Abstract
We examined the effects of tigecycline on three types of exoproteins, α-type phenol-soluble modulins (PSMα1 to PSMα4), α-hemolysin, and protein A, in 13 methicillin-resistant Staphylococcus aureus isolates compared to those of clindamycin and linezolid. Paradoxical increases in PSMαs occurred in 77% of the isolates with tigecycline at 1/4 and 1/8 MICs and clindamycin at 1/8 MIC compared to only 23% of the isolates with linezolid at 1/8 MIC. Induction was specific to PSMα1 to PSMα4, as protein A and α-hemolysin production was decreased under the same conditions by all of the antibiotics used.
TEXT
Methicillin-resistant Staphylococcus aureus (MRSA) virulence in pneumonia and bacteremia has been attributed to exoproteins, specifically, α-hemolysin (Hla) and α-type phenol-soluble modulins (PSMα1 to PSMα4), which are produced by nearly all S. aureus strains and in excess by community-acquired MRSA strains (1). These exoproteins not only cause direct damage to target host cells but also exacerbate the host inflammatory response, contributing to acute lung injury. Additionally, recent reports have demonstrated the importance of protein A (Spa) in invasive diseases such as pneumonia (2, 3).
In light of the impressive arsenal of virulence factors contributing to the success of MRSA as a pathogen, it is of keen interest to determine if anti-MRSA agents belonging to the antibiotic class of protein synthesis inhibitors provide the added antivirulence benefit of exoprotein inhibition. In the present study, we investigated the antivirulence potential of tigecycline, linezolid, and clindamycin which have been proven efficacious in the treatment of MRSA infections. Our goal was to determine whether antivirulence effects can be generalized across different clinical isolates, different agents that inhibit protein synthesis, and different exoproteins. Specifically, we tested the effects of the above three antibiotics at subinhibitory concentrations on formylated PSMα1 to PSMα4, Hla, and Spa production by invasive MRSA isolates.
Eleven invasive MRSA isolates were tested under the following conditions. A modified CLSI broth macrodilution assay was used to determine MICs after 24 h of incubation at 37°C and shaking at 250 rpm in tryptic soy broth. Supernatants were then analyzed by liquid chromatography-tandem mass spectrometry, and Hla and Spa were analyzed by Western blotting as previously described (4, 5). Measured exotoxin values were normalized to the cell optical density at 600 nm (OD600) at the time the supernatant was harvested. PSMα concentrations under various test conditions were compared by analysis of variance with Dunn's correction using GraphPad Prism version 5.0 software (GraphPad, San Diego, CA).
Table 1 depicts the SCCmec type, PVL status, and baseline PSMα production characteristics of the 13 isolates studied (11 clinical isolates and two control strains). The PSMα1 to PSMα4 peptides have been shown to cause concentration-dependent neutrophil lysis (6, 7). A PSMα3 concentration of 5 μg/ml has been shown to lyse nearly 50% of human polymorphonuclear neutrophils (PMNs), while 10 μg/ml of PSMα1 and PSMα4 can cause 60% and 10% PMN lysis, respectively (8, 9). Thus, isolates were grouped on the basis of the amount of the most potent peptide, PSMα3, produced at the baseline as very low to low (≤5 μg/ml), medium (6 to 15 μg/ml), or high (>15 μg/ml) producers.
Table 1.
Characteristics of the 11 clinical isolates and two control strains used in this study
| Isolate or strain | mec type | PVLa | Infection type | PSMb baseline | MIC (μg/ml)c |
||
|---|---|---|---|---|---|---|---|
| TYG | CL | LZ | |||||
| 1 | IV | + | Blood | Low | 0.125 | 0.188 | 3 |
| 2 | IV | + | Blood | Medium | 0.125 | 0.25 | 3 |
| 3 | IV | + | Blood | Low | 0.125 | 0.188 | 2 |
| 4 | IV | + | Blood | Medium | 0.125 | 0.25 | 2 |
| 5 | IV | + | Blood | Medium | 0.125 | 0.188 | 2 |
| 6 | IV | + | Pneumonia | Low | 0.188 | 0.125 | 2 |
| 7 | IV | + | Pneumonia | Medium | 0.125 | 0.188 | 3 |
| 8 | IV | + | Necrotizing pneumonia | High | 0.125 | 0.25 | 3 |
| 9 | II | − | Pneumonia | Very low | 0.125 | >256 | 3 |
| 10 | II | − | Pneumonia | Very low | 0.125 | >256 | 1 |
| 11 | II | − | Pneumonia | Very low | 0.125 | 0.188 | 2 |
| Control strains | |||||||
| USA300 (LAC) | IV | + | Wound | Medium | 0.25 | 0.25 | 2 |
| USA600 | II | − | Blood | Low | 0.125 | >256 | 2 |
A plus or minus sign denotes the presence or absence, respectively, of the lukF/S gene, which encodes the Panton-Valentine leukocidin.
The PSMα3 production level at the baseline was arbitrarily defined as very low (<1 μg/ml), low (1 to 5 μg/ml), medium (6 to 15 μg/ml), or high (>15 μg/ml).
TYG, tigecycline; CL, clindamycin; LZ, linezolid. Resistance is defined as a MIC of >256 μg/ml (not tested for PSMα production at subinhibitory concentration).
Bacterial growth at 1/2 MICs of all three antibiotics was altered in half of the isolates tested by as much as 50% of the final OD compared to a no-antibiotic control, while no significant difference in the growth of any isolates was observed at 1/4 and 1/8 MICs. Thus, subsequent discussions will focus on the effect of antibiotics at the latter subinhibitory concentrations that did not affect growth. Measured PSMα values were normalized to the OD600 at the time the supernatant was harvested.
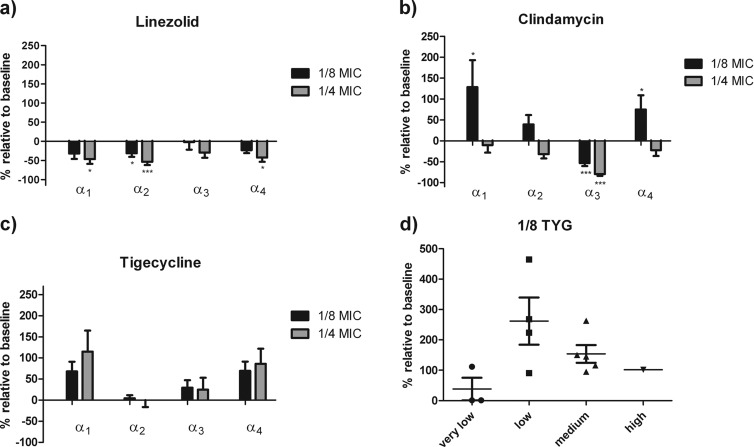
Tigecycline appears to have the least inhibitory potential overall, while linezolid has the greatest (Fig. 1a to c). Increased production of all four PSMα peptides was the primary response observed in the presence of tigecycline at 1/4 and 1/8 MICs, while with clindamycin this occurred only at 1/8 MIC (Fig. 1b and c). Notably, compared to clindamycin at 1/8 MIC, which significantly induced PSM production in seven isolates, linezolid at 1/8 MIC modestly induced PSM production in only three isolates (Fig. 1a).
Fig 1.
Overall effects of linezolid, clindamycin, and tigecycline on PSMα1 to PSMα4 production. (a to c) Effects of antibiotics on the production of PSMα1 to PSMα4 (α1, α2, α3, and α4, respectively). All measured PSM values (μg/ml) were normalized to OD600 and then calculated as percentages relative to the baseline. Isolates (n = 10) included only those that produced measureable PSMα peptides at the baseline (***, P < 0.0001; **, P < 0.001; *, P < 0.05). (d) Strain-to-strain variability of PSMα1 production at 1/8 MIC of tigecycline (TYG). Strains were grouped according to their baseline PSMα1 production as very low (<1 μg/ml), low (1 to 5 μg/ml), medium (6 to 15 μg/ml), and high (>15 μg/ml) producers.
Strain-specific responses to the presence of subinhibitory antibiotic concentrations in PSMα production were observed. Drug concentrations that induced PSMα production in some isolates did not do so in others, independently of the baseline production level. Specifically, with tigecycline at both 1/4 and 1/8 MICs (Fig. 1d), PSMα1 was induced at least 1.5 times above the baseline in 77% (11/13) of the isolates, while induction did not occur in two isolates at any of the concentrations tested. Similar results were observed with clindamycin, where 54% (7/13) of the isolates were induced by greater than 150%; however, this was observed only at 1/8 MIC. In those isolates, PSMα1 production was induced to greater than 10 μg/ml, which has been shown to cause significant PMN lysis (9). Linezolid at the same concentration resulted in increases in PSMα1 in only 23% (3/13) of the isolates tested, was inhibitory in 5 isolates, and had no significant effects on the remaining isolates. Of additional interest is the observation that nonproducers at the baseline produced PSMα peptides in the presence of subinhibitory concentrations of both linezolid and tigecycline. However, it is noteworthy that PSMα production that was induced in those two isolates did not exceed 4 μg/ml and thus would not be expected to have a significant impact on host PMNs. In contrast to results observed with the PSMα1 to PSMα4 peptides, we found that the production of both Hla and Spa did not increase under any condition and was inhibited in a dose-dependent manner at the concentrations of all three antibiotics tested regardless of whether PSMα peptides were induced or suppressed in those isolates (Fig. 2).
Fig 2.
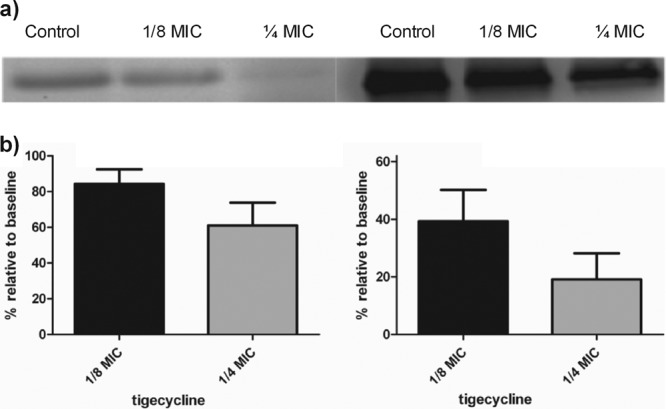
Effects of tigecycline on Hla and Spa production. (a) Representative images of Western blot assays of Hla and Spa, respectively. (b and c) Production of Hla and Spa in the presence of 1/8 and 1/4 MICs of tigecycline relative to the baseline. In no case was Hla or Spa production increased by subinhibitory concentrations of any of the antibiotics tested.
To our knowledge, we are the first to investigate the effect of tigecycline on MRSA PSMα1 to PSMα4 peptide production. We also included clindamycin and linezolid for comparison and tested their antivirulence potential against two other key exotoxins, Hla and Spa. We found that at sub-MICs, agents in this class of antibiotics have pleiotropic effects on toxin production that are dependent on the drug, strain, and toxin tested.
All three of the antibiotics tested are known to have large volumes of distribution and would be expected to be present at high concentrations within tissues. However, the concentrations tested reflect clinical scenarios where sub-MICs might be achieved at sites of infection because of inadequate dosing, altered pharmacokinetic parameters of the patient, and differential drug distribution to various body sites. Specifically, compared to the concentrations achieved at other body sites, tigecycline achieves relatively low levels in serum (0.11 to 0.19 μg/ml) and lung epithelial lining fluid (0.11 to 0.31 μg/ml), which are relevant to bacteremia and pneumonia, respectively, which are below the 0.5-μg/ml FDA MIC breakpoint of S. aureus susceptibility (10–12).
Low concentrations (1/4 and 1/8 MICs) of the antibiotics tested were more likely to induce PSMα production overall, with tigecycline having the greatest induction potential, clindamycin having less, and linezolid even less than that. Furthermore, the magnitudes of induction and inhibition differed among isolates, where the same drug could induce PSMα production in one isolate while inhibiting production in another. The observation of PSMα induction by protein synthesis-inhibiting antibiotics (e.g., linezolid and clindamycin) at sub-MICs is contrary to published literature on other exotoxins, where studies have consistently found toxin suppression under this class of antibiotics (13–16). The production of both Hla and Spa was suppressed by antibiotics tested at subinhibitory concentrations among the study isolates, independently of whether PSMα was induced in the same isolates. Hence, our results indicate that the induction effects may be specific for PSMα peptides. In contrast to our findings where significant inhibition was observed, a recent article showed tigecycline to be minimally inhibitory of Hla and Spa production (17); however, this difference may be due to the different strains and testing methods used.
Our study has several limitations. First, while we accounted for changes in cell counts by normalizing the amount of toxin measured to CFU counts, other factors, such as initial slowing of growth, could also affect toxin production. In addition, our observations are strictly in vitro and may not reflect how MRSA strains express virulence in the host environment, where the effects of toxin induction may be mitigated by host defenses that are present.
Further investigation is needed to examine if any other major toxins are induced, as PSMα peptides did when exposed to subinhibitory concentrations of different antibiotics. Elucidating the mechanism that underlies the induction of PSMα peptides is of great interest and has implications for the future development of new antibiotics. In light of the recent evidence implicating tigecycline in excess deaths and treatment failure in severe infections (18), the enhanced toxin production observed in this study may have clinical relevance and deserves further study. For now, our data caution against the general assumption by clinicians that all protein synthesis inhibitors possess inhibitory potential against all of the exotoxins produced by S. aureus and affirm the importance of adequate dosing.
ACKNOWLEDGMENTS
This study was supported by a research grant from Pfizer.
We thank Bixin Xi for his technical assistance in the development and optimization of the mass spectrometry assay for PSMα measurement. We also thank the Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA) and The Los Angeles County Public Health Department for providing the control strains used.
Footnotes
Published ahead of print 1 July 2013
REFERENCES
- 1.Li M, Diep BA, Villaruz AE, Braughton KR, Jiang X, DeLeo FR, Chambers HF, Lu Y, Otto M. 2009. Evolution of virulence in epidemic community-associated methicillin-resistant Staphylococcus aureus. Proc. Natl. Acad. Sci. U. S. A. 106:5883–5888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soong G, Martin FJ, Chun JR, Cohen TS, Ahn DS, Prince A. 2011. Staphylococcus aureus protein A mediates invasion across airway epithelial cells through activation of RhoA GTPase signaling and proteolytic activity. J. Biol. Chem. 286:35891–35898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin FJ, Gomez MI, Wetzel DM, Memmi G, O'Seaghdha M, Soong G, Schindler C, Prince A. 2009. Staphylococcus aureus activates type I IFN signaling in mice and humans through the Xr repeated sequences of protein A. J. Clin. Invest. 119:1931–1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamaki J, Synold T, Wong-Beringer A. 2011. Antivirulence potential of TR-700 and clindamycin on clinical isolates of Staphylococcus aureus producing phenol-soluble modulins. Antimicrob. Agents Chemother. 55:4432–4435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montgomery CP, Boyle-Vavra S, Daum RS. 2009. The arginine catabolic mobile element is not associated with enhanced virulence in experimental invasive disease caused by the community-associated methicillin-resistant Staphylococcus aureus USA300 genetic background. Infect. Immun. 77:2650–2656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang R, Braughton KR, Kretschmer D, Bach THL, Queck SY, Li M, Kennedy AD, Dorward DW, Klebanoff SJ, Peschel A, Deleo FR, Otto M. 2007. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat. Med. 13:1510–1514 [DOI] [PubMed] [Google Scholar]
- 7.Loffler B, Hussain M, Grundmeier M, Bruck M, Holzinger D, Varga G, Roth J, Kahl BC, Proctor RA, Peters G. 2010. Staphylococcus aureus Panton-Valentine leukocidin is a very potent cytotoxic factor for human neutrophils. PLoS Pathog. 6(1):e1000715. 10.1371/journal.ppat.1000715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hongo I, Baba T, Oishi K, Morimoto Y, Ito T, Hiramatsu K. 2009. Phenol-soluble modulin alpha 3 enhances the human neutrophil lysis mediated by Panton-Valentine leukocidin. J. Infect. Dis. 200:715–723 [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez DJ, Okumura CY, Hollands A, Kersten R, Akong-Moore K, Pence MA, Malone CL, Derieux J, Moore BS, Horswill AR, Dixon JE, Dorrestein PC, Nizet V. 2012. Novel phenol-soluble modulin derivatives in community-associated methicillin-resistant Staphylococcus aureus identified through imaging mass spectrometry. J. Biol. Chem. 287:13889–13898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodvold KA, Gotfried MH, Cwik M, Korth-Bradley JM, Dukart G, Ellis-Grosse EJ. 2006. Serum, tissue and body fluid concentrations of tigecycline after a single 100 mg dose. J. Antimicrob. Chemother. 58:1221–1229 [DOI] [PubMed] [Google Scholar]
- 11.Brink AJ, Bizos D, Boffard KD, Feldman C, Grolman DC, Pretorius J, Richards GA, Senekal M, Steyn E, Welkovic N. 2010. Guideline: appropriate use of tigecycline. S. Afr. Med. J. 100:388–394 [DOI] [PubMed] [Google Scholar]
- 12.Conte JE, Golden JA, Kelly MG, Zurlinden E. 2005. Steady-state serum and intrapulmonary pharmacokinetics and pharmacodynamics of tigecycline. Int. J. Antimicrob. Agents 25:523–529 [DOI] [PubMed] [Google Scholar]
- 13.Dumitrescu O, Badiou C, Bes M, Reverdy ME, Vandenesch F, Etienne J, Lina G. 2008. Effect of antibiotics, alone and in combination, on Panton-Valentine leukocidin production by a Staphylococcus aureus reference strain. Clin. Microbiol. Infect. 14:384–388 [DOI] [PubMed] [Google Scholar]
- 14.Dumitrescu O, Boisset S, Badiou C, Bes M, Benito Y, Reverdy ME, Vandenesch F, Etienne J, Lina G. 2007. Effect of antibiotics on Staphylococcus aureus producing Panton-Valentine leukocidin. Antimicrob. Agents Chemother. 51:1515–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stevens DL, Ma Y, Salmi DB, McIndoo E, Wallace RJ, Bryant AE. 2007. Impact of antibiotics on expression of virulence-associated exotoxin genes in methicillin-sensitive and methicillin-resistant Staphylococcus aureus. J. Infect. Dis. 195:202–211 [DOI] [PubMed] [Google Scholar]
- 16.Ohlsen K, Ziebuhr W, Koller KP, Hell W, Wichelhaus TA, Hacker J. 1998. Effects of subinhibitory concentrations of antibiotics on alpha-toxin (hla) gene expression of methicillin-sensitive and methicillin-resistant Staphylococcus aureus isolates. Antimicrob. Agents Chemother. 42:2817–2823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Otto MP, Martin E, Badiou C, Lebrun S, Bes M, Vandenesch F, Etienne J, Lina G, Dumitrescu O. 2013. Effects of subinhibitory concentrations of antibiotics on virulence factor expression by community-acquired methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 68:1524–1532 [DOI] [PubMed] [Google Scholar]
- 18.Prasad P, Sun J, Danner RL, Natanson C. 2012. Excess deaths associated with tigecycline after approval based on noninferiority trials. Clin. Infect. Dis. 54:1699–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]