Abstract
The membrane-associated drug transporter P-glycoprotein (P-gp) plays an essential role in drug efflux from the brain. Induction of this protein at the blood-brain barrier (BBB) could further affect the ability of a drug to enter the brain. At present, P-gp induction mediated by antiretroviral drugs at the BBB has not been fully investigated. Since P-gp expression is regulated by ligand-activated nuclear receptors, i.e., human pregnane X receptor (hPXR) and human constitutive androstane receptor (hCAR), these receptors could represent potential pathways involved in P-gp induction by antiretroviral drugs. The aims of this study were (i) to determine whether antiretroviral drugs currently used in HIV pharmacotherapy are ligands for hPXR or hCAR and (ii) to examine P-gp function and expression in human brain microvessel endothelial cells treated with antiretroviral drugs identified as ligands of hPXR and/or hCAR. Luciferase reporter gene assays were performed to examine the activation of hPXR and hCAR by antiretroviral drugs. The hCMEC/D3 cell line, which is known to display several morphological and biochemical properties of the BBB in humans, was used to examine P-gp induction following 72 h of exposure to these agents. Amprenavir, atazanavir, darunavir, efavirenz, ritonavir, and lopinavir were found to activate hPXR, whereas abacavir, efavirenz, and nevirapine were found to activate hCAR. P-gp expression and function were significantly induced in hCMEC/D3 cells treated with these drugs at clinical concentrations in plasma. Together, our data suggest that P-gp induction could occur at the BBB during chronic treatment with antiretroviral drugs identified as ligands of hPXR and/or hCAR.
INTRODUCTION
In the last decade, the use of combination antiretroviral therapy has led to a significant decline in the morbidity and mortality of people infected by human immunodeficiency virus (HIV). At present, although severe forms of HIV-associated neurocognitive disorders (e.g., HIV-associated dementia) have almost disappeared from clinical practice, the prevalence of other mild neurocognitive disorders (e.g., asymptomatic neurocognitive impairment) remains unchanged, suggesting that virus suppression in the brain is suboptimal (1). These disorders have been associated with a lower survival rate and can greatly affect the quality of life of individuals infected with HIV (2). One potential explanation for the high prevalence of these disorders is the limited ability of several antiretroviral drugs to enter the brain. A ranking system for the effectiveness with which drugs penetrate the central nervous system has been proposed by S. Letendre to evaluate whether brain penetration by antiretroviral drugs is associated with cerebrospinal fluid viral loads, a clinical indication of brain virus control (1). Letendre reported that HIV patients generally exhibited a lighter viral load in the cerebrospinal fluid while receiving antiretroviral drugs with greater brain-penetrating ability (1). In addition, further evidence suggested that the use of antiretroviral drugs that display a high ability to enter the brain can lead to better neurocognitive outcomes (3). These findings support the concept that the use of antiretroviral regimens with an improved ability to enter the brain could reduce viral loads in the brain and ultimately prevent HIV-associated neurological complications.
The presence of an intact blood-brain barrier (BBB) has long been known to restrict antiretroviral drug entry into the brain (4). In addition to the presence of tight junctions, one mechanism by which the BBB restricts drug entry is the expression of several membrane-associated drug efflux transporters in brain microvessel endothelial cells (5). In particular, P-glycoprotein (P-gp) expression at the luminal membrane of these endothelial cells can actively transport substrates (i.e., xenobiotics and drugs) back into the blood following the initial diffusion of these substrates across the luminal membrane (6–8). Substrates of P-gp include most HIV protease inhibitors (PIs), the nucleoside reverse transcriptase inhibitor abacavir, the chemokine CCR5 coreceptor antagonist maraviroc, and the HIV integrase inhibitor raltegravir (9). Therefore, induction of the functional expression of this protein at the BBB is expected to further restrict the ability of antiretroviral drugs known to serve as substrates of this transporter to enter the brain. Our group and others have previously demonstrated that the HIV PIs ritonavir and atazanavir can induce P-gp expression in human and rodent brain microvessel endothelial cells that constitute the BBB (10–12). However, little is known about the ability of other antiretroviral drugs to induce P-gp expression at this site. Induction of P-gp and other drug transporters (e.g., MRP2) in peripheral organs, such as the liver and intestine, has been demonstrated to be mediated through the activity of the xenobiotic-activating nuclear receptors pregnane X receptor (PXR) and constitutive androstane receptor (CAR) (13). At the BBB, PXR and CAR have also been demonstrated to regulate P-gp expression in porcine and rodent brain microvessel endothelial cells or isolated brain capillaries (14–18). Recently, our group has shown that human PXR (hPXR) and human CAR (hCAR) are actively involved in the regulation of P-gp in a human brain microvessel endothelial cell (hCMEC/D3) culture system (19). These two nuclear receptors have been identified as xenobiotic sensors that are able to interact with a wide array of pharmaceutical agents (20). At present, a few HIV PIs (i.e., ritonavir, amprenavir, and lopinavir) have been identified as ligands of hPXR by reporter-based assays (21, 22). In addition, efavirenz, a nonnucleotide reverse transcriptase inhibitor (NNRTI), is capable of inducing the promoter activity of the phase I drug-metabolizing cytochrome P450 enzyme 3A4 when transfected with a full-length hPXR plasmid (23). Moreover, Svärd et al. used a panel of antiretroviral drugs to screen for CYP3A4 and CYP2B6 promoter activation mediated by hPXR and hCAR; however, they reported several discrepancies in receptor activation by several HIV PIs (i.e., nelfinavir, ritonavir, and atazanavir) compared to previously published reports (22, 24). The aims of this study were (i) to determine whether several antiretroviral drugs currently used in first-line and alternative regimens during HIV pharmacotherapy are ligands for hPXR or hCAR and (ii) to examine P-gp function and expression in human brain microvessel endothelial cells following treatment with antiretroviral drugs identified as ligands of hPXR and hCAR. In this work, we used an in vitro cell culture system of brain microvessel endothelial cells (hCMEC/D3) that has been demonstrated to display several morphological and biochemical properties of the human brain microvascular endothelium, such as the expression of tight-junction and adhesion proteins, endothelial cell markers, drug efflux transporters (e.g., P-gp), and nuclear receptors (e.g., PXR and CAR) (12, 19, 25).
MATERIALS AND METHODS
Materials and reagents.
Cell culture medium (Opti-MEM and Dulbecco's modified Eagle's medium [DMEM]), fetal bovine serum (FBS), penicillin-streptomycin, sterile Dulbecco's phosphate-buffered saline (PBS), Hanks balanced salt solution, 0.25% trypsin, and liver digest medium were purchased from Invitrogen (Grand Island, NY). Rat tail type I collagen was purchased from Becton, Dickinson (San Jose, CA). Heat shock fraction V bovine serum albumin (BSA), dimethyl sulfoxide (DMSO), and acrylamide solution were obtained from Bioshop Canada Inc., (Burlington, ON, Canada). 6-(4-Chlorophenyl)-imidazo[2,1-b][1,3]thiazole-5-carbaldehyde O-(3,4-dichlorobenzyl)oxime (CITCO) and [[3,5-bis(1,1-dimethylethyl)-4-hydroxyphenyl]ethenylidene]bis-phosphonic acid tetraethyl ester (SR12813) were obtained from BIOMOL Research Laboratories (Plymouth Meeting, PA). Rifampin, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT), rhodamine-6G (R-6G), phenylmethanesulfonyl fluoride, protease inhibitor cocktail, and anti-actin (mouse monoclonal) antibody were purchased from Sigma-Aldrich (Oakville, ON, Canada). Antiretroviral drugs were kindly provided by the National Institutes of Health AIDS Research and Reference Reagent Program (Germantown, MD). Anti-P-gp (mouse monoclonal) antibody was obtained from ID Labs Inc. (London, ON, Canada). The goat anti-mouse horseradish peroxidase-conjugated secondary antibody was ordered from Jackson ImmunoResearch Laboratories Inc. (West Grove, PA). pRL-CMV was purchased from Promega (Madison, WI). UAS-luciferase, GAL4-CMX, GAL4-hPXR ligand binding domain (LBD), CMX, and β-galactosidase plasmids were provided by David Mangelsdorf (University of Texas Southwestern Medical Center, Dallas, TX). Gal4-hCAR LBD was a generous gift from David Moore (Baylor College of Medicine, Houston, TX).
Cell culture and luciferase reporter assays.
Monkey kidney fibroblast CV-1 cells, purchased from the American Type Culture Collection, were maintained in DMEM supplemented with 10% FBS and 1% penicillin-streptomycin (Invitrogen). Transfections were performed with FuGENE HD transfection reagent (Roche Diagnostics Corp.) in 48-well plates in DMEM supplemented with 10% charcoal-treated FBS (Invitrogen). The total amount of plasmid DNA was kept constant at 400 ng per well. Cells were cotransfected with a GAL4-responsive luciferase reporter (UAS-luc, 200 ng) and the GAL4 DNA binding domain fused to the LBD of hPXR (residues 184 to 433; 100 ng). Data were normalized to the β-galactosidase control (100 ng) and expressed in relative light units (RLU). As a negative control for the receptor, the cytomegalovirus-based (CMX) expression plasmid containing GAL4 alone was used as a negative control (GAL4-CMX). Antiretroviral drugs were added in fresh medium 6 h posttransfection, and cells were harvested 14 to 16 h later and assayed for luciferase and β-galactosidase activities. Primary cultures of mouse hepatocytes were isolated and cultured as previously described (26). In brief, wild-type 129/SvEv mouse liver was perfused with liver digestion medium (3 ml/min) and viable hepatocytes were purified by centrifugation three times at 50 × g. Freshly prepared hepatocytes were seeded into attachment medium (Williams E medium [10% charcoal-treated FBS, 1× penicillin-streptomycin, 10 nM insulin]) at a final density of 1.2 × 104/well of type I collagen-coated 48-well plates. Transfections were performed with 250 ng DNA per well with Lipofectamine 2000 reagent (Invitrogen) in Opti-MEM without FBS. Cells were cotransfected with a GAL4-responsive luciferase reporter (UAS-luc, 125 ng) and the GAL4 DNA binding domain fused to the LBD of hCAR (residues 101 to 348; 25 ng) and a pRL-CMV Renilla control (50 ng). The transfection efficiency in the primary cultures of mouse hepatocytes was approximately 80%, as determined by the transfection of a green fluorescent protein (GFP) control plasmid into some cultures. At 24 h posttransfection, cells were exposed to M199 medium without FBS containing antiretroviral drugs or positive-control ligands. Cells were harvested after an additional 14 to 16 h and assayed for luciferase and Renilla activities. Luciferase values were normalized for transfection efficiency to Renilla luciferase and expressed in RLU.
hCMEC/D3 cell culture system and ligand treatment.
The immortalized human brain microvessel endothelial cell line hCMEC/D3 was kindly provided by P. O. Couraud (Institut Cochin, Département de Biologie Cellulaire and INSERM, Paris, France) (25). Cells were cultured in rat tail type I collagen (BD)-coated 75-mm flasks, on 60-mm dishes, or on 24-well plates and maintained at 37°C in 5% CO2 and 95% humidified air in EGM-2 medium supplemented with the EGM-2 SingleQuot growth factor kit, which includes vascular endothelial growth factor, insulin-like growth factor 1, epidermal growth factor, fibroblast growth factors, hydrocortisone, ascorbate, gentamicin, and 2.5% FBS, as recommended (Lonza, Walkersville, MD). Once monolayers of hCMEC/D3 cells reached 80% confluence, the culture medium was aspirated and fresh medium containing hPXR or hCAR ligands was added to the cells for 72 h. All ligands were dissolved in DMSO. To ensure that the cells would remain viable during treatment, all of the ligand concentrations used were tested with the tetrazolium salts (MTT) assay as described previously (19). In brief, cells were incubated in PBS containing 2.5 mg/ml MTT at 37°C for 2 h following 72 h of ligand treatment. Cell viability was assessed by comparing the absorbance (580 nm) of cellular reduced MTT in ligand-treated cells to that of vehicle-treated cells with a SpectraMax 384 microplate reader (Molecular Devices, Sunnyvale, CA).
Immunoblot analysis.
P-gp and actin protein expression was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) according to previously published protocols (19). In brief, monolayers of hCMEC/D3 cells were washed with warm PBS once and collected by scraping in ice-cold PBS. Following centrifugation at 1,000 × g, whole-cell lysates were prepared by exposing cell pellets to lysis buffer (1% [vol/vol] NP-40, 20 mM Tris, 150 mM NaCl, 5 mM EDTA, 1 mM phenylmethanesulfonyl fluoride, 0.1% [vol/vol] protease inhibitor cocktail) for 15 min at 4°C. Cell lysates were sonicated for 10 s and centrifuged at 20,000 × g for 5 min at 4°C. Supernatants containing 50 μg of protein were resolved by SDS-PAGE and electrotransferred onto a polyvinylidene difluoride (PVDF) membrane (GE Healthcare Life Sciences, Piscataway, NJ). Membranes were blocked for 2 h in Tris buffer (15 mM Tris, 0.1% Tween 20, 5% skim milk) and incubated with a primary antibody overnight at 4°C prior to further incubation in an anti-mouse secondary antibody (Jackson ImmunoResearch Laboratories Inc.). P-gp expression was detected with a 1:500 dilution of mouse monoclonal C219 antibody (ID Labs, London, ON, Canada), which recognizes the highly conserved internal amino acid sequences VQEALD and VQAALD of P-gp. β-Actin expression was detected with a 1:3,000 dilution of mouse monoclonal C-4 antibody (Santa Cruz Biotechnology, Inc.). Densitometric analysis was performed with AlphaDigiDoc RT2 software (Alpha Innotech, San Leandro, CA) to quantify protein expression detected by an enhanced chemiluminescence kit (Pierce Thermo Fisher Scientific Inc., Waltham, MA). Whole-cell lysates of MDA435/LCC6/MDR1 cells (cultured in α minimum essential medium supplemented with 10% FBS and 1% penicillin-streptomycin) served as a positive control for P-gp expression.
R-6G transport assay.
The cellular accumulation of a well-established fluorescence P-gp substrate, R-6G, was measured to determine the activity of P-gp in hCMEC/D3 cells according to previously published protocols (12). Following 72 h of ligand (i.e., antiretroviral drug or hPXR or hCAR agonist) treatment of hCMEC/D3 cell monolayers grown on 24-well plates, cells were rinsed with fresh medium without ligands three times before being incubated with transport buffer (1× Hanks balanced salt solution, 10 mM HEPES, 0.01% BSA, pH 7.4) for 30 min. Cells were exposed to transport buffer containing 1 μM R-6G in the presence or absence of the P-gp inhibitor PSC-833 (5 μM) at 37°C for 30 min and then washed with ice-cold PBS three times to stop the reaction. Triton X-100 (1%) was added to the wells, and cellular R-6G accumulation was measured at excitation and emission wavelengths of 530 and 560 nm, respectively, with a SpectraMax plate reader (Molecular Devices, Sunnyvale, CA). Data are reported as the ratio of (i) the relative R-6G emission signal normalized to the protein content from corresponding wells of the ligand-treated group to (ii) that of the vehicle control.
Statistical analysis.
All experiments were repeated at least three times with different passages in cells. Results are reported as means ± standard deviations (SDs). Comparisons between groups were performed by one-way analysis of variance (ANOVA) with Dunnett's post hoc t test at a significance level of P < 0.05. Data were analyzed by SPSS software (SPSS Inc., Chicago, IL).
RESULTS
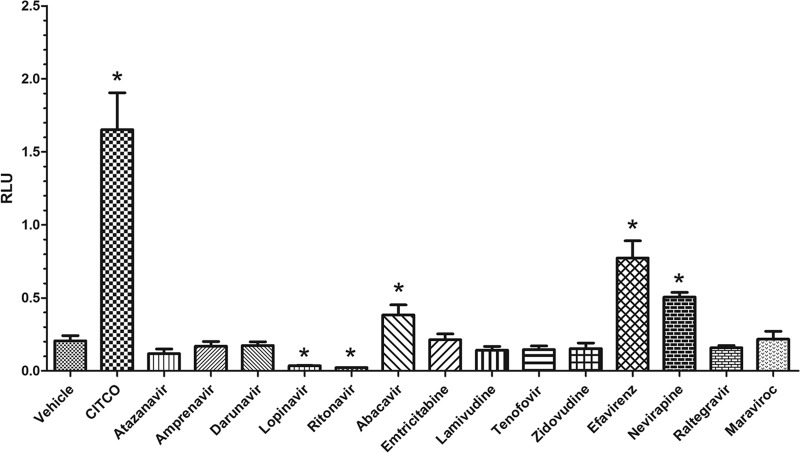
Luciferase reporter gene assays performed with CV-1 cells and primary cultures of mouse hepatocytes were used to examine the abilities of antiretroviral drugs to serve as ligands of hPXR and hCAR. GAL4-hPXR chimeric receptor activity was induced (n-fold induction normalized to DMSO) in the presence of positive controls, i.e., rifampin (4.5 ± 0.5) and SR12813 (17 ± 4.7), and the antiretroviral drugs lopinavir (8.1 ± 0.2), amprenavir (6.1 ± 0.4), efavirenz (3.2 ± 0.3), darunavir (3.2 ± 0.2), ritonavir (2.5 ± 0.1), and atazanavir (2.1 ± 0.2) (Fig. 1). GAL4-hCAR chimeric receptor activity was induced (n-fold induction normalized to DMSO) in the presence of the positive control, CITCO (7.9 ± 1.2), and the antiretroviral drugs efavirenz (3.7 ± 0.5), abacavir (2.4 ± 0.1), and nevirapine (1.8 ± 0.3) (Fig. 2). Data from a representative experiment are presented. Each screen was performed on three independent occasions with CV-1 cells of different passage numbers and independent isolations of primary mouse hepatocytes.
Fig 1.
Activation of hPXR by antiretroviral drugs. Representative data from three independent luciferase reporter gene assays performed with transfected CV-1 cells after 14 to 16 h of treatment with antiretroviral drugs (10 μM) are presented as means ± SDs of triplicates. Luciferase activity (representing ligand activation) was normalized to constitutive β-galactosidase activity (to control for transfection efficiency) and is reported in RLU. Statistically significant differences in receptor activation (normalized to the vehicle control) were determined by one-way ANOVA with Dunnett's post hoc test at a significance level of P < 0.05 (*).
Fig 2.
Activation of hCAR by antiretroviral drugs. Representative data from three independent dual-luciferase reporter gene assays performed with transfected primary cultures of mouse hepatocytes after 14 to 16 h of treatment with antiretroviral drugs (10 μM) are presented as means ± SDs of triplicates. Luciferase activity (representing ligand activation) was normalized to constitutive Renilla luciferase activity (to control for transfection efficiency) and is reported in RLU. Statistically significant differences in receptor activation (normalized to the vehicle control) were determined by one-way ANOVA with Dunnett's post hoc test at a significance level of P < 0.05 (*).
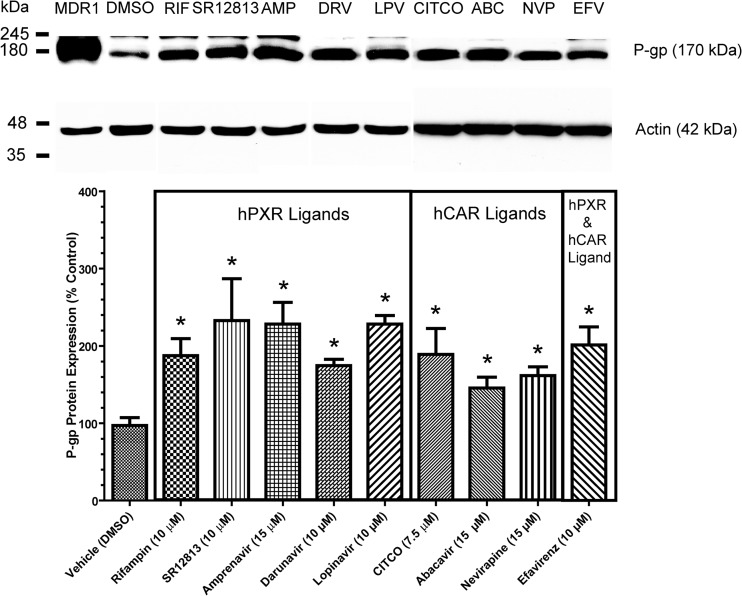
The hCMEC/D3 human brain microvessel endothelial cell culture system was used to examine P-gp induction mediated by antiretroviral drugs that exhibited hPXR and/or hCAR ligand properties. Previously, in the same culture system, our group demonstrated that 72 h of cell exposure to 10 μM atazanavir or 10 μM ritonavir could induce functional P-gp expression by approximately 2-fold compared to the vehicle control (DMSO) (12). In the present study, hCMEC/D3 cells were exposed to antiretroviral drugs for 72 h at clinically relevant concentrations in plasma (i.e., lopinavir, 8.7 to 15 μM [27]; amprenavir, 11 to 19 μM [28]; darunavir, 3.3 to 24 μM [29]; efavirenz, 9.2 to 17 μM [30]; abacavir, 5.2 to 15 μM [31]; nevirapine, 7.5 to 21 μM [32]). Lopinavir (10 μM), amprenavir (15 μM), and darunavir (10 μM), which served as ligands of hPXR, induced P-gp expression in hCMEC/D3 cells by approximately 2.3-fold ± 0.1-fold, 2.3-fold ± 0.3-fold, and 1.7-fold ± 0.1-fold, respectively, compared to the vehicle control (Fig. 3). Abacavir (15 μM) and nevirapine (15 μM), identified as ligands of hCAR, induced P-gp expression in hCMEC/D3 cells by approximately 1.5-fold ± 0.1-fold and 1.6-fold ± 0.1-fold, respectively, compared to the vehicle control. Efavirenz (10 μM), the only drug that served as a ligand of both hPXR and hCAR, induced P-gp expression in hCMEC/D3 cells by 2.0-fold ± 0.2-fold compared to the vehicle control. Rifampin and SR12813 (established agonists of hPXR) and CITCO (established agonist of hCAR) served as positive controls for P-gp induction. Consistent with previous findings, these ligands produced approximately 2-fold increases in P-gp expression (19). Drug accumulation assays with R-6G (a fluorescent P-gp substrate) were performed to assess changes in P-gp transport function in hCMEC/D3 cells following 72 h of treatment with antiretroviral drugs that mediated P-gp induction as depicted in Fig. 3. We observed that treatment of hCMEC/D3 cells with antiretroviral drugs significantly reduced the cellular accumulation of R-6G by approximately 20 to 30% compared to the vehicle control (Fig. 4), suggesting an increase in the R6G efflux process. Moreover, PSC833 (a potent inhibitor of P-gp) at 5 μM abolished the differences in R-6G accumulation between the vehicle control and treatment groups, further confirming the involvement of P-gp in the efflux of R-6G. Together, these data demonstrate that P-gp induction mediated by antiretroviral drugs can result in an increase in P-gp transport function in human brain microvessel endothelial cells.
Fig 3.
P-gp immunoblotting and densitometric analysis. Shown is P-gp expression normalized to the vehicle control (DMSO) in hCMEC/D3 cells after 72 h of treatment with (i) the hPXR ligands rifampin (10 μM; hPXR agonist), SR12813 (10 μM; potent hPXR agonist), amprenavir (15 μM; HIV PI), darunavir (10 μM; HIV PI), and lopinavir (10 μM; HIV PI); (ii) the hCAR ligands CITCO (7.5 μM; synthetic potent hCAR agonist), abacavir (15 μM; NRTI), and nevirapine (15 μM; NNRTI); or (iii) the dual hPXR and hCAR ligand efavirenz (10 μM; NNRTI). A representative immunoblot assay of P-gp expression is shown at the top. Whole-cell lysates prepared from a P-gp-overexpressing human breast carcinoma cell culture system (MDA-MDR1, 2 μg) served as a P-gp positive control. A 50-μg load of whole-cell lysate of hCMEC/D3 cells was resolved by 10% SDS-PAGE and subsequently transferred to a PVDF membrane. Densitometric analysis was performed to determine relative P-gp expression. P-gp was detected with the monoclonal antibody C219 (1:500) and an anti-mouse secondary antibody (1:3,000). Data represent n-fold percentage changes normalized to the vehicle control and are means ± SDs obtained from three experiments from cells of different passages. Statistically significant differences from the control in P-gp expression, as determined by one-way ANOVA with Dunnett's post hoc t test at a significance level of P < 0.05, are indicated (*).
Fig 4.
R-6G accumulation in hCMEC/D3 cells. R-6G accumulation in cell monolayers was measured after 72 h of cell treatment with (i) the hPXR ligands rifampin, SR12813, amprenavir, darunavir, and lopinavir; (ii) the hCAR ligands CITCO, abacavir, and nevirapine; or (iii) the dual hPXR and hCAR ligand efavirenz. For the group without PSC833 treatment (open bars), results are expressed as the mean percent change in R-6G accumulation versus the control (vehicle without PSC-833) ± the standard error of the mean obtained from three separate experiments with different passages of cells. For the PSC-833 group (solid bars), results are expressed as the mean percent change in R-6G accumulation normalized to the corresponding treatment without PSC-833 ± the standard error of the mean. In each experiment, each of the treatment and vehicle control groups was treated in triplicate. One-way ANOVA with Dunnett's post hoc t test at a significance level of P < 0.05 was performed. Symbols: *, statistically significant difference in R-6G accumulation between cells treated with antiretroviral drugs and cells treated with the vehicle control; **, statistically significant difference in R-6G accumulation between cells exposed to PSC-833 and cells not exposed to PSC-833.
DISCUSSION
The use of combination antiretroviral therapy has significantly increased the survival rate of individuals infected with HIV. Most of the antiretroviral drugs currently used in first-line and alternative regimens during HIV pharmacotherapy are known to be metabolized by phase I enzymes (i.e., cytochrome P450 enzymes) and/or transported by several drug efflux transporters (e.g., P-gp) (9, 33). Induction of these systems during prolonged antiretroviral treatment may profoundly alter the pharmacokinetic parameters of antiretroviral drugs and ultimately impair their effectiveness at target organs, contributing to the development of sanctuary sites and cellular reservoirs (34). At the BBB, the functional expression of P-gp has been recognized to restrict the entry of antiretroviral drugs into the brain, in particular for the HIV PI (4). P-gp induction at the BBB is expected to further limit the ability of these agents to enter the brain, preventing therapeutic concentrations from being achieved in the brain parenchyma. It has been proposed that antiretroviral regimens containing drugs with a better ability to enter the brain may improve the clinical outcomes of patients living with HIV-associated neurocognitive disorders (1). Therefore, knowledge of the ability of antiretroviral drugs to induce P-gp expression at the BBB can be useful in the design of drug regimens with limited P-gp induction during HIV pharmacotherapy, ultimately improving the ability of antiretroviral drugs that are known to be P-gp substrates to enter the brain.
Our group has recently demonstrated that well-established ligands of hPXR and hCAR can induce P-gp expression in hCMEC/D3 cells an in vitro representative system of the human BBB (12, 19). Luciferase reporter gene assays were used in the present study to examine the abilities of antiretroviral drugs to serve as ligands for hPXR and/or hCAR. Our results demonstrate that most members of the HIV PI pharmacotherapy class and efavirenz, an NNRTI, can serve as ligands of hPXR. This is consistent with earlier publications that demonstrated that ritonavir, lopinavir, and efavirenz were able to trigger hPXR activity (21–23). In addition, we provide the first evidence that amprenavir, darunavir, and atazanavir can also serve as ligands of hPXR (Fig. 1). Furthermore, there is evidence suggesting that lopinavir and abacavir can serve as ligands of hCAR in transfected human liver hepatocellular carcinoma cell line (HepG2) cells (22). In the present work, we performed a reporter gene assay of primary cultures of mouse hepatocytes that has previously been identified to exhibit enhanced CAR-inducible signaling (i.e., nuclear translocation) compared to HepG2 cultures (35, 36). We found that abacavir, which belongs to the nucleoside/nucleotide reverse transcriptase inhibitor (NRTI) class, as well as efavirenz and nevirapine of the NNRTI class, can serve as a ligand of hCAR (Fig. 2). In addition, none of the members of the HIV PI class were found to serve as ligands of hCAR. It is also interesting that lopinavir and ritonavir consistently reduced the activity of hCAR below its basal level in three experimental replicates at drug concentrations that were not found to be toxic to mouse hepatocytes. In the present study, the activation of hPXR and hCAR by antiretroviral drugs could be due to specific binding between ligands and the ligand binding pocket of the nuclear receptors. Several X-ray crystal structures of the ligand binding pocket of hPXR and hCAR have been shown to be large and flexible, with hydrophobic sites consisting of multiple polar residues (37, 38). At present, structural information on how antiretroviral drugs interact with the ligand binding pocket of hPXR and hCAR is unclear. Interestingly, a recent study has successfully predicted ligand activation of hPXR by efavirenz by an in silico ligand-docking approach; however, the specific interactions between chemical structures of other antiretroviral drugs and the ligand binding pocket of hPXR and hCAR have not been reported (39).
The effect of prolonged treatment of antiretroviral drugs on P-gp expression at the human BBB is unclear. Perloff et al. demonstrated that ritonavir can induce P-gp in isolated rat and bovine brain microvessel endothelial cells (10, 11). In addition, our group has previously shown that ritonavir and atazanavir can induce functional P-gp expression in hCMEC/D3 cells (12). To further examine this effect, we treated hCMEC/D3 cells with antiretroviral drugs that we identified as ligands of hPXR and hCAR. Our data show that prolonged exposure (72 h) to these drugs can induce functional expression of P-gp in hCMEC/D3 cells (Fig. 3 and 4). As well, results of MTT cell viability assays suggest that the concentrations of these agents used do not affect cell viability (data not shown). In the present study, P-gp induction in hCMEC/D3 cells mediated by treatment with ritonavir, atazanavir, lopinavir, darunavir, nevirapine, and efavirenz supports previous findings demonstrating that these drugs were able to induce P-gp expression in lymphocytes and intestinal and hepatic tissues (24, 40–46). Interestingly, efavirenz is the only drug that appears to serve as a ligand of both hPXR and hCAR. However, the induction effect was not significantly greater than those of other antiretroviral drugs that served as ligands for only one of the two receptors (hPXR or hCAR), suggesting that there is a lack of synergistic effects on P-gp induction mediated by hPXR and hCAR at the efavirenz concentration we used. Our present study cannot exclude the possibility that other ligand-activated nuclear receptors (e.g., vitamin D receptor and/or peroxisome proliferator-activated receptors) play a role in the regulation of P-gp in hCMEC/D3 cells. Further studies are required to examine the interactions of antiretroviral drugs with these other nuclear receptor pathways, which are also known to regulate P-gp at the BBB.
In conclusion, several antiretroviral drugs currently used as first-line and alternative regimens in HIV pharmacotherapy can serve as ligands of the nuclear receptors hPXR and hCAR. In particular, most PIs are hPXR ligands, while abacavir and nevirapine can serve as hCAR ligands and efavirenz is a ligand of both hPXR and hCAR. In addition, an increase in functional P-gp expression mediated by these antiretroviral drugs in hCMEC/D3 cells, an in vitro model of human brain microvessel endothelial cells, suggests that chronic antiretroviral pharmacotherapy with these agents could potentially lead to P-gp induction at the human BBB. This effect could further restrict the ability of antiretroviral drugs that are P-gp substrates to enter the brain. In our study, efavirenz, shown to be a P-gp inducer in hCMEC/D3 cells, is currently recommended for administration with abacavir as an alternative NNRTI-based regimen to pharmacotherapy-naïve, HIV-infected patients. Since abacavir is a known P-gp substrate (47), the potential P-gp induction mediated by efavirenz could further reduce abacavir concentrations in the brain. Positron emission tomography imaging with [11C]verapamil (a P-gp substrate) in humans could be used to investigate P-gp transport activity at the BBB and further examine the clinical effect of chronic efavirenz administration on functional P-gp expression at the BBB (48–50). The results of these studies could guide clinical recommendations of drug regimens that avoid potential P-gp induction at the BBB and improve the entry of antiretroviral drugs that are known P-gp substrates (i.e., abacavir) into the brain.
ACKNOWLEDGMENTS
This research was funded in part by a grant from the Canadian Institutes of Health Research (CIHR grant MOP56976) awarded to Reina Bendayan and in part by a grant from the Natural Sciences and Research Council of Canada (NSERC RGPIN 356873-08) awarded to Carolyn L. Cummins. Reina Bendayan is the recipient of a Career Scientist Award from the Ontario HIV Treatment Network, Ministry of Health of Ontario.
We have no conflicts of interest to declare.
Footnotes
Published ahead of print 8 July 2013
REFERENCES
- 1.Letendre S. 2011. Central nervous system complications in HIV disease: HIV-associated neurocognitive disorder. Top. Antivir. Med. 19:137–142 [PMC free article] [PubMed] [Google Scholar]
- 2.Vivithanaporn P, Heo G, Gamble J, Krentz HB, Hoke A, Gill MJ, Power C. 2010. Neurologic disease burden in treated HIV/AIDS predicts survival: a population-based study. Neurology 75:1150–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cysique LA, Brew BJ. 2009. Neuropsychological functioning and antiretroviral treatment in HIV/AIDS: a review. Neuropsychol. Rev. 19:169–185 [DOI] [PubMed] [Google Scholar]
- 4.Ene L, Duiculescu D, Ruta SM. 2011. How much do antiretroviral drugs penetrate into the central nervous system? J. Med. Life 4:432–439 [PMC free article] [PubMed] [Google Scholar]
- 5.Löscher W, Potschka H. 2005. Role of drug efflux transporters in the brain for drug disposition and treatment of brain diseases. Prog. Neurobiol. 76:22–76 [DOI] [PubMed] [Google Scholar]
- 6.Tsuji A, Terasaki T, Takabatake Y, Tenda Y, Tamai I, Yamashima T, Moritani S, Tsuruo T, Yamashita J. 1992. P-glycoprotein as the drug efflux pump in primary cultured bovine brain capillary endothelial cells. Life Sci. 51:1427–1437 [DOI] [PubMed] [Google Scholar]
- 7.Schinkel AH, Wagenaar E, Mol CA, van Deemter L. 1996. P-glycoprotein in the blood-brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J. Clin. Invest. 97:2517–2524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee CA, Cook JA, Reyner EL, Smith DA. 2010. P-glycoprotein related drug interactions: clinical importance and a consideration of disease states. Expert Opin. Drug Metab. Toxicol. 6:603–619 [DOI] [PubMed] [Google Scholar]
- 9.Kis O, Robillard K, Chan GNY, Bendayan R. 2010. The complexities of antiretroviral drug-drug interactions: role of ABC and SLC transporters. Trends Pharmacol. Sci. 31:22–35 [DOI] [PubMed] [Google Scholar]
- 10.Perloff MD, von Moltke LL, Greenblatt DJ. 2004. Ritonavir and dexamethasone induce expression of CYP3A and P-glycoprotein in rats. Xenobiotica 34:133–150 [DOI] [PubMed] [Google Scholar]
- 11.Perloff MD, von Moltke LL, Fahey JM, Greenblatt DJ. 2007. Induction of P-glycoprotein expression and activity by ritonavir in bovine brain microvessel endothelial cells. J. Pharm. Pharmacol. 59:947–953 [DOI] [PubMed] [Google Scholar]
- 12.Zastre JA, Chan GNY, Ronaldson PT, Ramaswamy M, Couraud PO, Romero IA, Weksler B, Bendayan M, Bendayan R. 2009. Up-regulation of P-glycoprotein by HIV protease inhibitors in a human brain microvessel endothelial cell line. J. Neurosci. Res. 87:1023–1036 [DOI] [PubMed] [Google Scholar]
- 13.Urquhart BL, Tirona RG, Kim RB. 2007. Nuclear receptors and the regulation of drug-metabolizing enzymes and drug transporters: implications for interindividual variability in response to drugs. J. Clin. Pharmacol. 47:566–578 [DOI] [PubMed] [Google Scholar]
- 14.Bauer B, Hartz AMS, Fricker G, Miller DS. 2004. Pregnane X receptor up-regulation of P-glycoprotein expression and transport function at the blood-brain barrier. Mol. Pharmacol. 66:413–419 [DOI] [PubMed] [Google Scholar]
- 15.Bauer B, Yang X, Hartz AMS, Olson ER, Zhao R, Kalvass JC, Pollack GM, Miller DS. 2006. In vivo activation of human pregnane X receptor tightens the blood-brain barrier to methadone through P-glycoprotein up-regulation. Mol. Pharmacol. 70:1212–1219 [DOI] [PubMed] [Google Scholar]
- 16.Ott M, Fricker G, Bauer B. 2009. Pregnane X receptor (PXR) regulates P-glycoprotein at the blood-brain barrier: functional similarities between pig and human PXR. J. Pharmacol. Exp. Ther. 329:141–149 [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Sykes DB, Miller DS. 2010. Constitutive androstane receptor-mediated up-regulation of ATP-driven xenobiotic efflux transporters at the blood-brain barrier. Mol. Pharmacol. 78:376–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lemmen J, Tozakidis IEP, Bele P, Galla HJ. 2013. Constitutive androstane receptor upregulates Abcb1 and Abcg2 at the blood-brain barrier after CITCO activation. Brain Res. 1501:68–80 [DOI] [PubMed] [Google Scholar]
- 19.Chan GN, Hoque MT, Cummins CL, Bendayan R. 2011. Regulation of P-glycoprotein by orphan nuclear receptors in human brain microvessel endothelial cells. J. Neurochem. 118:163–175 [DOI] [PubMed] [Google Scholar]
- 20.Chang TKH, Waxman DJ. 2006. Synthetic drugs and natural products as modulators of constitutive androstane receptor (CAR) and pregnane X receptor (PXR). Drug Metab. Rev. 38:51–73 [DOI] [PubMed] [Google Scholar]
- 21.Dussault I, Lin M, Hollister K, Wang EH, Synold TW, Forman BM. 2001. Peptide mimetic HIV protease inhibitors are ligands for the orphan receptor SXR. J. Biol. Chem. 276:33309–33312 [DOI] [PubMed] [Google Scholar]
- 22.Svärd J, Spiers JP, Mulcahy F, Hennessy M. 2010. Nuclear receptor-mediated induction of CYP450 by antiretrovirals: functional consequences of NR1I2 (PXR) polymorphisms and differential prevalence in whites and sub-Saharan Africans. J. Acquir. Immune Defic. Syndr. 55:536–549 [DOI] [PubMed] [Google Scholar]
- 23.Hariparsad N, Nallani SC, Sane RS, Buckley DJ, Buckley AR, Desai PB. 2004. Induction of CYP3A4 by efavirenz in primary human hepatocytes: comparison with rifampin and phenobarbital. J. Clin. Pharmacol. 44:1273–1281 [DOI] [PubMed] [Google Scholar]
- 24.Gupta A, Mugundu GM, Desai PB, Thummel KE, Unadkat JD. 2008. Intestinal human colon adenocarcinoma cell line LS180 is an excellent model to study pregnane X receptor, but not constitutive androstane receptor, mediated CYP3A4 and multidrug resistance transporter 1 induction: studies with anti-human immunodeficiency virus protease inhibitors. Drug Metab. Dispos 36:1172–1180 [DOI] [PubMed] [Google Scholar]
- 25.Weksler BB, Subileau EA, Perrière N, Charneau P, Holloway K, Leveque M, Tricoire-Leignel H, Nicotra A, Bourdoulous S, Turowski P, Male DK, Roux F, Greenwood J, Romero IA, Couraud PO. 2005. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 19:1872–1874 [DOI] [PubMed] [Google Scholar]
- 26.Patel R, Patel M, Tsai R, Lin V, Bookout AL, Zhang Y, Magomedova L, Li T, Chan JF, Budd C, Mangelsdorf DJ, Cummins CL. 2011. LXRβ is required for glucocorticoid-induced hyperglycemia and hepatosteatosis in mice. J. Clin. Invest. 121:431–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Capparelli EV, Holland D, Okamoto C, Gragg B, Durelle J, Marquie-Beck J, Van Den Brande G, Ellis R, Letendre S. 2005. Lopinavir concentrations in cerebrospinal fluid exceed the 50% inhibitory concentration for HIV. AIDS 19:949–952 [DOI] [PubMed] [Google Scholar]
- 28.Croteau D, Letendre S, Best BM, Rossi SS, Ellis RJ, Clifford DB, Collier AC, Gelman BB, Marra CM, McArthur J, McCutchan JA, Morgello S, Simpson DM, Way L, Capparelli E, Grant I. 2012. Therapeutic amprenavir concentrations in cerebrospinal fluid. Antimicrob. Agents Chemother. 56:1985–1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yilmaz A, Izadkhashti A, Price RW, Mallon PW, De Meulder M, Timmerman P, Gisslén M. 2009. Darunavir concentrations in cerebrospinal fluid and blood in HIV-1-infected individuals. AIDS Res. Hum. Retroviruses 25:457–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tashima KT, Caliendo AM, Ahmad M, Gormley JM, Fiske WD, Brennan JM, Flanigan TP. 1999. Cerebrospinal fluid human immunodeficiency virus type 1 (HIV-1) suppression and efavirenz drug concentrations in HIV-1-infected patients receiving combination therapy. J. Infect. Dis. 180:862–864 [DOI] [PubMed] [Google Scholar]
- 31.McDowell JA, Chittick GE, Ravitch JR, Polk RE, Kerkering TM, Stein DS. 1999. Pharmacokinetics of [14C]abacavir, a human immunodeficiency virus type 1 (HIV-1) reverse transcriptase inhibitor, administered in a single oral dose to HIV-1-infected adults: a mass balance study. Antimicrob. Agents Chemother. 43:2855–2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antinori A, Perno CF, Giancola ML, Forbici F, Ippolito G, Hoetelmans RM, Piscitelli SC. 2005. Efficacy of cerebrospinal fluid (CSF)-penetrating antiretroviral drugs against HIV in the neurological compartment: different patterns of phenotypic resistance in CSF and plasma. Clin. Infect. Dis. 41:1787–1793 [DOI] [PubMed] [Google Scholar]
- 33.Pal D, Kwatra D, Minocha M, Paturi DK, Budda B, Mitra AK. 2011. Efflux transporters- and cytochrome P-450-mediated interactions between drugs of abuse and antiretrovirals. Life Sci. 88:959–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pomerantz RJ. 2003. Reservoirs, sanctuaries, and residual disease: the hiding spots of HIV-1. HIV Clin. Trials 4:137–143 [DOI] [PubMed] [Google Scholar]
- 35.Maglich JM, Parks DJ, Moore LB, Collins JL, Goodwin B, Billin AN, Stoltz CA, Kliewer SA, Lambert MH, Willson TM, Moore JT. 2003. Identification of a novel human constitutive androstane receptor (CAR) agonist and its use in the identification of CAR target genes. J. Biol. Chem. 278:17277–17283 [DOI] [PubMed] [Google Scholar]
- 36.Li H, Chen T, Cottrell J, Wang H. 2009. Nuclear translocation of adenoviral-enhanced yellow fluorescent protein-tagged-human constitutive androstane receptor (hCAR): a novel tool for screening hcar activators in human primary hepatocytes. Drug Metab. Dispos. 37:1098–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xue Y, Chao E, Zuercher WJ, Willson TM, Collins JL, Redinbo MR. 2007. Crystal structure of the PXR-T1317 complex provides a scaffold to examine the potential for receptor antagonism. Bioorg. Med. Chem. 15:2156–2166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu RX, Lambert MH, Wisely BB, Warren EN, Weinert EE, Waitt GM, Williams JD, Collins JL, Moore LB, Willson TM, Moore JT. 2004. A structural basis for constitutive activity in the human CAR/RXRα heterodimer. Mol. Cell 16:919–928 [DOI] [PubMed] [Google Scholar]
- 39.Khandelwal A, Krasowski MD, Reschly EJ, Sinz MW, Swaan PW, Ekins S. 2008. Machine learning methods and docking for predicting human pregnane X receptor activation. Chem. Res. Toxicol. 21:1457–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perloff ES, Duan SX, Skolnik PR, Greenblatt DJ, von Moltke LL. 2005. Atazanavir: effects on P-glycoprotein transport and CYP3A metabolism in vitro. Drug Metab. Dispos. 33:764–770 [DOI] [PubMed] [Google Scholar]
- 41.Chandler B, Almond L, Ford J, Owen A, Hoggard P, Khoo S, Back D. 2003. The effects of protease inhibitors and nonnucleoside reverse transcriptase inhibitors on P-glycoprotein expression in peripheral blood mononuclear cells in vitro. J. Acquir. Immune Defic. Syndr. 33:551–556 [DOI] [PubMed] [Google Scholar]
- 42.Weiss J, Weis N, Ketabi-Kiyanvash N, Storch CH, Haefeli WE. 2008. Comparison of the induction of P-glycoprotein activity by nucleotide, nucleoside, and non-nucleoside reverse transcriptase inhibitors. Eur. J. Pharmacol. 579:104–109 [DOI] [PubMed] [Google Scholar]
- 43.Dixit V, Hariparsad N, Li F, Desai P, Thummel KE, Unadkat JD. 2007. Cytochrome P450 enzymes and transporters induced by anti-human immunodeficiency virus protease inhibitors in human hepatocytes: implications for predicting clinical drug interactions. Drug Metab. Dispos. 35:1853–1859 [DOI] [PubMed] [Google Scholar]
- 44.Perloff MD, von Moltke LL, Fahey JM, Daily JP, Greenblatt DJ. 2000. Induction of P-glycoprotein expression by HIV protease inhibitors in cell culture. AIDS 14:1287–1289 [DOI] [PubMed] [Google Scholar]
- 45.Vishnuvardhan D, von Moltke LL, Richert C, Greenblatt DJ. 2003. Lopinavir: acute exposure inhibits P-glycoprotein; extended exposure induces P-glycoprotein. AIDS 17:1092–1094 [DOI] [PubMed] [Google Scholar]
- 46.König SK, Herzog M, Theile D, Zembruski N, Haefeli WE, Weiss J. 2010. Impact of drug transporters on cellular resistance towards saquinavir and darunavir. J. Antimicrob. Chemother. 65:2319–2328 [DOI] [PubMed] [Google Scholar]
- 47.Shaik N, Giri N, Pan G, Elmquist WF. 2007. P-glycoprotein-mediated active efflux of the anti-HIV1 nucleoside abacavir limits cellular accumulation and brain distribution. Drug Metab. Dispos. 35:2076–2085 [DOI] [PubMed] [Google Scholar]
- 48.Muzi M, Mankoff DA, Link JM, Shoner S, Collier AC, Sasongko L, Unadkat JD. 2009. Imaging of cyclosporine inhibition of P-glycoprotein activity using 11C-verapamil in the brain: studies of healthy humans. J. Nucl. Med. 50:1267–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bauer M, Zeitlinger M, Karch R, Matzneller P, Stanek J, Jäger Böhmdorfer WM, Wadsak W, Mitterhauser M, Bankstahl JP, Löscher W, Koepp M, Kuntner C, Müller M, Langer O. 2012. Pgp-mediated interaction between (R)-[11C]verapamil and tariquidar at the human blood-brain barrier: a comparison with rat data. Clin. Pharmacol. Ther. 91:227–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Syvänen S, Eriksson J. 2013. Advances in PET imaging of P-glycoprotein function at the blood-brain barrier. ACS Chem. Neurosci. 4:225–237 [DOI] [PMC free article] [PubMed] [Google Scholar]