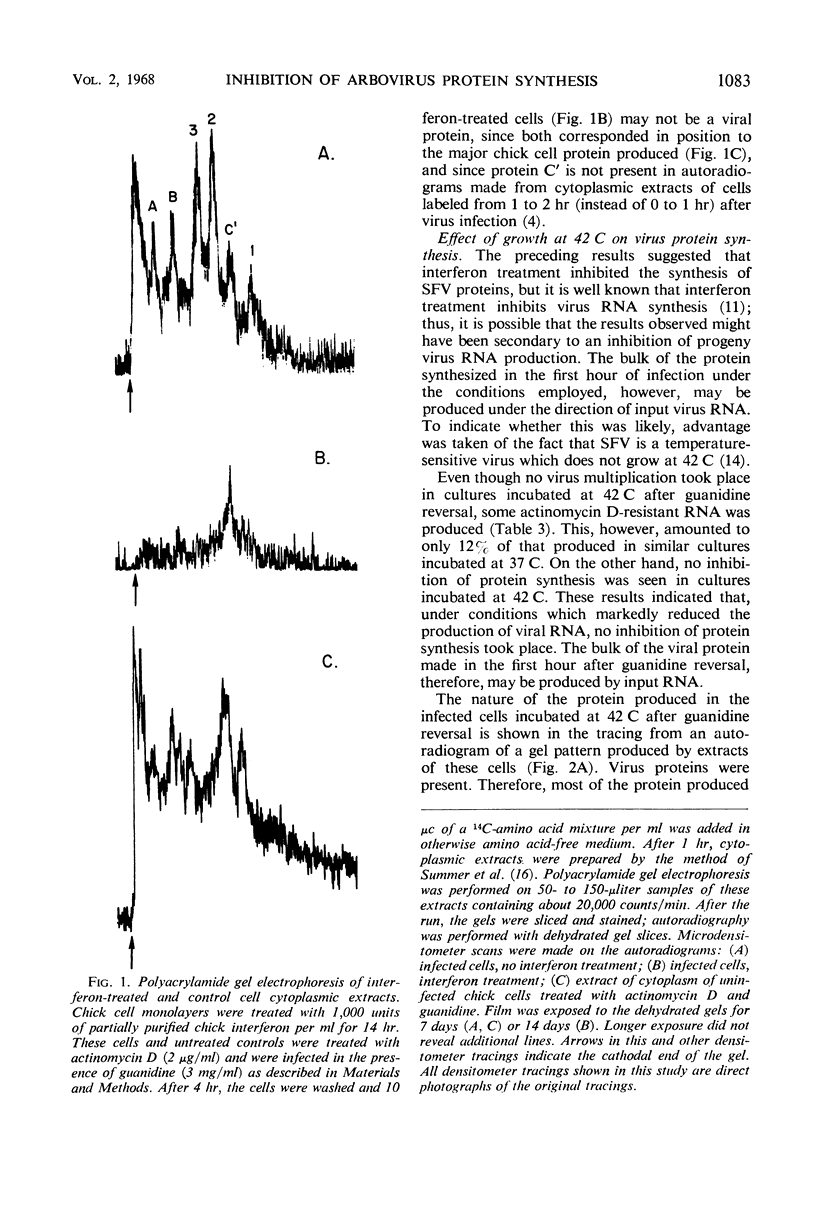
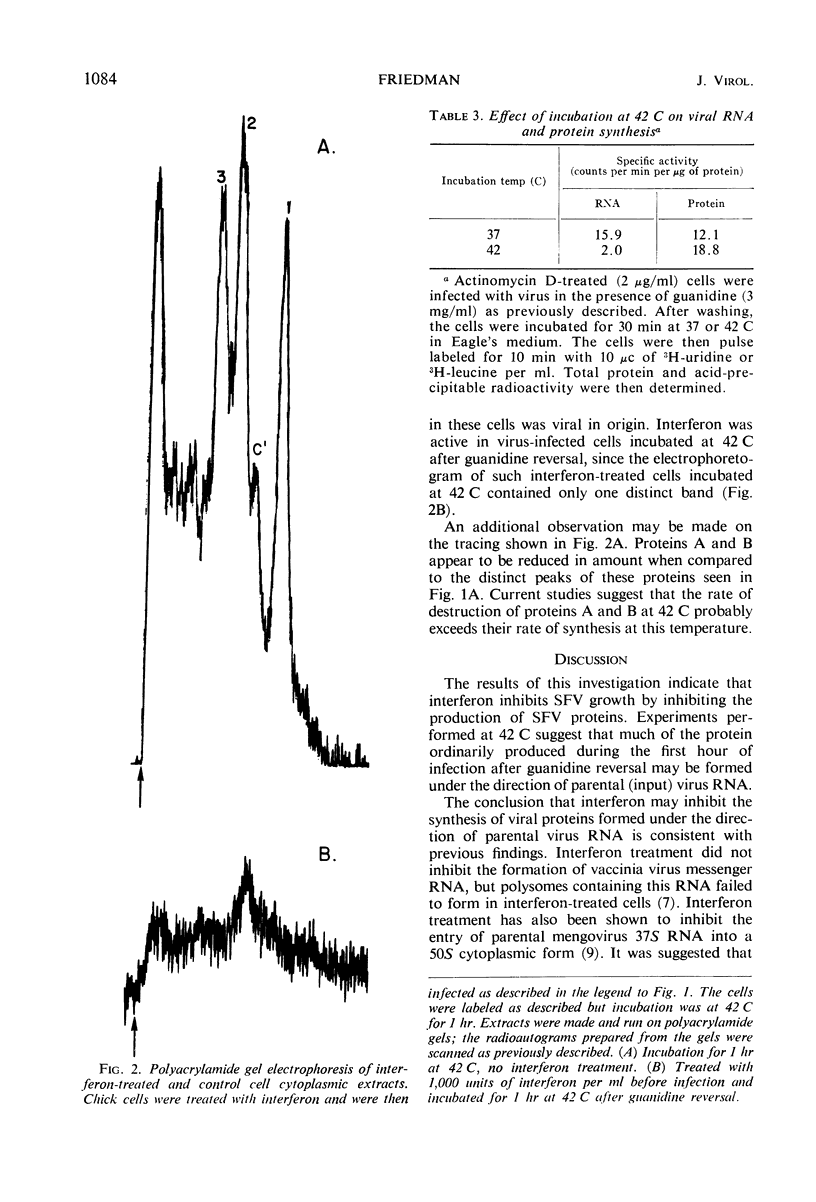
Abstract
Infection of cells treated with guanidine and actinomycin D and then washed to remove the guanidine inhibition of virus growth had no effect on antiviral activity already established by interferon. Protein synthesis in interferon-treated cells infected under these conditions was decreased as compared to control cells similarly treated but not exposed to interferon. In these control cells, analysis by polyacrylamide gel electrophoresis indicated that six proteins were produced during the first hour after guanidine reversal. Five of these proteins have been previously identified as probably being viral in origin. In interferon-treated cells, only a single major protein was produced. Ribonucleic acid (RNA) synthesis by Semliki Forest virus during the first hour after guanidine reversal was markedly depressed by incubation at 42 C, but no inhibition of total virus protein synthesis was seen; this finding suggested that much of the virus protein produced in the first hour after guanidine reversal was carried out by input virus RNA. Interferon was fully active in cells incubated at 42 C. The results suggested that interferon inhibits the production of Semliki Forest virus proteins ordinarily produced under the direction of the virus genome.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burge B. W., Pfefferkorn E. R. Isolation and characterization of conditional-lethal mutants of Sindbis virus. Virology. 1966 Oct;30(2):204–213. doi: 10.1016/0042-6822(66)90096-1. [DOI] [PubMed] [Google Scholar]
- Carter W. A., Levy H. B. Ribosomes: effect of interferon on their interaction with rapidly labeled cellular and viral RNA's. Science. 1967 Mar 10;155(3767):1254–1257. doi: 10.1126/science.155.3767.1254. [DOI] [PubMed] [Google Scholar]
- FRIEDMAN R. M., SONNABEND J. A. INHIBITION OF INTERFERON ACTION BY P-FLUOROPHENYLALANINE. Nature. 1964 Jul 25;203:366–367. doi: 10.1038/203366a0. [DOI] [PubMed] [Google Scholar]
- Fantes K. H. Further purification of chick interferon. Nature. 1965 Sep 18;207(5003):1298–1298. doi: 10.1038/2071298a0. [DOI] [PubMed] [Google Scholar]
- Friedman R. M., Fantes K. H., Levy H. B., Carter W. B. Interferon action on parental Semliki forest virus ribonucleic acid. J Virol. 1967 Dec;1(6):1168–1173. doi: 10.1128/jvi.1.6.1168-1173.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M. Structural and nonstructural proteins of an arbovirus. J Virol. 1968 Oct;2(10):1076–1080. doi: 10.1128/jvi.2.10.1076-1080.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joklik W. K., Merigan T. C. Concerning the mechanism of action of interferon. Proc Natl Acad Sci U S A. 1966 Aug;56(2):558–565. doi: 10.1073/pnas.56.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINE S. EFFECT OF ACTINOMYCIN D AND PUROMYCIN DIHYDROCHLORIDE ON ACTION OF INTERFERON. Virology. 1964 Dec;24:586–588. doi: 10.1016/0042-6822(64)90211-9. [DOI] [PubMed] [Google Scholar]
- Levy H. B., Carter W. A. Molecular basis of the action of interferon. J Mol Biol. 1968 Feb 14;31(3):561–577. doi: 10.1016/0022-2836(68)90428-2. [DOI] [PubMed] [Google Scholar]
- Marcus P. I., Salb J. M. Molecular basis of interferon action: inhibition of viral RNA translation. Virology. 1966 Nov;30(3):502–516. doi: 10.1016/0042-6822(66)90126-7. [DOI] [PubMed] [Google Scholar]
- Miner N., Ray W. J., Jr, Simon E. H. Effect of interferon on the production and action of viral RNA polymerase. Biochem Biophys Res Commun. 1966 Jul 20;24(2):264–268. doi: 10.1016/0006-291x(66)90730-3. [DOI] [PubMed] [Google Scholar]
- Mécs E., Sonnabend J. A., Martin E. M., Fantes K. H. The effect of interferon on the synthesis of RNA in chick cells infected with Semliki forest virus. J Gen Virol. 1967 Jan;1(1):25–40. doi: 10.1099/0022-1317-1-1-25. [DOI] [PubMed] [Google Scholar]
- RUIZ-GOMEZ J., ISAACS A. Optimal temperature for growth and sensitivity to interferon among different viruses. Virology. 1963 Jan;19:1–7. doi: 10.1016/0042-6822(63)90017-5. [DOI] [PubMed] [Google Scholar]
- Revel M., Herzberg M., Becarevic A., Gros F. Role of protein factor in the functional binding of ribosomes to natural messenger RNA. J Mol Biol. 1968 Apr 14;33(1):231–249. doi: 10.1016/0022-2836(68)90291-x. [DOI] [PubMed] [Google Scholar]
- Sonnabend J. A., Martin E. M., Mécs E., Fantes K. H. The effect of interferon on the synthesis and activity of an RNA polymerase isolated from chick cells infected with Semliki forest virus. J Gen Virol. 1967 Jan;1(1):41–48. doi: 10.1099/0022-1317-1-1-41. [DOI] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr, Darnell J. E., Jr Evidence for virus-specific noncapsid proteins in poliovirus-infected HeLa cells. Proc Natl Acad Sci U S A. 1965 Aug;54(2):505–513. doi: 10.1073/pnas.54.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR J. STUDIES ON THE MECHANISM OF ACTION OF INTERFERON. I. INTERFERON ACTION AND RNA SYNTHESIS IN CHICK EMBRYO FIBROBLASTS INFECTED WITH SEMLIKI FOREST VIRUS. Virology. 1965 Mar;25:340–349. doi: 10.1016/0042-6822(65)90053-x. [DOI] [PubMed] [Google Scholar]



