Abstract
Tn5801, originally detected in Staphylococcus aureus Mu50, is a Tn916 family element in which a unique int gene (int5801) replaces the int and xis genes in Tn916 (int916 and xis916). Among 62 tet(M)-positive tetracycline-resistant Streptococcus agalactiae isolates, 43 harbored Tn916, whereas 19 harbored a Tn5801-like element (Tn5801.Sag, ∼20.6 kb). Tn5801.Sag was characterized (PCR mapping, partial sequencing, and chromosomal integration) and compared to other Tn5801-like elements. Similar to Tn5801 from S. aureus Mu50, tested in parallel, Tn5801.Sag was unable to undergo circularization and conjugal transfer.
TEXT
Tn916 family elements (1, 2) are broad-host-range elements, widespread in Gram-positive bacteria, that mostly exhibit the distinctive properties of integrative and conjugative elements (ICEs) (3). Their open reading frames (ORFs) are organized into functional modules (conjugation, recombination, transcriptional regulation, and accessory functions): albeit with well-known variations (1, 2), the recombination module mostly consists of an integrase (int916) gene and an excisionase (xis916) gene, and the accessory gene is typically the tetracycline (TET) resistance determinant tet(M).
The best-known Tn916 family element from Staphylococcus aureus is Tn5801 (∼25.8 kb) (2), which was detected in the genome of Mu50 (DDBJ accession no. BA000017) (4), a well-established methicillin-resistant and vancomycin-intermediate Japanese clinical isolate (5). Tn5801, regarded as one of the nine genomic islands in the Mu50 genome (6), shows a modular organization similar to that of Tn916 and has several similar ORFs. However, besides the presence of additional ORFs, whose functions are largely unknown, DNA identities are rather low except in the case of tet(M) (97.7%). In particular, the recombination module differs from that of Tn916, as it lacks the xis gene and shows very low DNA identity (38.6%) between int5801 and int916. This organization closely resembles that found in CW459tet(M), a genetic element from Clostridium perfringens CW459 (GenBank accession no. AF329848) (7).
Tn5801-like transposons have been detected in other human isolates of S. aureus (8, 9); in one case the element, Tn6014 from S. aureus 1680, was able to transfer, at low frequency, to S. aureus recipients (8). Among streptococci, a Tn5801-like element has been described for Streptococcus mitis B6 (EMBL accession no. FN568063) (10).
In the present study, we showed that a Tn5801-like transposon, designated Tn5801.Sag, is found in about 30% of TET-resistant clinical isolates of Streptococcus agalactiae, a species in which TET resistance is around 90% worldwide. The genetic organization of Tn5801.Sag was determined and compared with that of other Tn5801-like elements, and the putative core site was identified. Similar to Tn5801 from S. aureus Mu50, which was tested in parallel in this study, Tn5801.Sag was unable to undergo circularization and conjugal transfer.
All PCR primers used are shown in Table 1.
Table 1.
Oligonucleotide primer pairs used
| Procedure and gene/amplicon | Primer designation | Sequence (5′–3′) | Reference or source | Product size (bp) |
|---|---|---|---|---|
| Detection of TET resistance genes | ||||
| tet(M) | TETM3 | ATGGAAGCCCAGAAAGGAT | 11 | 740 |
| TETM2 | GAACTCGAACAAGAGGAAAGC | 11 | ||
| tet(O) | TETO1 | AACTTAGGCATTCTGGCTCAC | 11 | 519 |
| TETO2 | TCCCACTGTTCCATATCGTCA | 11 | ||
| PCR evidence of Tn916 | ||||
| int916 | int-for | GCGTGATTGTATCTCACT | 12 | 1,046 |
| int-rev | GACGCTCCTGTTGCTTCT | 12 | ||
| xis916 | xis-for | AAGCAGACTGAGATTCCTA | 13 | 194 |
| xis-rev | GCGTCCAATGTATCTATAA | 13 | ||
| orf7-orf8a | O15 | GTACGTCCACCAATGTGG | 14 | 902 |
| O16 | GCACGCTTCCACGAAAGGAG | 14 | ||
| orf20-IR18–19a | J12 | CCCATTGAAGACGCAGAAGT | 15 | 801 |
| J11 | AAAAATCCCTACCGCACT | 15 | ||
| orf24-orf20a | TN6-rev | CCATCAAACATTCATTCAGC | 15 | 3,358 |
| J13 | GGTTTTGTGGTTAGTTTT | 15 | ||
| PCR mapping of Tn5801.Sagb | ||||
| int5801 | 1812 | GTCCATACGTTCCTAAAGTCGTC | 8 | 726 |
| 1811 | CCGATATTGAGCCTATTGATGTG | 8 | ||
| sav400 | 400R | TCGTATTTCAAGGCTTCGTC | This study | 369 |
| 400F | TACCGAAGAGTCCATCAAAC | This study | ||
| sav408 | 408R | AATGTAGGGGCGACTTGATG | This study | 1,005 |
| 408F | ACTGGCTTATGGCGTTTCTC | This study | ||
| sav409 | 409R | GCAGACAAACCAAGATAAGC | This study | 940 |
| 409F | GAGAGCGAATCAAAGCCAAC | This study | ||
| sav413 | 413R | AACACCGTTGTCGTCTCCAC | This study | 743 |
| 413F | TTGCTAGTAATATAAGGGCGA | This study | ||
| sav414 | 414R | ATTAGATACACAACATCCTCATC | This study | 579 |
| 414F | ACAGGCAATCCCATCAGAAC | This study | ||
| sav415 | 415R | TAGATGAGGCTTGATACACC | This study | 677 |
| 415F | TTCTCGTAACGGCTCCTATG | This study | ||
| int5801-tet(M) | 1812 | GTCCATACGTTCCTAAAGTCGTC | 8 | 4,971 |
| TETM2 | GAACTCGAACAAGAGGAAAGC | 11 | ||
| tet(M)-sav400 | TETM3 | ATGGAAGCCCAGAAAGGAT | 11 | 3,362 |
| 400F | TACCGAAGAGTCCATCAAAC | This study | ||
| sav400-sav408 | 400R | TCGTATTTCAAGGCTTCGTC | This study | 9,331 |
| 408F | ACTGGCTTATGGCGTTTCTC | This study | ||
| sav408-sav409 | 408R | AATGTAGGGGCGACTTGATG | This study | 2,612 |
| 409F | GAGAGCGAATCAAAGCCAAC | This study | ||
| sav409-sav411 | 409R | GCAGACAAACCAAGATAAGC | This study | 2,142 |
| 411F | GAGATTAGCAGAAGGTATTGTG | This study | ||
| sav409-sav413 | 409R | GCAGACAAACCAAGATAAGC | This study | 3,874 |
| 413F | TTGCTAGTAATATAAGGGCGA | This study | ||
| sav413-sav414 | 413R | AACACCGTTGTCGTCTCCAC | This study | 1,968 |
| 414F | ACAGGCAATCCCATCAGAAC | This study | ||
| sav414-sav415 | 414R | ATTAGATACACAACATCCTCATC | This study | 3,295 |
| 415F | TTCTCGTAACGGCTCCTATG | This study | ||
| Tn5801.Sag chromosomal integration sitec | ||||
| SAG967 (guaA) | LJ967 | CGTGAAGAAATCGCTAAAG | This study | 1,228 |
| int5801 | 1811 | CCGATATTGAGCCTATTGATGTG | 8 | |
| sav411 | CF1 | TTCAAAGGAACAGAAGCGGG | This study | 1,414 |
| SAG964 | RJ964 | GAAGTAGAAGAGAGCCATAG | This study | |
| Search for circular form | ||||
| int5801 | 1811 | CCGATATTGAGCCTATTGATGTG | 8 | |
| sav411 | CF1 | TTCAAAGGAACAGAAGCGGG | This study |
ORFs numbered according to the reported organization of Tn916 (GenBank accession no. U09422).
Tn5801 from the genome of S. aureus Mu50 (DDBJ accession no. BA000017; sav genes) was used as the reference sequence. In PCR assays, S. aureus Mu50 (ATCC 700699) was used as a positive control and S. pneumoniae BM4200 (Pasteur Institute Collection), harboring the Tn916-like transposon Tn1545 (1), was used as a negative control.
The genome of S. agalactiae strain 2603V/R (GenBank accession no. AE009948; SAG genes) was used as the reference sequence.
Characterization of TET-resistant S. agalactiae isolates.
Sixty-nine clinical isolates of S. agalactiae, recovered in laboratories of central Italy in 2010–2011 and confirmed as being Lancefield group B using Slidex Strepto Plus (bioMérieux, Marcy l'Étoile, France), were used. Of them, 64 (93%) were TET resistant (MICs, ≥8 μg/ml). PCR assays demonstrated that tet(M) and tet(O) were the sole tet genes in 58 and 2 isolates, respectively; 4 isolates carried both determinants. Among the 62 tet(M)-positive isolates, 43 yielded positive PCRs for int916 and xis916; the remaining 19 were negative for both genes as well as for three additional regions of the transposon. However, sequence analysis of the tet(M) amplicon, performed in 3/19 randomly selected isolates, showed 100% DNA identity to the corresponding tet(M) portion of Tn5801 from S. aureus Mu50 (4). This finding prompted us to look for int5801, the integrase gene of Tn5801, which was found in all 19 isolates. The latter fell into several serotypes and pulsotypes (data not shown), thus excluding that they represented a clonal population.
Characterization and comparative analysis of Tn5801 from S. agalactiae (Tn5801.Sag).
The 19 int5801-positive isolates underwent PCR mapping using the primers and strategies summarized in Table 1 and Fig. 1A. All isolates yielded comparable results, with positive PCRs and amplicons of the expected sizes obtained with all but six of the relevant primer pairs. Specifically, negative reactions were obtained with those pairs in which at least one primer targeted one of the last three ORFs of Tn5801 from S. aureus Mu50 (sav413, sav414, and sav415), which thus appeared not to be found in Tn5801 from S. agalactiae (designated Tn5801.Sag).
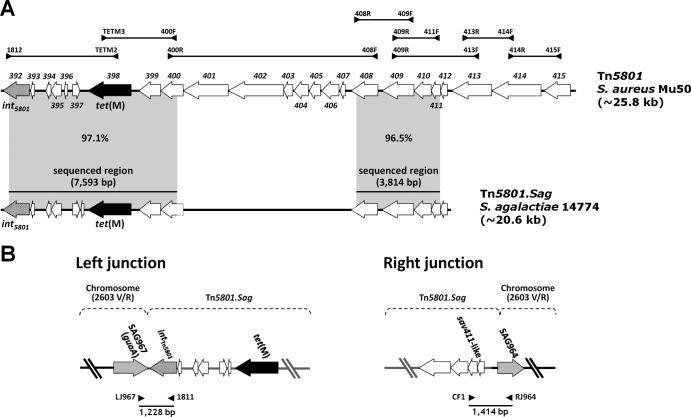
Fig 1.
Schematic representation of Tn5801.Sag from S. agalactiae strain 14774 (A) and its chromosomal integration (left and right junctions) (B). (A) Tn5801.Sag was determined by PCR mapping and sequencing of two regions. The primers used are listed in Table 1. The mapping strategy is outlined in the upper portion (the amplicons used to detect individual ORFs, i.e., obtained by pairing two primers internal to the same ORF, are not shown). The two regions sequenced initially (7,593 bp, left, and 3,814 bp, right) are indicated by horizontal bars. Tn5801.Sag is compared to Tn5801 from S. aureus Mu50, where ORFs are numbered sav392 to sav415 according to the original designations (DDBJ accession no. BA000017); percent DNA identities are reported in gray areas between sequenced regions. tet(M) and int5801 are represented as black and spotted arrows, respectively. (B) Tn5801.Sag was integrated at the 3′ end of the guaA gene. This gene, detected in all S. agalactiae genomes sequenced to date, corresponds to ORF967 from S. agalactiae 2603V/R (GenBank accession no. AE009948), from which chromosomal ORF designations derive. The amplicons obtained by pairing primers LJ967/1811 (left junction) and CF1/RJ964 (right junction), whose sequencing extended the two portions of Tn5801.Sag sequenced initially, are shown as bars. tet(M) and int5801 are represented as black and spotted arrows, respectively, and other Tn5801.Sag ORFs as white arrows; chromosomal ORFs are depicted as gray arrows.
One of the 19 isolates (strain 14774) was used in DNA sequencing experiments, performed as described elsewhere (16). Two amplicons, yielded by primer pairs 1812/400F [7,593 bp, encompassing the tet(M) gene and most int5801] and 408R/411F (3,814 bp), were sequenced (EMBL accession no. HF930766). The two sequenced regions of Tn5801.Sag displayed 97.1% [tet(M), 100%] and 96.5% DNA identities with the corresponding regions of Tn5801 from S. aureus Mu50 (Fig. 1A) and 96.9% [tet(M), 99.5%] and 96.5% with those of S. mitis B6; the former sequence displayed 96.1% identity [tet(M), 100%] with the corresponding region (the only one that has been sequenced; GenBank accession no. EU918655) of Tn6014 from S. aureus 1680. Greater identities [99.9% and 100%; tet(M), 100%] were recorded with the corresponding regions of E. faecalis 62, for which a “Tn916 element” (not identified as Tn5801-like) was reported (17) in the sequenced genome (GenBank accession no. CP002491). The latter element (∼20.6 kb) was very similar to Tn5801.Sag (∼20.6 kb based on sequencing and PCR mapping data) also as to ORF organization: in particular, the two elements share the lack of the last three ORFs of Tn5801 from S. aureus Mu50 (sav413, sav414, and sav415), located after the conjugation module and not present in Tn916. It is worth noting that the last ORF (sav415, a transposase gene) is also missing in the Tn5801-like element from S. mitis B6, in which sav413 and sav414 are present.
Chromosomal integration of Tn5801.Sag and identification of the putative core site.
The early study of CW459tet(M) (7) and later studies of genetic elements related to Tn5801 (10, 18, 19) concur in describing an integration site just downstream of guaA, a chromosomal gene encoding a GMP synthase that is consistently found adjacent to int5801 in the sequenced genomes containing a Tn5801 element. Using strategies refined in previous studies (16, 20–22), this site was thus explored in the 19 S. agalactiae isolates harboring Tn5801.Sag. The genome of S. agalactiae strain 2603V/R (GenBank accession no. AE009948) (23) was used as the reference sequence. As illustrated in Fig. 1B, pairing of primers LJ967/1811 (left junction) gave an ∼1.2-kb amplicon from all 19 S. agalactiae isolates; by pairing primers CF1/RJ964 (right junction), an ∼1.4-kb amplicon was obtained from all but one isolate, which yielded an ∼1-kb-larger amplicon; this was subsequently shown to reflect the presence of SAG965 and SAG966, encoding insertion sequences in the S. agalactiae 2603V/R genome that were not found in the other 18 isolates. By analyzing and comparing the two amplicon sequences from strain 14774, it was possible to determine the chromosomal junctions of Tn5801.Sag. The putative core site was an almost completely overlapping 11-bp sequence identified on the left (GAGTGGGAGTA) and right (GAGTGGGAATA) ends of the transposon; the latter sequence was identical to that found in both Tn5801 junctions of S. aureus Mu50.
Transferability studies.
Three isolates, including strain 14774, were used as donors in conjugal transfer experiments, performed as described elsewhere (24). No transconjugants were obtained with any of the three recipients used: S. agalactiae 1357RF (25), S. pyogenes 12RF (24), and S. aureus RN4220RF (26), used in the sole successful conjugative transfer of a Tn5801 element (Tn6014) reported so far (8). Similar negative results were obtained using S. aureus Mu50 (ATCC 700699) as the donor.
The apparent nontransferability of Tn5801.Sag and of Tn5801 from S. aureus Mu50 was consistent with the absence, in both cases, of an intermediate circular form, as resulting from the negative PCR obtained using the outward-directed primer pair 1811/CF1.
Conclusions.
Among the so-called Tn916-like elements (1, 2), major differences are found in the recombination module, where the prevailing two-gene organization (int916 and xis916) typical of Tn916 may be replaced by a single gene. This is the cases of tndX in Tn5397 from Clostridium difficile (7), a gene that in streptococci is commonly found in S. pyogenes in ICESp1116 (22); of int459 in CW459tet(M) from C. perfringens (7); and of int5801 (identical to int459) in Tn5801 from S. aureus (4). Now, the finding that in no less than 30% of TET-resistant clinical isolates of S. agalactiae—a species for which TET resistance rates are around 90%—resistance was mediated by the tet(M) gene carried by a Tn5801-like transposon (Tn5801.Sag) is a major result of this study. Accordingly, a sizable proportion (about 50/250) of S. agalactiae scaffolds and contigs currently found in GenBank harbors Tn5801.Sag. The frequent occurrence of Tn5801.Sag in S. agalactiae strengthens the notion of a composite organization of the chromosome of this species (27–29).
Subsequent to the original detection of Tn5801 in S. aureus Mu50, Tn5801-like transposons were detected in other human S. aureus isolates; one such transposon (Tn6014) was shown to be able to transfer to an S. aureus recipient (8). Conversely, Tn5801.Sag is apparently unable to transfer, like Tn5801 from S. aureus Mu50, whose actual transferability had not been tested before the present study.
As to the genetic organization of Tn5801.Sag, differences from other Tn5801-like transposons mainly involved the right terminus of the element, with the last three ORFs of Tn5801 from S. aureus Mu50 (sav413, sav414, and sav415, not present in Tn916) missing in Tn5801.Sag, while only the last one (sav415) is missing in Tn5801-like from S. mitis B6. In contrast, the left termini are very similar in all Tn5801-like transposons, and the adjacent chromosomal gene is unvaryingly guaA. Therefore, while Tn916 preferentially integrates into A·T-rich targets in a broad range of hosts (2), int5801 and related genes appear to code for integrases leading to site-specific recombination at the 3′ end of guaA.
Nucleotide sequence accession number.
Two new nucleotide sequences reported in this work have been deposited in the EMBL database under accession no. HF930766 .
ACKNOWLEDGMENTS
We are grateful to Andrea Brenciani and Claudio Palmieri for helpful discussions.
This work was partly supported by the Italian Ministry of Education, University and Research.
Footnotes
Published ahead of print 1 July 2013
REFERENCES
- 1.Roberts AP, Mullany P. 2009. A modular master on the move: the Tn916 family of mobile genetic elements. Trends Microbiol. 17:251–258 [DOI] [PubMed] [Google Scholar]
- 2.Roberts AP, Mullany P. 2011. Tn916-like genetic elements: a diverse group of modular mobile elements conferring antibiotic resistance. FEMS Microbiol. Rev. 35:856–871 [DOI] [PubMed] [Google Scholar]
- 3.Wozniak RA, Waldor MK. 2010. Integrative and conjugative elements: mosaic mobile genetic elements enabling dynamic lateral gene flow. Nat. Rev. Microbiol. 8:552–563 [DOI] [PubMed] [Google Scholar]
- 4.Kuroda M, Ohta T, Uchiyama I, Baba T, Yuzawa H, Kobayashi I, Cui L, Oguchi A, Aoki K, Nagai Y, Lian JQ, Ito T, Kanamori M, Matsumaru H, Maruyama A, Murakami H, Hosoyama A, Mizutani-Ui Y, Takahashi NK, Sawano T, Inoue R, Kaito C, Sekimizu K, Hirakawa H, Kuhara S, Goto S, Yabuzaki J, Kanehisa M, Yamashita A, Oshima K, Furuya K, Yoshino C, Shiba T, Hattori M, Ogasawara N, Hayashi H, Hiramatsu K. 2001. Whole genome sequencing of meticillin-resistant Staphylococcus aureus. Lancet 357:1225–1240 [DOI] [PubMed] [Google Scholar]
- 5.Hiramatsu K. 2001. Vancomycin-resistant Staphylococcus aureus: a new model of antibiotic resistance. Lancet Infect. Dis. 1:147–155 [DOI] [PubMed] [Google Scholar]
- 6.Gill SR, Fouts DE, Archer GL, Mongodin EF, Deboy RT, Ravel J, Paulsen IT, Kolonay JF, Brinkac L, Beanan M, Dodson RJ, Daugherty SC, Madupu R, Angiuoli SV, Durkin AS, Haft DH, Vamathevan J, Khouri H, Utterback T, Lee C, Dimitrov G, Jiang L, Qin H, Weidman J, Tran K, Kang K, Hance IR, Nelson KE, Fraser CM. 2005. Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. J. Bacteriol. 187:2426–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts AP, Johanesen PA, Lyras D, Mullany P, Rood JI. 2001. Comparison of Tn5397 from Clostridium difficile, Tn916 from Enterococcus faecalis and the CW459tet(M) element from Clostridium perfringens shows that they have similar conjugation regions but different insertion and excision modules. Microbiology 147:1243–1251 [DOI] [PubMed] [Google Scholar]
- 8.de Vries LE, Christensen H, Skov RL, Aarestrup FM, Agersø Y. 2009. Diversity of the tetracycline resistance gene tet(M) and identification of Tn916- and Tn5801-like (Tn6014) transposons in Staphylococcus aureus from humans and animals. J. Antimicrob. Chemother. 64:490–500 [DOI] [PubMed] [Google Scholar]
- 9.Li H, Zhao C, Chen H, Zhang F, He W, Wang X, Wang Q, Yang R, Zhou D, Wang H. 2013. Identification of gene clusters associated with host adaptation and antibiotic resistance in Chinese Staphylococcus aureus isolates by microarray-based comparative genomics. PLoS One 8:e53341. 10.1371/journal.pone.0053341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denapaite D, Brückner R, Nuhn M, Reichmann P, Henrich B, Maurer P, Schähle Y, Selbmann P, Zimmermann W, Wambutt R, Hakenbeck R. 2010. The genome of Streptococcus mitis B6—what is a commensal? PLoS One 5:e9426. 10.1371/journal.pone.0009426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olsvik B, Olsen I, Tenover FC. 1995. Detection of tet(M) and tet(O) using the polymerase chain reaction in bacteria isolated from patients with periodontal disease. Oral Microbiol. Immunol. 10:87–92 [DOI] [PubMed] [Google Scholar]
- 12.Doherty N, Trzcinski K, Pickerill P, Zawadzki P, Dowson CG. 2000. Genetic diversity of the tet(M) gene in tetracycline-resistant clonal lineages of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 44:2979–2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amezaga MR, Carter PE, Cash P, McKenzie H. 2002. Molecular epidemiology of erythromycin resistance in Streptococcus pneumoniae isolates from blood and noninvasive sites. J. Clin. Microbiol. 40:3313–3318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poyart C, Quesne G, Acar P, Berche P, Trieu-Cuot P. 2000. Characterization of the Tn916-like transposon Tn3872 in a strain of Abiotrophia defectiva (Streptococcus defectivus) causing sequential episodes of endocarditis in a child. Antimicrob. Agents Chemother. 44:790–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cochetti I, Tili E, Vecchi M, Manzin A, Mingoia M, Varaldo PE, Montanari MP. 2007. New Tn916-related elements causing erm(B)-mediated erythromycin resistance in tetracycline-susceptible pneumococci. J. Antimicrob. Chemother. 60:127–131 [DOI] [PubMed] [Google Scholar]
- 16.Brenciani A, Tiberi E, Bacciaglia A, Petrelli D, Varaldo PE, Giovanetti E. 2011. Two distinct genetic elements are responsible for erm(TR)-mediated erythromycin resistance in tetracycline-susceptible and tetracycline-resistant strains of Streptococcus pyogenes. Antimicrob. Agents Chemother. 55:2106–2112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brede DA, Snipen LG, Ussery DW, Nederbragt AJ, Nes IF. 2011. Complete genome sequence of the commensal Enterococcus faecalis 62, isolated from a healthy Norwegian infant. J. Bacteriol. 193:2377–2378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyd DA, Cabral T, Van Caeseele P, Wylie J, Mulvey MR. 2002. Molecular characterization of the vanE gene cluster in vancomycin-resistant Enterococcus faecalis N00-410 isolated in Canada. Antimicrob. Agents Chemother. 46:1977–1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smyth DS, Robinson DA. 2009. Integrative and sequence characteristics of a novel genetic element, ICE6013, in Staphylococcus aureus. J. Bacteriol. 191:5964–5975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brenciani A, Bacciaglia A, Vignaroli C, Pugnaloni A, Varaldo PE, Giovanetti E. 2010. Characterization of Φm46.1, the main Streptococcus pyogenes element carrying mef(A) and tet(O) genes. Antimicrob. Agents Chemother. 54:221–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giovanetti E, Brenciani A, Tiberi E, Bacciaglia A, Varaldo PE. 2012. ICESp2905, the erm(TR)-tet(O) element of Streptococcus pyogenes, is formed by two independent integrative and conjugative elements. Antimicrob. Agents Chemother. 56:591–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brenciani A, Tiberi E, Morici E, Oryasın E, Giovanetti E, Varaldo PE. 2012. ICESp1116, the genetic element responsible for erm(B)-mediated, inducible resistance to erythromycin in Streptococcus pyogenes. Antimicrob. Agents Chemother. 56:6425–6429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tettelin H, Masignani V, Cieslewicz MJ, Eisen JA, Peterson S, Wessels MR, Paulsen IT, Nelson KE, Margarit I, Read TD, Madoff LC, Wolf AM, Beanan MJ, Brinkac LM, Daugherty SC, DeBoy RT, Durkin AS, Kolonay JF, Madupu R, Lewis MR, Radune D, Fedorova NB, Scanlan D, Khouri H, Mulligan S, Carty HA, Cline RT, Van Aken SE, Gill J, Scarselli M, Mora M, Iacobini ET, Brettoni C, Galli G, Mariani M, Vegni F, Maione D, Rinaudo D, Rappuoli R, Telford JL, Kasper DL, Grandi G, Fraser CM. 2002. Complete genome sequence and comparative genomic analysis of an emerging human pathogen, serotype V Streptococcus agalactiae. Proc. Natl. Acad. Sci. U. S. A. 99:12391–12396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giovanetti E, Magi G, Brenciani A, Spinaci C, Lupidi R, Facinelli B, Varaldo PE. 2002. Conjugative transfer of the erm(A) gene from erythromycin-resistant Streptococcus pyogenes to macrolide-susceptible S. pyogenes, Enterococcus faecalis, and Listeria innocua. J. Antimicrob. Chemother. 50:249–252 [DOI] [PubMed] [Google Scholar]
- 25.Palmieri C, Magi G, Mingoia M, Bagnarelli P, Ripa S, Varaldo PE, Facinelli B. 2012. Characterization of a Streptococcus suis tet(O/W/32/O)-carrying element transferable to major streptococcal pathogens. Antimicrob. Agents Chemother. 56:4697–4702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kreiswirth BN, Lofdahl S, Betley MJ, O'Reilly M, Schlievert PM, Bergdoll MS, Novick RP. 1983. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature 305:709–712 [DOI] [PubMed] [Google Scholar]
- 27.Tettelin H, Masignani V, Cieslewicz MJ, Donati C, Medini D, Ward NL, Angiuoli SV, Crabtree J, Jones AL, Durkin AS, Deboy RT, Davidsen TM, Mora M, Scarselli M, Margarit y Ros I, Peterson JD, Hauser CR, Sundaram JP, Nelson WC, Madupu R, Brinkac LM, Dodson RJ, Rosovitz MJ, Sullivan SA, Daugherty SC, Haft DH, Selengut J, Gwinn ML, Zhou L, Zafar N, Khouri H, Radune D, Dimitrov G, Watkins K, O'Connor KJ, Smith S, Utterback TR, White O, Rubens CE, Grandi G, Madoff LC, Kasper DL, Telford JL, Wessels MR, Rappuoli R, Fraser CM. 2005. Genome analysis of multiple pathogenic isolates of Streptococcus agalactiae: implications for the microbial “pan-genome.” Proc. Natl. Acad. Sci. U. S. A. 102:13950–13955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brochet M, Rusniok C, Couv́ E, Dramsi Poyart C, Trieu-Cuot P, Kunst F, Glaser P. 2008. Shaping a bacterial genome by large chromosomal replacements, the evolutionary history of Streptococcus agalactiae. Proc. Natl. Acad. Sci. U. S. A. 105:15961–15966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brochet M, Couvé E, Glaser P, Guédon G, Payot S. 2008. Integrative conjugative elements and related elements are major contributors to the genome diversity of Streptococcus agalactiae. J. Bacteriol. 190:6913–6917 [DOI] [PMC free article] [PubMed] [Google Scholar]