Abstract
The combination of amphotericin B at a suboptimal dose (0.3 mg/kg) with voriconazole has shown efficacy in prolonging survival and reducing tissue burden in a murine model of disseminated infection by an isolate of Aspergillus fumigatus that had showed a poor in vivo response to the azole. The efficacy of the combined treatment was higher than that obtained with amphotericin B at 0.8 mg/kg.
TEXT
Aspergillus fumigatus produces severe infections with high morbidity and mortality worldwide, mainly in immunocompromised patients (1, 2). Voriconazole (VRC) is the first-line treatment for invasive aspergillosis (IA) (3); however, several isolates showing reduced susceptibility or resistance to azoles have been reported (4, 5). Since therapeutic alternatives are limited, combined therapy has been suggested as an option for treating refractory infections (6, 7). VRC combined with amphotericin B (AMB) has demonstrated clinical efficacy against several fungal pathogens (8–10), while the results obtained for clinical and experimental aspergillosis are inconclusive and divergent (11, 12). We have evaluated the therapeutic efficacy of AMB and VRC alone and in combination in a murine model of disseminated infection using an isolate of A. fumigatus (FMR 10528) that in a previous study showed a poor in vivo response to VRC (13).
The fungus was grown on potato dextrose agar (PDA) at 35°C for 48 h. The in vitro susceptibility determination was carried out using a microdilution reference method (14). Antifungal interactions were assessed in duplicate by a checkerboard microdilution method (15, 16). For the two drugs tested and their combination, a MIC-0 (100% growth inhibition) endpoint criterion was used. The fractional inhibitory concentration index (FICI) was used to classify drug interactions, which were defined as synergistic if the FICI was ≤0.5, antagonistic if the FICI was >4, and indifferent if the FICI was >0.5 but ≥4 (16). The isolate showed VRC and AMB MICs of 0.5 and 2 μg/ml, respectively, and a FICI value of 2. Tests were carried out in duplicate.
Male OF1 mice were immunosuppressed with a single intraperitoneal injection of cyclophosphamide at 200 mg/kg of body weight plus a single intravenous injection of 5-fluorouracil at 150 mg/kg 1 day before challenge (17). To prevent bacterial infection, all the animals received ceftazidime at 5 mg/kg/day subcutaneously, starting on the day of the infection. The procedure standards were approved by the Animal Welfare and Ethics Committee of the Universitat Rovira i Virgili.
Two inocula were tested, 1 × 104 and 1 × 106 CFU, in 0.2 ml of sterile saline solution injected via the lateral tail vein. Groups of 20 mice were established for each inoculum and each treatment. Ten mice from each group were randomly chosen for survival and the other 10 for tissue burden studies. The groups were treated once daily as follows: VRC at 25 mg/kg of body weight given orally by gavage (p.o.) (18), AMB at 0.3 or 0.8 mg/kg given intravenously (i.v.), and VRC at 25 mg/kg p.o. plus AMB at 0.3 mg/kg i.v. The selection of AMB 0.3 mg/kg to be combined with voriconazole was mainly in order to demonstrate the enhancement of the antifungal efficacy of the combination over the respective monotherapies and try to minimize the adverse effects of the polyene. The use of a highly effective amphotericin B dose combined with voriconazole could mask a significant improvement of the results obtained with the combination (19). All treatments began 24 h after challenge and lasted for 7 days. Animals treated with VRC received 50% grapefruit juice instead of water ad libitum, starting 1 day before the beginning of the treatment (20). Control animals received no antifungal treatment.
Mouse survival was checked daily for 28 days after challenge. For tissue burden studies, mice were euthanized by anoxia using a CO2 chamber on day 5 of treatment, and the kidneys and lungs were aseptically removed, weighed, and homogenized in 2 ml of sterile saline solution. Serial 10-fold dilutions of the homogenates were plated on PDA and incubated for 48 h at 25°C to determine the fungal load (CFU/g of tissue).
Mean survival time was estimated by the Kaplan-Meier test and compared among groups using the log rank test. Colony counts from tissue burden studies were analyzed using the Mann-Whitney U test. All the statistical analyses were made using the GraphPad Prism software, version 4.00 for MS Windows (GraphPad Software Inc., La Jolla, CA).
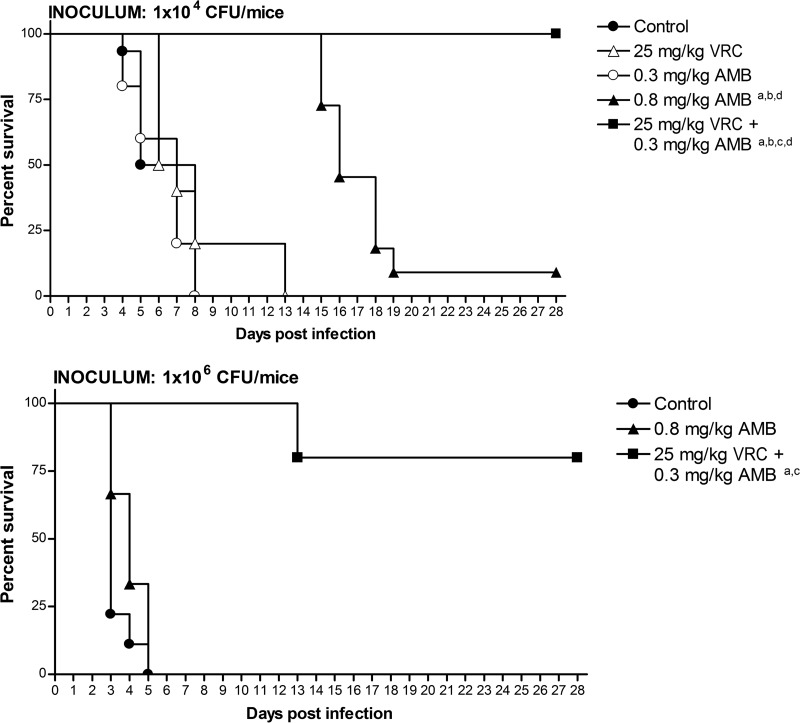
At the lowest inoculum, both AMB at 0.8 mg/kg and the combined therapy significantly prolonged survival, but only the combined treatment achieved 100% mouse survival (Fig. 1). VRC, AMB at 0.8 mg/kg, and the combination significantly reduced the fungal loads in the two tested organs, but VRC showed the lowest efficacy (Table 1).
Fig 1.
Cumulative survival of OF1 mice infected with A. fumigatus isolate FMR 10528 at two different inocula. VRC, voriconazole; AMB, amphotericin B deoxycholate. a, P < 0.05 versus control group; b, P < 0.05 versus VRC at 25 mg/kg; c, P < 0.05 versus AMB at 0.8 mg/kg; d, P < 0.05 versus AMB at 0.3 mg/kg.
Table 1.
Colony count results for kidneys and lungs of mice infected with A. fumigatus isolate FMR 10528 at two different inocula
| Inoculum (CFU/mouse) | Treatment groupa | Colony count (mean ± SD log10 CFU/g of tissue) in: |
|
|---|---|---|---|
| Kidney | Lung | ||
| 1 × 104 | Control | 4.03 ± 0.29 | 2.52 ± 1.00 |
| VRC 25 | 3.26 ± 0.21b | 1.31 ± 0.53b | |
| AMB 0.3 | 3.72 ± 0.43 | 2.73 ± 0.80 | |
| AMB 0.8 | 1.52 ± 0.35b,c,e | 0.01 ± 0.02b,c | |
| VRC 25 + AMB 0.3 | 2.61 ± 0.45b,c | 0.91 ± 0.85b,c | |
| 1 × 106 | Control | 4.81 ± 0.26 | 2.38 ± 0.27 |
| AMB 0.8 | 3.44 ± 0.14b | 2.30 ± 0.20 | |
| VRC 25 + AMB 0.3 | 3.53 ± 0.60b | 1.32 ± 0.40b,d | |
VRC 25, voriconazole at 25 mg/kg/day; AMB 0.3, amphotericin B deoxycholate at 0.3 mg/kg/day; AMB 0.8, amphotericin B deoxycholate at 0.8 mg/kg/day; VRC 25 + AMB 0.3, voriconazole at 25 mg/kg/day plus amphotericin B deoxycholate at 0.3 mg/kg/day.
P ≤ 0.05 versus control group.
P ≤ 0.05 versus VRC at 25 mg/kg.
P ≤ 0.05 versus AMB at 0.8 mg/kg.
P ≤ 0.05 versus VRC at 25 mg/kg plus amphotericin B at 0.3 mg/kg.
At the highest inoculum, the combined therapy significantly prolonged mouse survival in comparison to the control group. AMB at 0.8 mg/kg/day did not show efficacy (Fig. 1). The combination reduced the fungal load in both organs, but AMB at 0.8 mg/kg did so only in kidney (Table 1).
Although previous in vitro studies suggested possible antagonism between azoles and polyenes (21), clinical evidence does not support this concern, and in vivo data demonstrated the potential use of this combination for treating invasive fungal infections (9, 10, 19). As in previous studies (22), the combination of VRC plus AMB showed indifferent interaction in vitro; however, it worked in vivo better than the corresponding monotherapies, even using a suboptimal AMB dose. This suggests an important therapeutic benefit, since it minimizes the adverse effects that limit the use of AMB. Previous animal studies with this combination have given contradictory results. In a murine central nervous system infection model, AMB plus VRC showed efficacy superior to those of the corresponding monotherapies (19), while in two models of invasive pulmonary infection in mice and guinea pigs, this combination did not show significantly higher efficacy than the respective monotherapies (23, 24). However, the studies coincided in the absence of antagonism between these two drugs. The good in vivo response to the combination therapy observed in our experiment might be related to the improvement in voriconazole activity caused by a low concentration of amphotericin B, as has been reported before (19). The same phenomenon has been observed in vitro previously for the combination of amphotericin B plus posaconazole and could be explained by the destabilization of the fungal membrane by the polyene, which facilitates the entry of the azole into the fungal cell (25).
The lack of in vivo efficacy of the VRC treatment against the isolate used in our study is interesting because it showed a MIC below the epidemiological cutoff value proposed for this species (1 μg/ml) (26). This issue has been discussed in a recent study which demonstrated that isolates of A. fumigatus with VRC MICs of ≥0.5 μg/ml show variable and unpredictable in vivo responses to this drug (13).
In conclusion, the combination of VRC plus AMB at a suboptimal dose improved the efficacy of the respective monotherapies in the experimental IA. Further studies involving more strains are warranted to establish the possible use of this combination in the treatment of IA refractory to VRC therapy.
Footnotes
Published ahead of print 24 June 2013
REFERENCES
- 1.Steinbach WJ, Marr KA, Anaissie EJ, Azie N, Quan SP, Meier-Kriesche HU, Apewokin S, Horn DL. 2012. Clinical epidemiology of 960 patients with invasive aspergillosis from the PATH Alliance registry. J. Infect. 65:453–464 [DOI] [PubMed] [Google Scholar]
- 2.Mayr A, Lass-Flörl C. 2011. Epidemiology and antifungal resistance in invasive aspergillosis according to primary disease—review of the literature. Eur. J. Med. Res. 16:153–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Segal BH, Steinbach WJ, Stevens DA, van Burik JA, Wingard JR, Patterson TF. 2008. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin. Infect. Dis. 46:327–360 [DOI] [PubMed] [Google Scholar]
- 4.Pfaller M, Boyken L, Hollis R, Kroeger J, Messer S, Tendolkar S, Diekema D. 2011. Use of epidemiological cutoff values to examine 9-year trends in susceptibility of Aspergillus species to the triazoles. J. Clin. Microbiol. 49:586–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krishnan-Natesan S, Wu W, Cutright JL, Chandrasekar PH. 2012. In vitro-in vivo correlation of voriconazole resistance due to G448S mutation (cyp51A gene) in Aspergillus fumigatus. Diagn. Microbiol. Infect. Dis. 74:272–274 [DOI] [PubMed] [Google Scholar]
- 6.Beiras-Fernandez A, Bigdeli AK, Nickel T, Michel S, Ueberfuhr P, Reichart B, Kaczmarek I. 2011. Combination antifungal therapy for invasive pulmonary aspergillosis in a heart transplant recipient. Exp. Clin. Transplant. 4:279–283 [PubMed] [Google Scholar]
- 7.Baddley JW, Andes DR, Marr KA, Kauffman C, Kontoyiannis DP, Ito JI, Schuster MG, Brizendine KD, Patterson TF, Lyon GM, Boeckh M, Oster RA, Chiller T, Pappas PG. 2013. Antifungal therapy and length of hospitalization in transplant patients with invasive aspergilosis. Med. Mycol. 51:128–135 [DOI] [PubMed] [Google Scholar]
- 8.Durand-Joly I, Alfandari S, Benchikh Z, Rodrigue M, Espinel-Ingroff A, Catteau B, Cordevant C, Camus D, Dei-Cas E, Bauters F, Delhaes L, De Botton S. 2003. Successful outcome of disseminated Fusarium infection with skin localization treated with voriconazole and amphotericin B-lipid complex in a patient with acute leukemia. J. Clin. Microbiol. 41:4898–4900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crum-Cianflone NF. 2007. Voriconazole in combination with amphotericin B for salvage therapy of coccidioidomycosis. Case report and review of the literature. Infect. Dis. Clin. Pract. 15:265–268 [Google Scholar]
- 10.Loyse A, Wilson D, Meintjes G, Jarvis JN, Bicanic T, Bishop L, Rebe K, Williams A, Jaffar S, Bekker LG, Wood R, Harrison T. 2012. Comparison of the early fungicidal activity of high-dose fluconazole, voriconazole, and flucytosine as second-line drugs given in combination with amphotericin B for the treatment of HIV-associated cryptococcal meningitis. Clin. Infect. Dis. 54:121–128 [DOI] [PubMed] [Google Scholar]
- 11.Cuenca-Estrella M. 2004. Combinations of antifungal agents in therapy—what value are they? J. Antimicrob. Chemother. 54:854–869 [DOI] [PubMed] [Google Scholar]
- 12.Mukherjee PK, Sheehan DJ, Hitchcock CA, Ghannoum MA. 2005. Combination treatment of invasive fungal infections. Clin. Microbiol. Rev. 18:163–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salas V, Pastor FJ, Calvo E, Sutton DA, Fothergill AW, Guarro J. 2013. Evaluation of in vitro activity of voriconazole as predictive of in vivo outcome in a murine Aspergillus fumigatus infection model. Antimicrob. Agents Chemother. 57:1404–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clinical and Laboratory Standards Institute 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard, 2nd ed. Document M38-A2 CLSI, Wayne, PA [Google Scholar]
- 15.Dannaoui E, Lortholary O, Dromer F. 2004. In vitro evaluation of double and triple combinations of antifungal drugs against Aspergillus fumigatus and Aspergillus terreus. Antimicrob. Agents Chemother. 48:970–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Odds FC. 2003. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 52:1. [DOI] [PubMed] [Google Scholar]
- 17.Sun QN, Najvar LK, Bocanegra R, Loebenberg D, Graybill JR. 2002. In vivo activity of posaconazole against Mucor spp. in an immunosuppressed mouse model. Antimicrob. Agents Chemother. 46:2310–2312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warn PA, Sharp A, Mosquera J, Spickermann J, Schmitt-Hoffmann A, Heep M, Denning DW. 2006. Comparative in vivo activity of BAL4815, the active component of the prodrug BAL8557, in a neutropenic murine model of disseminated Aspergillus flavus. J. Antimicrob. Chemother. 58:1198–1207 [DOI] [PubMed] [Google Scholar]
- 19.Clemons KV, Espiritu M, Parmar R, Stevens DA. 2005. Comparative efficacies of conventional amphotericin B, liposomal amphotericin B (AmBisome), caspofungin, micafungin, and voriconazole alone and in combination against experimental murine central nervous system aspergillosis. Antimicrob. Agents Chemother. 49:4867–4875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sugar Liu AM XP. 2000. Effect of grapefruit juice on serum voriconazole concentrations in the mouse. Med. Mycol. 38:209–212 [DOI] [PubMed] [Google Scholar]
- 21.Meletiadis J, Petraitis V, Petraitiene R, Lin P, Stergiopoulou T, Kelaher AM, Sein T, Schaufele RL, Bacher J, Walsh TJ. 2006. Triazole-polyene antagonism in experimental invasive pulmonary aspergillosis: in vitro and in vivo correlation. J. Infect. Dis. 194:1008–1018 [DOI] [PubMed] [Google Scholar]
- 22.Cuenca-Estrella M, Gomez-Lopez A, Garcia-Effron G, Alcazar-Fuoli L, Mellado E, Buitrago MJ, Rodriguez-Tudela JL. 2005. Combined activity in vitro of caspofungin, amphotericin B, and azole agents against itraconazole-resistant clinical isolates of Aspergillus fumigatus. Antimicrob. Agents Chemother. 49:1232–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chandrasekar PH, Cutright JL, Manavathu EK. 2004. Efficacy of voriconazole plus amphotericin B or micafungin in a guinea-pig model of invasive pulmonary aspergillosis. Clin. Microbiol. Infect. 10:925–928 [DOI] [PubMed] [Google Scholar]
- 24.Clemons KV, Schwartz JA, Stevens DA. 2011. Therapeutic and toxicologic studies in a murine model of invasive pulmonary aspergillosis. Med. Mycol. 49:834–847 [DOI] [PubMed] [Google Scholar]
- 25.Perkhofer S, Lugger H, Dierich MP, Lass-Flörl C. 2007. Posaconazole enhances the activity of amphotericin B against Aspergillus hyphae in vitro. Antimicrob. Agents Chemother. 51:791–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Espinel-Ingroff A, Diekema DJ, Fothergill A, Johnson E, Pelaez T, Pfaller MA, Rinaldi MG, Cantón E, Turnidge J. 2010. Wild-type MIC distributions and epidemiological cutoff values for the triazoles and six Aspergillus spp. for the CLSI broth microdilution method (M38-A2 document). J. Clin. Microbiol. 48:3251–3257 [DOI] [PMC free article] [PubMed] [Google Scholar]