Abstract
Streptomycin binds to the bacterial ribosome and disrupts protein synthesis by promoting misreading of mRNA. Restrictive mutations on the ribosomal subunit protein S12 confer a streptomycin resistance (Strr) phenotype and concomitantly increase the accuracy of the decoding process and decrease the rate of translation. Spontaneous Strr mutants of Escherichia coli O157:H7 have been generated for in vivo studies to promote colonization and to provide a selective marker for this pathogen. The locus of enterocyte effacement (LEE) of E. coli O157:H7 encodes a type III secretion system (T3SS), which is required for attaching and effacing to the intestinal epithelium. In this study, we observed decreases in both the expression and secretion levels of the T3SS translocated proteins EspA and EspB in E. coli O157:H7 Strr restrictive mutants, which have K42T or K42I mutations in S12. However, mildly restrictive (K87R) and nonrestrictive (K42R) mutants showed slight or indistinguishable changes in EspA and EspB secretion. Adherence and actin staining assays indicated that restrictive mutations compromised the formation of attaching and effacing lesions in E. coli O157:H7. Therefore, we suggest that E. coli O157:H7 strains selected for Strr should be thoroughly characterized before in vivo and in vitro experiments that assay for LEE-directed phenotypes and that strains carrying nonrestrictive mutations such as K42R make better surrogates of wild-type strains than those carrying restrictive mutations.
INTRODUCTION
Streptomycin (Str), produced by the actinobacterium Streptomyces griseus, was the first discovered antibiotic of the aminoglycosides class (1). Str is bactericidal and targets the ribosome, resulting in misreading of mRNA. Several biochemical and structural studies have demonstrated that this miscoding occurs at the step of ternary complex (EF-Tu-GTP-amino acid tRNA) binding to the ribosomal A site (2). In the presence of Str, the forward rate constant of the next kinetic step, GTPase activation and hydrolysis, is indistinguishable for both cognate or near-cognate ternary complexes, resulting in reduced accuracy of amino acid-tRNA selection (2–4).
Streptomycin-resistant (Strr) bacteria were discovered shortly after clinical introduction of the antibiotic (5, 6). Spontaneous mutations conferring Strr in E. coli and other bacteria are typically found within the rpsL-encoded 30S ribosomal subunit protein S12 (7, 8), which is involved in the inspection of codon-anticodon pairings in the A site (9). Strr-conferring mutations within S12 are divided into two classes: restrictive and nonrestrictive (7). Mutations conferring restrictive decoding accuracy, including K42T (9) and K42N (10), decrease the rate of translation (7, 9, 11, 12) and increase cell doubling time (9, 13–16). Nonrestrictive mutations, such as K42R (10), are similar to wild-type strains in terms of translational accuracy and rate of peptide elongation (7). “Mildly restrictive” mutations have also been described (17).
Spontaneous Strr mutants of the food-borne pathogen enterohemorrhagic E. coli O157:H7 have been used for genetic (18) and animal colonization (19–22) studies. E. coli O157:H7 causes serious diseases, including hemorrhagic colitis and hemolytic-uremic syndrome (HUS) (23). Many of the key virulence factors of O157:H7 are encoded within the locus of enterocyte effacement (LEE), which is also found in both enteropathogenic E. coli (EPEC) (24, 25) and in the mouse pathogen Citrobacter rodentium (26). The LEE facilitates the intimate adherence between bacteria and epithelial cells and directs the effacement of microvilli, which collectively is called the attaching and effacing (A/E) phenotype (27). The protein products encoded by the LEE include an outer membrane protein (intimin), structural components of a type III secretion system (T3SS), and secreted effector proteins (28). During A/E lesion formation, LEE-encoded EspA (E. coli-secreted protein A) assembles into the T3SS syringe, forming the physical conduit between the bacterium and the eukaryotic cell surface (29). At the distal end of this conduit, EspB and EspD make 3- to 5-nm pores in the host epithelial cell membrane, completing a channel for protein translocation into host cells (30, 31). A protein designated the translocated intimin receptor (Tir) inserts within the host membrane and binds to intimin, mediating the intimate attachment of bacteria to the epithelium (32, 33). Subsequently, the cytoplasmic domains of Tir can trigger a marked rearrangement of the host actin cytoskeleton, resulting in the formation of a pedestal structure beneath the adherent bacterium (34, 35).
In the current study, we isolated 7 unique spontaneous Strr E. coli O157:H7 mutants that can be classified as restrictive, mildly restrictive, or nonrestrictive. We observed a striking decrease in EspA and EspB secretion levels with the restrictive mutants as well as compromised adherence and A/E lesion formation in vitro. We propose that restrictive Strr mutations in E. coli O157:H7 may impact in vivo and in vitro phenotypes that depend upon a functional T3SS.
MATERIALS AND METHODS
Bacterial strains and culture media.
The bacterial strains and plasmids used in this study are described in Table 1. Strains were routinely grown in liquid or solid lysogeny broth medium (LB) (36, 37). All stocks were maintained at −80°C in 10% glycerol. Strr mutants of E. coli O157:H7 strains were screened by plating overnight cultures of the wild-type strains on LB agar supplemented with Str (100 μg/ml). Putative Strr colonies were streaked on the same medium for purification. In addition to the prototypical E. coli O157:H7 strains EDL933 (38) and Sakai (39), three previously characterized O157:H7 clinical isolates obtained from the Pennsylvania Department of Health (40) were selected for comparative purposes.
Table 1.
Strains used in this study
| Strain | Relevant characteristics | Reference |
|---|---|---|
| Sakai | E. coli O157:H7; stx1+ stx2+ | W. Zhanga |
| Sakai-strR | Spontaneous Strr mutant of Sakai | This study |
| EDL933 | E. coli O157:H7; stx1+ stx2+ | 68 |
| EDL933-strR1 | Spontaneous Strr mutant of EDL933 | This study |
| EDL933-strR2 | Spontaneous Strr mutant of EDL933 | This study |
| EDL933-strR3 | Spontaneous Strr mutant of EDL933 | This study |
| PA4 | E. coli O157:H7; stx1+stx2+ | 40 |
| PA4-strR | Spontaneous Strr mutant of PA4 | This study |
| PA5 | E. coli O157:H7; stx1+stx2+ | 40 |
| PA5-strR | Spontaneous Strr mutant of PA5 | This study |
| PA11 | E. coli O157:H7; stx2+ | 40 |
| PA11-strR | Spontaneous Strr mutant of PA11 | This study |
Wei Zhang, Department of Biological and Chemical Sciences, Illinois Institute of Technology.
Analysis of secreted and intracellular proteins.
Quantification of secreted EspA and EspB was performed as previously reported (41). E. coli O157:H7 strains were incubated statically at 37°C for 8 h in both high-glucose Dulbecco's modified Eagle's medium (DMEM; with 4.5 g/liter glucose and l-glutamine and without sodium pyruvate; Cellgro, Manassas, VA) and low-glucose DMEM (with 1 g/liter glucose l-glutamine and sodium pyruvate; Gibco, Carlsbad, CA). Upon harvesting, no significant differences in cell density were observed between the wild type and mutants. Prior to precipitation, 2 μg/ml bovine serum albumin (BSA) was added to the supernatants as a control for the efficiency of the protein precipitation. BSA was visualized using a Coomassie brilliant blue staining. After SDS-PAGE, the gel was photographed using a transilluminator (UVP, Upland, CA). All experiments were conducted with at least two biological replicates.
For total intracellular protein, 2 ml of each culture was centrifuged (16,000 × g, 2 min, 20°C), and the pellet was resuspended in 100 μl of 1× Laemmli buffer (12 mM Tris-Cl [pH 6.8], 0.4% SDS, 2% glycerol, 1% β-mercaptoethanol, and 0.002% bromophenol blue). To lyse the bacterial cells, the samples were incubated at 100°C for 10 min. The Western blot method referenced above was used to visualize secreted EspB and EspA and was also used to detect and quantify the intracellular proteins. RNA polymerase (Pol) was used as an internal control and was detected using mouse anti-RNA Pol α (Santa Cruz Biotechnology, Santa Cruz, CA), followed by IRDye 800CW goat anti-mouse IgG (Licor, Lincoln, NE). The relative intracellular levels of EspA or EspB (in arbitrary units) from EDL933 derivatives (Espmutant), compared with the wild-type strain (ESPWT), were calculated as follows: (Espmutant/RNA Polmutant)/(EspWT/RNA PolWT). The experiments were performed in three biological replicates.
Growth curve and plating assay.
To profile the bacterial growth in high-glucose DMEM, overnight cultures of each strain were diluted to an optical density at 600 nm (OD600) of 0.05 and incubated statically at 37°C for 8 h. The OD600 was taken over the time course to profile the changes in cell densities. Measurements between 20 min and 2.5 h postinoculation were used to calculate the bacterial growth rates during the logarithmic phase. The experiments were done in three biological replicates. Cultures were sampled at the end of incubation, and the CFU/ml was determined by plating serial dilutions onto LB agar. Each plating assay was performed in two biological replicates with two experimental replicates.
Sequencing of the rpsL gene.
Identification of rpsL mutations in the genomes of O157:H7 isolates was accomplished by PCR amplification using the primer pair rpsL-L and rpsL-R (see Table S1 in the supplemental material), followed by DNA sequencing at the Penn State Genomics Core Facility (University Park, PA).
Cell culture and adherence assays.
HeLa cells were maintained in T75 flasks containing high-glucose DMEM at 37°C and 5% CO2. DMEM was supplemented with 10% fetal bovine serum (FBS; Life Technologies, Carlsbad, CA) and 1% antibiotic/antimycotic (Life Technologies, Carlsbad, CA). For adhesion assays, 24-well plates were seeded with 105 HeLa cells per well and incubated as described above until monolayers were confluent. Before use, HeLa cells were washed twice with 1 ml of sterile phosphate-buffered saline (PBS, pH 7.4). Cells were then replenished with DMEM containing no additives. Bacteria were grown in LB broth overnight at 37°C, and Strr mutants were grown in LB with the addition of Str. A 1:25 dilution of the overnight culture was made, and bacteria were grown to the logarithmic phase. Triplicate replicates of each strain were infected with a multiplicity of infection (MOI) of 10. Inoculum inputs were verified by dot plating onto LB plates containing selective antibiotics. Infected monolayers were incubated for 2 h at 37°C and 5% CO2. Nonadherent bacteria were removed by three consecutive 1-ml PBS washes. Monolayers were then incubated with 200 μl of PBS containing 0.1% Triton X-100 until monolayers detached. Recovered bacteria were then serially diluted and plated via drop plate method onto LB plates containing selective antibiotic. Results are from three independent experiments. The percentage of bacterial recovery was calculated as the recovered cell density divided by that of the inocula and then normalized against that of the respective wild-type strain. In order to estimate the bacterial cell density after 2 h of incubation in high-glucose DMEM, EDL933 and its derivatives were grown under the same conditions but in the absence of HeLa cells, and the CFU/ml was determined in a plating assay. This experiment was performed with two biological replicates, each with two technical repeats.
Fluorescent actin staining assays.
Fluorescent actin staining assays (FAS) were performed as previously described (42), using low-glucose DMEM supplemented with 10% FBS during infection. The medium was changed 3 h postinoculation, and an additional 3 h of incubation was allowed for the actin rearrangement to occur. After staining, pedestal formation was quantified as the number of HeLa cells with pedestals divided by the total number of HeLa cells within one field. The experiments were run with four biological replicates, and a total of 9 fields (2 or 3 in each replicate experiment) were counted for each strain. In order to estimate the bacterial cell density before the medium change, we inoculated E. coli O157:H7 strains into the same medium in the absence of HeLa cells. After 3 h of incubation, each culture was sampled and plated on LB agar. This experiment was performed with two biological replicates, each with two technical repeats.
RNA preparation and reverse transcription-qPCR.
E. coli cultures were grown under similar conditions as described previously for the protein secretion assays, and the total RNA was harvested after an 8-h static incubation in high-glucose DMEM, using the mirVana miRNA isolation kit (Life Technologies, Carlsbad, CA). The protocol from the manufacturer was generally followed, with one exception: RLT buffer (Qiagen, Valencia, CA) plus 1% β-mercaptoethanol was used instead of the original lysis/binding solution for higher bacterial cell lysis efficiency. Reverse transcription (RT) was performed using the ThermoScript RT-PCR system (Invitrogen, Grand Island, NY) and the reverse primers espA-R, espB-R, and rrsA-R (see Table S1 in the supplemental material). Quantitative PCR (qPCR) was used to quantify the transcription levels of espA, espB, and the internal control, rrsA. Each reaction mixture (20 μl) contained 10 μl PerfeCta SYBR green FastMix for iQ (Quanta, Gaithersburg, MD), forward and reverse primers (espA-F/R and espB-F/R or rrsA-F/R, 0.1 μM [see Table S1]), and 2.5 μl of the cDNA as the template. When necessary, the cDNA templates were diluted 100-fold prior to qPCR, so that the resulting CT (threshold cycle) was in the linear range of the assay. The relative mRNA levels were then determined as follows: CT of Esp − CT of RrsA. Each qPCR assay was run in triplicate, and extracted E. coli O157:H7 Sakai genome DNA (positive control), as well as no-template (negative-control) reactions were included. A total of three biological replicates were performed. A no-reverse transcriptase control was conducted for each RNA sample.
Statistical analysis.
Statistical analyses (Student's t test, one-way analysis of variance [ANOVA] and Tukey's multiple-comparison test) were conducted using the Minitab 16.2.0 F-test, within Microsoft Excel, to determine equal variance of the means.
RESULTS
The nature of rpsL mutations in Strr strains affects EspA/EspB secretion.
To determine whether Strr-conferring mutations in the S12 subunit affect the production of virulence proteins, we isolated spontaneous mutants of the prototypical strain E. coli O157:H7 EDL933 and screened them for changes in secreted levels of the T3SS proteins EspA and EspB. Most Strr mutants had reduced secretion levels of EspA and EspB compared to the Strs wild-type strain. We confirmed that our observation was reproducible by streaking our laboratory stock of EDL933 for isolated colonies, generating new Strr mutants from these isolates, and comparing EspA and EspB secretion levels between the new wild-type and Strr strains (data not shown).
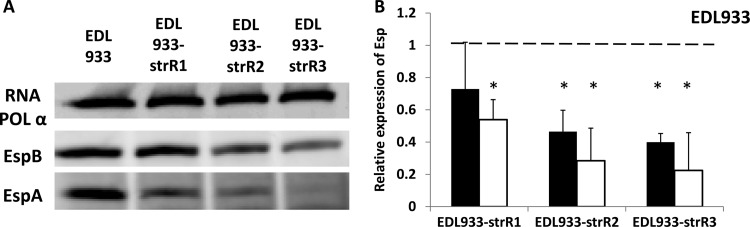
Next, we selected three spontaneous Strr mutants of E. coli O157:H7 EDL933 (Table 1) and characterized them by using a combination of Western blotting and DNA sequencing of rpsL. Three different mutations were identified: the isolates designated EDL933-strR1, -strR2, and -strR3 contained K42R, K42T, and K42N mutations in rpsL, respectively (Table 2). Secretion levels of EspA/B from EDL933-strR1 were indistinguishable from that from the wild-type strain; however, secretion levels for both EDL933-strR2 and -strR3 were dramatically reduced (Fig. 1). These phenotypes were observed in both high-glucose (Fig. 1) and low-glucose (data not shown) DMEM.
Table 2.
Point mutations identified in E. coli O157:H7 Strr strains
| Strain | S12 alterationa |
Phenotype | |
|---|---|---|---|
| Site 42 | Site 87 | ||
| Wild typeb | AAA(Lys) | AAA(Lys) | |
| EDL933-strR1 | AGA(Arg) | Nonrestrictive | |
| EDL933-strR2 | ACA(Thr) | Restrictive | |
| EDL933-strR3 | AAC(Asn) | Restrictive | |
| Sakai-strR | ACA(Thr) | Restrictive | |
| PA4-strR | ACA(Thr) | Restrictive | |
| PA5-strR | ACA(Thr) | Restrictive | |
| PA11-strR | AGA(Arg) | Mildly restrictive | |
The mutated bases are underlined.
The sequences of the wild-type allele of rpsL were the same in all E. coli O157:H7 strains included in this study.
Fig 1.
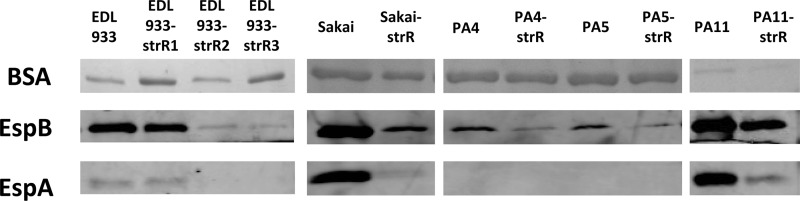
Secretion of EspA and EspB from E. coli O157:H7 strains and their respective Strr mutants. Total secreted protein was collected after 8 h of incubation in high-glucose DMEM and separated by SDS-PAGE using a 4-to-20% linear gradient polyacrylamide gel. BSA was detected by Coomassie blue staining. EspB and EspA were detected by Western blotting.
In order to determine whether the observed defects in secretion were specific to the EDL933 strain, we isolated Strr derivatives from a second prototypical O157:H7 strain, Sakai (39), and from three clinical isolates previously designated PA4, PA5, and PA11 (40) (Table 1). Three isolates (Sakai-strR, PA4-strR, and PA5-strR) carried the K42T mutation in S12 (Table 2), and all three showed reduced EspA and EspB secretion levels compared to their respective Strs wild-type strains (Fig. 1). The isolate PA11-strR, containing a K87R mutation previously defined as “mildly restrictive” (10, 17, 43), exhibited only a slight reduction of EspA and EspB compared to the wild-type strain (Fig. 1).
Restrictive Strr mutations alter the bacterial growth rate.
It was previously reported that restrictive mutations negatively impact the growth rate of E. coli (13–16). Hence, we compared the growth profiles of the three EDL933 derivatives with the wild type in high-glucose DMEM (see Fig. S1 in the supplemental material). There were no obvious differences in the lag times between EDL933, EDL933-strR1, EDL933-strR2, and EDL933-strR3; however, the doubling times during logarithmic growth were 63.0 ± 0.8, 63.3 ± 2.4, 68.5 ± 2.7, and 72.9 ± 0.8 (mean ± standard deviation) minutes, respectively. The OD600 of the two restrictive mutants, EDL933-strR2 and -strR3, were consistently below those of EDL933 and EDL933-strR1 for the first 5 h; however, no differences in cell density were observed between these strains 6 h postinoculation.
Intracellular expression of EspA and EspB in E. coli O157:H7 EDL933 and its Strr mutants.
We next asked whether defects in EspA and EspB secretion were mediated at the translational and/or transcriptional levels. To test the first hypothesis, we prepared whole-cell extracts of EDL933 and the three mutants after 8 h of incubation, when all cultures achieved the same optical density (see Fig. S1). Western blot analysis showed that the intracellular levels of EspB from EDL933-strR2 and -strR3 and of EspA from all three mutants were significantly lower than that seen with the wild-type strain (P < 0.05, Student's t test) (Fig. 2A). The reduction in EspA/B accumulation was greater for EDL933-strR2 and -strR3 than for EDL933-strR1.
Fig 2.
The effect of Strr mutations on EspA and EspB expression. (A) Representative Western blots of RNA Pol subunit α, EspB, and EspA of E. coli O157:H7 EDL933 and its mutants. (B) The intensities of Esp protein bands (closed boxes, EspB; open boxes, EspA) were analyzed using the Odyssey application software. The relative Esp expression levels of the derivative strains compared to wild-type EDL933 were calculated as described in the text. *, significantly less EspA or EspB was recovered from the whole-cell extract (P < 0.05, Student's t test). All results for Esp quantification are averages from three biological repeats, and error bars represent 1 standard deviation.
It is well recognized that restrictive Strr mutations lower the rate of protein synthesis (7, 9, 11, 12). However, a variety of proteins have also been identified that directly or indirectly impact transcription of LEE-encoded proteins (44). Therefore, our next question was whether the defects in EspA/B accumulation were due to decreased synthesis of one or more of these regulators. Using qPCR, we determined that the relative transcription levels (CT of Esp − CT of RrsA) for espA were 15.25 ± 1.28 (mean ± standard deviation), 14.70 ± 1.03, 15.29 ± 1.02, and 14.64 ± 1.12 for EDL933, EDL933-strR1, -strR2, and -strR3, respectively. In the case of espB, the values were 12.40 ± 0.15, 13.08 ± 1.14, 11.43 ± 2.02, and 12.70 ± 2.36, respectively. Although slight differences in espA/B transcription levels were seen among EDL933 and its mutants, the differences were not significant (P = 0.833 for espA, P = 0.665 for espB [one-way ANOVA]). This experiment supported a model where translational but not transcriptional defects are primarily responsible for the reductions in EspA/B accumulation described above.
Effect of Strr mutation on adherence of E. coli O157:H7 to HeLa cells.
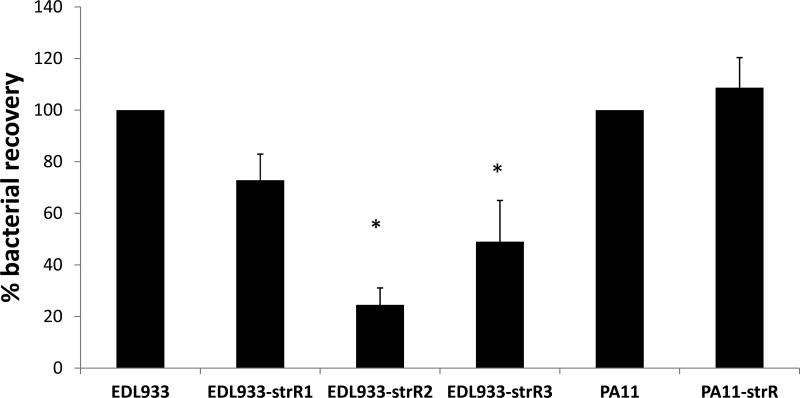
EspA and EspB are critical for T3SS-mediated attachment to human cell lines (45). Therefore, we next hypothesized that our Strr strains would have reduced abilities to adhere to HeLa cells compared to the wild type. Adherence of EDL933-strR1, EDL933-strR2, and EDL933-strR3 was decreased by 27%, 76%, and 51% from the wild-type EDL933, respectively (Fig. 3); the values for EDL933-strR2 and -strR3 were significantly decreased (P < 0.05, one-way ANOVA with Tukey's test). Hence, the decrease in adherence correlated with reduced EspA/B secretion levels (Fig. 1). Plate counts taken 2 h postinoculation for the wild-type strain, EDL933-strR1, -strR2, and -strR3 were estimated to be (1.50 ± 0.33) × 107 (mean ± standard deviation), (1.49 ± 0.27) × 107, (1.05 ± 0.17) × 107, and (0.54 ± 0.23) × 107 CFU/ml, respectively. Adherence levels were comparable for the mildly restrictive strain PA11-strR and its wild-type control (P = 0.50, Student's t test) (Fig. 3).
Fig 3.
Relative adherence levels of E. coli O157:H7 strains and their respective Strr mutants. HeLa cells grown as monolayers were inoculated with bacteria to an MOI of 10 and incubated at 37°C and 5% CO2 for 2 h. HeLa cells were lysed, and 10-fold serial dilutions were plated on LB agar to determine the CFU/ml. After calculating the percentages of bacterial recovery (final number of CFU per ml divided by the initial number of CFU/ml, times 100), the data from all the mutants were normalized against those for the parental wild-type strain. The averages from three biological replicates are presented, and the error bars represent 1 standard error. Asterisks indicate significantly different results from the respective wild type (P < 0.05, Student's t test).
Strr mutations affect actin reorganization.
The secretion of EspA (29) and EspB (30) are essential for the formation of A/E lesions on epithelial cells, which is observed as an accumulation of the host actin cytoskeleton beneath the bacterial attachment site (25, 46). To test whether different Strr mutations impacted actin polymerization, FAS assays were performed with E. coli O157:H7 EDL933 and its mutants (Fig. 4). Fluorescence microscopy revealed the formation of typical actin pedestals on HeLa cells upon infection (Fig. 4A). Actin polymerization was observed as a characteristic cup-like structure (bright green) underneath the bacterium (red). By quantifying the percentage of HeLa cells with at least one pedestal, per field, we observed that all 3 mutants were defective in promoting actin polymerization (Fig. 4B). Compared to the wild-type strain, EDL933-strR1, -strR2, and -strR3 showed a 12%, 35%, and 80% reduction in pedestal formation, and the differences for EDL933-strR2 and -strR3 were statistically significant (P < 0.05, one-way ANOVA with Tukey's multiple comparison test). The decrease in A/E lesion formation was consistent with the reduction of EspA/B seen in the EDL933 Strr mutants (Fig. 1). In addition, after the first 3 h of incubation, the cell densities for the wild-type strain, EDL933-strR1, -strR2, and -strR3 were estimated to be (1.67 ± 0.18) × 107 (mean ± standard deviation), (1.89 ± 0.64) × 107, (1.04 ± 0.21) × 107, and (0.72 ± 0.23) × 107 CFU/ml, respectively.
Fig 4.
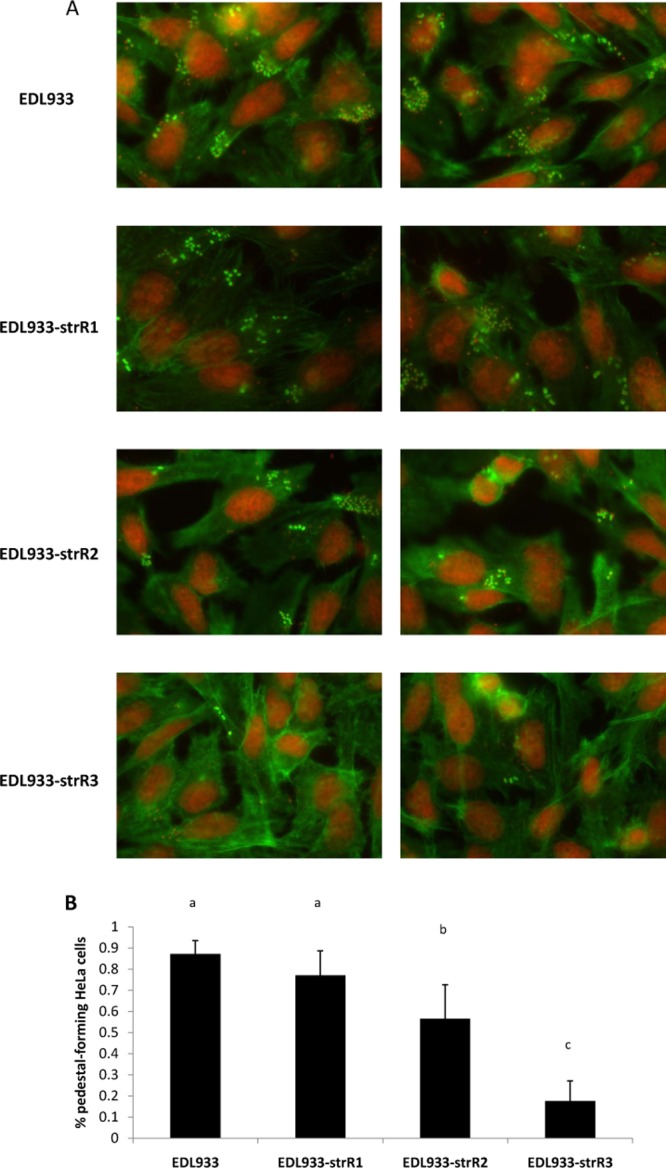
Strr mutations compromise A/E lesion formation of E. coli O157:H7 EDL933. A FAS assay was performed to assess the pedestal formation capabilities of EDL933 and its mutants. (A) Two fields for each strain are presented. Pedestals are bright green (actin) cups beneath the red bacteria. (B) Quantification of pedestal formation. The percentage of pedestal-forming cells per field was quantified. The result for each strain is an average from 9 fields (approximately 50 cells per field), and the error bar represents 1 standard deviation. Values assigned with different letters were significantly different (P < 0.05, one-way ANOVA with Tukey's multiple-comparison test).
DISCUSSION
The Str-treated mouse model was proposed by Myhal et al. as a method for studying E. coli intestinal colonization (47). The antibiotic was reported to decrease the abundance of facultative anaerobes within the gut microflora, although another study has suggested that streptomycin treatment decreases colonization resistance through altering the pH and volatile fatty acid composition of the ecosystem (48). Str treatment has additionally been used to overcome colonization resistance when studying E. coli colonization in cattle (19–22), in mice (49–52), and for in vivo infection models of Salmonella (53, 54), Aeromonas (55), Klebsiella (56), and Vibrio (57) species. This experimental design remains common despite the earlier observations that restrictive Strr mutations compromise the fitness of Salmonella in mice (58, 59). These studies revealed that restrictive Strr mutants are outcompeted 100- to 1,000-fold by the wild-type strain 4 days after coinjection intraperitoneally into mice at a 1:1 ratio. The investigators argued that this effect was due to the slight decrease in the growth rates of the mutants. In our study, we also observed 8.9% (K42T in S12) and 15.7% (K42N) decreases in the growth rates of Strr mutants of E. coli O157:H7 (see Fig. S1 in the supplemental material), but more importantly, we also observed reduced secretion of two critical components of the T3SS that are required for optimal colonization of cattle (60–62), humans (63), and pigs (64).
We analyzed 7 spontaneous Strr mutants from 5 E. coli O157:H7 strains and observed 4 different mutations in rpsL (Table 2). All mutations were located within either site 42 or 87. These were previously reported to be hot spots for Strr mutations (16), likely because they provide cells with higher levels of antibiotic resistance than other mutations. Based on previous studies (9, 10, 17), these 4 mutations could be divided into three groups: restrictive (K42T and K42N), mildly restrictive (K87R), and nonrestrictive (K42R) (Table 2). Mutations conferring restrictive decoding accuracy concomitantly decrease the rate of translation (7, 9, 11, 12), while the translational efficiency of nonrestrictive mutants is indistinguishable from the wild-type strain (7). In the current study, we observed a striking correspondence between the predicted translational efficiency and the EspA and EspB secretion levels. A dramatic reduction in secretion levels was observed in restrictive mutants (Sakai-strR, PA4-strR, PA5-strR, EDL933-strR2, and EDL933-strR3), a slight reduction with the mildly restrictive mutant (PA11-strR), and no difference in the nonrestrictive mutant (EDL933-strR1) (Fig. 1). Admittedly, this correspondence was observed when we used a small set of mutants, and the effects of Strr mutations on EspA/B secretion may vary depending upon the E. coli O157:H7 strain and specific Strr-conferring mechanism. We plan to address these questions in the future by designing more-comprehensive studies.
EspA is a major component in the T3SS syringe and is required for intimate physical interaction with host cells (29). EspB is translocated through the EspA filament (29) and forms pores in the host membrane. Both of these proteins are required for the translocation of Tir (65). Tir facilitates the bacterial intimate adherence and triggers the actin polymerization in the epithelia (34, 45). Reduced secretion of EspA and EspB in the restrictive mutants (EDL933-strR2 and -strR3) decreased bacterial adherence (Fig. 3), consistent with the previously reported study (66) in which espA and espB null mutants were used. We noticed that PA11-strR secreted markedly less EspA than the wild type (Fig. 1), although no significant differences in adherence to HeLa cells were observed. We noted that PA11-strR secretes EspA/B to levels that are comparable to wild-type EDL933 and higher than those seen with strains PA4 and PA5 (Fig. 1), suggesting that perhaps the amount of EspA/B secreted by PA11-strR is sufficient to promote optimal adherence in this assay. The reduced secretion of the two LEE-encoded proteins also had a remarkable impact on pedestal formation (Fig. 4). These observations collectively suggest a link between Strr mutations and the T3SS-related virulence potential of E. coli O157:H7. Hence, our results suggest that restrictive Strr mutants may not always be reliable surrogates for their wild-type parents, although this argument requires further in vivo experiments. Our work also highlights the fact that independently generated Strr strains may not be phenotypically equivalent.
The negative impact of restrictive mutations on bacterial growth has been previously reported (13–16), and we noticed growth differences as well in high-glucose DMEM during the first 5 h of incubation (see Fig. S1A in the supplemental material). The reduced growth rate of restrictive mutants likely contributed to the results in the adherence assay (Fig. 3) and FAS assay (Fig. 4), in which results were analyzed 2 and 3 h postinfection, respectively. Importantly, however, our results demonstrated for the first time that restrictive mutations also affect adherence and actin accumulation through decreased secretion of LEE-encoded proteins (Fig. 2). A variety of genes have been identified that regulate the transcription of espA and espB, yet the results from our qPCR experiments and Western blotting analysis of intracellular EspA and EspB (Fig. 2) strongly suggested that the secretion defect is primarily the result of a reduced rate of EspA and EspB protein synthesis. Why synthesis of EspA and EspB specifically is decreased needs to be further explored. The translation rate has been reported to impact the folding of protein domains (67), providing one mechanism worth investigating in the future.
In conclusion, our results clearly indicate that Strr-conferring mutations are not always neutral with regard to virulence phenotypes and may affect the progression of the colonization and pathogenesis of E. coli O157:H7. We suggest that the rpsL mutation imparting the Strr phenotype should be identified before using strains in assays that depend upon a functioning T3SS. Strains carrying nonrestrictive mutations, such as K42R, likely make better surrogates for the wild type than strains carrying restrictive mutations.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by USDA-NIFA grant 2009-03611, by start-up funds through the Penn State University Department of Food Science and College of Agricultural Sciences, and by the Casida Development Professorship to E.G.D.
We thank the Penn State Genomics Core Facility (University Park, PA) for help in generating DNA sequence information. We thank Sarah Forester (Department of Food Science, the Penn State University) for help with equipment settings and James Kaper and Jane Michalski (University of Maryland, School of Medicine) for providing EspA and EspB antibodies.
Footnotes
Published ahead of print 24 June 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00709-13.
REFERENCES
- 1.Schatz A, Bugle E, Waksman SA. 1944. Streptomycin, a substance exhibiting antibiotic activity against Gram-positive and Gram-negative bacteria. Proc. Soc. Exp. Biol. Med. 55:66–69 [DOI] [PubMed] [Google Scholar]
- 2.Gromadski KB, Rodnina MV. 2004. Kinetic determinants of high-fidelity tRNA discrimination on the ribosome. Mol. Cell 13:191–200 [DOI] [PubMed] [Google Scholar]
- 3.Rodnina MV, Daviter T, Gromadski K, Wintermeyer W. 2002. Structural dynamics of ribosomal RNA during decoding on the ribosome. Biochimie 84:745–754 [DOI] [PubMed] [Google Scholar]
- 4.Demirci H, Murphy Ft Murphy E, Gregory ST, Dahlberg AE, Jogl G. 2013. A structural basis for streptomycin-induced misreading of the genetic code. Nat. Commun. 4:1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller CP, Bohnhoff M. 1947. Two streptomycin-resistant variants of Meningococcus. J. Bacteriol. 54:467–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newcombe HB, Hawirko R. 1949. Spontaneous mutation to streptomycin resistance and dependence in Escherichia coli. J. Bacteriol. 57:565–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bohman K, Ruusala T, Jelenc PC, Kurland CG. 1984. Kinetic impairment of restrictive streptomycin-resistant ribosomes. Mol. Gen. Genet. 198:90–99 [DOI] [PubMed] [Google Scholar]
- 8.Holberger LE, Hayes CS. 2009. Ribosomal protein S12 and aminoglycoside antibiotics modulate A-site mRNA cleavage and transfer-messenger RNA activity in Escherichia coli. J. Biol. Chem. 284:32188–32200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agarwal D, Gregory ST, O'Connor M. 2011. Error-prone and error-restrictive mutations affecting ribosomal protein S12. J. Mol. Biol. 410:1–9 [DOI] [PubMed] [Google Scholar]
- 10.Tubulekas I, Buckingham RH, Hughes D. 1991. Mutant ribosomes can generate dominant kirromycin resistance. J. Bacteriol. 173:3635–3643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogle JM, Ramakrishnan V. 2005. Structural insights into translational fidelity. Annu. Rev. Biochem. 74:129–177 [DOI] [PubMed] [Google Scholar]
- 12.Stelzl U, Connell S, Nierhaus KH, Wittmann-Liebold B. 2001. Ribosomal proteins: role in ribosomal functions. Encyclopedia of Life Sciences. John Wiley & Sons, Ltd, Hoboken, NJ. 10.1038/npg.els.0000687 [DOI] [Google Scholar]
- 13.Ruusala T, Andersson D, Ehrenberg M, Kurland CG. 1984. Hyper-accurate ribosomes inhibit growth. EMBO J. 3:2575–2580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hosaka T, Tamehiro N, Chumpolkulwong N, Hori-Takemoto C, Shirouzu M, Yokoyama S, Ochi K. 2004. The novel mutation K87E in ribosomal protein S12 enhances protein synthesis activity during the late growth phase in Escherichia coli. Mol. Genet. Genomics 271:317–324 [DOI] [PubMed] [Google Scholar]
- 15.Ehrenberg M, Kurland CG. 1984. Costs of accuracy determined by a maximal growth rate constraint. Q. Rev. Biophys. 17:45–82 [DOI] [PubMed] [Google Scholar]
- 16.Chumpolkulwong N, Hori-Takemoto C, Hosaka T, Inaoka T, Kigawa T, Shirouzu M, Ochi K, Yokoyama S. 2004. Effects of Escherichia coli ribosomal protein S12 mutations on cell-free protein synthesis. Eur. J. Biochem. 271:1127–1134 [DOI] [PubMed] [Google Scholar]
- 17.Gregory ST, Cate JHD, Dahlberg AE. 2001. Streptomycin-resistant and streptomycin-dependent mutants of the extreme thermophile Thermus thermophilus1. J. Mol. Biol. 309:333–338 [DOI] [PubMed] [Google Scholar]
- 18.Shakhnovich EA, Davis BM, Waldor MK. 2009. Hfq negatively regulates type III secretion in EHEC and several other pathogens. Mol. Microbiol. 74:347–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snider TA, Fabich AJ, Washburn KE, Sims WP, Blair JL, Cohen PS, Conway T, Clinkenbeard KD. 2006. Evaluation of a model for Escherichia coli O157:H7 colonization in streptomycin-treated adult cattle. Am. J. Vet. Res. 67:1914–1920 [DOI] [PubMed] [Google Scholar]
- 20.Snider TA, Fabich AJ, Conway T, Clinkenbeard KD. 2009. E. coli O157:H7 catabolism of intestinal mucin-derived carbohydrates and colonization. Vet. Microbiol. 136:150–154 [DOI] [PubMed] [Google Scholar]
- 21.Dean-Nystrom EA, Stoffregen WC, Bosworth BT, Moon HW, Pohlenz JF. 2008. Early attachment sites for Shiga-toxigenic Escherichia coli O157:H7 in experimentally inoculated weaned calves. Appl. Environ. Microbiol. 74:6378–6384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cornick NA, Booher SL, Moon HW. 2002. Intimin facilitates colonization by Escherichia coli O157:H7 in adult ruminants. Infect. Immun. 70:2704–2707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffin PM, Tauxe RV. 1991. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol. Rev. 13:60–98 [DOI] [PubMed] [Google Scholar]
- 24.McDaniel TK, Kaper JB. 1997. A cloned pathogenicity island from enteropathogenic Escherichia coli confers the attaching and effacing phenotype on E. coli K-12. Mol. Microbiol. 23:399–407 [DOI] [PubMed] [Google Scholar]
- 25.McDaniel TK, Jarvis KG, Donnenberg MS, Kaper JB. 1995. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc. Natl. Acad. Sci. U. S. A. 92:1664–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deng W, Li Y, Vallance BA, Finlay BB. 2001. Locus of enterocyte effacement from Citrobacter rodentium: sequence analysis and evidence for horizontal transfer among attaching and effacing pathogens. Infect. Immun. 69:6323–6335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jerse AE, Yu J, Tall BD, Kaper JB. 1990. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc. Natl. Acad. Sci. U. S. A. 87:7839–7843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frankel G, Phillips AD, Rosenshine I, Dougan G, Kaper JB, Knutton S. 1998. Enteropathogenic and enterohaemorrhagic Escherichia coli: more subversive elements. Mol. Microbiol. 30:911–921 [DOI] [PubMed] [Google Scholar]
- 29.Knutton S, Rosenshine I, Pallen MJ, Nisan I, Neves BC, Bain C, Wolff C, Dougan G, Frankel G. 1998. A novel EspA-associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithelial cells. EMBO J. 17:2166–2176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolff C, Nisan I, Hanski E, Frankel G, Rosenshine I. 1998. Protein translocation into host epithelial cells by infecting enteropathogenic Escherichia coli. Mol. Microbiol. 28:143–155 [DOI] [PubMed] [Google Scholar]
- 31.Gauthier A, Puente JL, Finlay BB. 2003. Secretin of the enteropathogenic Escherichia coli type III secretion system requires components of the type III apparatus for assembly and localization. Infect. Immun. 71:3310–3319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delahay RM, Frankel G, Knutton S. 2001. Intimate interactions of enteropathogenic Escherichia coli at the host cell surface. Curr. Opin. Infect. Dis. 14:559–565 [DOI] [PubMed] [Google Scholar]
- 33.Creuzburg K, Middendorf B, Mellmann A, Martaler T, Holz C, Fruth A, Karch H, Schmidt H. 2011. Evolutionary analysis and distribution of type III effector genes in pathogenic Escherichia coli from human, animal and food sources. Environ. Microbiol. 13:439–452 [DOI] [PubMed] [Google Scholar]
- 34.Gruenheid S, DeVinney R, Bladt F, Goosney D, Gelkop S, Gish GD, Pawson T, Finlay BB. 2001. Enteropathogenic E. coli Tir binds Nck to initiate actin pedestal formation in host cells. Nat. Cell Biol. 3:856–859 [DOI] [PubMed] [Google Scholar]
- 35.Unsworth KE, Mazurkiewicz P, Senf F, Zettl M, McNiven M, Way M, Holden DW. 2007. Dynamin is required for F-actin assembly and pedestal formation by enteropathogenic Escherichia coli (EPEC). Cell. Microbiol. 9:438–449 [DOI] [PubMed] [Google Scholar]
- 36.Bertani G. 1951. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 62:293–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bertani G. 2004. Lysogeny at mid-twentieth century: P1, P2, and other experimental systems. J. Bacteriol. 186:595–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perna NT, Plunkett G, III, Burland V, Mau B, Glasner JD, Rose DJ, Mayhew GF, Evans PS, Gregor J, Kirkpatrick HA, Posfai G, Hackett J, Klink S, Boutin A, Shao Y, Miller L, Grotbeck EJ, Davis NW, Lim A, Dimalanta ET, Potamousis KD, Apodaca J, Anantharaman TS, Lin J, Yen G, Schwartz DC, Welch RA, Blattner FR. 2001. Genome sequence of enterohaemorrhagic Escherichia coli O157:H7. Nature 409:529–533 [DOI] [PubMed] [Google Scholar]
- 39.Hayashi T, Makino K, Ohnishi M, Kurokawa K, Ishii K, Yokoyama K, Han CG, Ohtsubo E, Nakayama K, Murata T, Tanaka M, Tobe T, Iida T, Takami H, Honda T, Sasakawa C, Ogasawara N, Yasunaga T, Kuhara S, Shiba T, Hattori M, Shinagawa H. 2001. Complete genome sequence of enterohemorrhagic Escherichia coli O157:H7 and genomic comparison with a laboratory strain K-12. DNA Res. 8:11–22 [DOI] [PubMed] [Google Scholar]
- 40.Hartzell A, Chen C, Lewis C, Liu K, Reynolds S, Dudley EG. 2011. Escherichia coli O157:H7 of genotype lineage-specific polymorphism assay 211111 and clade 8 are common clinical isolates within Pennsylvania. Foodborne Pathog. Dis. 8:763–768 [DOI] [PubMed] [Google Scholar]
- 41.Chen C, Lewis CR, Goswami K, Roberts EL, DebRoy C, Dudley EG. 2013. Identification and characterization of spontaneous deletions within the Sp11-Sp12 prophage region of Escherichia coli O157:H7 Sakai. Appl. Environ. Microbiol. 79:1934–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Njoroge JW, Nguyen Y, Curtis MM, Moreira CG, Sperandio V. 2012. Virulence meets metabolism: Cra and KdpE gene regulation in enterohemorrhagic Escherichia coli. mBio 3(5):e00280-00212. 10.1128/mBio.00280-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vila-Sanjurjo A, Lu Y, Aragonez JL, Starkweather RE, Sasikumar M, O'Connor M. 2007. Modulation of 16S rRNA function by ribosomal protein S12. Biochim. Biophys. Acta 1769:462–471 [DOI] [PubMed] [Google Scholar]
- 44.Mellies JL, Barron AM, Carmona AM. 2007. Enteropathogenic and enterohemorrhagic Escherichia coli virulence gene regulation. Infect. Immun. 75:4199–4210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hartland EL, Daniell SJ, Delahay RM, Neves BC, Wallis T, Shaw RK, Hale C, Knutton S, Frankel G. 2000. The type III protein translocation system of enteropathogenic Escherichia coli involves EspA-EspB protein interactions. Mol. Microbiol. 35:1483–1492 [DOI] [PubMed] [Google Scholar]
- 46.Finlay BB, Rosenshine I, Donnenberg MS, Kaper JB. 1992. Cytoskeletal composition of attaching and effacing lesions associated with enteropathogenic Escherichia coli adherence to HeLa cells. Infect. Immun. 60:2541–2543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Myhal ML, Laux DC, Cohen PS. 1982. Relative colonizing abilities of human fecal and K-12 strains of Escherichia coli in the large intestines of streptomycin-treated mice. Eur. J. Clin. Microbiol. 1:186–192 [DOI] [PubMed] [Google Scholar]
- 48.Hentges D, Pongpech P, Que J. 1990. Hypothesis: how streptomycin treatment compromises colonisation resistance against enteric pathogens in mice. Microb. Ecol. Health Dis. 3:105–111 [Google Scholar]
- 49.Lindgren SW, Melton AR, O'Brien AD. 1993. Virulence of enterohemorrhagic Escherichia coli O91:H21 clinical isolates in an orally infected mouse model. Infect. Immun. 61:3832–3842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fujii J, Kita T, Yoshida S, Takeda T, Kobayashi H, Tanaka N, Ohsato K, Mizuguchi Y. 1994. Direct evidence of neuron impairment by oral infection with verotoxin-producing Escherichia coli O157:H− in mitomycin-treated mice. Infect. Immun. 62:3447–3453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miranda RL, Conway T, Leatham MP, Chang DE, Norris WE, Allen JH, Stevenson SJ, Laux DC, Cohen PS. 2004. Glycolytic and gluconeogenic growth of Escherichia coli O157:H7 (EDL933) and E. coli K-12 (MG1655) in the mouse intestine. Infect. Immun. 72:1666–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uhlich GA, Keen JE, Elder RO. 2002. Variations in the csgD promoter of Escherichia coli O157:H7 associated with increased virulence in mice and increased invasion of HEp-2 cells. Infect. Immun. 70:395–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bohnhoff M, Miller CP. 1962. Enhanced susceptibility to Salmonella infection in streptomycin-treated mice. J. Infect. Dis. 111:117–127 [DOI] [PubMed] [Google Scholar]
- 54.Barthel M, Hapfelmeier S, Quintanilla-Martinez L, Kremer M, Rohde M, Hogardt M, Pfeffer K, Russmann H, Hardt WD. 2003. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect. Immun. 71:2839–2858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sanderson K, Ghazali FM, Kirov SM. 1996. Colonization of streptomycin-treated mice by Aeromonas species. J. Diarrhoeal Dis. Res. 14:27–32 [PubMed] [Google Scholar]
- 56.Favre-Bonté S, Licht TR, Forestier C, Krogfelt KA. 1999. Klebsiella pneumoniae capsule expression is necessary for colonization of large intestines of streptomycin-treated mice. Infect. Immun. 67:6152–6156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olivier V, Queen J, Satchell KJ. 2009. Successful small intestine colonization of adult mice by Vibrio cholerae requires ketamine anesthesia and accessory toxins. PLoS One 4:e7352. 10.1371/journal.pone.0007352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bjorkman J, Andersson DI. 2000. The cost of antibiotic resistance from a bacterial perspective. Drug Resist. Updat. 3:237–245 [DOI] [PubMed] [Google Scholar]
- 59.Bjorkman J, Hughes D, Andersson DI. 1998. Virulence of antibiotic-resistant Salmonella typhimurium. Proc. Natl. Acad. Sci. U. S. A. 95:3949–3953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dziva F, van Diemen PM, Stevens MP, Smith AJ, Wallis TS. 2004. Identification of Escherichia coli O157:H7 genes influencing colonization of the bovine gastrointestinal tract using signature-tagged mutagenesis. Microbiology 150:3631–3645 [DOI] [PubMed] [Google Scholar]
- 61.Sheng H, Lim JY, Knecht HJ, Li J, Hovde CJ. 2006. Role of Escherichia coli O157:H7 virulence factors in colonization at the bovine terminal rectal mucosa. Infect. Immun. 74:4685–4693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dean-Nystrom EA, Bosworth BT, Moon HW, O'Brien AD. 1998. Escherichia coli O157:H7 requires intimin for enteropathogenicity in calves. Infect. Immun. 66:4560–4563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Donnenberg MS, Tacket CO, James SP, Losonsky G, Nataro JP, Wasserman SS, Kaper JB, Levine MM. 1993. Role of the eaeA gene in experimental enteropathogenic Escherichia coli infection. J. Clin. Invest. 92:1412–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Donnenberg MS, Tzipori S, McKee ML, O'Brien AD, Alroy J, Kaper JB. 1993. The role of the eae gene of enterohemorrhagic Escherichia coli in intimate attachment in vitro and in a porcine model. J. Clin. Invest. 92:1418–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kenny B, Abe A, Stein M, Finlay BB. 1997. Enteropathogenic Escherichia coli protein secretion is induced in response to conditions similar to those in the gastrointestinal tract. Infect. Immun. 65:2606–2612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abe A, Heczko U, Hegele RG, Finlay BB. 1998. Two enteropathogenic Escherichia coli type III secreted proteins, EspA and EspB, are virulence factors. J. Exp. Med. 188:1907–1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marin M. 2008. Folding at the rhythm of the rare codon beat. Biotech. J. 3:1047–1057 [DOI] [PubMed] [Google Scholar]
- 68.Wells JG, Davis BR, Wachsmuth IK, Riley LW, Remis RS, Sokolow R, Morris GK. 1983. Laboratory investigation of hemorrhagic colitis outbreaks associated with a rare Escherichia coli serotype. J. Clin. Microbiol. 18:512–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.