Abstract
We evaluated the activity of solithromycin against 196 clinical gonococcal isolates collected at the Public Health Ontario Laboratories, Toronto, Canada, including isolates with different levels of azithromycin resistance, as well as the role of pH in MIC determinations using pH-adjusted agar plates (pH range, 5.6 to 7.6). In vitro invasion assays were performed using monolayers of HeLa epithelial cells and clinical gonococci displaying different azithromycin MICs; infected cultures were treated with solithromycin, and its intracellular activity was determined by CFU assays after 3 and 20 h of exposure. Solithromycin displayed a MIC50 and MIC90 of 0.0625 and 0.125 μg/ml, respectively, making its activity at least 4-fold higher than that of azithromycin. Clinical isolates with elevated MICs for azithromycin (MICs of ≥2,048 μg/ml and 4 to 8 μg/ml) showed solithromycin MIC values of 8 and 0.5 μg/ml, respectively. In contrast to azithromycin, solithromycin MICs were not significantly affected by acidic pHs, suggesting more stability at lower pH. Moreover, when intracellular Neisseria gonorrhoeae isolates were incubated with solithromycin at 4 times, 1 times, and one-fourth of the MIC, the exposure to solithromycin resulted in the progressive loss of viability of most isolates over time. The intracellular activity of solithromycin, combined with the low MICs to this agent, indicates that it may be an attractive option for gonorrhea treatment if clinical trials in development reveal that this drug can be used safely in adult indications, especially when multidrug-resistant clinical isolates are now emerging.
INTRODUCTION
Among the few newer antimicrobials developed in recent years, solithromycin (CEM-101), a potent novel fluoroketolide currently in clinical development, has a reported high potency against Gram-positive pathogens, including macrolide-resistant isolates (1–3), and various fastidious Gram-negative pathogens (4–6). In vitro studies have shown that solithromycin has activity comparable or superior to that of telithromycin, erythromycin, azithromycin, and clarithromycin against different bacterial species (4, 7). Moreover, Golparian et al. have reported that the in vitro activity of solithromycin is superior to that of azithromycin and many other antimicrobials against international reference strains and clinical gonococcal isolates, including some with high-level antimicrobial resistance (5). In this study, we investigated the in vitro activity of solithromycin at different pHs against a gonococcal collection including azithromycin-susceptible and -resistant isolates. Using the carcinoembryonic antigen cell adhesion molecule (CEACAM)-mediated uptake of colony opacity-associated adhesin (Opa)-expressing Neisseria gonorrhoeae as a well-characterized model of bacterial invasion (8), we analyzed the intracellular activity of solithromycin on gonococcal invasion into cervical epithelial cells. This study was initiated to provide comprehensive data on the activity of solithromycin and facilitate its development as a potential alternative chemotherapeutic agent in the eradication of N. gonorrhoeae.
(Part of this study was presented at the 52nd Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, 2012, and at IDWeek, San Diego, CA, 2012.)
MATERIALS AND METHODS
Antimicrobial susceptibility testing.
Public Health Ontario (PHO) Laboratories provide primary testing for dedicated sexually transmitted infection clinics throughout the province of Ontario, Canada. This includes both culture and nucleic acid amplification testing (NAAT) for N. gonorrhoeae. PHO also performs susceptibility testing for all N. gonorrhoeae isolates identified at other hospital and private laboratories across the province. In that context, a total of 196 N. gonorrhoeae isolates were selected from the clinical isolates collected from 2008 to 2011. Among those isolates, 67 were reported in our previous susceptibility profiles study (8 antibiotics, including penicillin, tetracycline, ciprofloxacin, erythromycin, azithromycin, spectinomycin, ceftriaxone, and cefixime) (9). Isolates susceptible to, with reduced susceptibility to, and resistant to azithromycin were also included. Primary specimens and isolates received for confirmation of N. gonorrhoeae were subcultured on New York City (NYC) agar (10) and incubated for 24 to 72 h in 5% CO2 at 35 to 37°C. Gram stain, oxidase, and carbohydrate utilization tests (glucose, maltose, sucrose, and O-nitrophenyl-β-d-galactopyranoside [ONPG]) were performed. Identification of N. gonorrhoeae also included testing growth on blood agar at 22°C and on nutrient agar at 35.5°C. All isolates were cultured on GC agar and 1% defined growth supplement at 37°C in 5% CO2 for 20 to 24 h and stored at −86°C. Each sample was subcultured twice on NYC agar before antimicrobial testing. The MICs of solithromycin and azithromycin were determined by the agar dilution method according to the Clinical and Laboratory Standards Institute (CLSI) guidelines (11). Since CLSI does not include breakpoints for azithromycin, we used the European Committee on Antimicrobial Susceptibility Testing (EUCAST) breakpoints for interpretation of the results (susceptible [S], ≤0.25 μg/ml; resistant [R], ≥1 μg/ml) (available at http://www.eucast.org/clinical_breakpoints/). N. gonorrhoeae strains WHO L (intermediate resistance to azithromycin; MIC of 0.5 μg/ml) and P (resistant to azithromycin; MIC of 2 μg/ml) were used as quality control strains (12). For susceptibility testing of selected N. gonorrhoeae isolates against solithromycin and azithromycin at different pHs, GC agar with pHs ranging from 5.6 to 7.6 was in-house prepared and buffered using 0.1 M potassium phosphate buffers (5.6 to 7.6). The final pH values of the GC agar plates were confirmed using a flat pH surface electrode.
Molecular characterization of macrolide resistance.
The presence of acquired 23S rRNA methylases (ermA, ermB, ermC, and ermF genes), efflux pump (mefA/E gene), macrolide 2′-phosphotransferase (mphA gene), and erythromycin esterases (ereA and ereB genes) and possible mutations in the chromosomal mtrR gene/promoter and riboproteins L4 and L22 (rplD and rplV genes) were tested as described previously (9) in selected isolates used for intracellular activity assays. Mutations in the 4 copies of 23S rRNA rrl genes identified in N. gonorrhoeae were also analyzed by DNA sequencing of PCR amplicons. Briefly, for each 23S rRNA copy, a common primer inside each copy of the rrl genes and specific external primers on the closer open reading frame downstream to each rrl genes were designed from the complete N. gonorrhoeae NCCP11945 genome (accession number NC_011035) (Table 1). Using the first PCR amplicons as a DNA template, nested PCRs were next performed using a set of common primers (NG23s1905-F and NG23s2769-R) (Table 1) internal to the 23S rRNA gene (including the peptidyl-transferase loop of the domain V). The second-round PCR amplicons were sequenced to identify point mutations associated with macrolide resistance. The same approach was used for analysis of domain II of the 23S rRNA gene, particularly the position A752 (NG752-R and NG752-F) (Table 1) potentially involved in solithromycin binding.
Table 1.
Oligonucleotides used for amplification and sequencing of domains II and V of the 23S rRNA gene
| 23S rRNA domain and locus | Primer | Sequence (5′–3′) | Reference |
|---|---|---|---|
| Domain V | |||
| External-specific primersa | NG23S1-F | GGCTATGAAGGCGGCGATT | 9 |
| NG23S2-F | TTTCAGATGAGTAATGTACACC | 9 | |
| NG23S3-F | CAATCCGCAAGTCTGCCGA | 9 | |
| NG23S4-F | CTCTCCGATCCCGAACTCG | 9 | |
| Internal common primera | NG23S-R | GAAGATGTGCAAGCATCGGA | 9 |
| Nested PCR | NG23s1905-F | ACGGTCCTAAGGTAGCGA | 9 |
| NG23s2769-R | TCTCATCTTCAGGCGAGTT | 9 | |
| Domain II | |||
| Internal common primera | NG752-R | CAACGACTTACATTCAGTAGC | This study |
| Seminested PCRb | NG752-F | TTCTGATACCTCCAGCACAC | This study |
External-specific primers were used for specific amplification of each copy of the 23S rRNA gene (4 copies in N. gonorrhoeae) together with each internal common primer (NG23S-R for amplification of domain V and NG752-R for amplification of domain II). The obtained amplicons were used for the subsequent nested or seminested assay.
For the seminested PCR, primers NG752-F and NG752-R were used.
Intracellular activity of solithromycin.
Five N. gonorrhoeae clinical isolates demonstrating susceptibility (GN48) and resistance (GN640, GN641, GN642, and GN726) to azithromycin were used for the experiments and grown as described above. For infection assays, stably transfected HeLa line (human endocervical epithelial) cells expressing the human CEACAM1 receptor (8, 13, 14) were grown to 70 to 80% confluence in 24-well plates (Costar 24-well flat-bottom plate; Corning Incorporated, Lowell, MA) containing 1 ml/well of RPMI 1640 medium (Life Technologies, Burlington, Ontario, Canada) supplemented with 10% heat-inactivated fetal calf serum and 1% glutamine (Life Technologies) and incubated at 37°C in the presence of 5% CO2 for 48 h. Cells were washed three times in serum-free RPMI 1640 prior to infection. Gonococci were scraped from fresh overnight culture plates, resuspended in 1 ml of phosphate-buffered saline (PBS) containing 1 mM MgCl2 and 0.5 mM CaCl2 (pH 7.0), washed once by pelleting, and resuspended to a concentration of 3.2 × 107 cells/ml. Gonococci were added to the cells at a multiplicity of infection (MOI) of 80:1 in a volume of 1 ml per well. Samples were subjected to centrifugation for 5 min at 500 rpm to promote bacterial association with HeLa cells. Then the cultures were incubated for 1 h at 37°C in 5% CO2. After 3 washes in serum-free RPMI to remove nonadherent bacteria, gentamicin was added (50-μg/ml final concentration) for 1 h to eradicate extracellular bacteria. To confirm that the gentamicin treatment was sufficient to kill extracellular gonococci in our infection assays, the MICs for gentamicin were initially determined on bacterial suspensions of the five clinical isolates studied. MIC values ranged from 3 to 8 μg/ml, suggesting that all of the isolates should be killed by the 50-μg/ml concentration of gentamicin used in the infection assay. Cells were washed three times in serum-free RPMI 1640 to remove extracellular bacteria and gentamicin and were incubated with various concentrations of solithromycin (4 times, 1 times, and one-fourth of the MIC) or medium/buffer alone at 37°C in 5% CO2. After 0, 3, and 20 h of incubation, cells were washed 3 times with PBS containing 1 mM MgCl2 and 0.5 mM CaCl2 (pH 7.0), and 1% saponin was then added to permeabilize and lyse HeLa cells for 15 min at 37°C in 5% CO2 before dilution and plating onto GC agar. Plates were incubated overnight to quantify viable intracellular bacteria (13). All assays were performed in quadruplicate with two independent biological duplicates.
RESULTS
Antigonococcal activity of solithromycin.
Overall, solithromycin demonstrated superior potency against N. gonorrhoeae compared to azithromycin, with lower MICs than azithromycin against the vast majority of isolates evaluated (Fig. 1). Most of the isolates (96%) showed MICs for solithromycin ranging from ≤0.015 μg/ml to 0.125 μg/ml, whereas 75% of the isolates showed MICs for azithromycin ranging from 0.125 μg/ml to 0.5 μg/ml. None of the tested isolates had solithromycin MICs greater than 8 μg/ml. N. gonorrhoeae MICs for solithromycin ranged from ≤0.015 to 8 μg/ml compared to the MICs of azithromycin, which ranged from ≤0.031 to ≥2,048 μg/ml. Solithromycin had a MIC50 of 0.0625 μg/ml and a MIC90 of 0.125 μg/ml, making its activity 4-fold higher than that of azithromycin (MIC50 of 0.25 μg/ml and MIC90 of 0.5 μg/ml). Clinical isolates with elevated MICs for azithromycin (MICs of ≥2,048 and 4 to 8 μg/ml) showed solithromycin MICs of 8 μg/ml (≥256-fold difference) and 0.25 to 0.5 μg/ml (16-fold difference), respectively (Fig. 1).
Fig 1.
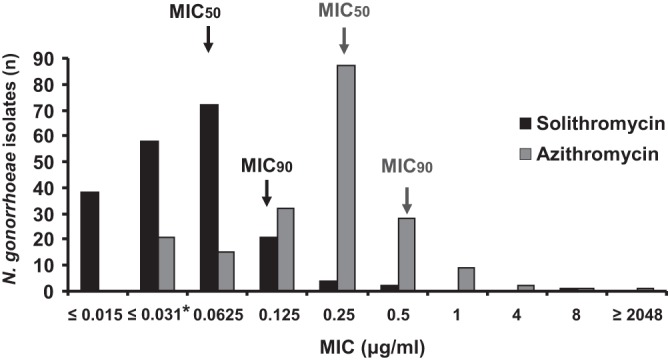
MIC distribution of solithromycin and azithromycin for a collection of 196 clinical N. gonorrhoeae isolates. *, lowest dilution tested for azithromycin.
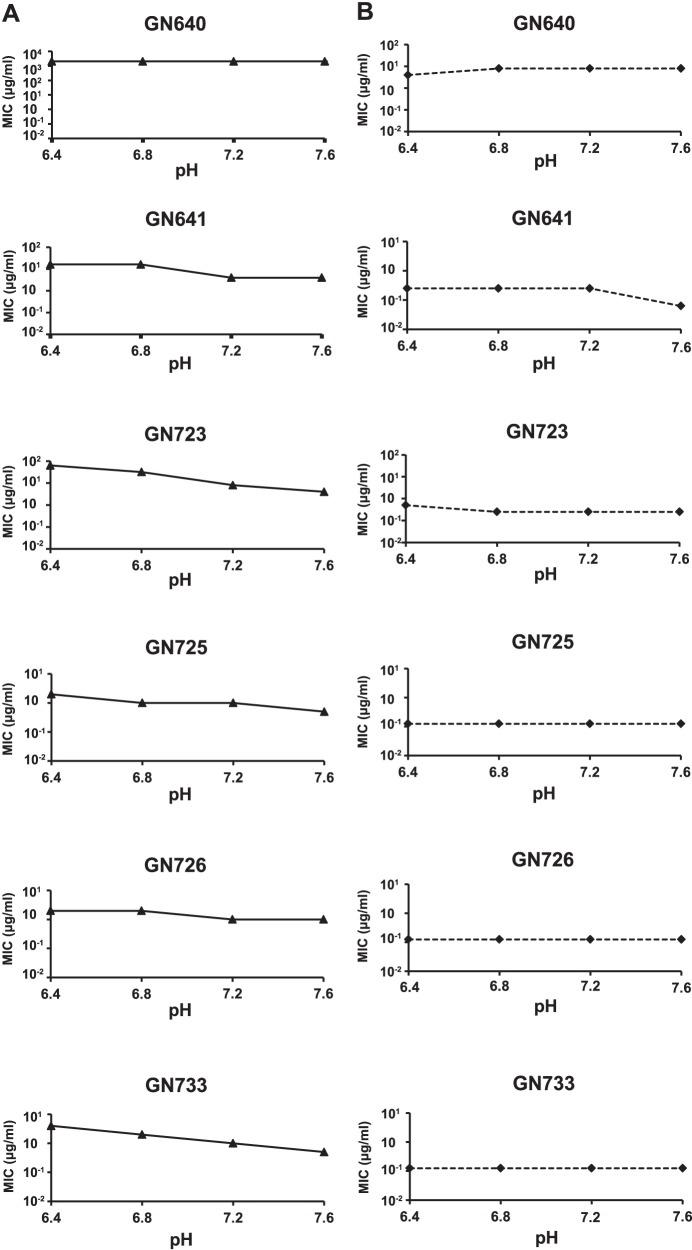
Role of pH in the activity of solithromycin and azithromycin.
We investigated the effect of pH on the activity of solithromycin and azithromycin against N. gonorrhoeae using pH-adjusted agar plates (pH range, 5.6 to 7.6). This range was selected to cover the values at which the antibiotics can be exposed in the extracellular milieu or intracellularly for N. gonorrhoeae. Unlike erythromycin, the 15-member azalide ring of azithromycin is acid stable (15). For the short duration of the assay, azithromycin and solithromycin are both stable at the pHs tested, but azithromycin is less stable at an acid pH for longer periods of time as the cladinose sugar may be lost (Cempra Inc., unpublished data). We compared the susceptibilities of six N. gonorrhoeae isolates which displayed the highest solithromycin and azithromycin MIC values at different pHs. Control strains and clinical isolates were not able to grow on plates with pHs 5.6 and 6.0 in the absence of antibiotics. Therefore, data are presented only for plates prepared at pH 6.4, 6.8, 7.2, and 7.6. All isolates with intermediate susceptibility (0.5 μg/ml) or resistance (≥1 μg/ml) to azithromycin displayed 2- to 16-fold-higher MICs at lower pHs than at pH 7.2 (control plate). In contrast, solithromycin MICs remained low throughout the pH range evaluated and were not significantly affected by acidic pHs (Fig. 2).
Fig 2.
Susceptibilities to azithromycin (A) and solithromycin (B) of N. gonorrhoeae isolates grown on pH-adjusted GC agar plates. MIC determinations were performed in pH-adjusted GC plates. EUCAST breakpoints for azithromycin were used: S, ≤0.25 μg/ml; I, 0.5 μg/ml; R, ≥1 μg/ml.
Macrolide resistance mechanisms.
Three different mechanisms involved in macrolide resistance have been described in N. gonorrhoeae: efflux systems (16, 17), modification of the ribosomal target by methylases (18), and ribosomal modification by point mutations in the macrolide targets (19). The presence of these mechanisms was investigated on five representative N. gonorrhoeae clinical isolates displaying susceptibility (GN48) and various resistance levels (GN640, GN641, GN642, and GN726) to azithromycin (Table 2). High-level macrolide resistance (strain GN640) was associated with target mutation (A2143G in all 4 copies of the 23S rRNA rrl gene) (20, 21). Low-level resistance was linked to a different mutation in the target (C2599T) but also in the mtrR gene and riboprotein L22 (Table 2). No mutations in domain II of the 23S rRNA gene, position A752, were detected in any isolate. Isolates GN726 and GN48 were wild type for all the genes tested. Macrolide resistance mediated by acquired efflux pump, methylases, and esterases was not detected in either isolate. Mutations affecting MtrR (the repressor of the chromosome-encoded MtrCDE efflux pump) was found only in strain GN640.
Table 2.
Characterization of macrolide resistance mechanisms on five N. gonorrhoeae clinical isolates
| Strain | MIC (μg/ml)a |
Mutation | Gene affected | ||
|---|---|---|---|---|---|
| AZM | ERY | SOLI | |||
| GN48 | 0.062 (S) | ND | ≤0.015 | Noneb | Noneb |
| GN640 | ≥2,048 (R) | ≥256 | 8 | A2143Gc | rrl (23S rRNA)d |
| G45D | mtrR | ||||
| GN641 | 3 (R) | 64 | 0.25 | C2599Tc | rrl (23S rRNA)d |
| GN642 | 1 (R) | 3 | 0.5 | A89D | rplV (riboprotein L22) |
| GN726 | 1 (R) | 3 | 0.0625 | Noneb | Noneb |
AZM, azithromycin; ERY, erythromycin; SOLI, solithromycin; ND, not determined; S, susceptible; R, resistant. EUCAST breakpoints are as follows: S, ≤0.25 μg/ml; I, 0.5 μg/ml; R, ≥1 μg/ml.
All the chromosomal targets studied were wild type.
N. gonorrhoeae numbering. Positions A2143 and C2599 correspond to A2059 and C2611 in Escherichia coli numbering, respectively.
The four rrl alleles contained the same mutation.
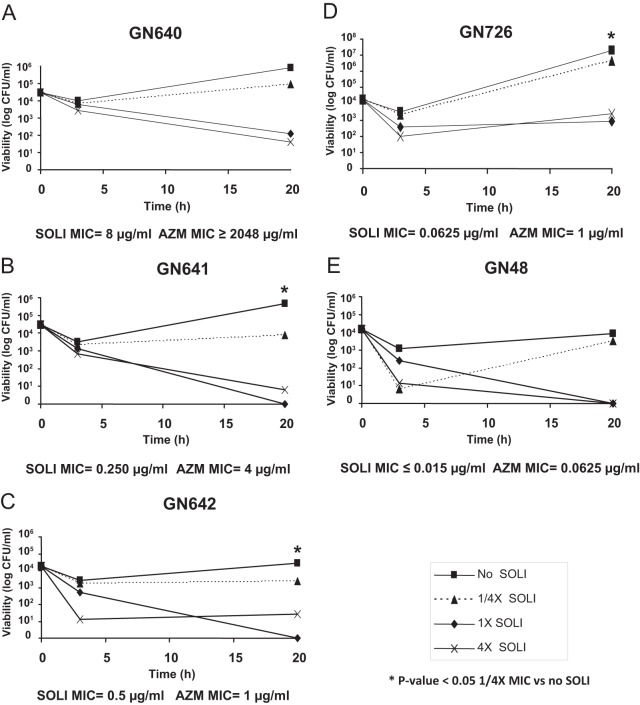
Intracellular activity of solithromycin against gonococci.
The intracellular activity of solithromycin against these five N. gonorrhoeae isolates was next tested. Following incubation within HeLa cells expressing the CEACAM1 receptor, internalized N. gonorrhoeae isolates were exposed to solithromycin at 4 times, 1 times, and one-fourth of their respective MICs. To rule out a possible growth-suppressive effect of solithromycin on epithelial cells in our experimental conditions, we first verified if increasing concentrations of solithromycin can affect the viability of epithelial cells. Solithromycin concentrations ranging from 0.0625 to 32 μg/ml were shown to not affect the viability of HeLa cells when evaluated by trypan blue staining (data not shown). As presented in Fig. 3, all isolates were internalized with similar efficiency at time zero. Exposure to solithromycin at 4 times and 1 times the MIC resulted in the progressive loss of viability of all isolates at 20 h compared to time zero with the exception of GN726, for which viability remained stable at 20 h compared to 3 h (Fig. 3D). The loss of viability for all other isolates ranged from 99% to 100% of CFU per milliliter. Interestingly, in the untreated culture controls, GN726 replicated by 7 log units at 20 h while GN48, GN642, GN640, and GN641 ranged from 3 to 5 log units. Concentrations of solithromycin equal to one-fourth of the MIC for each isolate were uniformly ineffective in killing intracellular organisms. However, 3 of the N. gonorrhoeae clinical isolates (GN641, GN642, and GN726) displayed a significantly lower viability at one-fourth of the solithromycin MIC than with no antibiotic at 20 h (P value < 0.05).
Fig 3.
Intracellular activity of solithromycin (SOLI) against five N. gonorrhoeae clinical isolates. N. gonorrhoeae isolates internalized within the HeLa cell line were exposed to various concentrations of solithromycin. At indicated time points, samples were harvested and bacteria were enumerated by CFU assays. 4×, exposure to 4 times the solithromycin MIC; 1×, exposure to 1 times the MIC; 1/4×, exposure to one-fourth of the MIC. *, Student's t test P value of <0.05 for no solithromycin versus one-fourth of the MIC of solithromycin. AZM, azithromycin.
DISCUSSION
Since the early 1990s, clinical isolates with decreased susceptibility to azithromycin have been observed in several countries (22, 23). Interestingly, the Gonococcal Isolate Surveillance Project data for 2007 showed that the distribution of azithromycin MICs shifted toward the higher MICs in the United States (24). However, the use of a single dose of 1 g azithromycin in combination therapy (with cephalosporins) is still the recommended treatment. The emergence of multidrug-resistant and untreatable N. gonorrhoeae highlights the importance of identifying alternatives using known antibiotics or new antimicrobials for effective treatment of gonorrhea infections. Among known antibiotics, gentamicin and ertapenem have been evaluated as potential drugs for the treatment of gonorrhea (25–27).
Recently, solithromycin, a new fluoroketolide with high affinity for bacterial ribosomes, has been evaluated as a potential new antimicrobial for gonorrhea treatment (5). In the present study, we measured for the first time the potency of solithromycin against a variety of Canadian clinical isolates recently collected in Ontario and displaying a broad range of susceptibility to azithromycin, including a high level of resistance (e.g., MIC of ≥2,048 μg/ml). We found that solithromycin has higher intrinsic antigonococcal activity (lower MICs) than azithromycin. It is known that the C5 desosamine sugar residues in macrolides and ketolides interact with positions A2058 and A2059 (Escherichia coli nucleotide numbering; A2142 and A2143 in N. gonorrhoeae numbering) of the bacterial 23S rRNA (28). This explains why mutations in these nucleotides can cause resistance to these antibiotics, and it is consistent with the high azithromycin MIC observed with the N. gonorrhoeae GN640 isolate (A2143G) of our study. In the case of ketolides, the alkyl-aryl side chain attached at the C-11 and C-12 carbon atoms also interacts with the A752-U2609 bp (E. coli nucleotide numbering; A750-U2597 in N. gonorrhoeae numbering), and in the particular case of solithromycin, the fluorine atom positioned near the glycosidic bond (atom N-1) of C2611 (E. coli numbering; C2599 in N. gonorrhoeae numbering) can potentially contribute to the drug binding (29). Compared to azithromycin, these multiple interactions may explain the better anchoring of solithromycin to the ribosome, even in the presence of mutations in key positions (i.e., A2142 and A2143). Because of that, lower MICs are expected, as observed in the isolate GN640 (MIC of ≥2,048 μg/ml for azithromycin versus 8 μg/ml for solithromycin). The mutation C2599T may affect the interaction of the solithromycin fluorine atom with the ribosome, reducing the susceptibility to solithromycin (from ≤0.015 μg/ml in the wild-type GN48 to 0.25 μg/ml in GN641). No mutations affecting the binding of the solithromycin alkyl-aryl side chain were detected by sequencing.
Moreover, with a subset of selected isolates, we also investigated the pH stability and the intracellular activity of solithromycin using a tissue culture model of cervical cell lines. Our results also showed stable activity of solithromycin at different pH values against N. gonorrhoeae, whereas azithromycin showed a marked decrease in potency against all isolates tested when the pH was decreased from 7.6 to 6.4 (Fig. 2). Several studies have previously demonstrated that pH affects azithromycin activity in vitro (30–32). In contrast, our results showed stable antigonococcal activity of solithromycin between pH 6.4 and 7.4, suggesting that solithromycin would be likely to have potent activity in acidic compartments such as endosomes/lysosomes compared to that of azithromycin. In agreement with our results, a previous study has demonstrated that acidic pH has a reduced inhibitory effect on solithromycin activity compared to azithromycin activity (30). This same study also showed that solithromycin has high intracellular activity against Staphylococcus aureus, Listeria monocytogenes, and Legionella pneumophila due to high intracellular accumulation.
In intracellular assays, solithromycin demonstrated efficient activity against all tested isolates with the exception of GN726 (Fig. 3). When exposed to 4 times the MIC, this specific clinical isolate did not show a significant decrease of viability at 20 h compared to that at 3 h. This phenotype is not explained by an increase in resistance to macrolides since GN726 does not present any mutations in the drug target, the 4 copies of the 23S rRNA gene, and does not express acquired efflux pumps or methylases associated with macrolide resistance. The reduced intracellular activity of solithromycin against this specific isolate may be explained by an elevated intracellular fitness, as it replicated more efficiently by 2 to 3 log units within epithelial cells than did other isolates in the untreated culture controls at 20 h. To rule out viable but not internalized gonococci affecting the intracellular assay results, we used gentamicin treatment, which is a standard method to quantify viable intracellular N. gonorrhoeae (13, 33). Considering the gonococcal MICs, the used concentration (50 μg/ml) was sufficient to kill all not-internalized isolates, consistent with a previous study showing that, upon gentamicin treatment, nonviable N. gonorrhoeae isolates fail to recruit F-actin and are not internalized by host cells (34).
In conclusion, solithromycin was demonstrated to be stable and potent against N. gonorrhoeae, even against isolates with high azithromycin MICs. In phase 2 of a clinical trial, solithromycin has been found to be 100% effective against N. gonorrhoeae in all cultures tested (urethra, oropharynx, and rectal) using 1.2 g and 1 g as a single dose (Cempra Inc., unpublished data). Our in vitro results and these phase 2 studies show the potential value of solithromycin for use in the treatment of gonorrhea, especially when multidrug-resistant clinical isolates displaying full resistance to azithromycin and extended-spectrum cephalosporins are now emerging.
ACKNOWLEDGMENTS
We thank Vanessa Allen (Public Health Ontario) for providing the gonococcal isolates used in this study and Scott D. Gray-Owen (Department of Molecular Genetics, University of Toronto, Toronto, ON, Canada) for the stably transfected HeLa epithelial cells used in this study and for expert technical assistance.
This work was supported by Cempra Inc.
P.F. is the CEO of Cempra Inc. All other authors have no conflicts of interest to declare.
Footnotes
Published ahead of print 24 June 2013
REFERENCES
- 1.Putnam SD, Sader HS, Farrell DJ, Biedenbach DJ, Castanheira M. 2011. Antimicrobial characterisation of solithromycin (CEM-101), a novel fluoroketolide: activity against staphylococci and enterococci. Int. J. Antimicrob. Agents 37:39–45 [DOI] [PubMed] [Google Scholar]
- 2.McGhee P, Clark C, Kosowska-Shick KM, Nagai K, Dewasse B, Beachel L, Appelbaum PC. 2010. In vitro activity of CEM-101 against Streptococcus pneumoniae and Streptococcus pyogenes with defined macrolide resistance mechanisms. Antimicrob. Agents Chemother. 54:230–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farrell DJ, Sader HS, Castanheira M, Biedenbach DJ, Rhomberg PR, Jones RN. 2010. Antimicrobial characterisation of CEM-101 activity against respiratory tract pathogens, including multidrug-resistant pneumococcal serogroup 19A isolates. Int. J. Antimicrob. Agents 35:537–543 [DOI] [PubMed] [Google Scholar]
- 4.Waites KB, Crabb DM, Duffy LB. 2009. Comparative in vitro susceptibilities of human mycoplasmas and ureaplasmas to a new investigational ketolide, CEM-101. Antimicrob. Agents Chemother. 53:2139–2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Golparian D, Fernandes P, Ohnishi M, Jensen JS, Unemo M. 2012. In vitro activity of the new fluoroketolide solithromycin (CEM-101) against a large collection of clinical Neisseria gonorrhoeae isolates and international reference strains, including those with high-level antimicrobial resistance: potential treatment option for gonorrhea? Antimicrob. Agents Chemother. 56:2739–2742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Putnam SD, Castanheira M, Moet GJ, Farrell DJ, Jones RN. 2010. CEM-101, a novel fluoroketolide: antimicrobial activity against a diverse collection of Gram-positive and Gram-negative bacteria. Diagn. Microbiol. Infect. Dis. 66:393–401 [DOI] [PubMed] [Google Scholar]
- 7.Farrell DJ, Castanheira M, Sader HS, Jones RN. 2010. The in vitro evaluation of solithromycin (CEM-101) against pathogens isolated in the United States and Europe (2009). J. Infect. 61:476–483 [DOI] [PubMed] [Google Scholar]
- 8.Gray-Owen SD, Dehio C, Haude A, Grunert F, Meyer TF. 1997. CD66 carcinoembryonic antigens mediate interactions between Opa-expressing Neisseria gonorrhoeae and human polymorphonuclear phagocytes. EMBO J. 16:3435–3445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen VG, Farrell DJ, Rebbapragada A, Tan J, Tijet N, Perusini SJ, Towns L, Lo S, Low DE, Melano RG. 2011. Molecular analysis of antimicrobial resistance mechanisms in Neisseria gonorrhoeae isolates from Ontario, Canada. Antimicrob. Agents Chemother. 55:703–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faur YC, Weisburd MH, Wilson ME, May PS. 1973. A new medium for the isolation of pathogenic Neisseria (NYC medium). I Formulation and comparisons with standard media. Health Lab. Sci. 10:44–54 [PubMed] [Google Scholar]
- 11.Clinical and Laboratory Standards Institute 2009. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—eighth edition. CLSI M07-A8. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 12.Unemo M, Fasth O, Fredlund H, Limnios A, Tapsall J. 2009. Phenotypic and genetic characterization of the 2008 WHO Neisseria gonorrhoeae reference strain panel intended for global quality assurance and quality control of gonococcal antimicrobial resistance surveillance for public health purposes. J. Antimicrob. Chemother. 63:1142–1151 [DOI] [PubMed] [Google Scholar]
- 13.Gray-Owen SD, Lorenzen DR, Haude A, Meyer TF, Dehio C. 1997. Differential Opa specificities for CD66 receptors influence tissue interactions and cellular response to Neisseria gonorrhoeae. Mol. Microbiol. 26:971–980 [DOI] [PubMed] [Google Scholar]
- 14.McCaw SE, Liao EH, Gray-Owen SD. 2004. Engulfment of Neisseria gonorrhoeae: revealing distinct processes of bacterial entry by individual carcinoembryonic antigen-related cellular adhesion molecule family receptors. Infect. Immun. 72:2742–2752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiese EF, Steffen SH. 1990. Comparison of the acid stability of azithromycin and erythromycin A. J. Antimicrob. Chemother. 25(Suppl A):39–47 [DOI] [PubMed] [Google Scholar]
- 16.Luna VA, Cousin S, Jr, Whittington WL, Roberts MC. 2000. Identification of the conjugative mef gene in clinical Acinetobacter junii and Neisseria gonorrhoeae isolates. Antimicrob. Agents Chemother. 44:2503–2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shafer WM, Veal WL, Lee EH, Zarantonelli L, Balthazar JT, Rouquette C. 2001. Genetic organization and regulation of antimicrobial efflux systems possessed by Neisseria gonorrhoeae and Neisseria meningitidis. J. Mol. Microbiol. Biotechnol. 3:219–224 [PubMed] [Google Scholar]
- 18.Roberts MC, Chung WO, Roe D, Xia M, Marquez C, Borthagaray G, Whittington WL, Holmes KK. 1999. Erythromycin-resistant Neisseria gonorrhoeae and oral commensal Neisseria spp. carry known rRNA methylase genes. Antimicrob. Agents Chemother. 43:1367–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ng LK, Martin I, Liu G, Bryden L. 2002. Mutation in 23S rRNA associated with macrolide resistance in Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 46:3020–3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galarza PG, Abad R, Canigia LF, Buscemi L, Pagano I, Oviedo C, Vazquez JA. 2010. New mutation in 23S rRNA gene associated with high level of azithromycin resistance in Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 54:1652–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chisholm SA, Dave J, Ison CA. 2010. High-level azithromycin resistance occurs in Neisseria gonorrhoeae as a result of a single point mutation in the 23S rRNA genes. Antimicrob. Agents Chemother. 54:3812–3816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Workowski KA, Berman SM, Douglas JM., Jr 2008. Emerging antimicrobial resistance in Neisseria gonorrhoeae: urgent need to strengthen prevention strategies. Ann. Intern. Med. 148:606–613 [DOI] [PubMed] [Google Scholar]
- 23.Newman LM, Moran JS, Workowski KA. 2007. Update on the management of gonorrhea in adults in the United States. Clin. Infect. Dis. 44(Suppl 3):S84–S101 [DOI] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention 2009. Sexually transmitted disease surveillance 2007. US Department of Health and Human Services, Atlanta, GA [Google Scholar]
- 25.Unemo M, Golparian D, Limnios A, Whiley D, Ohnishi M, Lahra MM, Tapsall JW. 2012. In vitro activity of ertapenem versus ceftriaxone against Neisseria gonorrhoeae isolates with highly diverse ceftriaxone MIC values and effects of ceftriaxone resistance determinants: ertapenem for treatment of gonorrhea? Antimicrob. Agents Chemother. 56:3603–3609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chisholm SA, Quaye N, Cole MJ, Fredlund H, Hoffmann S, Jensen JS, van de Laar MJ, Unemo M, Ison CA. 2011. An evaluation of gentamicin susceptibility of Neisseria gonorrhoeae isolates in Europe. J. Antimicrob. Chemother. 66:592–595 [DOI] [PubMed] [Google Scholar]
- 27.Brown LB, Krysiak R, Kamanga G, Mapanje C, Kanyamula H, Banda B, Mhango C, Hoffman M, Kamwendo D, Hobbs M, Hosseinipour MC, Martinson F, Cohen MS, Hoffman IF. 2010. Neisseria gonorrhoeae antimicrobial susceptibility in Lilongwe, Malawi, 2007. Sex. Transm. Dis. 37:169–172 [DOI] [PubMed] [Google Scholar]
- 28.Schlunzen F, Zarivach R, Harms J, Bashan A, Tocilj A, Albrecht R, Yonath A, Franceschi F. 2001. Structural basis for the interaction of antibiotics with the peptidyl transferase centre in eubacteria. Nature 413:814–821 [DOI] [PubMed] [Google Scholar]
- 29.Llano-Sotelo B, Dunkle J, Klepacki D, Zhang W, Fernandes P, Cate JH, Mankin AS. 2010. Binding and action of CEM-101, a new fluoroketolide antibiotic that inhibits protein synthesis. Antimicrob. Agents Chemother. 54:4961–4970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lemaire S, Van Bambeke F, Tulkens PM. 2009. Cellular accumulation and pharmacodynamic evaluation of the intracellular activity of CEM-101, a novel fluoroketolide, against Staphylococcus aureus, Listeria monocytogenes, and Legionella pneumophila in human THP-1 macrophages. Antimicrob. Agents Chemother. 53:3734–3743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Retsema JA, Brennan LA, Girard AE. 1991. Effects of environmental factors on the in vitro potency of azithromycin. Eur. J. Clin. Microbiol. Infect. Dis. 10:834–842 [DOI] [PubMed] [Google Scholar]
- 32.Barry AL, Fuchs PC. 1991. In-vitro potency of azithromycin against gram-negative bacilli is method-dependent. J. Antimicrob. Chemother. 28:607–610 [DOI] [PubMed] [Google Scholar]
- 33.Shaw JH, Falkow S. 1988. Model for invasion of human tissue culture cells by Neisseria gonorrhoeae. Infect. Immun. 56:1625–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bish SE, Song W, Stein DC. 2008. Quantification of bacterial internalization by host cells using a β-lactamase reporter strain: Neisseria gonorrhoeae invasion into cervical epithelial cells requires bacterial viability. Microbes Infect. 10:1182–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]