Abstract
Trough (predose) voriconazole concentrations in plasma and pulmonary epithelial lining fluid (ELF) of lung transplant recipients receiving oral voriconazole preemptive treatment were determined. The mean (± standard deviation [SD]) ELF/plasma ratio was 12.5 ± 6.3. A strong positive linear relationship was noted between trough plasma and ELF voriconazole concentrations (r2 = 0.87), suggesting the feasibility of using trough plasma voriconazole concentration as a surrogate to estimate the corresponding concentration in ELF of lung transplant recipients.
TEXT
Invasive fungal infections (IFIs) are a major cause of morbidity and mortality in lung transplant recipients. While IFIs caused by Aspergillus spp. are relatively uncommon (6 to 16%) (1), noninvasive Aspergillus colonization occurs in approximately one-third of lung transplant recipients (2). These colonized patients may be predisposed to a high risk of IFIs due to failure of anastomosis healing, neutrophil dysfunction, and, to a lesser degree, T-cell immunosuppression. Even in the absence of fungal invasion, Aspergillus colonization and bronchitis may lead to graft dysfunction and increase the risk of bronchiolitis obliterans syndrome in lung transplant recipients (2). A preemptive antifungal approach is an attractive strategy where antifungal treatment is administered to high-risk patients with pre- and/or posttransplant bronchial Aspergillus colonization (3). Voriconazole (VRC) is widely employed (4), but its oral bioavailability is significantly lower in lung transplant recipients (24 to 64%) than healthy adults (96%) (5). The risk of suboptimal VRC exposure is of concern, as predose (trough) serum concentrations below 1.5 μg/ml have been correlated with increased likelihood of breakthrough IFIs and fungal airway colonization (6). For treatment of established IFIs, a higher therapeutic range (1.5 to 4.5 μg/ml in plasma) is required for >85% probability of clinical success (7). As ∼78% of IFI cases are limited to the lungs (8), it is imperative to ensure adequate antifungal concentrations at the airway interface for eradication of the colonizing fungal isolate(s), or at least to prevent fungal invasion.
The concentration of VRC within the lung epithelial lining fluid (ELF) may provide a better estimate of antifungal exposure for prophylaxis and treatment of the early stage of pulmonary IFI (9); however, routine monitoring of VRC concentration in the ELF via bronchoscopy is not practical. The use of plasma VRC concentration as a surrogate for the ELF concentration is therefore an attractive option. A study investigated the bronchopulmonary penetration of VRC following intravenous administration for 3 days to healthy adults; bronchoalveolar lavage (BAL) fluid and plasma samples were collected at 4, 8, and 12 h (10). The relevance of this study to lung transplant patients with pulmonary colonization or IFIs is unclear. Another study reported the concentration of VRC in ELF and plasma (samples collected simultaneously) of lung transplant recipients, but only two out of 12 patients had trough concentrations measured (11). The current study explored the relationship between trough plasma and ELF VRC concentrations in lung transplant recipients.
This was a prospective observational pilot study. Participants aged ≥18 years, receiving oral VRC for preemptive treatment and undergoing scheduled bronchoscopy with BAL after lung transplantation, were enrolled, and their consent was obtained. Every patient was sampled predose after at least 1 week of oral VRC therapy and followed up to 3 months post-BAL. Human ethics committees of Alfred Hospital and Monash University approved the study. Fiber-optic bronchoscopy was performed per standard procedure at the Alfred Hospital. Sterile 20-ml aliquots of normal saline were sequentially instilled into the right middle lobe and immediately aspirated into collection traps. The total volume of pooled BAL fluid aspirates was measured and recorded. The BAL fluid sample was placed on ice prior to centrifugation (800 × g, 5 min, 4°C), as described previously (10). A blood sample (3 ml) was collected during bronchoscopy and centrifuged (1,505 × g, 10 min, 4°C). The BAL fluid supernatant and plasma samples were stored at −80°C and −20°C, respectively, until analysis.
VRC concentrations in plasma and BAL fluid were measured using a high-performance liquid chromatography–fluorescence method (12), modified slightly for plasma in the sample preparation and chromatographic conditions. Normal saline (surrogate matrix) was used to prepare the calibration curves for the BAL fluid assay and validated using VRC-free blank BAL fluid from lung transplant recipients. The calibration curves were linear from 2.5 to 500 ng/ml and 50 to 10,000 ng/ml for BAL fluid and plasma assays, respectively. Urea concentrations in the BAL fluid supernatant and plasma were determined using the QuantiChrom urea assay kit. The volume of ELF and the VRC concentration in the ELF were calculated using the urea dilution method as previously reported (13, 14). The relationship between plasma and ELF VRC concentrations was examined using linear regression.
Between October 2011 and July 2012, 12 consecutive patients were enrolled (Table 1). BAL fluid samples from three patients were excluded from analysis due to either gross contamination with blood or interference in the analytical measurement of VRC concentration. All except one patient reported full adherence to VRC during the previous 7 days. An average (standard deviation [SD]) of 78 (32) ml of saline was instilled, with 36 ± 9 ml of BAL fluid recovered. The calculated volume (mean ± SD) of ELF recovered was 0.45 ± 0.21 ml, or 1.3% of total BAL fluid recovered. Table 1 showed that VRC concentrations in ELF were higher than the reported MIC90 (i.e., 0.5 μg/ml) against most Aspergillus and yeast isolates in all patients (15). A strong positive linear relationship was noted between ELF and plasma VRC concentrations (r2 = 0.868, P < 0.001) (Fig. 1), which can be described by the regression equation [VRC]ELF = 15.44[VRC]plasma − 1.16, where [VRC]ELF was the VRC concentration in ELF and [VRC]plasma was the plasma VRC concentration. The VRC ELF/plasma ratio (mean ± SD) was 12.5 ± 6.3 (coefficient of variation [CV], 50%).
Table 1.
Characteristics and outcome of lung transplant recipients receiving VRCd
| Patient no. | Age (yr)/sex | Type of transplant | Underlying disease | Timing of bronchoscopy posttransplant (days) | VRC dose regimen (mg b.i.d.) | Timing of sampling after last dose (h) | Treatment duration of VRC prior to BAL (mo) | Plasma (μg/ml) | ELF (μg/ml) | ELF/plasma ratioe | Outcome | Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 53/M | Singlea | IPF | 54 | 200 | 13.2 | 1.0 | 1.67 | 42.3 | 25.2 | Successb | |
| 2 | 57/M | Double | IPF | 197 | 200 | 14.1 | 3.5 | 3.18 | 37.9 | 11.9 | Failure | Persistent Paecilomyces variotiic colonization |
| 3 | 63/F | Double | COPD | 14 | 200 | 13.0 | 2.3 | 0.15 | 1.56 | 10.4 | Failure | Breakthrough Aspergillus fumigatus colonization |
| 4 | 49/F | Double | Sarcoidosis | 3,285 | 200 | 11.8 | 12 | 0.57 | 5.75 | 10.0 | Successb | |
| 5 | 60/F | Double | COPD | 227 | 150 | 14.0 | 7.5 | 3.87 | 59.5 | 15.4 | Failure | Persistent yeast colonization |
| 6 | 41/F | Double | Bronchiectasis | 2,920 | 200 | 25.8 | 36 | 0.12 | NA | NA | Failure | Persistent Scedosporium prolificans colonization |
| 7 | 55/F | Singlea | IPF | 120 | 200 | 16.0 | 0.75 | 0.67 | 2.98 | 4.5 | Successb | |
| 8 | 46/F | Double | IPF | 180 | 150 | 16.0 | 2.5 | 0.09 | NA | NA | Successb | |
| 9 | 61/F | Double | COPD | 99 | 200 | 13.0 | 0.38 | 3.99 | NA | NA | Successb | |
| 10 | 38/M | Double | ILD and PAH | 95 | 200 | 13.8 | 1.25 | 0.48 | 2.92 | 6.1 | Successb | |
| 11 | 58/F | Double | COPD | 60 | 250 | 16.7 | 2.0 | 1.56 | 27.4 | 17.6 | Successb | |
| 12 | 52/F | Double | Emphysema | 480 | 200 | 12.7 | 0.5 | 0.70 | 7.66 | 11.0 | Successb |
BAL fluid sampling was performed at the transplanted lung.
Successful eradication of airway fungal colonization and no breakthrough infection within 3 months post-BAL.
Voriconazole-resistant strain but sensitive to amphotericin B, posaconazole, and caspofungin.
Abbreviations: M, male; F, female; IPF, idiopathic pulmonary fibrosis; COPD, cronic obstructive pulmonary disease; ILD, interstitial lung disease; PAH, pulmonary arterial hypertension; b.i.d., twice daily; NA, not available.
Mean ± SD, 12.5 ± 6.3.
Fig 1.
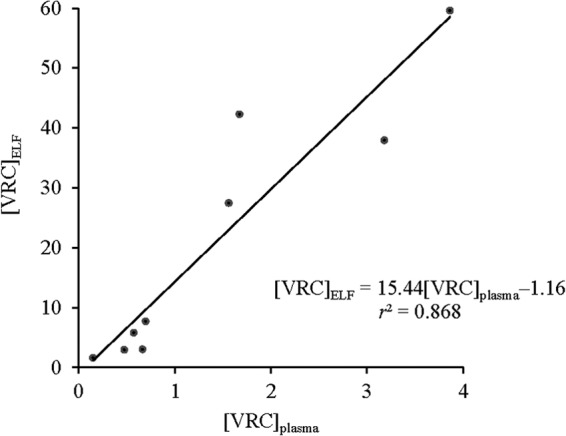
Linear model predicting the relationship between VRC concentrations in plasma ([VRC]plasma) and ELF ([VRC]ELF); P < 0.001.
In this pilot study, we have observed a strong linear relationship between trough plasma and ELF VRC concentrations, which provides a basis to support the use of trough plasma VRC concentration as a surrogate for the corresponding concentration in ELF. Despite differences in study design (trough versus random sampling, early versus late postoperative time) between the present and previous study in lung transplant recipients (11), the mean ELF/plasma ratio of VRC noted in this study was similar to, but less variable than, that in the earlier study (mean ± SD, 11 ± 8; CV, 73%). The exact mechanism for the higher VRC concentration in ELF than in plasma is not fully understood. One possible mechanism relates to ion trapping of VRC, which is a weak organic base (pKa, 1.76), in the lower-pH environment of lung fluid (16). However, ion trapping will have its greatest impact on a weak organic base with a pKa in the range of 7.5 to 10.5 and is therefore unlikely to be a major contributor to our observed high ELF/plasma concentration ratio. Second, higher binding to proteins in ELF than in plasma could, in theory, also contribute to an ELF/plasma concentration ratio greater than unity. However, the albumin concentration in ELF has been reported to be only ∼9% of that in plasma (14), and there is a paucity of information about the types and concentrations of other proteins. Thus, differential protein binding in ELF and plasma is not likely to be an explanation for the high concentration ratio. Third, a relatively high intracellular concentration of VRC within alveolar macrophages (10), likely to be important in treating fungal infections, could result in in vitro efflux of VRC from these cells during BAL fluid sample collection and processing, thereby contributing to the high ELF/plasma ratio (17), an effect that is inherent with the BAL procedure.
In conclusion, the current study found a close relationship between trough plasma and ELF VRC concentrations, suggesting that trough plasma VRC concentration could serve as a potential surrogate of the concentration in ELF. Prospective evaluation of the use of trough plasma VRC concentration as an aid to clinical decision making around VRC dosage regimens is under way.
ACKNOWLEDGMENTS
We thank the participants who provided BAL fluid and plasma samples, the clinicians and nursing staff in the Lung Transplant Service of the Alfred Hospital for assistance in sample collection, and Lauren Mitchell for help in flagging eligible participants.
An Endeavor Postgraduate Award to S.-C. Heng is gratefully acknowledged.
Footnotes
Published ahead of print 1 July 2013
REFERENCES
- 1.Singh N, Paterson DL. 2005. Aspergillus infections in transplant recipients. Clin. Microbiol. Rev. 18:44–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weigt SS, Elashoff RM, Huang C, Ardehali A, Gregson AL, Kubak B, Fishbein MC, Saggar R, Keane MP, Lynch JP, III, Zisman DA, Ross DJ, Belperio JA. 2009. Aspergillus colonization of the lung allograft is a risk factor for bronchiolitis obliterans syndrome. Am. J. Transplant. 9:1903–1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sole A, Salavert M. 2008. Fungal infections after lung transplantation. Transplant. Rev. 22:89–104 [DOI] [PubMed] [Google Scholar]
- 4.Neoh CF, Snell GI, Kotsimbos T, Levvey B, Morrissey CO, Slavin MA, Stewart K, Kong DC. 2011. Antifungal prophylaxis in lung transplantation—a world-wide survey. Am. J. Transplant. 11:361–366 [DOI] [PubMed] [Google Scholar]
- 5.Han K, Capitano B, Bies R, Potoski BA, Husain S, Gilbert S, Paterson DL, McCurry K, Venkataramanan R. 2010. Bioavailability and population pharmacokinetics of voriconazole in lung transplant recipients. Antimicrob. Agents Chemother. 54:4424–4431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitsani D, Nguyen MH, Shields RK, Toyoda Y, Kwak EJ, Silveira FP, Pilewski JM, Crespo MM, Bermudez C, Bhama JK, Clancy CJ. 2012. Prospective, observational study of voriconazole therapeutic drug monitoring among lung transplant recipients receiving prophylaxis: factors impacting levels of and associations between serum troughs, efficacy, and toxicity. Antimicrob. Agents Chemother. 56:2371–2377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pascual A, Csajka C, Buclin T, Bolay S, Bille J, Calandra T, Marchetti O. 2012. Challenging recommended oral and intravenous voriconazole doses for improved efficacy and safety: population pharmacokinetics-based analysis of adult patients with invasive fungal infections. Clin. Infect. Dis. 55:381–390 [DOI] [PubMed] [Google Scholar]
- 8.Pappas PG, Alexander BD, Andes DR, Hadley S, Kauffman CA, Freifeld A, Anaissie EJ, Brumble LM, Herwaldt L, Ito J, Kontoyiannis DP, Lyon GM, Marr KA, Morrison VA, Park BJ, Patterson TF, Perl TM, Oster RA, Schuster MG, Walker R, Walsh TJ, Wannemuehler KA, Chiller TM. 2010. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin. Infect. Dis. 50:1101–1111 [DOI] [PubMed] [Google Scholar]
- 9.Hope WW, Drusano GL. 2009. Antifungal pharmacokinetics and pharmacodynamics: bridging from the bench to bedside. Clin. Microbiol. Infect. 15:602–612 [DOI] [PubMed] [Google Scholar]
- 10.Crandon JL, Banevicius MA, Fang AF, Crownover PH, Knauft RF, Pope JS, Russomanno JH, Shore E, Nicolau DP, Kuti JL. 2009. Bronchopulmonary disposition of intravenous voriconazole and anidulafungin given in combination to healthy adults. Antimicrob. Agents Chemother. 53:5102–5107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Capitano B, Potoski BA, Husain S, Zhang S, Paterson DL, Studer SM, McCurry KR, Venkataramanan R. 2006. Intrapulmonary penetration of voriconazole in patients receiving an oral prophylactic regimen. Antimicrob. Agents Chemother. 50:1878–1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heng SC, Nation RL, Levvey B, Snell GI, Slavin MA, Kong DC. 2013. Quantification of voriconazole in human bronchoalveolar lavage fluid using high-performance liquid chromatography with fluorescence detection. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 913-914:171–175 [DOI] [PubMed] [Google Scholar]
- 13.Capitano B, Mattoes HM, Shore E, O'Brien A, Braman S, Sutherland C, Nicolau DP. 2004. Steady-state intrapulmonary concentrations of moxifloxacin, levofloxacin, and azithromycin in older adults. Chest 125:965–973 [DOI] [PubMed] [Google Scholar]
- 14.Rennard SI, Basset G, Lecossier D, O'Donnell KM, Pinkston P, Martin PG, Crystal RG. 1986. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J. Appl. Physiol. 60:532–538 [DOI] [PubMed] [Google Scholar]
- 15.Sabatelli F, Patel R, Mann PA, Mendrick CA, Norris CC, Hare R, Loebenberg D, Black TA, McNicholas PM. 2006. In vitro activities of posaconazole, fluconazole, itraconazole, voriconazole, and amphotericin B against a large collection of clinically important molds and yeasts. Antimicrob. Agents Chemother. 50:2009–2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng AW, Bidani A, Heming TA. 2004. Innate host defense of the lung: effects of lung-lining fluid pH. Lung 182:297–317 [DOI] [PubMed] [Google Scholar]
- 17.Kiem S, Schentag JJ. 2008. Interpretation of antibiotic concentration ratios measured in epithelial lining fluid. Antimicrob. Agents Chemother. 52:24–36 [DOI] [PMC free article] [PubMed] [Google Scholar]


