Abstract
The prevalence of heterogeneous vancomycin-intermediate Staphylococcus aureus (hVISA) infections varies in the literature, a problem complicated by the lack of routine screening procedures; however, limited data suggest that hVISA has been associated with persistent bloodstream infections (BSI) and vancomycin failure, yet these studies have been confounded by design issues. We conducted this study to compare the characteristics of patients with BSI caused by hVISA with those with vancomycin-susceptible Staphylococcus aureus (VSSA) treated with vancomycin. This retrospective, multicenter matched (1:1) cohort study compared the clinical characteristics and outcomes of hVISA and VSSA. Patients with hVISA methicillin-resistant Staphylococcus aureus (MRSA) BSI from 2004 to 2012 were matched to VSSA-MRSA BSI patients. The primary outcome was failure of vancomycin treatment, defined as a composite of persistent bacteremia (≥7 days), persistent signs and symptoms, change of MRSA antibiotic, recurrent BSI, or MRSA-related mortality. We identified 122 matched cases. The overall vancomycin failure rate was 57% (82% hVISA versus 33% VSSA; P < 0.001). The individual components of failure in hVISA versus VSSA were persistent bacteremia, 59% versus 21% (P < 0.001); change in MRSA therapy, 54% versus 25% (P = 0.001); MRSA-related mortality, 21% versus 10% (P = 0.081); and recurrence of BSI, 26% versus 2% (P < 0.001). Using logistic regression analysis and adjusting for covariates, hVISA (adjusted odds ratio [aOR], 11.1; 95% confidence interval [CI], 4.3 to 28.7) and intensive care unit (ICU) admission (aOR, 4.5; 95% CI, 1.8 to 11.6) were still independently associated with vancomycin failure. Relative to VSSA BSI, patients with hVISA were more likely to experience failure of vancomycin treatment, including persistent bacteremia and recurrence. Our results indicate that hVISA was responsible for considerable morbidity.
INTRODUCTION
Methicillin-resistant Staphylococcus aureus (MRSA) infections continue to pose a dilemma in the hospital environment as well as in the community setting (1, 2). Vancomycin has been the primary treatment for infections caused by MRSA; however, reduced susceptibility to vancomycin has emerged in vancomycin-intermediate S. aureus (VISA) and vancomycin-resistant S. aureus (VRSA) as well as heterogeneous vancomycin-intermediate S. aureus (hVISA), compromising the utility of this antibiotic (3–5). In previous studies, the prevalence of the hVISA phenotype has ranged from 0 to 29.2% of MRSA isolates. Several factors may influence the wide variance in prevalence, including geographical area, site of infection, and screening/detection methods (macro-Etest versus population analysis profile) (6–11). In contrast to VISA or VRSA strains, hVISA strains are often undetected since these organisms are considered vancomycin susceptible on the basis of traditional MIC testing performed by clinical microbiology laboratories (12–14). The gold standard for detecting hVISA is the modified population analysis profile (mPAP), referencing the area under the curve (AUC) of the test strain to hVISA control strain Mu3 (15). Due to the labor-intensive nature of the PAP method and the lack of standardization by the Clinical and Laboratory Standards Institute (CLSI), it is not routinely performed as a screening/detection method for hVISA. The inability to detect subpopulations with reduced vancomycin susceptibility in routine practice makes accurate estimation of the prevalence and clinical impact of hVISA difficult to establish.
Understanding and managing patients with hVISA is a major therapeutic challenge, because it has been shown that patients with hVISA infections are likely to fail therapy with vancomycin (14, 16, 17). A recent systematic review and meta-analysis evaluated the significance of hVISA and reported a 2.37-fold-increased risk of glycopeptide failure (95% confidence interval [CI], 1.53 to 3.67) compared to vancomycin-susceptible S. aureus (VSSA) (8). This is even more concerning considering that hVISA has been linked to high bacterial load infections, prolonged fever and bacteremia, and increased hospital length of stay (10, 18, 19). Other clinical characteristics that have been correlated with hVISA are elevated vancomycin MICs (e.g., >1 mg/liter) and prior vancomycin use (6, 17, 19, 20).
Prior studies have associated hVISA with poorer outcomes; however, these studies differed in detection methods and outcome analysis, making it difficult to determine the true significance of hVISA. Further, the severity of illness and frequency of high-risk infection sources among hVISA patients complicated the ability to directly compare their outcomes to those of patients with VSSA infections. Therefore, we investigated the morbidity and mortality of a multicenter cohort of patients with bloodstream infection (BSI) caused by hVISA and compared the results to those of patients with VSSA treated with vancomycin by matching patients according to infection source. A secondary aim was to correlate success or failure on the basis of organism characteristics such as vancomycin susceptibility including heteroresistance, MIC, genetic polymorphism (agr and staphylococcal cassette mec [SCCmec] type), delta-hemolysin activity, and vancomycin trough concentrations in serum.
(An abstract containing results from this study was presented in platform format at the 52nd Interscience Conference on Antimicrobial Agents and Chemotherapy [ICAAC] in San Francisco, CA, on 10 September 2012.)
MATERIALS AND METHODS
Study design and population.
This was a retrospective, multicenter (the Detroit Medical Center, the Ohio State University Medical Center, the Albany Medical Center, the University of Pittsburgh Medical Center, and the Veterans Affairs Medical Center in Providence, RI), matched (1:1) cohort study comparing the outcomes of hVISA infections versus VSSA infections in patients with MRSA BSI treated with vancomycin from January 2004 to August 2012. The institutional review board at each of the participating medical centers approved the study. MRSA BSI patients were defined as patients with MRSA in blood cultures that met the Centers for Disease Control and Prevention (CDC) criteria for primary bloodstream infection (21). Adult patients aged ≥18 years identified from clinical blood cultures positive for MRSA with susceptible vancomycin MIC (≤2 mg/liter) and who received vancomycin for ≥72 h were eligible for inclusion in this study. Patients with hVISA were matched 1:1 to VSSA patients based on age group (<50 years, 50 to 70 years, and >70 years), site of infection (including concomitant site of MRSA infection), and year of infection ± 2 years.
Microbiological and molecular data.
For patients with multiple MRSA blood cultures, the first MRSA isolate for each patient was obtained for extensive microbiology evaluation at the Anti-Infective Research Laboratory (Detroit, MI). Vancomycin MIC was determined by broth microdilution performed in agreement with CLSI guidelines using the current susceptible breakpoint of ≤2 mg/liter and the Etest method according to the manufacturer's instructions (bioMérieux, Durham, NC) (22).
A random selection of 1,249 MRSA bloodstream isolates from all involved institutions were initially screened using the Macro Etest method described by Wootton and colleagues (15, 23) and confirmed by vancomycin mPAP. Vancomycin mPAP was determined on all strains at an inoculum of approximately 1 × 108 CFU/ml. Fifty microliters of this suspension was plated on vancomycin containing BHI at increasing concentrations (0, 0.5, 1, 1.5, 2, 3, 4, and 8 mg/liter) using an automated spiral dispenser (Whitley Automated Spiral Plater, Bon Whitley Scientific, West Yorkshire, United Kingdom). After incubation at 35°C for 48 h, colony counts (log10 CFU/ml) were determined using a laser colony counter (ProtoCOL; Synbiosis, Cambridge, United Kingdom). The AUC was determined for each test isolate and compared to the AUC of the reference strain, Mu3 (ATCC 700698). The test isolate was considered an hVISA or VSSA if the population analysis profile area under the curve (PAP-AUC) ratio of the test isolate to Mu3 was ≥0.9 or <0.9, respectively, as previously described by Wootton and colleagues (6, 15).
The staphylococcal cassette mec (SCCmec) type and the agr group for the isolates were characterized using multiplex PCR, as described elsewhere (24, 25). The expression of the agr gene cluster was determined by quantitating delta-hemolysin production utilizing a previously described method by cross-streaking test strains perpendicular to RN4220 as previously described (26).
Data collection.
Patient data collection included baseline demographics such as age, sex, comorbidities, hospitalization history within 180 days before admission, receipt of prior antibiotic therapy (>48 h in the previous 30 days), use of concomitant antibiotics, concurrent sites of MRSA infection, acute physiology and chronic health evaluation II (APACHE II) score, and Charlson comorbidity index at the first positive blood culture. Additional data collected were duration of bacteremia, pharmacokinetic variables (dosing and vancomycin concentrations in serum), length of hospital stay, and infection outcomes including duration of signs and symptoms and 30-day mortality. Calculation of vancomycin AUC/MIC was estimated from the daily dose given during the first 24 h of therapy divided by clearance derived from previous literature (27). The concomitant site with MRSA infection was determined by assessment of other MRSA-positive cultures at the time of onset of MRSA and/or clinical description by the treating physician in the medical record.
Outcome assessment.
For the purposes of data analysis, patients were stratified into 2 groups according to the presence of hVISA (hVISA versus non-hVISA [VSSA]). Thirty-day MRSA-related mortality, time to clearance of MRSA from the bloodstream, resolution of signs and symptoms of infection, and length of hospital stay after the first positive blood culture were assessed. The length of hospital stay was defined as the number of days after the onset of infection. Failure of vancomycin treatment was defined by the following criteria: (i) nonresolving bacteremia for at least 7 days while on vancomycin (17, 20, 28); (ii) new onset or worsening of signs or symptoms of infection while on vancomycin therapy (7, 28); (iii) either change of MRSA intravenous antibiotic or addition to vancomycin of a second antimicrobial agent targeted against MRSA, necessitated by lack of resolution (2, 29, 30); and (iv) recurrent BSI defined as a positive blood culture for MRSA within 60 days of completion of antimicrobial therapy (30). Death was considered to be attributed to MRSA infection as described previously (28, 31) if one of the following criteria was present: (i) blood cultures were positive for MRSA at the time of death, (ii) death occurred before resolution of signs and symptoms of MRSA infection, (iii) death occurred 14 days after the onset of MRSA without another explanation, (iv) autopsy findings indicated MRSA as a cause of death, and (v) MRSA was indicated as a cause of death on the death certificate. Length of hospital stay after the onset of infection was defined as the time from the first MRSA-positive culture until discharge or death (31).
Statistical analysis.
Categorical variables were compared by McNemar's test, continuous variables were compared by paired Student's t test, and ordinal variables were compared by the Wilcoxon signed rank test. Appropriateness of age matching was examined by paired Student's t test. Multivariable analyses were performed to determine the independent association of vancomycin treatment failure while adjusting for confounding variables. All variables significantly associated with the outcome on univariate analysis (P ≤ 0.2) were included in the explanatory multivariable model using a backward stepwise approach. All tests were two-tailed, and P values of <0.05 were considered to be statistically significant. SPSS Statistics, IBM SPSS software, version 20.0 (SPSS Inc., Chicago, IL), was used for all calculations.
RESULTS
We identified and included 61 matched pairs during the study period that met the criteria, for a total of 122 BSI. All 122 patients received vancomycin for initial treatment for MRSA BSI for at least 72 h, and the median duration of vancomycin treatment was 9 days (interquartile range [IQR], 5 to 14). The primary sources of infection for these cases were as follows: infective endocarditis, 48 patients (39.3%); pneumonia, 10 patients (8.2%); intravenous (IV) catheter-related infection, 30 patients (24.6%); bone and joint infections, 18 patients (14.8%); skin and soft tissue infection, 12 patients (9.8%); and unknown source, 4 patients (3.3%). Overall, 40 (32.8%) patients received source control, and there was no difference between hVISA and VSSA groups in source control: 32.8% versus 32.8%, respectively (P > 0.99). Within the specific primary source of infection, no statistical difference was found between hVISA and VSSA. There were no differences in baseline demographics and clinical characteristics of patients with hVISA and VSSA except for two characteristics (Table 1). Presence of hVISA was significantly associated with decubitus ulcers (P = 0.044) and a vancomycin MIC of >1 mg/liter by the Etest method (P = 0.038) compared to VSSA. However, there was no statistical difference between patients with hVISA and VSSA with respect to age, severity of illness (APACHE II), Charlson comorbidity index, or history of vancomycin use, hospitalization, and MRSA infection. Vancomycin concentrations in serum did not differ between the two groups, with median initial vancomycin trough concentrations in serum of 12.1 mg/liter (IQR, 6.8 to 18.3 mg/liter) and 11.7 mg/liter (IQR, 8.4 to 16 mg/liter) for the hVISA and VSSA groups, respectively (P = 0.790). The vancomycin MIC distribution determined by broth microdilution and Etest susceptibility methods and the hVISA frequency are presented in Fig. 1 and 2, respectively.
Table 1.
Baseline demographic and clinical features of MRSA associated with hVISA compared to VSSAa
| Feature | hVISA (n = 61) | VSSA (n = 61) | P value |
|---|---|---|---|
| Baseline demographics | |||
| Age (mean yr ± SD) | 57.5 ± 14.3 | 56.5 ± 16.7 | 0.745 |
| Weight (kg) | 72.6 (63–87.3) | 75 (67.9–85) | 0.278 |
| Creatinine clearance (ml/min) | 41.1 (18–82.1) | 55 (29.6–95.3) | 0.134 |
| APACHE II score | 12 (8–18) | 10 (7–15) | 0.251 |
| Charlson index | 3 (1–4) | 2 (1–4) | 0.404 |
| Female sex | 30 (49.2) | 21 (34.4) | 0.099 |
| ICU admission | 29 (47.5) | 23 (37.7) | 0.272 |
| Previous hospitalization | 32 (52.5) | 34 (55.7) | 0.716 |
| Previous surgery | 4 (6.6) | 10 (16.4) | 0.088 |
| Previous antibiotics | 30 (49.2) | 24 (39.3) | 0.274 |
| Previous VAN | 13 (21.3) | 11 (18) | 0.649 |
| Prior MRSA infection | 9 (14.8) | 8 (13.1) | 0.794 |
| Renal disease | 28 (45.9) | 26 (42.6) | 0.715 |
| Chronic kidney disease | 6 (9.8) | 8 (13.1) | 0.570 |
| Hemodialysis | 17 (27.9) | 10 (16.4) | 0.127 |
| Diabetes | 20 (32.8) | 16 (26.2) | 0.427 |
| Decubitus ulcer | 11 (18) | 3 (4.9) | 0.044 |
| Injection drug user | 17 (27.9) | 19 (31.1) | 0.691 |
| Prosthetic device/hardware | 4 (6.6) | 4 (6.6) | 1.00 |
| Clinical characteristics | |||
| VAN BMD MIC, 2 mg/liter | 18 (29.5) | 7 (11.7) | 0.015 |
| VAN Etest MIC, >1 mg/liter | 43 (71.7) | 32 (53.3) | 0.038 |
| VAN duration (days) | 8 (5–15) | 9 (5–15) | 0.640 |
| VAN initial trough (n = 109) | 12.1 (6.8–18.3) (n = 56) | 11.7 (8.4–16) (n = 53) | 0.790 |
| Initial VAN trough, ≥15 mg/liter | 20 (35.7) | 14 (26.4) | 0.295 |
| AUC/MIC | 445.4 (282.3–855) | 467 (344–728.4) | 0.605 |
| AUC/MIC, ≥400 | 34 (55.7) | 39 (63.9) | 0.356 |
| SCCmec type II | 28 (46.7) | 20 (33.3) | 0.136 |
| agr group II | 29 (48.3) | 28 (46.7) | 0.855 |
| agr dysfunctional activity | 11 (19.3) | 11 (19.3) | 1.000 |
| Clinical outcomes | |||
| Duration of bacteremia (days) | 7 (3–11) | 4 (2–7) | 0.003 |
| Duration of signs/symptoms (days) | 3 (1–10) | 2 (1–3) | 0.031 |
| Length of stay (days) | 24 (14–44.5) | 16 (9.5–30.5) | 0.022 |
| Length of stay after first positive blood culture (days) | 22 (12–39) | 13 (9–28) | 0.021 |
Values are followed by percentage in parentheses for categorical data, unless otherwise specified; median values are followed by the interquartile range in parentheses. BMD, broth microdilution; VAN, vancomycin; AUC, area under the concentration-time curve over 24 h.
Fig 1.
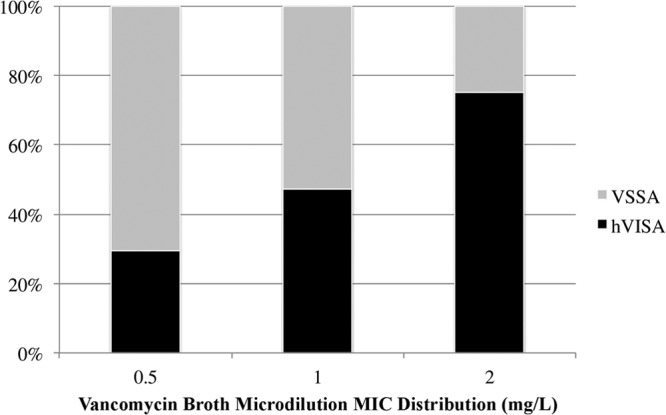
Vancomycin MIC distribution by the broth microdilution method and hVISA frequency for all MRSA isolates.
Fig 2.
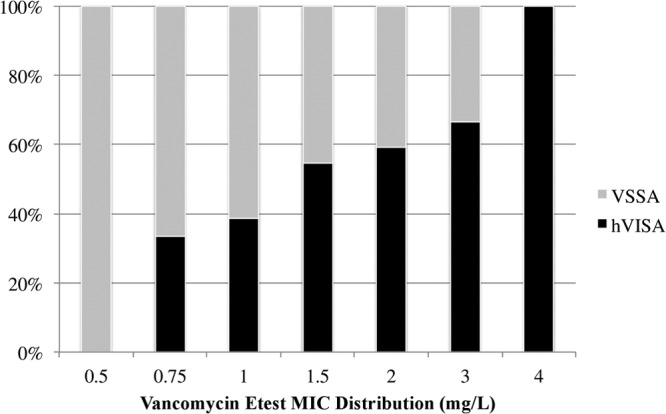
Vancomycin MIC distribution by the Etest method and hVISA frequency for all MRSA isolates.
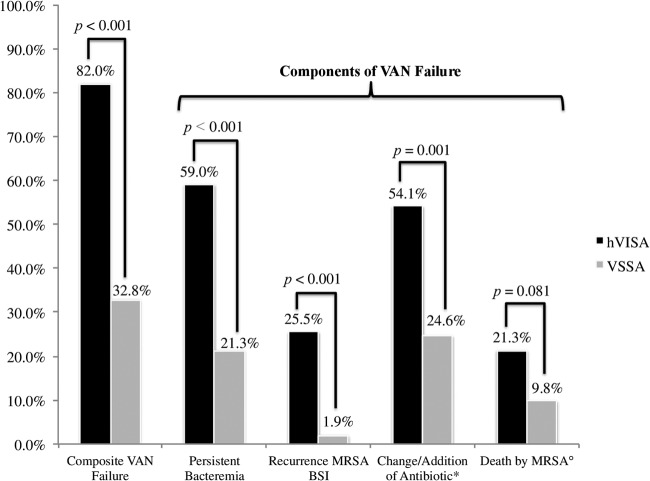
Overall vancomycin treatment failure was 57.3% (70/122), and patients with hVISA were associated with a significantly higher vancomycin treatment failure (82%) than patients with VSSA (32.8%; P < 0.001) (Fig. 3). Of interest, each criterion for vancomycin treatment failure was more common with hVISA: persistent bacteremia (59% versus 21.3%, P < 0.001), change or addition of anti-MRSA antibiotic (54.1% versus 24.6%, P = 0.001), and recurrence of MRSA BSI after 60 days from end of treatment (25.5% versus 1.9%, P < 0.001). Signs and symptoms were more prolonged, by an average of 1 day, in the hVISA group than in the VSSA group with a median of 3 days (IQR, 1 to 10) versus 2 days (IQR, 1 to 3), respectively (P = 0.031). Median duration of bacteremia was 3 days longer in the hVISA group than in the VSSA group: 7 days (IQR, 3 to 11.5) versus 4 days (IQR, 2 to 7), respectively (P = 0.003). Thirty-day MRSA infection-related mortality was doubled in the group with hVISA infection, yet its incidence was not statistically significant compared to VSSA-related mortality (21.3% versus 9.8%, respectively; P = 0.081). Likewise, no difference in all-cause 30-day mortality was detected between hVISA and VSSA patients (24.6% versus 11.5%, respectively; P = 0.076). Compared to VSSA, hVISA was associated with longer total hospital length of stay (median difference of 8 days, P = 0.022) as well as longer length of stay after the onset of infection (average difference of 9 days, P = 0.021). Overall, a total of 47 cases (38.5%) were treated with an alternative agent to vancomycin or with combination therapy. Of these, 33 cases (70.2%) switched to daptomycin as an alternative agent; 23 (48.9%) of these were in the hVISA group versus 10 (21.3%) in the VSSA group. The remaining alternative agents used were intravenous trimethoprim-sulfamethoxazole (4 cases, 8.5%), intravenous linezolid (5 cases, 10.6%), and gentamicin in combination with vancomycin (5 cases, 10.6%).
Fig 3.
Comparison of hVISA and VSSA for composite vancomycin treatment failure. VAN, vancomycin; MRSA, methicillin-resistant Staphylococcus aureus; BSI, bloodstream infection; *, change of MRSA intravenous antibiotic or addition to vancomycin of a second antimicrobial agent targeted against MRSA; °, 30-day mortality MRSA infection related.
Characteristics associated with vancomycin treatment failure in univariate and multivariable analyses are shown in Tables 2 and 3. No relationship with vancomycin treatment failure was identified, with initial vancomycin trough concentrations in serum of <15 mg/liter compared to ≥15 mg/liter, 60% versus 55.9%, respectively (P = 0.686). Additionally, there was no relationship of vancomycin treatment failure, with an AUC24/MIC (ratio of area under the concentration curve over 24 h divided by the MIC) of <400 compared to ≥400, 59.2% versus 56.2%, respectively, (P = 0.741). There was a higher frequency of hVISA and ICU admission among the patients with vancomycin treatment failure than of successful treatment in the univariate analyses. These covariates along with APACHE II score, SCCmec type II, renal disease, and high-risk infection source (31) (infective endocarditis, pneumonia, or bone and joint infection) were evaluated in the logistic regression model, and three covariates remained in the model. Heterogeneous vancomycin-intermediate Staphylococcus aureus (adjusted odds ratio [aOR], 11.1; 95% CI, 4.3 to 28.7) and intensive care unit admission (aOR, 4.5; 95% CI, 1.8 to 11.6) were independently associated with vancomycin treatment failure.
Table 2.
Univariate analysis of clinical features of MRSA associated with vancomycin treatment failurea
| Feature | Success (n = 52) | Failure (n = 70) | P value |
|---|---|---|---|
| Patient characteristics | |||
| Age (mean yr ± SD) | 56.7 ± 17.9 | 57.2 ± 13.5 | 0.868 |
| Weight (kg) | 75.3 (67.2–86.4) | 72.6 (63–86.8) | 0.303 |
| Creatinine clearance (ml/min) | 52.8 (28.1–93.9) | 49 (19.8–89) | 0.669 |
| APACHE II score | 10 (6–14.8) | 11.5 (8–18) | 0.174 |
| Charlson index | 2 (0.5–3) | 3 (1–5) | 0.090 |
| Female sex | 22 (42.3) | 29 (41.4) | 0.922 |
| ICU admission | 13 (25) | 39 (55.7) | 0.001 |
| Previous hospitalization | 27 (51.9) | 39 (55.7) | 0.678 |
| Previous surgery | 8 (15.4) | 6 (8.6) | 0.243 |
| Previous antibiotics | 22 (42.3) | 32 (45.7) | 0.708 |
| Previous VAN | 9 (17.3) | 15 (21.4) | 0.571 |
| Prior MRSA infection | 8 (15.4) | 9 (12.9) | 0.690 |
| Renal disease | 19 (36.5) | 35 (50) | 0.120 |
| Chronic kidney disease | 8 (15.4) | 6 (8.6) | 0.243 |
| Hemodialysis | 10 (19.2) | 17 (24.3) | 0.506 |
| Diabetes | 14 (26.9) | 22 (31.4) | 0.589 |
| Decubitus ulcer | 4 (7.7) | 10 (14.3) | 0.390 |
| Injection drug user | 15 (28.8) | 21 (30) | 0.890 |
| Prosthetic device/hardware | 2 (3.8) | 6 (8.6) | 0.464 |
| Infective endocarditis | 18 (34.6) | 30 (42.9) | 0.357 |
| Pneumonia | 3 (5.8) | 7 (10) | 0.400 |
| Bone or joint infection | 7 (13.5) | 11 (15.7) | 0.729 |
| High-risk infection groupb | 28 (53.8) | 48 (68.6) | 0.097 |
| MRSA isolate characteristics | |||
| hVISA | 11 (21.2) | 50 (71.4) | <0.001 |
| VAN BMD MIC, 2 mg/liter | 9 (17.6) | 16 (22.9) | 0.485 |
| VAN Etest MIC, >1 mg/liter | 29 (56.9) | 46 (66.7) | 0.273 |
| SCCmec type II | 16 (31.4) | 32 (46.4) | 0.097 |
| agr group II | 24 (47.1) | 33 (47.8) | 0.934 |
| agr dysfunctional activity | 7 (14.9) | 15 (22.7) | 0.300 |
| Clinical characteristics | |||
| VAN duration (days) | 9.5 (6–14) | 8 (4–15) | 0.226 |
| VAN initial trough (n = 109) | 11.7 (8.3–18) (n = 45) | 11.7 (7.8–16.6) (n = 64) | 0.638 |
| Initial VAN trough, ≥ 15 mg/liter | 15 (34.1) | 19 (29.7) | 0.628 |
| AUC/MIC | 446.9 (334.9–717.6) | 466.1 (289.3–853.5) | 0.979 |
| AUC/MIC, ≥400 | 32 (61.5) | 41 (58.6) | 0.741 |
| Length of stay (days) | 15.5 (9–29.8) | 24 (13–45) | 0.003 |
| Length of stay after first positive blood culture (days) | 11 (8–26.3) | 22 (13–42) | <0.001 |
Values are followed by percentage in parentheses for categorical data, unless otherwise specified; median values are followed by the interquartile range in parentheses. BMD, broth microdilution; VAN, vancomycin; AUC, area under the concentration-time curve over 24 h.
Infection caused by infective endocarditis, pneumonia, or bone and joint infection.
Table 3.
Logistic regression analysis of risk factors associated with vancomycin treatment failure
| Factor | Adjusted OR (95% CI) | P value |
|---|---|---|
| hVISA | 11.138 (4.316–28.742) | <0.001 |
| Admission to ICU | 4.507 (1.751–11.597) | 0.002 |
| High-risk infectiona | 2.528 (1.000–6.391) | 0.050 |
Infection caused by infective endocarditis, pneumonia, or bone and joint infection.
DISCUSSION
Our study implies that the odds of vancomycin treatment failure were 11 times greater for a patient with hVISA BSI than for a patient with VSSA BSI. Similar to what was reported in previous literature, vancomycin treatment failure was common in MRSA bacteremia and was more pronounced in patients infected with hVISA. Prior studies reported comparable failure proportions with a range from 68.4% to 100% of hVISA infections (10, 17, 32–34), using similar definitions of vancomycin treatment failure. The most frequently observed component of failure was persistent bacteremia, which was a commonly used criterion in other studies (16–18, 20, 33). Change of vancomycin to an alternative therapy (e.g., daptomycin) or addition of another anti-MRSA antibiotic (e.g., gentamicin) was also a common contributor to the vancomycin failure rate. This criterion of the composite for vancomycin treatment failure has been included in a previous study (29) but has not always been considered in the MRSA bacteremia literature. All of our 33 patients with hVISA and 13/15 patients with VSSA that changed in MRSA therapy were also deemed to have experienced failure of vancomycin treatment by persistent bacteremia. The remaining 2 VSSA cases that had a change in MRSA therapy occurred before 2008, indicating that the change may be due to an overall poor clinical response to vancomycin and not necessarily to an elevated high vancomycin MIC as noted in the recommendations of the 2011 MRSA clinical practice guidelines by the Infectious Diseases Society of America (2). Another important outcome in patients with hVISA for vancomycin treatment failure was recurrence of MRSA BSI 60 days from the end of therapy. The percentage of 60-day recurrence that we observed was similar to that reported by Lodise and colleagues (13%) in a single-center cohort of MRSA bacteremia (30). However, this is the first study that evaluated recurrence and found an association with hVISA infection.
Despite the association with prolonged bacteremia, prolonged symptoms, and recurrence, this study did not observe a difference in 30-day MRSA-related mortality associated with hVISA. The overall 30-day MRSA infection-related mortality in this group of MRSA BSI was 15.6%, which is lower than rates reported by other studies, which range from 17.3% to 28.4% (7, 17, 20). While patients with hVISA infection had a higher mortality rate than patients with VSSA, this did not reach statistical significance. Since hVISA is associated with factors known to influence mortality such as high vancomycin MIC, persistent bacteremia, and recurrence of BSI, one might expect an increased mortality compared to that of VSSA BSI. One explanation for the lack of a mortality difference in our study is that the sample size was chosen to detect a difference in vancomycin failure and not specifically infection-related mortality. However, the observed phenomenon may also be related to hVISA strain characteristics that contribute to persistence, rather than virulence. It has been suggested that hVISA and VISA strains have reduced virulence factors, leading to a decreased host immune response, resulting in reduced mortality but an increase in persistent infections (35, 36). van Hal and colleagues recently reported that the presence of hVISA is an independent predictor of survival, specifically in ST239 MRSA strains (19). In contrast, our current findings did not find hVISA to be correlated with survival. No molecular typing (e.g., spa typing or pulsed-field gel electrophoresis) was completed for this study, as the clonal relatedness of isolates is unlikely to have a direct influence on response to therapy; however, identification of specific MRSA clones would be advantageous for epidemiological purposes.
High vancomycin MIC has also been associated with hVISA in the literature (6, 19, 20) and is consistent with our findings indicating that the frequency of hVISA increases with vancomycin MIC by both broth microdilution and Etest methods. Our Etest method displayed statistical significance with patients with hVISA when MICs were greater than 1 mg/liter, analogous to the results of Musta and colleagues (20). Prior literature in MRSA bacteremia suggests that high vancomycin MIC is associated with treatment failure (37). However, our data indicated that a vancomycin Etest MIC of >1 mg/liter had higher treatment failure but the difference was not statistically significant (66.7% versus 56.9%; P = 0.273) compared to treatment success; therefore, these data may imply that hVISA BSI irrespective of vancomycin MIC may fail on vancomycin treatment. Patients with hVISA had similar proportions of having adequate vancomycin trough concentrations and an AUC24/MIC of >400. However, these patients still had a higher proportion of treatment failure. Rose and colleagues have previously demonstrated using in vitro pharmacokinetics and pharmacodynamics models that even high-dose vancomycin exposure (5 g IV every 12 h; AUC24/MIC for the free, unbounded fraction of vancomycin [ƒAUC24/MIC], 799) did not result in a significant impact on hVISA strains (38).
We controlled for potential confounding variables by matching in this retrospective multicenter cohort study; however, this study does have some limitations. First, all of these MRSA isolates were obtained from the first positive culture for screening and identification of hVISA by mPAP, which could overlook hVISA arising in subsequent cultures. Second, although the macro Etest method for screening for hVISA has been found to have a high specificity (39), it is possible that some hVISA strains may be misclassified as negative; however, all strains were confirmed by mPAP for matching purposes if the macro Etest initially identified the strain as VSSA or hVISA. It was not possible to complete the removal of the source of infection in all patients, and this confounding factor may affect our primary outcome since it was not controlled by our matching design. The small sample size precludes extensive analysis of confounding factors; nonetheless, this multicenter study is the first matched-cohort comparative study of hVISA confirmed by the gold standard of mPAP, representing a significant advancement in the available evidence (15). Finally, selection bias is a potential concern with clinical outcomes in retrospective studies when vancomycin susceptibility has been identified; however, clinicians were unaware of hVISA presence, thus not altering therapy or management during hospitalization.
In conclusion, the results propose that patients with MRSA BSI caused by hVISA are at increased risk of vancomycin treatment failure. Irrespective of high vancomycin MIC, hVISA is independently associated with vancomycin treatment failure. One of the major issues for identifying patients infected with this organism is the lack of a reliable and minimized-labor commercial product for routine screening. Current methods are expensive, lack sufficient sensitivity, are labor-intensive, and are not standardized. It is still considered difficult to have a prospective study without proper and rapid identification of hVISA. This may be only the tip of the iceberg, as larger surveillance studies are needed to evaluate the true prevalence of hVISA. Our data would support that hVISA identification is important based on the overall impact on patient outcomes including the association of prolonged days of bacteremia, longer days of persistent signs and symptoms, extended length of hospitalization, higher frequencies of MRSA BSI recurrence, and an 11-times-greater risk of vancomycin treatment failure.
ACKNOWLEDGMENTS
No financial support was obtained for the preparation of this article.
A.M.C. has received grant support from Cubist, Forest, and Michigan Department of Community Health. S.N.L. has received grant support from Astellas, Cubist, and Theravance. S.L.D. has served on the advisory board for Forest. T.P.L. has received grant support from Cubist, Forest, Pfizer, has served as a consultant to Cubist, Forest, Trius, Theravance, Pfizer, and Medicines Company, and served on speaker's bureau for Forest and Pfizer. D.A.G. has received grant support from Merck, has served as a Scientific Advisor to Cepheid, Forest, Optimer, Cubist, Cepheid, and AdvanDx, and has served on speaker's bureau for Merck. K.L.L. has received grant support from Cubist, Pfizer, and Astellas and has served as a Scientific Advisor to Cubist, Forest, Cepheid, TheraDoc, and Davol, Inc. B.A.P. has received grant support from Cubist. M.J.R. has received grant support from Cubist, Forest, Clinical Therapeutics, Cerexa, NIH, and the Michigan Department of Community Health, has served as a consultant to Cubist, Forest, and Cepheid, and has served on speaker's bureau for Cubist, Novartis, Forest, and Theravance. N.P. has nothing to declare.
Footnotes
Published ahead of print 24 June 2013
REFERENCES
- 1.Sakoulas G, Gold HS, Cohen RA, Venkataraman L, Moellering RC, Eliopoulos GM. 2006. Effects of prolonged vancomycin administration on methicillin-resistant Staphylococcus aureus (MRSA) in a patient with recurrent bacteraemia. J. Antimicrob. Chemother. 57:699–704 [DOI] [PubMed] [Google Scholar]
- 2.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BEJRM, Talan DA, Chambers HF. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 52:e18–e55 [DOI] [PubMed] [Google Scholar]
- 3.Hiramatsu K, Hanaki H, Ino T, Yabuta K, Oguri T, Tenover FC. 1997. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J. Antimicrob. Chemother. 40:135–136 [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention 2002. Staphylococcus aureus resistant to vancomycin—United States, 2002. MMWR Morb. Mortal. Wkly. Rep. 51:565–567 [PubMed] [Google Scholar]
- 5.Fridkin SK, Hageman J, McDougal LK, Mohammed J, Jarvis WR, Perl TM, Tenover FC. 2003. Epidemiological and microbiological characterization of infections caused by Staphylococcus aureus with reduced susceptibility to vancomycin, United States, 1997-2001. Clin. Infect. Dis. 36:429–439 [DOI] [PubMed] [Google Scholar]
- 6.Rybak MJ, Leonard SN, Rossi KL, Cheung CM, Sader HS, Jones RN. 2008. Characterization of vancomycin-heteroresistant Staphylococcus aureus from the metropolitan area of Detroit, Michigan, over a 22-year period (1986 to 2007). J. Clin. Microbiol. 46:2950–2954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horne KC, Howden BP, Grabsch EA, Graham M, Ward PB, Xie S, Mayall BC, Johnson PD, Grayson ML. 2009. Prospective comparison of the clinical impacts of heterogeneous vancomycin-intermediate methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-susceptible MRSA. Antimicrob. Agents Chemother. 53:3447–3452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Hal SJ, Paterson DL. 2011. Systematic review and meta-analysis of the significance of heterogeneous vancomycin-intermediate Staphylococcus aureus isolates. Antimicrob. Agents Chemother. 55:405–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sader HS, Jones RN, Rossi KL, Rybak MJ. 2009. Occurrence of vancomycin-tolerant and heterogeneous vancomycin-intermediate strains (hVISA) among Staphylococcus aureus causing bloodstream infections in nine U. S. A. hospitals. J. Antimicrob. Chemother. 64:1024–1028 [DOI] [PubMed] [Google Scholar]
- 10.Bae IG, Federspiel JJ, Miro JM, Woods CW, Park L, Rybak MJ, Rude TH, Bradley S, Bukovski S, de la Maria CG, Kanj SS, Korman TM, Marco F, Murdoch DR, Plesiat P, Rodriguez-Creixems M, Reinbott P, Steed L, Tattevin P, Tripodi MF, Newton KL, Corey GR, Fowler VG., Jr 2009. Heterogeneous vancomycin-intermediate susceptibility phenotype in bloodstream methicillin-resistant Staphylococcus aureus isolates from an international cohort of patients with infective endocarditis: prevalence, genotype, and clinical significance. J. Infect. Dis. 200:1355–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richter SS, Satola SW, Crispell EK, Heilmann KP, Dohrn CL, Riahi F, Costello AJ, Diekema DJ, Doern GV. 2011. Detection of Staphylococcus aureus isolates with heterogeneous intermediate-level resistance to vancomycin in the United States. J. Clin. Microbiol. 49:4203–4207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu C, Chambers HF. 2003. Staphylococcus aureus with heterogeneous resistance to vancomycin: epidemiology, clinical significance, and critical assessment of diagnostic methods. Antimicrob. Agents Chemother. 47:3040–3045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howden BP, Davies JK, Johnson PD, Stinear TP, Grayson ML. 2010. Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin-intermediate and heterogeneous vancomycin-intermediate strains: resistance mechanisms, laboratory detection, and clinical implications. Clin. Microbiol. Rev. 23:99–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howden BP, Johnson PD, Ward PB, Stinear TP, Davies JK. 2006. Isolates with low-level vancomycin resistance associated with persistent methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob. Agents Chemother. 50:3039–3047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wootton M, Howe RA, Hillman R, Walsh TR, Bennett PM, MacGowan AP. 2001. A modified population analysis profile (PAP) method to detect hetero-resistance to vancomycin in Staphylococcus aureus in a UK hospital. J. Antimicrob. Chemother. 47:399–403 [DOI] [PubMed] [Google Scholar]
- 16.Howden BP, Ward PB, Charles PG, Korman TM, Fuller A, du Cros P, Grabsch EA, Roberts SA, Robson J, Read K, Bak N, Hurley J, Johnson PD, Morris AJ, Mayall BC, Grayson ML. 2004. Treatment outcomes for serious infections caused by methicillin-resistant Staphylococcus aureus with reduced vancomycin susceptibility. Clin. Infect. Dis. 38:521–528 [DOI] [PubMed] [Google Scholar]
- 17.Charles PG, Ward PB, Johnson PD, Howden BP, Grayson ML. 2004. Clinical features associated with bacteremia due to heterogeneous vancomycin-intermediate Staphylococcus aureus. Clin. Infect. Dis. 38:448–451 [DOI] [PubMed] [Google Scholar]
- 18.Maor Y, Hagin M, Belausov N, Keller N, Ben-David D, Rahav G. 2009. Clinical features of heteroresistant vancomycin-intermediate Staphylococcus aureus bacteremia versus those of methicillin-resistant S. aureus bacteremia. J. Infect. Dis. 199:619–624 [DOI] [PubMed] [Google Scholar]
- 19.van Hal SJ, Jones M, Gosbell IB, Paterson DL. 2011. Vancomycin heteroresistance is associated with reduced mortality in ST239 methicillin-resistant Staphylococcus aureus blood stream infections. PLoS One 6:e21217. 10.1371/journal.pone.0021217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Musta AC, Riederer K, Shemes S, Chase P, Jose J, Johnson LB, Khatib R. 2009. Vancomycin MIC plus heteroresistance and outcome of methicillin-resistant Staphylococcus aureus bacteremia: trends over 11 years. J. Clin. Microbiol. 47:1640–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. 1988. CDC definitions for nosocomial infections, 1988. Am. J. Infect. Control 16:128–140 [DOI] [PubMed] [Google Scholar]
- 22.Clinical and Laboratory Standards Institute 2010. Performance standards for antimicrobial susceptibility testing, 16th informational supplement. CLSI document M100-S20 ed CLSI, Wayne, PA [Google Scholar]
- 23.Walsh TR, Bolmstrom A, Qwarnstrom A, Ho P, Wootton M, Howe RA, MacGowan AP, Diekema D. 2001. Evaluation of current methods for detection of staphylococci with reduced susceptibility to glycopeptides. J. Clin. Microbiol. 39:2439–2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milheirico C, Oliveira DC, de Lencastre H. 2007. Update to the multiplex PCR strategy for assignment of mec element types in Staphylococcus aureus. Antimicrob. Agents Chemother. 51:3374–3377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilot P, Lina G, Cochard T, Poutrel B. 2002. Analysis of the genetic variability of genes encoding the RNA III-activating components Agr and TRAP in a population of Staphylococcus aureus strains isolated from cows with mastitis. J. Clin. Microbiol. 40:4060–4067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Traber K, Novick R. 2006. A slipped-mispairing mutation in AgrA of laboratory strains and clinical isolates results in delayed activation of agr and failure to translate delta- and alpha-haemolysins. Mol. Microbiol. 59:1519–1530 [DOI] [PubMed] [Google Scholar]
- 27.Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. 2004. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin. Pharmacokinet. 43:925–942 [DOI] [PubMed] [Google Scholar]
- 28.Kullar R, Davis SL, Levine DP, Rybak MJ. 2011. Impact of vancomycin exposure on outcomes in patients with methicillin-resistant Staphylococcus aureus bacteremia: support for consensus guidelines suggested targets. Clin. Infect. Dis. 52:975–981 [DOI] [PubMed] [Google Scholar]
- 29.Kopp BJ, Nix DE, Armstrong EP. 2004. Clinical and economic analysis of methicillin-susceptible and -resistant Staphylococcus aureus infections. Ann. Pharmacother. 38:1377–1382 [DOI] [PubMed] [Google Scholar]
- 30.Lodise TP, Graves J, Evans A, Graffunder E, Helmecke M, Lomaestro BM, Stellrecht K. 2008. Relationship between vancomycin MIC and failure among patients with methicillin-resistant Staphylococcus aureus bacteremia treated with vancomycin. Antimicrob. Agents Chemother. 52:3315–3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lodise TP, McKinnon PS, Swiderski L, Rybak MJ. 2003. Outcomes analysis of delayed antibiotic treatment for hospital-acquired Staphylococcus aureus bacteremia. Clin. Infect. Dis. 36:1418–1423 [DOI] [PubMed] [Google Scholar]
- 32.Ariza J, Pujol M, Cabo J, Pena C, Fernandez N, Linares J, Ayats J, Gudiol F. 1999. Vancomycin in surgical infections due to methicillin-resistant Staphylococcus aureus with heterogeneous resistance to vancomycin. Lancet 353:1587–1588 [DOI] [PubMed] [Google Scholar]
- 33.Fong RK, Low J, Koh TH, Kurup A. 2009. Clinical features and treatment outcomes of vancomycin-intermediate Staphylococcus aureus (VISA) and heteroresistant vancomycin-intermediate Staphylococcus aureus (hVISA) in a tertiary care institution in Singapore. Eur. J. Clin. Microbiol. Infect. Dis. 28:983–987 [DOI] [PubMed] [Google Scholar]
- 34.Neoh HM, Hori S, Komatsu M, Oguri T, Takeuchi F, Cui L, Hiramatsu K. 2007. Impact of reduced vancomycin susceptibility on the therapeutic outcome of MRSA bloodstream infections. Ann. Clin. Microbiol. Antimicrob. 6:13. 10.1186/1476-0711-6-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howden BP, Smith DJ, Mansell A, Johnson PD, Ward PB, Stinear TP, Davies JK. 2008. Different bacterial gene expression patterns and attenuated host immune responses are associated with the evolution of low-level vancomycin resistance during persistent methicillin-resistant Staphylococcus aureus bacteraemia. BMC Microbiol. 8:39. 10.1186/1471-2180-8-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peleg AY, Monga D, Pillai S, Mylonakis E, Moellering RC, Jr, Eliopoulos GM. 2009. Reduced susceptibility to vancomycin influences pathogenicity in Staphylococcus aureus infection. J. Infect. Dis. 199:532–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Hal SJ, Lodise TP, Paterson DL. 2012. The clinical significance of vancomycin minimum inhibitory concentration in Staphylococcus aureus infections: a systematic review and meta-analysis. Clin. Infect. Dis. 54:755–771 [DOI] [PubMed] [Google Scholar]
- 38.Rose WE, Leonard SN, Rossi KL, Kaatz GW, Rybak MJ. 2009. Impact of inoculum size and heterogeneous vancomycin-intermediate Staphylococcus aureus (hVISA) on vancomycin activity and emergence of VISA in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 53:805–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wootton M, MacGowan AP, Walsh TR, Howe RA. 2007. A multicenter study evaluating the current strategies for isolating Staphylococcus aureus strains with reduced susceptibility to glycopeptides. J. Clin. Microbiol. 45:329–332 [DOI] [PMC free article] [PubMed] [Google Scholar]