Abstract
Extended-spectrum-beta-lactamase (ESBL)-producing Escherichia coli (ESBL E. coli) strains are of major concern because few antibiotics remain active against these bacteria. We investigated the association between the fecal relative abundance (RA) of ESBL-producing E. coli (ESBL-RA) and the occurrence of ESBL E. coli urinary tract infections (UTIs). The first stool samples passed after suspicion of UTI from 310 women with subsequently confirmed E. coli UTIs were sampled and tested for ESBL-RA by culture on selective agar. Predictive values of ESBL-RA for ESBL E. coli UTI were analyzed for women who were not exposed to antibiotics when the stool was passed. ESBL E. coli isolates were characterized for ESBL type, phylogroup, relatedness, and virulence factors. The prevalence of ESBL E. coli fecal carriage was 20.3%, with ESBL E. coli UTIs being present in 12.3% of the women. The mean ESBL-RA (95% confidence interval [CI]) was 13-fold higher in women exposed to antibiotics at the time of sampling than in those not exposed (14.3% [range, 5.6% to 36.9%] versus 1.1% [range, 0.32% to 3.6%], respectively; P < 0.001) and 18-fold higher in women with ESBL E. coli UTI than in those with another E. coli UTI (10.0% [range, 0.54% to 100%] versus 0.56% [range, 0.15% to 2.1%[, respectively; P < 0.05). An ESBL-RA of <0.1% was 100% predictive of a non-ESBL E. coli UTI. ESBL type, phylogroup, relatedness, and virulence factors were not found to be associated with ESBL-RA. In conclusion, ESBL-RA was linked to the occurrence of ESBL E. coli UTI in women who were not exposed to antibiotics and who had the same clone of E. coli in urine samples and fecal samples. Especially, a low ESBL-RA appeared to be associated with a low risk of ESBL E. coli infection.
INTRODUCTION
Escherichia coli is a commensal bacterium of the human intestinal tract, with a normal density of colonization (DC) of 107 to 108 CFU per gram of feces. The E. coli intestinal population includes one or more clones, and the relative abundance (RA) of each clone varies (1). Usually, antibiotic-susceptible E. coli bacteria form the dominant population, with resistant E. coli being subdominant (2). However, the proportion of resistant clones increases with antibiotic exposure (3, 4). E. coli is also a major pathogen and is the leading cause of urinary tract infections (UTIs) (5). E. coli strains that cause UTIs are commonly accepted to originate from the intestine (6). However, which of the intestinal E. coli strains will cause infection remains unclear (5). The prominent E. coli clones typically colonize the urethra more often than the subdominant ones (7), but it has been suggested that subdominant E. coli strains may overcome their disadvantage for causing UTI when carrying specific virulence factors, such as adhesins or siderophores, which may help the bacteria to survive and multiply in the urinary tract (8). This hypothesis has hardly been confirmed in clinical studies due to the difficulty of quantifying the infecting E. coli clones within the total intestinal population when the clone is subdominant. However, this issue can be resolved by focusing on antibiotic-resistant E. coli strains, which are easily detectable in feces by using selective agar, even when present in small numbers (9).
Extended-spectrum cephalosporins are often used for the treatment of upper UTIs (10). Resistance to this class of antibiotics has been increasing among E. coli strains that cause UTIs, both in hospitals and in the community, as a result of a worldwide dissemination of extended-spectrum-beta-lactamase (ESBL)-producing strains, particularly those of the CTX-M type (11, 12). This increase has been fueled by intestinal colonization, which occurs more frequently than actual infections (4). In our study, we assessed the relationship between the fecal RA of ESBL E. coli (ESBL-RA) and the occurrence of ESBL E. coli UTIs in women in the community.
(These results have partly been presented at the 22nd European Congress of Clinical Microbiology and Infectious Diseases [ECCMID], London, United Kingdom, 2012.)
MATERIALS AND METHODS
Patients and strains.
We performed a cross-sectional study. Patients were included from 5 centers (Table 1) located in countries with a high prevalence of ESBL E. coli (http://www.ecdc.europa.eu/en/activities/surveillance/EARS-Net), including the National Center for Preventive Medicine, Chişinău, Moldova (May 2009 to June 2010); The Modus Vivendi community-based laboratory, Chişinău, Moldova (February 2009 to September 2010); the Cantacuzino Institute, Bucharest, Romania (October 2008 to July 2009); the Infectious Diseases Polyclinic of the Ege University Teaching Hospital, Izmir, Turkey (September 2010 to March 2011); and the Internal Medicine Polyclinic of the Attikon University Teaching Hospital, Athens, Greece (January 2010 to February 2011). A local investigator from each center was trained at the central laboratory (Bacteriology Laboratory, Bichat-Claude Bernard Hospital, Paris, France) in order to ensure experimental homogeneity among sites.
Table 1.
Characteristics of the populations included in this study
| Parameter | Value for country |
P value | |||
|---|---|---|---|---|---|
| Moldova | Turkey | Romania | Greece | ||
| Type of center | Primary care | Tertiary hospital | Primary care | Tertiary hospital | |
| No. of patients | 184 | 76 | 39 | 11 | |
| Median age of patients (yr) (range) | 31 (17–79) | 50 (19–92) | 61 (22–81) | 49 (30–76) | <0.001 |
| No. (%) of patients with exposure to antibiotics | 128 (69.6) | 42 (55.3) | 8 (20.5) | 3 (27.3) | <0.001 |
| No. (%) of pregnant patients | 94 (51.1) | 6 (7.9) | 0 (0) | 0 (0) | <0.001 |
| No. (%) of patients with antecedent UTI | 160 (87.0) | 55 (72.4) | 38 (97.4) | 10 (90.9) | <0.01 |
| No. (%) of patients with diabetes | 15 (8.2) | 17 (22.4) | 4 (10.3) | 0 (0) | <0.01 |
| No. (%) of patients with hospitalization for <3 mo | 68 (37.0) | 21 (27.6) | 7 (17.9) | 0 (0) | <0.05 |
Female outpatients consulting these centers for UTI symptoms throughout the study period were considered for enrollment after an appropriate urine sample had been taken. They were asked to store the first stool passed after their initial visit at 4°C in a specifically provided container and to bring it back when returning for definitive urine test results. Patients returning after 72 h were excluded from the study. The same standardized questionnaire was used at all sites to record demographic data, antecedent UTI, recent antibiotic use, hospitalization in the last 3 months, pregnancy, and diabetes. All ethical and informed consent rules from each country were followed. Patients diagnosed with UTI caused by E. coli upon return were further included in the study.
Approximately 100 mg of each stool sample was diluted in 1 ml of brain heart infusion broth supplemented with 10% glycerol and maintained at −80°C by the local investigators. Stool samples and UTI strains (one per patient) were frozen and kept on dry ice during transport to the central laboratory, where they were kept frozen until use. There, UTI strains were retested for antibiotic susceptibility; ESBL presence was detected by the disc diffusion method, as recommended by the French Society for Microbiology (http://www.sfm-microbiologie.org/). Batches of stool samples were defrosted at 4°C, and 100 μl of each broth was plated onto Drigalski agar plates, with or without 1 mg/liter of cefotaxime, and cultured for 48 h at 37°C. All CFU with distinct morphotypes were further identified by matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry (Bruker Daltonics, Bremen, Germany) and tested for antibiotic susceptibility by the disc diffusion method. Densities of total enterobacteria and of ESBL E. coli were determined by plating serial dilutions of the broth onto Drigalski agar, with or without 1 mg/liter cefotaxime. CFU were counted in decimal logarithms at the dilution in which 1 to 100 CFU grew. ESBL-RA was calculated as the ratio of the ESBL E. coli counts divided by the total number of enterobacteria, expressed as a percentage. For women who carried more than one ESBL E. coli clone, the ESBL-RA of the dominant clone was considered.
Antibiotic exposure.
No reliable information on previous exposure to antibiotics was available, except for the current episode. The women were categorized as being exposed to antibiotics at the time of fecal sampling if they declared that they had taken antibiotics for the current infection or if antibiotic activity was detected in their fecal samples. Fecal antibiotic activity was detected by using a simple microbiological assay, performed as described previously (13). Briefly, 10 μl of each defrosted stool sample was placed onto antibiotic-free, sterile, 6-mm-diameter paper discs (Dutscher, Brumath, France). The discs were then placed onto Mueller-Hinton agar containing a 105-CFU/ml suspension of a fully susceptible E. coli strain (ATCC 25922; American Type Culture Collection, Manassas, VA, USA). Stool samples for which a zone of inhibition was observed around the disc, following overnight incubation at 37°C, were determined to have antibacterial activity. Studies in which volunteers were experimentally exposed to antibiotics have shown that this test is always negative for samples taken before drug exposure began (14).
Molecular characterization of ESBL strains.
All ESBL E. coli strains from urine samples and fecal samples were further characterized. DNA was extracted by using a Triton-based lysis buffer combined with microbeads and further quantified with a NanoDrop quantifier (Thermo Scientific, Waltham, MA, USA). Group 1 and 9 blaCTX-M genes were amplified and sequenced by using previously described primers (15). If negative, other ESBL-encoding genes (group 2, 8, and 25 CTX-M, TEM, and SHV) were sought (15). Phylogroups were determined by triplex PCR, as described previously (16). Virulence factors harboring the sfa/foc, iroN, cnf1, hlyC, aer, fyuA, irp2, ireA, iha, ibeA, sat, neuC, and usp genes were detected by PCR, as described previously (17), and virulence scores were calculated (18).
Concordance between E. coli strains from urine and fecal samples in ESBL E. coli carriers.
The relatedness between ESBL E. coli strains from urine and fecal samples was assessed by repetitive element palindromic PCR (rep-PCR) using primers REP1R (5′-NNNGCGCCGNCATCAGGC-3′) and REP2R (5′-ACGTCTTATCAGGCCTAC-3′) (19). Rep-PCR products were migrated on an Agilent 2100 Bioanalyzer with DiversiLab software (bioMérieux, Marcy l'Etoile, France). “Concordant” women were those with urine and fecal samples that contained ESBL E. coli strains that had ≥95% similarity. “Discordant” women refers to those for whom the ESBL E. coli strains from urine and fecal samples had <95% similarity or for whom there was no ESBL E. coli in the urine sample.
Statistical analysis.
Data were analyzed by using GraphPad PRISM version 5.04. Qualitative variables were tested by χ2 or Fisher's exact test. Associations between continuous variables and qualitative variables were tested by Student's t tests or by analysis of variance. The significance level was set at a value of 0.05.
RESULTS
ESBL E. coli prevalence in urine and fecal samples.
In total, 310 women with E. coli UTIs returned a stool sample within 72 h after their first visit, including 184 from Moldova, 76 from Turkey, 39 from Romania, and 11 from Greece (Table 1). The median age of the women was 35 years (range, 17 to 92 years). The overall prevalence of fecal carriage of ESBL E. coli among all sites was 63/310 (20.3%) (Fig. 1). These rates varied by country, with 9.8% in Moldova, 47.4% in Turkey, 17.9% in Romania, and 18.2% in Greece. Among the 63 carriers, 58 (92.1%) and 5 (7.9%) carried 1 and 2 ESBL E. coli strains, respectively. The ESBL E. coli prevalence in UTI was 12.3% (38/310), with 63.2% (24/38) of women being fecal carriers at the time of sampling. Thus, there were 14 women with an ESBL E. coli UTI who were not detected as ESBL fecal carriers. Among them, 1 carried an SHV-2-producing Klebsiella pneumoniae isolate, which may have outnumbered an ESBL E. coli isolate on the plates; 9 were exposed to antibiotics at the time of sampling; and 2 had <103 enterobacteria per gram of feces, which may have interfered with bacterial counts. No explanation was found for the remaining 2 women.
Fig 1.
Flow chart of the study. Dashed boxes represent discordant women, and solid boxes represent concordant women (see Materials and Methods).
CTX-M-15 was the predominant ESBL allele (69.8%), followed by CTX-M-14 (13.2%) and CTX-M-3 (11.3%), without significant differences in type distribution between urine and fecal strains, yet the numbers in the subgroups were low (Table 2). In contrast, ESBL E. coli phylogroups were unevenly distributed (P < 0.05), with phylogroup A (69.1%) being dominant over phylogroups B2 (17.7%) and D (13.2%) among fecal strains, whereas phylogroups B2, A, and D were evenly represented among the urine strains (Table 2). Among ESBL E. coli fecal carriers, 51.6% (32/63) (Fig. 1) were exposed to antibiotics at the time of sampling, either because they declared having taken antibiotics for the current episode of UTI (n = 30) or because antibacterial activity was detected in their feces (n = 2), without an affirmative declaration.
Table 2.
Characterization of ESBL E. coli from urine and stool samples from the 63 ESBL E. coli-carrying women
| ESBL | No. of samples with ESBL |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Urine (n = 38) |
Stool (n = 63)a |
Total | |||||||||
| Phylogroup A | Phylogroup B1 | Phylogroup B2 | Phylogroup D | Total | Phylogroup A | Phylogroup B1 | Phylogroup B2 | Phylogroup D | Total | ||
| Group 1 CTX-M | |||||||||||
| CTX-M-15 | 10 | 0 | 11 | 7 | 28 | 32 | 0 | 8 | 6 | 46 | 74 |
| CTX-M-1 | 1 | 0 | 0 | 0 | 1 | 4 | 0 | 0 | 0 | 4 | 5 |
| CTX-M-3 | 1 | 0 | 0 | 4 | 5 | 4 | 0 | 0 | 3 | 7 | 12 |
| Group 9 CTX-M | |||||||||||
| CTX-M-14 | 0 | 0 | 3 | 0 | 3 | 7 | 0 | 4 | 0 | 11 | 14 |
| CTX-M-16 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Total | 12 | 0 | 14 | 12 | 38 | 47 | 0 | 12 | 9 | 68 | 106 |
Including 5 women with 2 different ESBL E. coli strains producing the respective CTX-M alleles (1 woman each): CTX-M alleles 15 and 1, 15 and 3, 15 and 14, 15 and 15, and 1 and 1.
Associations with ESBL-RA.
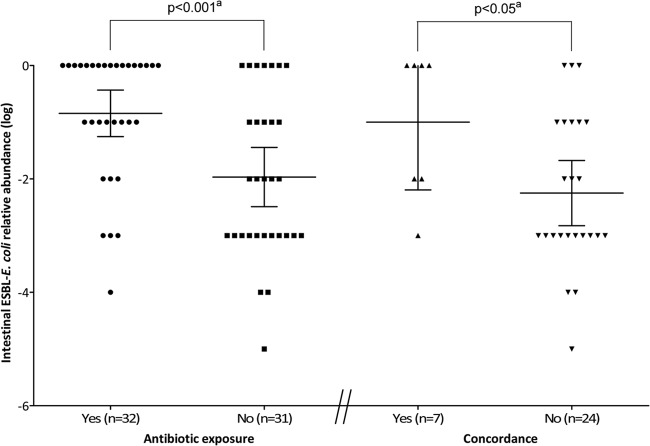
The mean ESBL-RA (95% confidence interval [CI]) was 13-fold higher in women exposed to antibiotics when the stool was passed than in those not exposed (14.3% [range, 5.6% to 36.9%] versus 1.1% [range, 0.32% to 3.6%], respectively; P < 0.001) (Fig. 2). Thus, because antibiotic exposure influenced ESBL-RA, the relationship between ESBL-RA and the occurrence of ESBL E. coli UTI was analyzed only among the 31 women (11 with ESBL E. coli UTI and 20 with non-ESBL E. coli UTI) (Fig. 1) who were not exposed to antibiotics when the stool was passed. ESBL-RA did not vary significantly as a function of the day on which the stool sample was returned. Stool samples returned on day 0 (n = 5), day 1 (n = 5), day 2 (n = 17), and day 3 (n = 4) showed mean ESBL-RAs of 2.5% (range, 0.10% to 65.4%), 1.0% (range, 0.017% to 57.0%), 1.0% (range, 0.16% to 6.2%), and 0.56% (range, 0.0011% to 34.1%), respectively (see Fig. S1 in the supplemental material). There was no significant difference in ESBL-RA between carriers of CTX-M-15 E. coli (n = 19) or another type of CTX-M (n = 12) (mean, 0.79% [range, 0.18% to 3.4%] versus 1.8% [range, 0.17% to 18.5%], respectively) (see Fig. S2 in the supplemental material). The mean ESBL-RA was also not correlated with the virulence factor score of the fecal ESBL strains (0.56% [range, 0.050% to 6.4%], 3.2% [range, 0.51% to 19.5%], and 0.52% [range, 0.036% to 7.5%] for virulence scores of 0 to 1, 2 to 3, and 4 to 7, respectively) (see Fig. S2 in the supplemental material). Likewise, the mean ESBL-RA did not correlate with the phylogroup of the fecal ESBL strains (0.90% [range, 0.20% to 4.1%], 0.56% [range, 0.0023% to 73.0%], and 4.0% [range, 0.15 to 96.4%] for phylogroups A, B2, and D, respectively) (see Fig. S2 in the supplemental material). A total of 24 of the 31 women (77.4%) were discordant, either because they had a non-ESBL E. coli UTI (n = 20) or because the ESBL E. coli isolates from their fecal and urine samples had dissimilar rep-PCR patterns (n = 4), whereas 7 (22.6%) were concordant (Fig. 1). The mean ESBL-RA was 18-fold higher for the concordant women than for the discordant ones (10.0% [range, 0.54% to 100%] versus 0.56% [range, 0.15% to 2.1%], respectively; P < 0.05) (Fig. 2). In contrast, the mean virulence factor scores for the fecal ESBL E. coli strains were not significantly different between concordant and discordant women (1.7 [range, 0.050 to 3.4] versus 2.3 [range, 1.4 to 3.1]) (data not shown).
Fig 2.
Intestinal ESBL-RA in 63 women according to antibiotic exposure and to the concordance of urinary and fecal ESBL strains (concordance, ≥95% similarity between rep-PCR patterns [see Materials and Methods]) among the 31 women not exposed to antibiotics. The main horizontal bar represents the mean. Error bars represent 95% confidence intervals. a, determined by a two-tailed, unpaired t test.
The sensitivity, specificity, and predictive values of ESBL-RA for the presence of ESBL E. coli in urine are shown in Table 3. When the ESBL-RA was <0.1%, the negative predictive value for ESBL E. coli UTI was 100%. In contrast, positive predictive values (PPVs) reached a maximum of 57% when the ESBL-RA was ≥10%.
Table 3.
ESBL-RA as a predictor of UTI caused by ESBL E. coli for the 31 women not exposed to antibiotics
| ESBL-RA value (%) | Concordance determined according toa: |
|||
|---|---|---|---|---|
| Sens | Spec | PPV | NPV | |
| 10–100 | 0.57 | 0.77 | 0.57 | 0.88 |
| 1–10 | 0.57 | 0.61 | 0.33 | 0.84 |
| 0.1–1 | 0.86 | 0.45 | 0.35 | 0.93 |
| 0.01–0.1 | 1.00 | 0.10 | 0.26 | 1.00 |
| 0.001–0.01 | 1.00 | 0.03 | 0.23 | 1.00 |
Concordance applied to women with urine and fecal samples that contained ESBL E. coli strains that displayed ≥95% similarity (see Materials and Methods). Sens, sensitivity; Spec, specificity; PPV, positive predictive value; NPV, negative predictive value.
DISCUSSION
Our most important result was that the fecal ESBL-RA was linked to the occurrence of UTI caused by ESBL E. coli in women who were not exposed to antibiotics and who had the same clone of E. coli in urine samples and fecal samples. In particular, we found that women with a low ESBL-RA (<0.1%) had no risk to develop ESBL E. coli UTI. Conversely, the risk increased along with the RA, but the PPV peaked to 57% only. This description of a quantitative link between ESBL-RA and ESBL E. coli UTI is novel. Indeed, if the predominant fecal E. coli strain had previously been reported to be more likely to cause a UTI (7) or to translocate to the bloodstream (20) than a subdominant one, no precise quantification of the phenomenon was available until now. We initially included 310 women in the study, but we performed the final analysis on a relatively small subset (10%) of them in order to ensure maximal homogeneity and eliminate as many biases as possible. In particular, we excluded from the analysis all women who might have taken antibiotics, even if the reported agent had in theory little or no activity against ESBL-RA, because we judged that the answers to the questionnaire might have been unreliable as to the type of antibiotic taken. In spite of this reduced sample size, our results were statistically significant and conclusive.
Another new result was the 13-fold increase in ESBL-RA in women exposed to antibiotics at the time of sampling. The increase in intestinal density of resistant Gram-negative bacilli during antibiotic administration was previously observed with other antibiotics, such as co-trimoxazole (3). However, to the best of our knowledge, it had not been previously quantified for ESBL strains. Whether a more distant exposure would have done the same, and to which extent, could not be tested here due to a lack of reliable information on previous antibiotics used. Since previous antibiotic treatment is also a known risk factor for the occurrence of UTI with resistant bacteria (21–23), our results suggest that, at least in some cases, ESBL E. coli UTI occurs as a 2-step phenomenon, which would include first an increase in fecal ESBL-RA following antibiotic exposure in low-count carriers and then the development of ESBL E. coli UTI in individuals with an already high ESBL-RA. If so, maintenance of a low ESBL-RA in the feces might help to minimize the probability of developing an ESBL E. coli UTI, even if complete eradication is not obtained. If this hypothesis was proven accurate in a larger and prospective study, attempts to prevent increases in ESBL-RA during antibiotic treatments (24) might prove to be very useful. Conversely, previous antibiotic exposure might have eradicated ESBL E. coli carriage in some women for whom an ESBL E. coli strain was found in urine samples. Indeed, it has been shown that low concentrations of antibiotics may be sufficient to eliminate fecal bacteria if they are over the MIC of the target strains (25). Also, during treatment with fluoroquinolones, fecal concentrations of antibiotics can reach very high levels that overcome the MIC of even resistant strains (26). In other women, antibiotics might have been taken at a time distant from the time of stool emission, which could explain why they were not detected by our test, despite a residual effect on the microbiota (3, 27). Obviously, further studies on the precise role of antibiotic exposure in ESBL-RA are needed.
We chose RA to measure the level of ESBL E. coli fecal carriage instead of bacterial DC because variations in the sizes of samples, transport conditions, and thaw cycles may occur despite standardization. We assumed that variations in these parameters would evenly affect counts of total enterobacteria and of ESBL E. coli. RA would thus be less subject to technical variations than DC. Also, because RA is a ratio, it can be determined by using rectal swabs for which the precise quantity of the fecal material is unknown, which is not possible with DC. This may be relevant for clinical research because swabs are easier to obtain, on demand, than fecal samples. Recently, rectal swabs were similarly used to assess the RA of KPC-producing K. pneumoniae rather than DC (28).
In our study, virulence factors were not observed to have an influence on the propensity of fecal ESBL E. coli to cause UTI, which suggests the role of dominance over virulence in the pathogenesis of UTI in women, at least for ESBL E. coli strains. Additionally, correlations were not found between phylogroups and ESBL-RA, suggesting that other traits might determine the level of ESBL E. coli within E. coli populations.
This study has some limitations, however. We assumed that the densities that we observed in the stool samples reflected the densities at the onset of UTI, even if the feces were passed shortly thereafter. We cannot rule out that the densities of ESBL E. coli and total enterobacteria might have changed between these time points. Another limitation was that, in the case of carriage of multiple ESBL E. coli strains, only the dominant strain could have been detectable. This might explain why we observed a substantial number of discordant women. Indeed, these limitations may have lowered the PPV, as dominant strains at high ESBL-RA were not found to cause UTI. Also, the populations studied were from countries with very diverse health systems, and the types of centers where the women were recruited differed (Table 1). However, this heterogeneity may also be viewed as a strength, since it decreased the possibility that the results resulted from specific characteristics present at a single center.
In conclusion, the level of ESBL E. coli intestinal carriage, as measured by fecal ESBL-RA, was linked to the presence of ESBL E. coli in the urine of infected patients. New perspectives for the clinical management of ESBL E. coli UTI might emerge from this observation.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by ECO-NET (an Egide program [http://www.egide.asso.fr/]), by the FP7 Health Program (EvoTAR project, contract no. 282004, and R-GNOSIS project, contract no. 282512), and by Oseo (http://www.oseo.fr/) (Nosobio program). T.K. was supported by a grant from the Federation of European Microbiological Societies (FEMS Research Fellowship).
We thank Irina Codita, Alexandru Decusar, Iurii Roscin, Ala Marina, Iulia Marusicenco, Oleg Benes, Feriha Çilli, Garyfallia Poulakou, Kiriaki Kanellakopoulou, Stavroula Kanellaki, and Florentia Pournou for their contribution to the patient recruitment and sampling processes. We thank Editage for providing editorial assistance.
We declare that we have no conflicts of interest.
Footnotes
Published ahead of print 8 July 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00238-13.
REFERENCES
- 1.Lidin-Janson G, Kaijser B, Lincoln K, Olling S, Wedel H. 1978. The homogeneity of the faecal coliform flora of normal school-girls, characterized by serological and biochemical properties. Med. Microbiol. Immunol. 164:247–253 [DOI] [PubMed] [Google Scholar]
- 2.Marshall B, Tachibana C, Levy SB. 1983. Frequency of tetracycline resistance determinant classes among lactose-fermenting coliforms. Antimicrob. Agents Chemother. 24:835–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray BE, Rensimer ER, DuPont HL. 1982. Emergence of high-level trimethoprim resistance in fecal Escherichia coli during oral administration of trimethoprim or trimethoprim-sulfamethoxazole. N. Engl. J. Med. 306:130–135 [DOI] [PubMed] [Google Scholar]
- 4.Tosh PK, McDonald LC. 2012. Infection control in the multidrug-resistant era: tending the human microbiome. Clin. Infect. Dis. 54:707–713 [DOI] [PubMed] [Google Scholar]
- 5.Hooton T. 2012. Uncomplicated urinary tract infection. N. Engl. J. Med. 366:1028–1037 [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto S, Tsukamoto T, Terai A, Kurazono H, Takeda Y, Yoshida O. 1997. Genetic evidence supporting the fecal-perineal-urethral hypothesis in cystitis caused by Escherichia coli. J. Urol. 157:1127–1129 [PubMed] [Google Scholar]
- 7.Moreno E, Andreu A, Pigrau C, Kuskowski M, Johnson J, Prats G. 2008. Relationships between Escherichia coli strains causing acute cystitis in women and the fecal E. coli population of the host. J. Clin. Microbiol. 46:2529–2534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plos K, Connell H, Jodal U, Marklund BI, Marild S, Wettergren B, Svanborg C. 1995. Intestinal carriage of P fimbriated Escherichia coli and the susceptibility to urinary tract infection in young children. J. Infect. Dis. 171:625–631 [DOI] [PubMed] [Google Scholar]
- 9.Bhalla A, Pultz NJ, Ray AJ, Hoyen CK, Eckstein EC, Donskey CJ. 2003. Antianaerobic antibiotic therapy promotes overgrowth of antibiotic-resistant, gram-negative bacilli and vancomycin-resistant enterococci in the stool of colonized patients. Infect. Control Hosp. Epidemiol. 24:644–649 [DOI] [PubMed] [Google Scholar]
- 10.Gupta A, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, Moran GJ, Nicolle LE, Raz R, Schaeffer AJ, Soper DE. 2011. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin. Infect. Dis. 52:103–120 [DOI] [PubMed] [Google Scholar]
- 11.Bush K, Fisher JF. 2011. Epidemiological expansion, structural studies, and clinical challenges of new beta-lactamases from gram-negative bacteria. Annu. Rev. Microbiol. 65:455–478 [DOI] [PubMed] [Google Scholar]
- 12.Pitout JD, Laupland KB. 2008. Extended-spectrum beta-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect. Dis. 8:159–166 [DOI] [PubMed] [Google Scholar]
- 13.Messer JW, Leslie JE, Houghtby GA, Peeler JT, Barnett JE. 1982. Bacillus stearothermophilus disc assay for detection of inhibitors in milk: collaborative study. J. Assoc. Off. Anal. Chem. 65:1208–1214 [PubMed] [Google Scholar]
- 14.Pletz MW, Rau M, Bulitta J, De Roux A, Burkhardt O, Kruse G, Kurowski M, Nord CE, Lode H. 2004. Ertapenem pharmacokinetics and impact on intestinal microflora, in comparison to those of ceftriaxone, after multiple dosing in male and female volunteers. Antimicrob. Agents Chemother. 48:3765–3772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saladin M, Cao VT, Lambert T, Donay JL, Herrmann JL, Ould-Hocine Z, Verdet C, Delisle F, Philippon A, Arlet G. 2002. Diversity of CTX-M beta-lactamases and their promoter regions from Enterobacteriaceae isolated in three Parisian hospitals. FEMS Microbiol. Lett. 209:161–168 [DOI] [PubMed] [Google Scholar]
- 16.Clermont O, Bonacorsi S, Bingen E. 2000. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 66:4555–4558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson JR, Clermont O, Menard M, Kuskowski MA, Picard B, Denamur E. 2006. Experimental mouse lethality of Escherichia coli isolates, in relation to accessory traits, phylogenetic group, and ecological source. J. Infect. Dis. 194:1141–1150 [DOI] [PubMed] [Google Scholar]
- 18.Lefort A, Panhard X, Clermont O, Woerther PL, Branger C, Mentre F, Fantin B, Wolff M, Denamur E. 2011. Host factors and portal of entry outweigh bacterial determinants to predict the severity of Escherichia coli bacteremia. J. Clin. Microbiol. 49:777–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruimy R, Genauzeau E, Barnabe C, Beaulieu A, Tibayrenc M, Andremont A. 2001. Genetic diversity of Pseudomonas aeruginosa strains isolated from ventilated patients with nosocomial pneumonia, cancer patients with bacteremia, and environmental water. Infect. Immun. 69:584–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berg RD. 1981. Promotion of the translocation of enteric bacteria from the gastrointestinal tracts of mice by oral treatment with penicillin, clindamycin, or metronidazole. Infect. Immun. 33:854–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez-Bano J, Navarro MD, Romero L, Martinez-Martinez L, Muniain MA, Perea EJ, Perez-Cano R, Pascual A. 2004. Epidemiology and clinical features of infections caused by extended-spectrum beta-lactamase-producing Escherichia coli in nonhospitalized patients. J. Clin. Microbiol. 42:1089–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calbo E, Romani V, Xercavins M, Gomez L, Vidal CG, Quintana S, Vila J, Garau J. 2006. Risk factors for community-onset urinary tract infections due to Escherichia coli harbouring extended-spectrum beta-lactamases. J. Antimicrob. Chemother. 57:780–783 [DOI] [PubMed] [Google Scholar]
- 23.Colodner R, Rock W, Chazan B, Keller N, Guy N, Sakran W, Raz R. 2004. Risk factors for the development of extended-spectrum beta-lactamase-producing bacteria in nonhospitalized patients. Eur. J. Clin. Microbiol. Infect. Dis. 23:163–167 [DOI] [PubMed] [Google Scholar]
- 24.Tarkkanen AM, Heinonen T, Jogi R, Mentula S, van der Rest ME, Donskey CJ, Kemppainen T, Gurbanov K, Nord CE. 2009. P1A recombinant beta-lactamase prevents emergence of antimicrobial resistance in gut microflora of healthy subjects during intravenous administration of ampicillin. Antimicrob. Agents Chemother. 53:2455–2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bourgeois-Nicolaos N, Massias L, Couson B, Butel MJ, Andremont A, Doucet-Populaire F. 2007. Dose dependence of emergence of resistance to linezolid in Enterococcus faecalis in vivo. J. Infect. Dis. 195:1480–1488 [DOI] [PubMed] [Google Scholar]
- 26.Fantin B, Duval X, Massias L, Alavoine L, Chau F, Retout S, Andremont A, Mentre F. 2009. Ciprofloxacin dosage and emergence of resistance in human commensal bacteria. J. Infect. Dis. 200:390–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dethlefsen L, Relman DA. 2011. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc. Natl. Acad. Sci. U. S. A. 108(Suppl 1):4554–4561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lerner A, Romano J, Chmelnitsky I, Navon-Venezia S, Edgar R, Carmeli Y. 2013. Rectal swabs are suitable for quantifying the carriage load of KPC-producing carbapenem-resistant Enterobacteriaceae. Antimicrob. Agents Chemother. 57:1474–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.