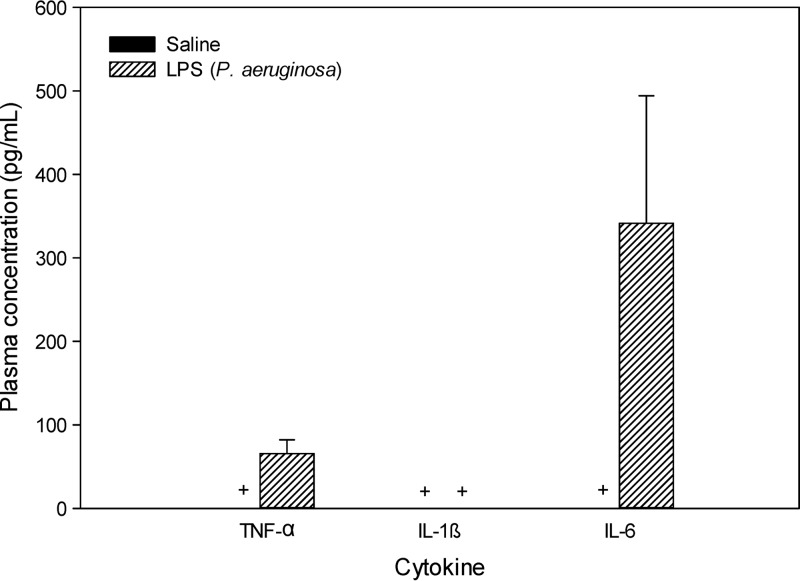
Plasma concentrations of TNF-α, IL-1β, and IL-6 following administration of P. aeruginosa LPS are shown in Fig. 2. The concentrations of all three cytokines were below the lower limit of quantification (LLQ; i.e., <8 pg/ml) in saline-treated mice. The IL-1β levels in plasma following administration of P. aeruginosa LPS were also below the LLQ. However, the concentrations of the other two cytokines (i.e., TNF-α and IL-6) were considerably higher in P. aeruginosa LPS-treated mice, although a statistically significant difference (relative to the control) could not be demonstrated, given that the corresponding cytokine concentrations in saline-treated mice were all below the LLQ.
Fig 2.
Plasma concentrations (pg/ml) of TNF-α, IL-1β, and IL-6 in Swiss outbred mice 4 h after the last dose of saline or P. aeruginosa LPS (3 mg/kg at 0, 6, and 24 h). Data are presented as the mean ± SD (n = 4). +, plasma concentrations were below the lower limit of quantification (8 pg/ml).
Effect of different LPS species on claudin-5 expression.
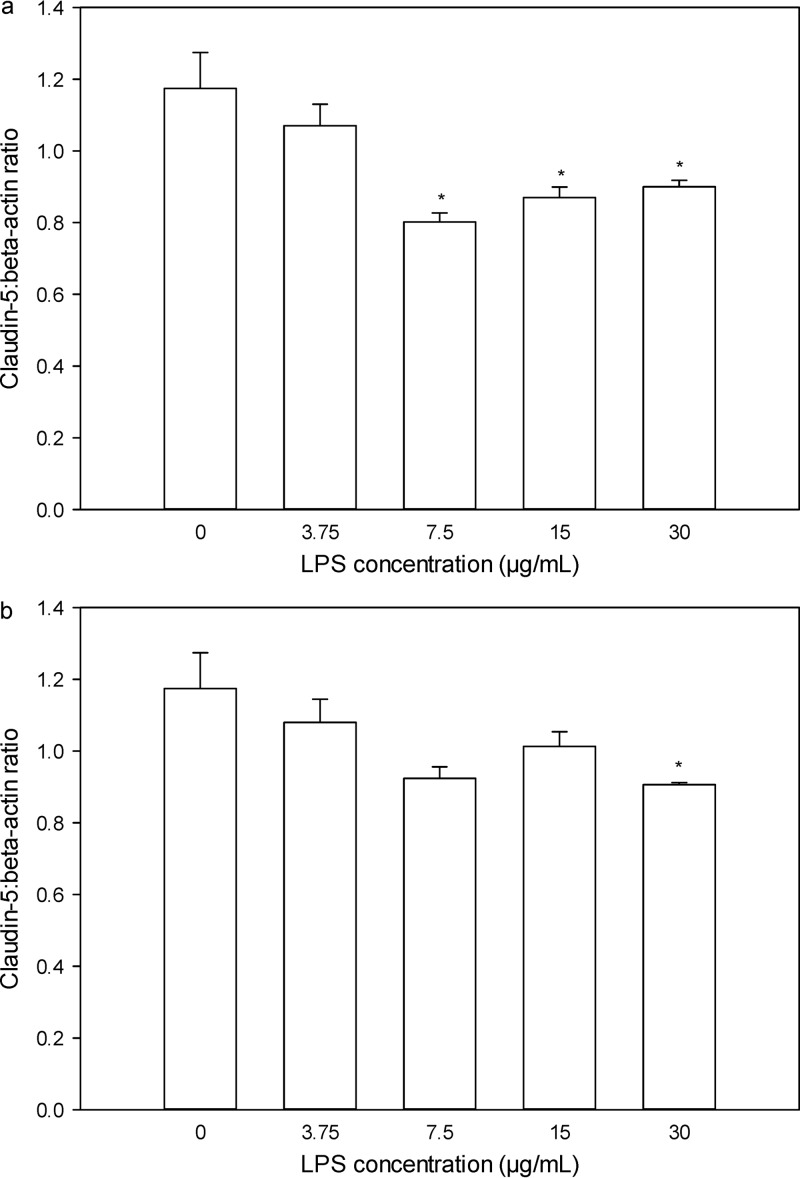
As shown in Fig. 3a, the claudin-5/β-actin ratios were not different between hCMEC/D3 cells treated with growth medium and those treated with S. enterica LPS at 3.75 μg/ml. However, as the concentration of S. enterica LPS increased to 7.5 μg/ml and above, the claudin-5/β-actin ratios decreased significantly relative to that for the medium-treated cells (P < 0.05). In contrast, no difference in the claudin-5/β-actin ratio was observed between cells treated with medium and those treated with P. aeruginosa LPS until the cells were treated with the highest concentration of LPS (30 μg/ml) (Fig. 3b).
Fig 3.
Claudin-5/β-actin ratios in hCMEC/D3 cells 28 h after treatment with S. enterica LPS (a) and P. aeruginosa LPS (b) at concentrations of 0, 3.75, 7.5, 15, and 30 μg/ml. Data are presented as the mean ± SD (n = 3). *, P < 0.05 compared to the control using a one-way analysis of variance.
Amelioration of the effect of LPS on BBB integrity by colistin.
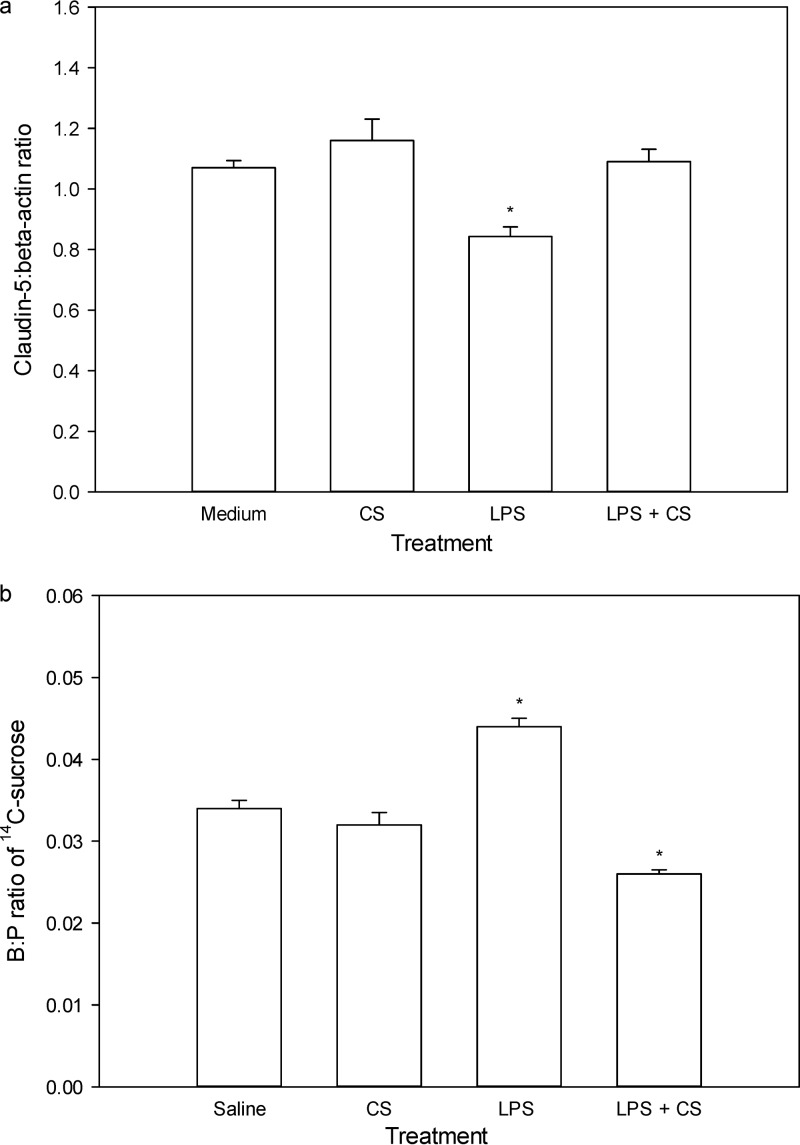
As shown in Fig. 4a, the claudin-5/β-actin ratio in hCMEC/D3 cells was not affected by treatment with colistin alone; however, treatment with S. enterica LPS at 15 μg/ml resulted in a significant (P < 0.05) reduction in the expression of claudin-5. Importantly, when LPS-treated hCMEC/D3 cells were also treated with colistin at 30 μg/ml, the expression of claudin-5 returned to the baseline level, being no different from that for the control treated hCMEC/D3 cells.
Fig 4.
(a) Claudin-5/β-actin ratios in hCMEC/D3 cells 28 h after treatment with serum-free medium, colistin sulfate (CS; 30 μg/ml in serum-free medium), LPS (S. enterica, 15 μg/ml in serum-free medium), and LPS (S. enterica, 15 μg/ml in serum-free medium) with colistin sulfate (30 μg/ml in serum-free medium) (n = 3); (b) B:P ratios of [14C]sucrose in Swiss outbred mice at 4 h after the last dose of the i.p. saline regimen and the s.c. saline regimen; i.p. saline and s.c. colistin sulfate (40 mg/kg) at 0, 6, and 24 h; i.p. S. enterica LPS (3 mg/kg) and s.c. saline at 0, 6, and 24 h; and i.p. S. enterica LPS (3 mg/kg) with s.c. colistin sulfate (40 mg/kg) at 0, 6, and 24 h. Data are presented as the mean ± SD (n = 4). *, P < 0.05 compared to medium (a) or saline (b) using a one-way analysis of variance.
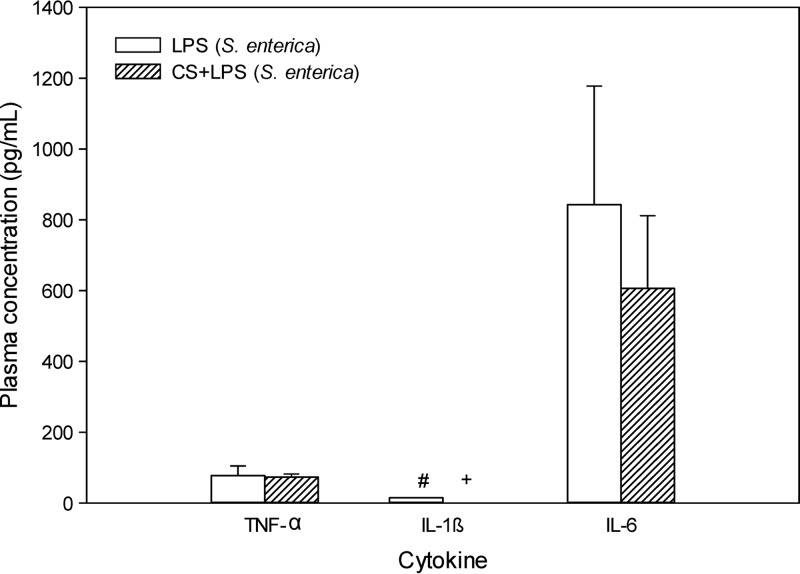
Similarly, the integrity of the BBB (as measured by the B:P ratio of [14C]sucrose) was no different between saline-treated (0.034 ± 0.003) and colistin-treated (0.032 ± 0.003) mice (Fig. 4b). As expected, the B:P ratio of [14C]sucrose was significantly increased following S. enterica LPS treatment (0.044 ± 0.002); however, when LPS was coadministered with colistin at 40 mg/kg, the brain uptake of [14C]sucrose returned to baseline levels (0.026 ± 0.001). This amelioration of LPS-induced BBB disruption mediated by colistin was not associated with alterations to plasma cytokine levels (Fig. 5), consistent with a direct protective effect of colistin on the BBB, as observed in the studies with hCMEC/D3 cells.
Fig 5.
Plasma concentrations (pg/ml) of TNF-α, IL-1β, and IL-6 in Swiss outbred mice at 4 h after the last dose of LPS alone (S. enterica, 3 mg/kg at 0, 6, and 24 h) or LPS (S. enterica, 3 mg/kg at 0, 6, and 24 h) with colistin sulfate (CS) (40 mg/kg at 0, 6, and 24 h). Data are presented as the mean ± SD (n = 4). +, plasma concentrations were below the lower limit of quantification (8 pg/ml); #, n = 2 (as the remaining replicates were below 8 pg/ml).
DISCUSSION
Due to the unique TJ structure exhibited by endothelial cells forming the BBB, the biochemical homeostasis within the CNS is well maintained (3). However, systemic inflammation and bacterial infections may cause disruption of the BBB TJs. The subsequent BBB hyperpermeability may potentially lead to neurotoxicity, given that the brain exposure to normally impenetrable compounds may be increased (20). For example, systemic challenge with LPS or lambda-carrageenan leads to enhanced brain uptake of BBB integrity markers, such as [125I]albumin and [14C]sucrose (5, 26). Similarly, we have assessed the impact of systemic inflammation on the CNS exposure of the poorly permeable antibiotic colistin (27) and have shown increased brain uptake following repeated i.p. injections of LPS from S. enterica (9). This suggested that the brain uptake of this antibiotic may also be enhanced during bacterial infection; however, with bacteremia caused by P. aeruginosa, BBB integrity and brain uptake of colistin remained unaltered in mice (15).
In order to address whether the disparity resulted from the differences between the bacterial species, the effect of LPS from P. aeruginosa on BBB dynamics was assessed. After being administered in the same dosing regimen used for LPS from S. enterica (i.e., 3 mg/kg at 0, 6, and 24 h) (15), the brain uptake of [14C]sucrose and colistin in mice treated with P. aeruginosa LPS was not different from that in saline-treated mice. In contrast, significantly higher [14C]sucrose and colistin B:P ratios (P < 0.05) were observed in LPS (S. enterica)-treated mice (9). We did not reassess the effects of S. enterica LPS on the brain uptake of [14C]sucrose or colistin in the current study, as previous results in our laboratory have demonstrated the effects of S. enterica LPS to be quite reproducible (with a coefficient of variation of 12.2% over 24 replicates) and consistent with reports from other laboratories (28, 29). The lack of effect of P. aeruginosa LPS is consistent with our previous observations demonstrating that inoculation of P. aeruginosa did not affect the paracellular integrity of the BBB (15), despite the plasma concentrations of IL-6 and TNF-α being substantially increased with both P. aeruginosa inoculation and P. aeruginosa LPS administration. These data point to the possibility that increases in the plasma concentrations of these cytokines may not be related to BBB disruption. This is further supported by the observations that plasma concentrations of TNF-α and IL-6 in P. aeruginosa LPS-treated mice were not significantly different (P > 0.05) from those in mice treated with S. enterica LPS (15), an LPS which we previously demonstrated induces significant BBB disruption. This is an interesting observation, given that these are the main cytokines involved in immune responses to infections (30) and have been reported to decrease the integrity of the BBB (11). Moreover, the fact that colistin was not able to decrease the plasma concentrations of TNF-α and IL-6 induced by S. enterica LPS but still ameliorate the BBB-disrupting effect of S. enterica LPS further supports the notion that S. enterica LPS may be causing a direct BBB-disrupting effect independently of TNF-α and IL-6. The results may appear to be in contrast to those of other studies showing that altered BBB integrity was observed in brain endothelial cells and animals following treatment with cytokines such as TNF-α, IL-1β, and IL-6 (11, 12). However, the BBB-disrupting effect of cytokines in those studies was observed only at concentrations far exceeding those that we have measured in vivo with both LPS administration and bacterial infection (10, 31). Therefore, the BBB-disrupting effects of cytokines observed in those studies are likely not to be a reflection of the effects observed in our in vivo studies.
It has been well reported that LPS can affect the integrity of the BBB and alter the paracellular permeability through binding to receptors expressed at the BBB, such as Toll-like receptor 4 (TLR4) or CD14 (32, 33). Several cytoplasmic signaling molecules, such as RhoA, nuclear factor kappa B, phosphoinositide 3-kinase kinase, myosin light chain kinase, protein kinase C, and mitogen-activated protein kinase, have been suggested to be involved in this process, either directly or indirectly via the rearrangement of the actin cytoskeleton (8, 18, 21). Given our in vivo findings that LPS could affect BBB paracellular function in a species-dependent manner, we assessed whether P. aeruginosa and S. enterica LPSs exhibited differences in their ability to alter TJ expression in a relevant in vitro BBB model. Recently generated by immortalizing primary human brain endothelial cells (34), the hCMEC/D3 cells show a morphology that closely resembles that of primary cells in culture, and they form confluent monolayers that express important in vivo BBB characteristics, such as TJ proteins (35). While this model does not serve as a direct model of the mouse BBB, characteristics of this cell line similar to those of mouse brain endothelial cells allow it to act as a sufficient model to corroborate the findings of our in vivo mouse brain uptake studies (36–38). As studies have shown that the level of LPS in the general circulation represents approximately 10% of the injected i.p. dose (25), the estimated plasma concentration of LPS in our animal studies following i.p. administration of a dose of 3 mg/kg was expected to be approximately 15 μg/ml. This concentration may be slightly higher than the concentrations of LPS observed in patients with severe sepsis; however, laboratory animals appear to be relatively insensitive to LPS, thus requiring higher doses to result in a systemic infectious state (39), and doses at the mg/kg level are not unusual (40). Therefore, with our doses of LPS administered to mice and the expected plasma concentrations of LPS from such doses, LPS concentrations ranging from 3.75 to 30 μg/ml were chosen to measure the impact of different LPS species on claudin-5 expression in the cell culture model. The results demonstrated that while S. enterica LPS reduced the expression of claudin-5 at a relatively low concentration, the concentration of P. aeruginosa LPS required to decrease claudin-5 expression was approximately four times higher. Our findings from the in vitro studies corroborate the results from our in vivo studies suggesting that S. enterica LPS exhibits a greater ability to decrease TJ function at the BBB.
The first step required for LPS to exert its direct BBB-disrupting effect is through binding to LPS receptors at the BBB, mainly mediated by the lipid A component of LPS (41). Therefore, the reason for the difference in the specific BBB-disrupting effect of S. enterica LPS relative to that of P. aeruginosa LPS may be differences in their structures, especially the lipid A component of LPS. Indeed, LPSs from different bacterial species exhibit varied biological activities. Zughaier et al. have shown that LPS from Escherichia coli has a TNF-α-inducing activity ∼8-fold greater than that of LPS from P. aeruginosa (42). It is interesting to note that lipid A, which adopts a conical conformation, is more active in inducing cytokine production through binding to receptors than endotoxins that exhibit a cylindrical molecular shape (43). There is evidence suggesting that lipid A of LPS from S. enterica exhibits a conical shape (44), while lipid A of LPS from P. aeruginosa exhibits a cylindrical shape (45). Moreover, lengths of lipid A side chains of 12 to 14 carbons have been shown to be optimal for stimulating efficient immunological responses (46, 47). Most side chains of lipid A from P. aeruginosa LPS contain 12 or even fewer carbons, whereas there are 14 carbon atoms in most side chains of lipid A from S. enterica LPS (48). Given these conformational and carbon-length characteristics of S. enterica LPS, which are desirable for LPS receptor binding, it is likely that LPS from S. enterica may bind to its receptors at the BBB more effectively than LPS from P. aeruginosa, triggering downstream signaling pathways leading to greater BBB disruption.
Given that colistin is able to bind to LPS and, indeed, that this is the initial step of its antibacterial effect (49), we investigated whether the BBB-disruptive effects of LPS from S. enterica could be ameliorated by coadministration of colistin. Treatment of hCMEC/D3 cells with S. enterica LPS led to a significant reduction in claudin-5 expression. However, when cotreated with colistin at 30 μg/ml, a concentration similar to that which we used in mice (9), the claudin-5-reducing effect of LPS was prevented. It should be noted that alterations of BBB function are not always accompanied by changes in the endothelial expression of TJ proteins (50, 51) and the increased expression of claudin-5 may not necessarily correlate with improved BBB function. Therefore, it was necessary to determine whether a similar effect would be observed in vivo. Thus, the BBB penetration of [14C]sucrose in mice was assessed under conditions involving a range of different pretreatments. The brain uptake of [14C]sucrose in mice treated with colistin alone did not differ from that in saline-treated mice, suggesting that repeated subcutaneous administrations of colistin did not affect BBB integrity. An opening of the BBB paracellular route was observed in S. enterica LPS-treated mice, as the B:P ratio of [14C]sucrose increased significantly; however, coadministration of colistin with LPS restored the BBB integrity, indicating that colistin negated the LPS-disruptive effect in vivo. The amelioration of the BBB-disrupting effects of S. enterica LPS induced by colistin may result from a direct binding of lipid A to colistin. As mentioned above, the lipid A component is the moiety of LPS responsible for binding to receptors and triggering the downstream signaling pathways responsible for the damage of TJ function (33, 52). Therefore, the avid binding of colistin to the lipid A portion of LPS (53) may decrease the free concentration of circulating LPS available to the receptors (i.e., TLR4), thus preventing the ability of LPS to decrease the BBB expression of claudin-5 in vivo. This observation may suggest a new potential therapeutic role for colistin, namely, prevention of the LPS-induced BBB disruption that is observed in various disorders such as sepsis.
Conclusion.
The ability of LPS to induce BBB disruption in vitro and in vivo has been demonstrated to be species dependent. Moreover, colistin has the ability to reduce the LPS-mediated BBB paracellular disruption potentially observed with Gram-negative bacterium-induced bacteremia.
ACKNOWLEDGMENTS
We thank Pierre-Olivier Couraud for kindly providing the hCMEC/D3 cells.
R. L. Nation and J. Li are supported by research grants from the National Institute of Allergy and Infectious Diseases (R01AI07896, R01AI079330, and R01AI098771). J. Li is an Australian National Health and Medical Research Council Senior Research Fellow.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Footnotes
Published ahead of print 24 June 2013
REFERENCES
- 1.Redzic Z. 2011. Molecular biology of the blood-brain and the blood-cerebrospinal fluid barriers: similarities and differences. Fluids Barriers CNS 8:3–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. 2003. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J. Cell Biol. 161:653–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonzalez-Mariscal L, Betanzos A, Nava P, Jaramillo BE. 2003. Tight junction proteins. Prog. Biophys. Mol. Biol. 81:1–44 [DOI] [PubMed] [Google Scholar]
- 4.Wolburg H, Lippoldt A. 2002. Tight junctions of the blood-brain barrier: development, composition and regulation. Vascul. Pharmacol. 38:323–337 [DOI] [PubMed] [Google Scholar]
- 5.McCaffrey G, Seelbach MJ, Staatz WD, Nametz N, Quigley C, Campos CR, Brooks TA, Davis TP. 2008. Occludin oligomeric assembly at tight junctions of the blood-brain barrier is disrupted by peripheral inflammatory hyperalgesia. J. Neurochem. 106:2395–2409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paul R, Lorenzl S, Koedel U, Sporer B, Vogel U, Frosch M, Pfister HW. 1998. Matrix metalloproteinases contribute to the blood-brain barrier disruption during bacterial meningitis. Ann. Neurol. 44:592–600 [DOI] [PubMed] [Google Scholar]
- 7.Tsao N, Hsu HP, Wu CM, Liu CC, Lei HY. 2001. Tumour necrosis factor-alpha causes an increase in blood-brain barrier permeability during sepsis. J. Med. Microbiol. 50:812–821 [DOI] [PubMed] [Google Scholar]
- 8.Dohgu S, Fleegal-DeMotta MA, Banks WA. 2011. Lipopolysaccharide-enhanced transcellular transport of HIV-1 across the blood-brain barrier is mediated by luminal microvessel IL-6 and GM-CSF. J. Neuroinflammation 8:167–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin L, Li J, Nation RL, Nicolazzo JA. 2011. Impact of P-glycoprotein inhibition and lipopolysaccharide administration on blood-brain barrier transport of colistin in mice. Antimicrob. Agents Chemother. 55:502–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abraham CS, Deli MA, Joo F, Megyeri P, Torpier G. 1996. Intracarotid tumor necrosis factor-alpha administration increases the blood-brain barrier permeability in cerebral cortex of the newborn pig: quantitative aspects of double-labelling studies and confocal laser scanning analysis. Neurosci. Lett. 208:85–88 [DOI] [PubMed] [Google Scholar]
- 11.de Vries HE, Blom-Roosemalen MC, van Oosten M, de Boer AG, van Berkel TJ, Breimer DD, Kuiper J. 1996. The influence of cytokines on the integrity of the blood-brain barrier in vitro. J. Neuroimmunol. 64:37–43 [DOI] [PubMed] [Google Scholar]
- 12.Farkas G, Marton J, Nagy Z, Mandi Y, Takacs T, Deli MA, Abraham CS. 1998. Experimental acute pancreatitis results in increased blood-brain barrier permeability in the rat: a potential role for tumor necrosis factor and interleukin 6. Neurosci. Lett. 242:147–150 [DOI] [PubMed] [Google Scholar]
- 13.Deli MA, Descamps L, Dehouck MP, Cecchelli R, Joo F, Abraham CS, Torpier G. 1995. Exposure of tumor necrosis factor-alpha to luminal membrane of bovine brain capillary endothelial cells cocultured with astrocytes induces a delayed increase of permeability and cytoplasmic stress fiber formation of actin. J. Neurosci. Res. 41:717–726 [DOI] [PubMed] [Google Scholar]
- 14.Saija A, Princi P, Lanza M, Scalese M, Aramnejad E, De Sarro A. 1995. Systemic cytokine administration can affect blood-brain barrier permeability in the rat. Life Sci. 56:775–784 [DOI] [PubMed] [Google Scholar]
- 15.Jin L, Li J, Nation RL, Nicolazzo JA. 2012. Effect of systemic infection induced by Pseudomonas aeruginosa on the brain uptake of colistin in mice. Antimicrob. Agents Chemother. 56:5240–5246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dohgu S, Banks WA. 2008. Lipopolysaccharide-enhanced transcellular transport of HIV-1 across the blood-brain barrier is mediated by the p38 mitogen-activated protein kinase pathway. Exp. Neurol. 210:740–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veszelka S, Pasztoi M, Farkas AE, Krizbai I, Ngo TK, Niwa M, Abraham CS, Deli MA. 2007. Pentosan polysulfate protects brain endothelial cells against bacterial lipopolysaccharide-induced damages. Neurochem. Int. 50:219–228 [DOI] [PubMed] [Google Scholar]
- 18.He F, Peng J, Deng XL, Yang LF, Wu LW, Zhang CL, Yin F. 2011. RhoA and NF-kappaB are involved in lipopolysaccharide-induced brain microvascular cell line hyperpermeability. Neuroscience 188:35–47 [DOI] [PubMed] [Google Scholar]
- 19.Boveri M, Kinsner A, Berezowski V, Lenfant AM, Draing C, Cecchelli R, Dehouck MP, Hartung T, Prieto P, Bal-Price A. 2006. Highly purified lipoteichoic acid from gram-positive bacteria induces in vitro blood-brain barrier disruption through glia activation: role of pro-inflammatory cytokines and nitric oxide. Neuroscience 137:1193–1209 [DOI] [PubMed] [Google Scholar]
- 20.Xaio H, Banks WA, Niehoff ML, Morley JE. 2001. Effect of LPS on the permeability of the blood-brain barrier to insulin. Brain Res. 896:36–42 [DOI] [PubMed] [Google Scholar]
- 21.Schreibelt G, Kooij G, Reijerkerk A, van Doorn R, Gringhuis SI, van der Pol S, Weksler BB, Romero IA, Couraud PO, Piontek J, Blasig IE, Dijkstra CD, Ronken E, de Vries HE. 2007. Reactive oxygen species alter brain endothelial tight junction dynamics via RhoA, PI3 kinase, and PKB signaling. FASEB J. 21:3666–3676 [DOI] [PubMed] [Google Scholar]
- 22.Afonso PV, Ozden S, Cumont MC, Seilhean D, Cartier L, Rezaie P, Mason S, Lambert S, Huerre M, Gessain A, Couraud PO, Pique C, Ceccaldi PE, Romero IA. 2008. Alteration of blood-brain barrier integrity by retroviral infection. PLoS Pathog. 4:e1000205. 10.1371/journal.ppat.1000205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mares J, Kumaran S, Gobbo M, Zerbe O. 2009. Interactions of lipopolysaccharide and polymyxin studied by NMR spectroscopy. J. Biol. Chem. 284:11498–11506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin L, Li J, Nation RL, Nicolazzo JA. 2009. Brain penetration of colistin in mice assessed by a novel high-performance liquid chromatographic technique. Antimicrob. Agents Chemother. 53:4247–4251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lenczowski MJ, Van Dam AM, Poole S, Larrick JW, Tilders FJ. 1997. Role of circulating endotoxin and interleukin-6 in the ACTH and corticosterone response to intraperitoneal LPS. Am. J. Physiol. 273:R1870–R1877 [DOI] [PubMed] [Google Scholar]
- 26.Nonaka N, Shioda S, Banks WA. 2005. Effect of lipopolysaccharide on the transport of pituitary adenylate cyclase activating polypeptide across the blood-brain barrier. Exp. Neurol. 191:137–144 [DOI] [PubMed] [Google Scholar]
- 27.Bergen PJ, Landersdorfer CB, Zhang J, Zhao M, Lee HJ, Nation RL, Li J. 2012. Pharmacokinetics and pharmacodynamics of ‘old' polymyxins: what is new? Diagn. Microbiol. Infect. Dis. 74:213–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salkeni MA, Lynch JL, Otamis-Price T, Banks WA. 2009. Lipopolysaccharide impairs blood-brain barrier P-glycoprotein function in mice through prostaglandin- and nitric oxide-independent pathways. J. Neuroimmune Pharmacol. 4:276–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jaeger LB, Dohgu S, Sultana R, Lynch JL, Owen JB, Erickson MA, Shah GN, Price TO, Fleegal-Demotta MA, Butterfield DA, Banks WA. 2009. Lipopolysaccharide alters the blood-brain barrier transport of amyloid beta protein: a mechanism for inflammation in the progression of Alzheimer's disease. Brain Behav. Immun. 23:507–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kielian T, Mayes P, Kielian M. 2002. Characterization of microglial responses to Staphylococcus aureus: effects on cytokine, costimulatory molecule, and Toll-like receptor expression. J. Neuroimmunol. 130:86–99 [DOI] [PubMed] [Google Scholar]
- 31.Desai TR, Leeper NJ, Hynes KL, Gewertz BL. 2002. Interleukin-6 causes endothelial barrier dysfunction via the protein kinase C pathway. J. Surg. Res. 104:118–123 [DOI] [PubMed] [Google Scholar]
- 32.Quan N, He L, Lai W. 2003. Endothelial activation is an intermediate step for peripheral lipopolysaccharide induced activation of paraventricular nucleus. Brain Res. Bull. 59:447–452 [DOI] [PubMed] [Google Scholar]
- 33.Singh AK, Jiang Y. 2004. How does peripheral lipopolysaccharide induce gene expression in the brain of rats? Toxicology 201:197–207 [DOI] [PubMed] [Google Scholar]
- 34.Weksler BB, Subileau EA, Perriere N, Charneau P, Holloway K, Leveque M, Tricoire-Leignel H, Nicotra A, Bourdoulous S, Turowski P, Male DK, Roux F, Greenwood J, Romero IA, Couraud PO. 2005. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 19:1872–1874 [DOI] [PubMed] [Google Scholar]
- 35.Poller B, Gutmann H, Krahenbuhl S, Weksler B, Romero I, Couraud PO, Tuffin G, Drewe J, Huwyler J. 2008. The human brain endothelial cell line hCMEC/D3 as a human blood-brain barrier model for drug transport studies. J. Neurochem. 107:1358–1368 [DOI] [PubMed] [Google Scholar]
- 36.Kooijmans SA, Senyschyn D, Mezhiselvam MM, Morizzi J, Charman SA, Weksler B, Romero IA, Couraud PO, Nicolazzo JA. 2012. The involvement of a Na+- and Cl−-dependent transporter in the brain uptake of amantadine and rimantadine. Mol. Pharm. 9:883–893 [DOI] [PubMed] [Google Scholar]
- 37.Vu K, Weksler B, Romero I, Couraud PO, Gelli A. 2009. Immortalized human brain endothelial cell line HCMEC/D3 as a model of the blood-brain barrier facilitates in vitro studies of central nervous system infection by Cryptococcus neoformans. Eukaryot. Cell 8:1803–1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu ZH, Hofman FM, Zlokovic BV. 2003. A simple method for isolation and characterization of mouse brain microvascular endothelial cells. J. Neurosci. Methods 130:53–63 [DOI] [PubMed] [Google Scholar]
- 39.Copeland S, Warren HS, Lowry SF, Calvano SE, Remick D. 2005. Acute inflammatory response to endotoxin in mice and humans. Clin. Diagn. Lab. Immunol. 12:60–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nemzek JA, Hugunin KM, Opp MR. 2008. Modeling sepsis in the laboratory: merging sound science with animal well-being. Comp. Med. 58:120–128 [PMC free article] [PubMed] [Google Scholar]
- 41.Dunn-Siegrist I, Tissieres P, Drifte G, Bauer J, Moutel S, Pugin J. 2012. Toll-like receptor activation of human cells by synthetic triacylated lipid A-like molecules. J. Biol. Chem. 287:1621–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zughaier SM, Ryley HC, Jackson SK. 1999. Lipopolysaccharide (LPS) from Burkholderia cepacia is more active than LPS from Pseudomonas aeruginosa and Stenotrophomonas maltophilia in stimulating tumor necrosis factor alpha from human monocytes. Infect. Immun. 67:1505–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Netea MG, van Deuren M, Kullberg BJ, Cavaillon JM, Van der Meer JW. 2002. Does the shape of lipid A determine the interaction of LPS with Toll-like receptors? Trends Immunol. 23:135–139 [DOI] [PubMed] [Google Scholar]
- 44.Schromm AB, Brandenburg K, Loppnow H, Moran AP, Koch MH, Rietschel ET, Seydel U. 2000. Biological activities of lipopolysaccharides are determined by the shape of their lipid A portion. Eur. J. Biochem. 267:2008–2013 [DOI] [PubMed] [Google Scholar]
- 45.Furchtgott L, Wingreen NS, Huang KC. 2011. Mechanisms for maintaining cell shape in rod-shaped Gram-negative bacteria. Mol. Microbiol. 81:340–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller SI, Ernst RK, Bader MW. 2005. LPS, TLR4 and infectious disease diversity. Nat. Rev. Microbiol. 3:36–46 [DOI] [PubMed] [Google Scholar]
- 47.Somerville JE, Jr, Cassiano L, Darveau RP. 1999. Escherichia coli msbB gene as a virulence factor and a therapeutic target. Infect. Immun. 67:6583–6590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ogawa T, Asai Y, Makimura Y, Tamai R. 2007. Chemical structure and immunobiological activity of Porphyromonas gingivalis lipid A. Front. Biosci. 12:3795–3812 [DOI] [PubMed] [Google Scholar]
- 49.Davis SD, Iannetta A, Wedgwood RJ. 1971. Activity of colistin against Pseudomonas aeruginosa: inhibition by calcium. J. Infect. Dis. 124:610–612 [DOI] [PubMed] [Google Scholar]
- 50.Kis B, Snipes JA, Deli MA, Abraham CS, Yamashita H, Ueta Y, Busija DW. 2003. Chronic adrenomedullin treatment improves blood-brain barrier function but has no effects on expression of tight junction proteins. Acta Neurochir. Suppl. 86:565–568 [DOI] [PubMed] [Google Scholar]
- 51.Simpson JE, Wharton SB, Cooper J, Gelsthorpe C, Baxter L, Forster G, Shaw PJ, Savva G, Matthews FE, Brayne C, Ince PG. 2010. Alterations of the blood-brain barrier in cerebral white matter lesions in the ageing brain. Neurosci. Lett. 486:246–251 [DOI] [PubMed] [Google Scholar]
- 52.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. 1999. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the LPS gene product. J. Immunol. 162:3749–3752 [PubMed] [Google Scholar]
- 53.Conly J, Johnston B. 2006. Colistin: the phoenix arises. Can. J. Infect. Dis. Med. Microbiol. 17:267–269 [DOI] [PMC free article] [PubMed] [Google Scholar]