Abstract
Chronic Schistosoma mansoni infections lead to severe tissue destruction of the gut wall and liver and can influence drug disposition. This study aimed to investigate the impact of a chronic S. mansoni infection on the pharmacokinetic (PK) parameters of two promising antischistosomal lead candidates (mefloquine and enpiroline) in mice. Studies were conducted in two different mouse cohorts (S. mansoni-infected and uninfected mice) for both drugs. Plasma samples were collected at various time points after oral treatment (200 mg/kg of body weight) with study drugs. A high-performance liquid chromatography (HPLC) method was validated to analyze enpiroline and mefloquine in plasma. Livers and intestines were collected from infected animals to determine the onset of action, hepatic shift, and worm burden reduction. Following mefloquine administration, hepatic shifting and significant worm burden reductions (79.2%) were observed after 72 h. At 1 week posttreatment with enpiroline, the majority of worms had migrated to the liver and significant worm burden reductions were observed (93.1%). The HPLC method was selective, accurate (87.8 to 111.4%), and precise (<10%) for the analysis of both drugs in plasma samples. The PK profiles revealed increased values for half-life (t1/2) and area under the concentration-time curve (AUC) for both drugs in infected animals compared to the t1/2 and AUC values in uninfected animals. Considerable changes were observed for mefloquine, with a 5-fold increase of t1/2 (182.7 h versus 33.6 h) and 2-fold increase of AUC (1,116,517.8 ng · h/ml versus 522,409.1 ng · h/ml). S. mansoni infections in mice influence the PK profiles of enpiroline and mefloquine, leading to delayed clearance. Our data confirm that drug disposition should be carefully studied in schistosomiasis patients.
INTRODUCTION
Schistosomiasis is one of the most important neglected tropical diseases (1). Based on the latest estimates, 780 million people are at risk of an infection with schistosomes, with the majority of cases in sub-Saharan Africa (2). Schistosomiasis is a chronic disease caused by the immunological response to eggs trapped in tissue and organs. For example, in the case of Schistosoma mansoni, eggs get trapped mainly in the gut wall and liver, leading to severe tissue destruction, hepatosplenomegaly, periportal liver fibrosis, and portal hypertension (3). Treatment with praziquantel is the mainstay of control and widely used in preventive chemotherapy programs (4).
The liver is one of the main organs in charge of drug metabolism, and the gut wall is significantly involved in the absorption process of drugs (5). It is therefore not surprising that pharmacokinetic (PK) changes occur in patients with schistosomiasis, depending on the extent of disease (6). For example, a study in Egyptian schistosomiasis patients treated with praziquantel demonstrated that PK parameters increased in proportion to the degree of hepatic insufficiency (7). In addition, PK studies undertaken with praziquantel in S. mansoni-infected mice documented elevated plasma levels in infected animals compared to the plasma levels in noninfected mice. Furthermore, the infection resulted in significant inhibition of microsomal cytochrome P450 activities compared with those in noninfected mice (8).
The areas of endemicity of schistosomiasis and some other infectious diseases, such as malaria and soil-transmitted helminths, overlap geographically (9). Coendemicity, as well as known similarities of both parasites, such as hemoglobin digestion (10, 11), were the key rationales for in-depth studies on the potential antischistosomal effects of antimalarials (9). In recent years, the antimalarial mefloquine emerged as a promising lead candidate. The drug presented remarkable in vitro and in vivo activities against major schistosome species (12) and high egg reduction rates in combination with artesunate in an exploratory clinical trial in Schistosoma haematobium-infected school-aged children (13). Recently, a clinical trial in pregnant malaria-infected women revealed a positive effect of mefloquine on S. haematobium coinfections (14). A population PK study of mefloquine observed altered PK profiles in malaria-infected persons compared to the PK profiles in uninfected people (P. Olliaro, personal communication). Nevertheless, the impact of a schistosome infection on the drug disposition of mefloquine has not been studied to date.
The aim of the present study was to investigate the PK parameters of mefloquine and enpiroline in the chronic S. mansoni infection mouse model. Enpiroline was included in this study since recent in vitro and in vivo studies on mefloquine-related arylmethanols identified enpiroline as a promising lead candidate with excellent activity on both S. mansoni and S. haematobium (12). Drug plasma levels were analyzed between 1 and 168 h after oral treatment with mefloquine and enpiroline. For that purpose, we adapted a previously established high-performance liquid chromatography (HPLC)-UV method (15), revalidated the method for mouse plasma samples, and expanded it for the quantification of enpiroline. Finally, we determined the onset of action of both drugs following administration to infected animals.
MATERIALS AND METHODS
Drugs and chemicals.
Mefloquine {[2,8-bis(trifluoromethyl)quinolin-4-yl](-piperidin-2-yl)methanol} was kindly provided by Cilag AG (Switzerland). Two mefloquine metabolites, one a human metabolite (Ro 21-5104) and one an animal metabolite (Ro 14-0518), were gifts of Hoffmann-La Roche (Switzerland). The Walter Reed Army Institute of Research (Silver Spring, MD) kindly provided us with enpiroline [threo-α-(2-piperidyl)-2-trifluoromethyl-6-(4-trifluoromethylphenyl)-4-pyri-dinemethanol]. Chlorpromazine [2-chlor-10-(3-dimethylaminopropyl) phenothiazin] was purchased from Sigma-Aldrich (Switzerland). Mefloquine and enpiroline were dissolved in dimethyl sulfoxide (DMSO) and chlorpromazine in ethanol (10 mg/ml stock solutions). All analytes are depicted in Fig. 1. Methanol (Sigma-Aldrich) and acetonitrile (Biosolve BV, Netherlands) were purchased in HPLC grade. Monobasic potassium phosphate and phosphoric acid (85%) were acquired from Sigma-Aldrich (Switzerland).
Fig 1.
Chemical structures of mefloquine, enpiroline, and chlorpromazine (internal standard).
Animals and parasites.
Animal studies were carried out at the Swiss Tropical and Public Health Institute (Basel, Switzerland) under protocols approved by Swiss national and cantonal animal welfare regulations. Three-week-old (weight, 14 g) female NMRI mice (n = 119) (Charles River, Sulzfeld, Germany) were allowed to adapt in the animal facilities for 1 week under controlled conditions (temperature, ca. 22°C; humidity, ca. 50%; 12-h-light and 12-h-dark cycle; and free access to rodent diet and water) before infection. Half of the mice (infected cohorts) were subcutaneously infected with 100 S. mansoni (Liberian strain) cercariae by following the standard procedures of our laboratory. Animals were then left under controlled conditions for 7 weeks to establish an early-stage chronic schistosome infection with visible impairment of liver (granulomatous tissue) and gut (swelling, inflammation). The remainder of the mice (uninfected cohorts) were likewise kept under controlled conditions for 7 weeks.
Pharmacokinetic studies.
Pharmacokinetic studies were conducted in two different mouse cohorts for both drugs, one cohort being S. mansoni-infected and the second being noninfected NMRI mice. Oral formulations of enpiroline and mefloquine were prepared 3 h before treatment as water-based suspensions in 7% (vol/vol) Tween 80 and 3% (vol/vol) ethanol. Groups of mice (n = 3 per group) were treated orally with 200 mg per kg of body weight of enpiroline or mefloquine by gavage and sacrificed by the CO2 method at selected time points posttreatment (1, 2, 4, 8, 12, 24, 48, 72, or 168 h). Whole-blood samples of 0.5 to 1 ml were collected by cardiac puncture of each mouse. Each cohort had untreated control mice (n = 8 infected and n = 3 noninfected), and each of the four treatment arms (infected and noninfected mice treated with mefloquine and infected and noninfected mice treated with enpiroline) consisted of 27 mice for 3 mice per sampling time point. Blood samples were collected into lithium-heparin-coated Microtainers (Sarstedt) and centrifuged to obtain plasma samples, which were stored at −80°C until analysis.
Studies on the onset of antischistosomal action in mice.
The onset of action was determined at different time points following treatment with single oral doses of enpiroline and mefloquine administered to mice (n = 3 per group). After each PK sampling point (1, 2, 4, 8, 12, 24, 48, 72, or 168 h posttreatment), the liver and gut, including the portal vein and mesenterial veins, were dissected from infected mice. Livers were pressed and examined under the microscope, and all worms were sexed and counted (16). In addition, worms within the portal vein and mesenterial vein system were picked and counted separately as described previously (16). The worm burdens (average numbers of live worms) of treated mice were compared to the worm burdens of control (nontreated) animals (average numbers of live worms), and the reductions of worm burden (reduction of live worms) calculated for each time point. Furthermore, the onset of action, visible as the migration of worms from the mesenteric veins to the liver, was determined based on the distribution of worms (live and dead) within portal and mesenterial veins and pressed livers (hepatic shift).
HPLC-UV analysis and sample extraction.
A validated high-performance liquid chromatography analytic method introduced by Lai and colleagues (15) for mefloquine in human plasma was used. The method was adapted in order to detect mefloquine and enpiroline simultaneously under similar conditions. Important validation parameters, such as selectivity, intra- and interday accuracy and precision, bench-top stability, and extraction recovery were determined according to the bioanalytical method validation guidance for industry of the Food and Drug Administration (FDA) (17).
Plasma samples (100 μl) were processed using protein precipitation with methanol containing an internal standard (IS) (chlorpromazine at a concentration of 5 μg/ml). Each mouse sample was extracted three times on three different days. Samples were vortex mixed and centrifuged (Eppendorf 5415C centrifuge) at 10,000 × g for 10 min. The supernatant was transferred to a microtube and evaporated to dryness in a Speedvac (Labcanco) at 38°C for 2 h. The residue was reconstituted in 100 μl acetonitrile–0.05 M KH2PO4 buffer (1:1) solution and transferred to an autosampler vial.
Analyte working solutions were prepared by serial dilution in acetonitrile-KH2PO4 buffer (0.05 M) (1:1) to final concentrations of 4, 8, 20, 40, 80, 200, 400, and 800 μg/ml. Calibration curves were established by diluting analyte working solutions with blank mouse plasma (purchased from Dunn Technik, Germany) (1:20 in a total volume of 100 μl). Each calibration set consisted of one blank plasma sample (plasma sample processed without IS), one zero sample (plasma sample spiked with IS), and 8 calibration samples (0.2, 0.4, 1, 2, 4, 10, 20, and 40 μg/ml). The lowest and highest calibrator corresponded to the lower and upper limit of quantification (LLOQ and ULOQ), respectively. The LLOQ was selected as the minimal concentration in plasma samples that could be analyzed with a precision of ≤20% (CV%) and accuracy of between 80% and 120% (signal/noise ratio of >5:1).
In addition, quality control (QC) samples (n = 6) were prepared at low (0.75 μg/ml), medium (7.5 μg/ml), and high (30 μg/ml) concentrations covering the entire calibration range.
Selectivity was determined by examination of blank mouse plasma obtained from different origins (noninfected NMRI mice and infected mice without treatment [n = 6 each] and commercially acquired mouse plasma) for interference by endogenous substances using the above-described extraction procedure but without adding the IS working solution.
The accuracy and precision of the method were evaluated by analyzing quality control (QC) samples (n = 6 per concentration). The intra- and interday accuracy/precision were determined within a single run and between different assays (n = 3), respectively. Freshly prepared calibration standards were used for the analyses. The precision was calculated using the coefficient of variation (CV [%]). The accuracy represented the measured concentration as the fraction of the nominal concentration expressed as a percentage. A precision of ±15% (LLOQ, ±20%) and accuracy of between 85% and 115% (LLOQ, 80 to 120%) were accepted in our study.
The relative recoveries of mefloquine and enpiroline were determined by comparing the absolute peak areas of blank plasma samples spiked before and after the extraction at 0.75, 7.5, and 30 μg/ml (n = 5 per concentration). The matrix effects of mefloquine and enpiroline were assessed as the ratios of the absolute peak areas of blank plasma samples spiked after the extraction to the absolute peak areas of the analytes solved in a mixture of acetonitrile-KH2PO4 buffer (1:1, vol/vol).
Autosampler stability and extended bench-top stability studies were included in our method validation. QC samples were used to test stabilities under different conditions. Autosampler stability was evaluated by the analysis of QC samples (n = 6 per concentration) over a period of 72 h. Extended bench-top stability was evaluated after samples were kept under bench-top conditions for 8 h. The concentrations of these samples were compared with the concentrations of freshly prepared QC samples. The drug solutions were considered stable with deviations of not more than ±15% and ±20% at the LLOQ.
For the HPLC-UV detection, an aliquot (50 μl) of each sample was injected onto an Inertsil 8-3 analytical column (2.1 mm by 30 mm, 3.5 μm; GL Sciences, Japan). Separation was carried out using an isocratic elution method with a mobile phase consisting of 35% methanol, 25% acetonitrile, and 40% KH2PO4 buffer (0.05 M) adjusted to pH 3.9 with 0.05% phosphoric acid. The flow rate was set at 1 ml/min and increased to 1.5 ml/min from 11 min onwards. UV signals were detected at 284 nm. The Agilent 1100 series HPLC system (Agilent Technologies, Inc.) consisted of binary pumps, a microvacuum degasser, an analytics autosampler, a column heater (temp, 25°C), and a UV-vis detector.
Pharmacokinetic analysis and statistics.
The analytical raw data (area under the concentration-time curve [AUC]) of the plasma samples were processed with ChemStation software (Agilent). Average values, as well as calibration lines and animal data, were calculated using Microsoft Excel 2007. Statsdirect (Statsdirect Ltd., Altrincham, Cheshire, United Kingdom) was used to determine whether worm burden reductions were significant (Kruskal-Wallis test). The mean value from the three animals at each time point was plotted against time to give plasma concentration-time profiles. The PK parameters of mefloquine and enpiroline were determined by noncompartmental analysis using WinNonLin (version 5.2, Pharsight Corporation, USA). The following parameters are presented here: maximal plasma concentration (Cmax; ng/ml), time to achieve maximal plasma concentration (Tmax; h), AUC from 0 to 168 h (AUC0-168; ng · ml/min), AUC extrapolated to infinity (AUC0-∞; ng · ml/min), and terminal elimination half-life (t1/2; h). Tmax and Cmax are the observed data from the mean concentration time curve. The terminal elimination rate constant is the first-order rate constant associated with the terminal elimination phase. It is estimated via linear regression of time versus log concentration. The extrapolated times and areas after the last data point are extrapolated to infinity. For any of the AUC calculation methods, the AUC rule after the last time point is the log rule.
RESULTS
Studies on the onset of antischistosomal action of enpiroline and mefloquine in mice.
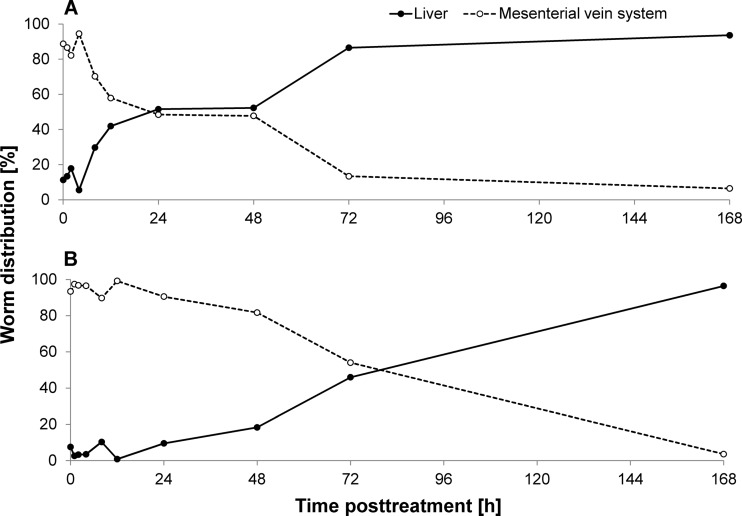
We studied the worm distribution from 1 h to 168 h after treatment. Following mefloquine administration, schistosomes started to migrate into the liver (hepatic shift) 8 to 12 h posttreatment. Approximately half of the worms (live and dead) were found within the liver 1 to 2 days posttreatment, and nearly all worms (86.5%) were observed in the liver from day 3 onwards (Fig. 2). A worm burden reduction of 79.2% was calculated at 72 h posttreatment, while a worm burden reduction of 46.9% was determined for the group of mice (n = 3) analyzed at 1 week posttreatment.
Fig 2.
Worm distribution (live and dead) between liver and mesenterial vein system presented over time posttreatment with 200 mg/kg mefloquine (A) or enpiroline (B).
Worms shifted into the liver slowly following enpiroline treatment, and an equal distribution between liver and mesenterial veins was reached 72 h posttreatment. The majority of worms had migrated to the liver 1 week posttreatment. Enpiroline evolved its full antischistosomal activity 1 week posttreatment, revealing a significant worm burden reduction of 93.1%.
Validation of the HPLC-UV method.
The calibration curves of mefloquine and enpiroline were linear from 200 to 40,000 ng/ml mouse plasma (R2 > 0.999). The applied method was found to be selective, since there was no interference between the retention times of analytes and any endogenous substances (signal/noise ratio of >5:1) or any mefloquine metabolites tested (human and animal metabolites). The mean extraction recoveries estimated for mefloquine and enpiroline were 76.6% and 71.3%, respectively, and were consistent over the whole calibration line with a CV of less than 12%. Only minor matrix effects (<20%) were present (Table 1).
Table 1.
Relative recovery and matrix effect of mefloquine and enpiroline extracted from mouse plasma samples by protein precipitation with methanol
| Analyte | Nominal concn (ng/ml) | Relative recovery (%) | Mean ± CVa (%) | Matrix effect (%) | Mean ± CVa (%) |
|---|---|---|---|---|---|
| Mefloquine | 750 | 78.4 | 76.6 ± 7.9 | 86.2 | 83.9 ± 7.1 |
| 7,500 | 72.9 | 77.5 | |||
| 30,000 | 78.3 | 88.1 | |||
| Enpiroline | 750 | 66.3 | 71.3 ± 12 | 90.7 | 84.3 ± 11.1 |
| 7,500 | 69.8 | 75.2 | |||
| 30,000 | 77.9 | 87.0 |
n = 5 samples.
The intraday accuracy estimated for both analytes ranged from 87.8% to 111.4%, with a precision of ≤10%. Interday accuracy was always within a 90 to 110% margin, with a maximal imprecision of 10.5% (Table 2). The LLOQ value of 200 ng/ml plasma was determined with interday accuracies of 97.4% ± 3.8% for mefloquine and 113.5% ± 4.4% for enpiroline.
Table 2.
Intraday and-interday accuracy and precisiona
| Analyte (regression model) | Nominal concn (ng/ml) | Intraday values for: |
Interday values for: |
||||
|---|---|---|---|---|---|---|---|
| Mean concn (ng/ml) | RSD (%) | Accuracy (%) | Mean concn (ng/ml) | RSD (%) | Accuracy (%) | ||
| Mefloquine (linear, R2 = 0.9995) | 750 | 835.3 | 1.8 | 111.4 | 732.0 | 9.5 | 97.6 |
| 7,500 | 6,779.9 | 2.9 | 90.4 | 7,625.3 | 8.9 | 101.7 | |
| 30,000 | 29,129.3 | 2.0 | 97.1 | 31,222.4 | 6.4 | 104.1 | |
| Enpiroline (linear, R2 = 0.9996) | 750 | 816.9 | 5.0 | 108.9 | 797.2 | 8.9 | 106.3 |
| 7,500 | 6,584.6 | 5.4 | 87.8 | 7,762.6 | 10.5 | 103.5 | |
| 30,000 | 28,809.1 | 2.2 | 96.0 | 31,905.2 | 8.0 | 106.4 | |
Intraday values are the mean values of 6 samples. The results of one representative experiment are shown. Interday values are the mean values of 3 independent sets of experiments. RSD, relative standard deviation.
The results obtained from stability experiments demonstrated that the samples were stable for increased times under bench-top conditions (t = 8 h) and autosampler conditions (10°C, t = 72 h). Variations of plasma samples following 72 h under autosampler conditions were below 2.9%, and accuracies ranged from 90.2 to 103.5%. Stability was likewise observed for our samples when left at room temperature (23 to 25°C) for 8 h.
Disposition of enpiroline and mefloquine in S. mansoni-infected and uninfected mice.
The established HPLC-UV method was used to study the pharmacokinetics of mefloquine and enpiroline in S. mansoni-infected and noninfected NMRI mice following the administration of a single oral dose of 200 mg/kg mefloquine or enpiroline. The pharmacokinetic parameters are summarized in Table 3.
Table 3.
Pharmacokinetic parameters of mefloquine and enpiroline following oral administration to mice infected or not infected with S. mansoni
| Parameter | Mean value (n = 3 mice/time point) for mice treated (200 mg/kg) with: |
|||
|---|---|---|---|---|
| Mefloquine |
Enpiroline |
|||
| S. mansoni infected | Noninfected | S. mansoni infected | Noninfected | |
| t1/2 (h) | 182.7 | 33.6 | 182.1 | 72.0 |
| AUC0-168 (ng · h/ml) | 567,845.5 | 503,623.5 | 819,557.5 | 859,603 |
| AUC0-∞ (ng · h/ml) | 1,116,517.8 | 522,409.1 | 1,790,287.1 | 1,107,982.3 |
| Cmax (ng/ml) | 7,238 | 10,223 | 5,746 | 7,641 |
| Tmax (h) | 12 | 12 | 24 | 48 |
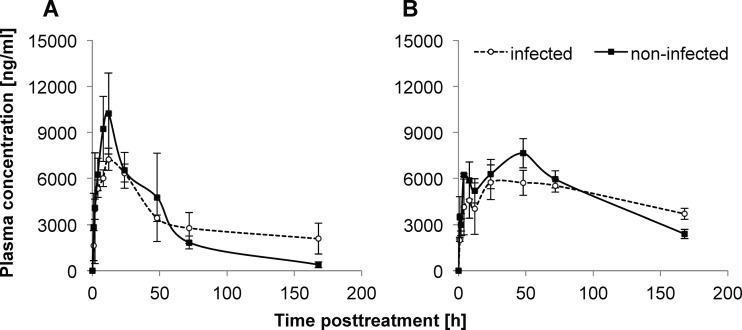
A 2-fold increase of the AUC0-∞ for mefloquine and enpiroline was observed in S. mansoni-infected mice. The Cmax levels were increased 30 to 40% within the noninfected cohorts (10,223 ng/ml versus 7,238 ng/ml for mefloquine [R2 = 0.94 to 0.98] and 7,641 ng/ml versus 5,746 ng/ml for enpiroline [R2 = 0.99]). The time to achieve maximal concentration (Tmax) altered only for enpiroline, with Tmax being reached 24 h later in the noninfected animals than in infected mice (24 versus 48 h). The half-lives (t1/2) of mefloquine and enpiroline were elevated 5- and 2.5-fold, respectively (182.7 h versus 33.6 h for mefloquine and 182.1 h versus 72 h for enpiroline), in the infected population. The plasma concentration-time profiles of both drugs in infected and uninfected mice are presented in Fig. 3.
Fig 3.
Mean plasma concentration-time profiles of mefloquine (A) and enpiroline (B) after oral administration of drug at 200 mg/kg to 3 mice per time point for either infected or noninfected animals. Error bars show standard deviations.
DISCUSSION
Chronic S. mansoni infections cause substantial pathological and physiological changes in infected patients, leading to clinical symptoms such as abdominal pain, diarrhea, blood in the stool, and finally, liver cirrhosis and portal hypertension (18).
The liver and gut, which are affected the most by S. mansoni infection, play important roles in drug metabolism and disposition (3, 19). Changes in the absorption, distribution, metabolism, and elimination of orally applied drugs are therefore very likely in S. mansoni-infected individuals. An altered drug disposition has been shown in several clinical trials in patients with schistosomiasis (6, 7).
In the past few years, drug metabolism and PK studies have increasingly been integrated into early stages of the drug discovery process. However, in the field of schistosomiasis, few studies have been carried out to date elucidating the pharmacokinetic/pharmacodynamic (PK/PD) relationships and studying the influence of the parasitic infection on drug disposition. It was therefore our aim to investigate the PK properties of the two antimalarials mefloquine and enpiroline in S. mansoni-infected and uninfected animals.
We successfully adapted an analytical HPLC-UV method, originally used to determine artesunate, its active metabolite dihydroartemisinin, and mefloquine in human plasma (15), to analyze enpiroline and mefloquine in mouse plasma. The accuracy, precision, and sensitivity values documented for our analytical method were similar to those obtained in the original work.
The speed of onset of action represents a key pharmacodynamic parameter. We determined the hepatic shift (a well-described parameter in antischistosomal drug discovery), which monitors the forced migration of schistosomes to the liver after treatment with active drugs (20). Interestingly, the hepatic shift of all worms started rather late, only 72 h posttreatment for mefloquine and 168 h following drug administration for enpiroline. The hepatic shift of mefloquine observed in our study was comparable to those in two previous studies in S. mansoni-infected mice using a single oral dose of 400 mg/kg (21, 22). Mefloquine acts slightly faster in Schistosoma japonicum-infected mice (22, 23). The slow onset of action of mefloquine on schistosomes in vivo has been reported in other studies (23). In contrast, praziquantel acts much faster; the hepatic shift was already completed 30 min after oral drug administration (unpublished observations), which might be explained by the drug's action on the muscles of schistosomes. In addition, in vivo studies showed that extensive structural changes to worms occurred within 15 min after treatment and adult worms died within 24 h following treatment with praziquantel (21, 24).
Interestingly, the majority of worms exposed to mefloquine were still not affected at 7 days (reduction in worm burden, 46.9%) posttreatment in our study. On the other hand, previous studies presented worm burden reductions of 74.1% already at 1 week posttreatment with mefloquine (400 mg/kg), and a worm burden reduction of 72.3% at 2 weeks posttreatment was observed for the dosage of 200 mg/kg (22). These differences in worm burden reductions might be explained by the rather small groups of three mice per time point in our study setup. Additionally, the speed of action of the 200 mg/kg dosage might be slower.
The rather late onsets of action might correlate with the long half-lives determined for mefloquine (t1/2, 182.7 h) and enpiroline (t1/2, 182.1 h) in infected animals, which are increased as much as 2.5- to 5-fold compared to those in noninfected animals. A previous PK study with mefloquine in uninfected mice revealed a half-life of 17 h after oral treatment with 8 to 10 mg/kg, which is in a range similar to that described from our estimation of 33.6 h (25). However, it is important to emphasize that a rather small sample size was used in the present work and interindividual variations between the animals are high. Furthermore, the estimated AUC is 2-fold higher in infected animals than in noninfected mice for both drugs. The slow clearance of both drugs might be attributed to a decreased activity of the cytochrome P450 enzyme system caused by the S. mansoni infection (8). It is known that the AUC and the elimination half-life of mefloquine in humans are significantly increased by inhibition of CYP3A4 with inhibitors (e.g., ketoconazole) (Lariam information leaflet). Surprisingly, both drugs presented rather decreased maximal plasma concentrations (Cmax) in infected mice. The heavy inflammation of the gut wall that was observed microscopically might result in decreased drug absorption, which could be an explanation for this finding. It is interesting to note that, contrary to our findings, increased Cmax values were determined for praziquantel in infected rodents (8).
Mefloquine presented plasma concentrations above the IC50 (half-maximal inhibitory concentration) value determined in vitro (4.7 ± 2.8 μg/ml at 24 h postexposure) for approximately 32 h (12). Interestingly, the plasma levels of enpiroline did not reach the IC50 determined at 24 h in vitro (7.4 ± 2.7 μg/ml). However, enpiroline is a slow-acting drug in vitro, presenting an IC50 of 3.1 μg/ml after 72 h of drug exposure (12). The plasma level remained above this concentration for up to 150 h. Since Cmax levels are reached much earlier (12 h for mefloquine and 24 h for enpiroline) than the onset of action (24 to 72 h for mefloquine and 168 h for enpiroline), one can speculate that the antischistosomal activities of both drugs are AUC rather than Cmax driven. However, further rigorous PK/PD studies are required to strengthen our hypothesis.
In conclusion, we showed that an S. mansoni infection triggers considerable changes in the drug disposition of mefloquine and enpiroline. Pharmacokinetic studies with antischistosomal lead candidates in infected animals should be integrated early in the drug discovery process. In addition, PK studies should be conducted in S. mansoni- and S. haematobium-infected children, the key target group of preventive chemotherapy, since children depend greatly on safe, efficacious, and controlled dosage regimens. Our findings might be of public health relevance, since mefloquine is widely used in areas where schistosome infections are common. Overdosing might occur and lead to toxic adverse events, which might be of relevance in light of the reported neurotoxicity of mefloquine (26). Furthermore, influences on the pharmacokinetic profiles of antimalarials could lead to prolonged plasma levels beneath the minimal inhibition concentration and, hence, result in suboptimal dosing and selection for drug-resistant parasites. Therefore, in-depth studies should also be conducted with antimalarials in patients coinfected with S. mansoni and Plasmodium falciparum.
ACKNOWLEDGMENTS
This work was supported by the Swiss National Science Foundation (project no. PPOOA3-114941 and PPOOP3_135170 to J.K., U.D., and M.V.) and the Scientific & Technological Cooperation Programme Switzerland-Russia (J.K. and K.I.).
Footnotes
Published ahead of print 8 July 2013
REFERENCES
- 1.WHO 2012. Research priorities for helminth infections. World Health Organ. Tech. Rep. Ser. 972:1–174 [PubMed] [Google Scholar]
- 2.Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. 2006. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect. Dis. 6:411–425 [DOI] [PubMed] [Google Scholar]
- 3.Gryseels B, Polman K, Clerinx J, Kestens L. 2006. Human schistosomiasis. Lancet 368:1106–1118 [DOI] [PubMed] [Google Scholar]
- 4.Fenwick A, Webster JP, Bosque-Oliva E, Blair L, Fleming FM, Zhang Y, Garba A, Stothard JR, Gabrielli AF, Clements AC, Kabatereine NB, Toure S, Dembele R, Nyandindi U, Mwansa J, Koukounari A. 2009. The Schistosomiasis Control Initiative (SCI): rationale, development and implementation from 2002-2008. Parasitology 136:1719–1730 [DOI] [PubMed] [Google Scholar]
- 5.Lin J, Sahakian DC, de Morais SM, Xu JJ, Polzer RJ, Winter SM. 2003. The role of absorption, distribution, metabolism, excretion and toxicity in drug discovery. Curr. Top. Med. Chem. 3:1125–1154 [DOI] [PubMed] [Google Scholar]
- 6.Wilby K, Gilchrist S, Ensom MH. 12 March 2013. A review of the pharmacokinetic implications of schistosomiasis. Clin. Pharmacokinet. 10.1007/s40262-013-0055-8 [DOI] [PubMed] [Google Scholar]
- 7.el Guiniady MA, el Touny MA, Abdel-Bary MA, Abdel-Fatah SA, Metwally A. 1994. Clinical and pharmacokinetic study of praziquantel in Egyptian schistosomiasis patients with and without liver cell failure. Am. J. Trop. Med. Hyg. 51:809–818 [DOI] [PubMed] [Google Scholar]
- 8.Botros SS, El-Din SH, El-Lakkany NM, Sabra AN, Ebeid FA. 2006. Drug-metabolizing enzymes and praziquantel bioavailability in mice harboring Schistosoma mansoni isolates of different drug susceptibilities. J. Parasitol. 92:1344–1349 [DOI] [PubMed] [Google Scholar]
- 9.Keiser J, Utzinger J. 2012. Antimalarials in the treatment of schistosomiasis. Curr. Pharm. Des. 18:3531–3538 [PubMed] [Google Scholar]
- 10.Correa Soares JB, Menezes D, Vannier-Santos MA, Ferreira-Pereira A, Almeida GT, Venancio TM, Verjovski-Almeida S, Zishiri VK, Kuter D, Hunter R, Egan TJ, Oliveira MF. 2009. Interference with hemozoin formation represents an important mechanism of schistosomicidal action of antimalarial quinoline methanols. PLoS Negl. Trop. Dis. 3:e477. 10.1371/journal.pntd.0000477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olliaro PL, Haynes RK, Meunier B, Yuthavong Y. 2001. Possible modes of action of the artemisinin-type compounds. Trends Parasitol. 17:122–126 [DOI] [PubMed] [Google Scholar]
- 12.Ingram K, Ellis W, Keiser J. 2012. Antischistosomal activities of mefloquine-related arylmethanols. Antimicrob. Agents Chemother. 56:3207–3215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keiser J, N′Guessan NA, Adoubryn KD, Silue KD, Vounatsou P, Hatz C, Utzinger J, N′Goran EK. 2010. Efficacy and safety of mefloquine, artesunate, mefloquine-artesunate, and praziquantel against Schistosoma haematobium: randomized, exploratory open-label trial. Clin. Infect. Dis. 50:1205–1213 [DOI] [PubMed] [Google Scholar]
- 14.Basra A, Mombo-Ngoma G, Capan Melser M, Akerey Diop D, Wurbel H, Mackanga JR, Furstenau M, Manego Zoleko R, Adegnika AA, Gonzalez R, Menendez C, Kremsner PG, Ramharter M. 2013. Efficacy of mefloquine intermittent preventive treatment in pregnancy against Schistosoma haematobium infection in Gabon: a nested randomized controlled assessor-blinded clinical trial. Clin. Infect. Dis. 56:e68–e75 [DOI] [PubMed] [Google Scholar]
- 15.Lai CS, Nair NK, Mansor SM, Olliaro PL, Navaratnam V. 2007. An analytical method with a single extraction procedure and two separate high performance liquid chromatographic systems for the determination of artesunate, dihydroartemisinin and mefloquine in human plasma for application in clinical pharmacological studies of the drug combination. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 857:308–314 [DOI] [PubMed] [Google Scholar]
- 16.Xiao SH, Keiser J, Chollet J, Utzinger J, Dong Y, Endriss Y, Vennerstrom JL, Tanner M. 2007. In vitro and in vivo activities of synthetic trioxolanes against major human schistosome species. Antimicrob. Agents Chemother. 51:1440–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Food and Drug Administration 2001. Guidance for industry: bioanalytical method validation. Center for Drug Evaluation and Research, U.S. Food and Drug Administration, Rockville, MD: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070107.pdf [Google Scholar]
- 18.Danso-Appiah A, Olliaro PL, Donegan S, Sinclair D, Utzinger J. 2013. Drugs for treating Schistosoma mansoni infection. Cochrane Database Syst. Rev. 2:CD000528. 10.1002/14651858.CD000528.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verbeeck RK. 2008. Pharmacokinetics and dosage adjustment in patients with hepatic dysfunction. Eur. J. Clin. Pharmacol. 64:1147–1161 [DOI] [PubMed] [Google Scholar]
- 20.Buttle GA, Khayyal MT. 1962. Rapid hepatic shift of worms in mice infected with Schistosoma mansoni after a single injection of tartar emetic. Nature 194:780–781 [DOI] [PubMed] [Google Scholar]
- 21.Manneck T, Haggenmuller Y, Keiser J. 2010. Morphological effects and tegumental alterations induced by mefloquine on schistosomula and adult flukes of Schistosoma mansoni. Parasitology 137:85–98 [DOI] [PubMed] [Google Scholar]
- 22.Keiser J, Chollet J, Xiao SH, Mei JY, Jiao PY, Utzinger J, Tanner M. 2009. Mefloquine—an aminoalcohol with promising antischistosomal properties in mice. PLoS Negl. Trop. Dis. 3:e350. 10.1371/journal.pntd.0000350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang CW, Xiao SH, Utzinger J, Chollet J, Keiser J, Tanner M. 2009. Histopathological changes in adult Schistosoma japonicum harbored in mice treated with a single dose of mefloquine. Parasitol. Res. 104:1407–1416 [DOI] [PubMed] [Google Scholar]
- 24.Shaw MK, Erasmus DA. 1983. Schistosoma mansoni: dose-related tegumental surface changes after in vivo treatment with praziquantel. Z. Parasitenkd. 69:643–653 [DOI] [PubMed] [Google Scholar]
- 25.Rozman RS, Molek NA, Koby R. 1978. The absorption, distribution, and excretion in mice of the antimalarial mefloquine, erythro-2,8-bis(trifluoromethyl)-alpha-(2-piperidyl)-4-quinolinemethanol hydrochloride. Drug Metab. Dispos. 6:654–658 [PubMed] [Google Scholar]
- 26.Toovey S. 2009. Mefloquine neurotoxicity: a literature review. Travel Med. Infect. Dis. 7:2–6 [DOI] [PubMed] [Google Scholar]