Abstract
Objectives:
To examine the impact of serum-derived bovine immunoglobulin, an oral medical food known to neutralize bacterial antigen and reduce intestinal inflammation, on restoration of mucosal immunity and gastrointestinal function in individuals with HIV enteropathy.
Design:
Open-label trial with intensive 8-week phase of bovine serum immunoglobulin (SBI) 2.5 g twice daily with a 4-week washout period and an optional 9-month extension study.
Methods:
HIV enteropathy was defined as chronic gastrointestinal symptoms including frequent loose or watery stools despite no identifiable, reversible cause. Upper endoscopy for tissue immunofluorescent antibody assay and disaccharide gut permeability/absorption studies were performed before and after 8 weeks of SBI to test mucosal immunity and gastrointestinal function. Blood was collected for markers of microbial translocation, inflammation, and collagen kinetics. A validated gastrointestinal questionnaire assessed changes in symptoms.
Results:
All eight participants experienced profound improvement in symptoms with reduced bowel movements/day (P = 0.008) and improvements in stool consistency (P = 0.008). Gut permeability was normal before and after the intervention, but d-xylose absorption increased in seven of eight participants. Mucosal CD4+ lymphocyte densities increased by a median of 139.5 cells/mm2 from 213 to 322 cells/mm2 (P = 0.016). Intestinal-fatty acid binding protein (I-FABP), a marker of enterocyte damage, initially rose in seven of eight participants after 8 weeks (P = 0.039), and then fell below baseline in four of five who continued receiving SBI (P = 0.12). Baseline serum I-FABP levels were negatively correlated with subsequent rise in mucosal CD4+ lymphocyte densities (r = −0.74, P = 0.046).
Conclusion:
SBI significantly increases intestinal mucosal CD4+ lymphocyte counts, improves duodenal function, and showed evidence of promoting intestinal repair in the setting of HIV enteropathy.
Keywords: bovine immunoglobulin, d-xylose absorption, gastrointestinal associated lymphoid tissue, gut permeability, HIV enteropathy, immune reconstitution, immunohistochemistry, intestinal fatty acid binding protein, monocyte chemotaxis protein-1
Introduction
Early in the HIV epidemic, gastrointestinal-related disease was a dominant presenting symptom complex. Jarry et al.[1] utilized histopathologic evaluations of the gut to demonstrate the profound immune depletion in gastrointestinal-associated lymphoid tissue (GALT) caused by HIV infection. HIV enteropathy became known as the syndrome of gastrointestinal complaints that persisted despite completion of appropriate therapy for any identified abnormalities [2,3]. It was believed to be due to HIV infection within the gastrointestinal mucosa, if not the enterocytes themselves, resulting in defects of mucosal function [4,5]. Despite the improvements in morbidity and mortality related to HIV infection [6], diarrhea and related gastrointestinal complaints remain in nearly 30% of patients on antiretroviral therapy (ART) [7,8].
GALT is recognized as a primary HIV target, irrespective of the mode of transmission, likely due to its constitutively high level of activation [9,10]. Within weeks of infection, human and nonhuman primate studies demonstrate infection of virtually all susceptible CD4+ T lymphocytes with HIV [11–13]. Despite effective ART, lymphocyte recovery in duodenal lamina propria lags far behind immune reconstitution in the peripheral blood compartment [14–16].
Depletion of lymphocytes in GALT leads to bacterial translocation, irrespective of the mechanism for cell loss [17,18]. Consequences of HIV-induced CD4+ T-cell depletion from GALT and microbial translocation as measured by plasma concentrations of lipopolysaccharide (LPS) and bacterial 16S ribosomal-RNA gene sequences (rDNA) include increased levels of proinflammatory cytokines and peripheral blood CD8+ T-cell subsets with an activated phenotype [19,20]. We have performed preliminary studies showing that the composition of the gut microbiota is correlated with both duodenal mucosal T-cell distribution and activation, as well as systemic immune activation in HIV patients both naïve or receiving ART [21].
Bovine serum immunoglobulin (SBI) is a specially formulated medical food that provides distinct nutritional support for the clinical dietary management of enteropathy under medical supervision. It is widely used in animal husbandry to reduce severe gastrointestinal manifestations of inflammation in young animals [22,23]. Animal model experiments of intestinal inflammation demonstrate that the immunoglobulins present in SBI neutralize LPS and other bacterial antigens and improve intestinal barrier function damaged by bacterial toxins [24–27]. This oral immunoglobulin undergoes reversible conformational changes under gastric pH and otherwise passes through the intestines unabsorbed. Available commercially, it has a long history of safety, including in children [28]. The hypothesis pursued in this clinical trial was that neutralization of proinflammatory bacterial antigen in the gut by SBI would lead to restoration of mucosal immunity and gastrointestinal function. We targeted study participants with a diagnosis of HIV enteropathy for this clinical trial as this population likely has an underlying disturbance in gut microbiota composition and altered gastrointestinal function as a feature of their immunopathology.
Methods
Study design
This open-label pilot study enrolled participants with the diagnosis of HIV enteropathy to receive 2.5 g of SBI (EnteraHealth, Akeny, Iowa, USA) twice daily for 8 weeks followed by a 4-week washout phase. HIV enteropathy was defined as chronic gastrointestinal symptoms including loose or watery stools and increased stool frequency (>3/day) despite no identifiable, reversible cause. Participants with gastrointestinal symptoms after the washout phase were invited to enter into an extension period for an additional 9 months. Screening studies included stool samples for Clostridium difficile toxin, enteric pathogens, antigen testing for giardia, cryptosporidium, Entamoeba histolytica, Helicobacter pylori, and stains for ova and parasites, acid-fast bacilli, microsporidia, and isospora. Screening tests performed for food intolerance included endomysial antibodies to exclude gluten allergy and a period of lactose-free diet if indicated. ART-associated gastrointestinal intolerance was assessed either by a history of symptoms predating treatment initiation or persistence of symptoms despite a change in regimen. Continuation of stable, chronic antimotility agents was permitted with the provision that increases in doses were avoided. Study entry was delayed at least 4 weeks following antibiotic administration except for chronic Pneumocystis pneumonia prophylaxis. Participants underwent phlebotomy and stool submission at preentry and entry visits, at weeks 7 and 8 while receiving SBI, at week 12 after a 4-week washout, and then again at the end of 48 weeks for those participants who entered an extension study. Plasma and serum was available from a cohort of controls for comparison (n = 8). Analysis performed on the last sample available for a participant (end of treatment – EOT) was week 8 or week 48. All participants signed an informed consent form approved by the UC Davis Institutional Review Board [Clinical Trial Registry (Clinicaltrials.gov): NCT01313910].
Gastrointestinal questionnaire and food diary
A licensed dietician met with participants to assess food intolerance prior to enrollment and throughout the first 12 weeks to review a food diary. A steady diet was maintained in order to not confound observed clinical results. A validated gastrointestinal questionnaire, completed at each study visit, recorded daily stool frequency, stool consistency relying on a pictorial scale from 1 (normal, formed) to 6 (water, puddle-like), and gastrointestinal symptoms over the previous week [7]. The questions assessed symptoms including cramping, urgency, incontinence, and nocturnal bowel movements (possible score from 0 to 39 with normal <4).
Gastrointestinal function assays: rhamnose/lactulose gut permeability and d-xylose absorption study
The disaccharide gut permeability and d-xylose absorption study were performed in the week preceding study entry and after 7 weeks of SBI treatment. Participants consumed a syrup consisting of lactulose (5 g), l-rhamnose (1 g), and d-xylose (500 mg; BCM Specials Products, London, UK) on an empty stomach and collected all urine over the subsequent 4 h [29]. Urine disaccharide levels were measured in the laboratory of Dr Roy Sherwood [30]. Results are reported as the ratio of lactulose/l-rhamnose (normal <0.05). Duodenal absorption function is reported as the amount of d-xylose collected in the urine over 4 h.
Upper endoscopy and tissue processing
Distal duodenum biopsies were obtained before and 8 weeks after initiating SBI. Tissue was either paraffin-embedded for routine histology and immunofluorescent antibody assay (IFA) or underwent digestion to single-cell suspension for polychromatic flow cytometry as previously described [31]. Peripheral blood mononuclear cells (PBMCs) were separated by Ficol-Hypaque and processed with the duodenal cells in identical fashion. Fluorescence-activated cell analysis was performed on a custom Becton-Dickinson LSRII cytometer and analyzed with FlowJo (TreeStar, Ashland, Oregon, USA).
For IFA analysis, the primary antibodies were polyclonal anti-CD3 rabbit serum (Dako Inc., Carpinteria, California, USA) and monoclonal anti-CD4 or CD8 mouse serum (Leica Microsystems, Buffalo Grove, Illinois, USA). Binding of CD3+ and CD4+ or CD8+ receptors was detected simultaneously using Alexafluor 488-labeled polyclonal goat antirabbit IgG and Alexafluor 568-labeled polyclonal goat antimouse IgG (both Molecular Probes, Eugene, Oregon, USA). Positive cells were counted by a single observer (Z.-M.M.) and presented as cells/mm2 of lamina propria or intraepithelial regions of duodenal mucosa [16,32,33] (Fig. 1).
Fig. 1.
Immunofluorescent antibody assay for T-lymphocyte populations in duodenal tissue.
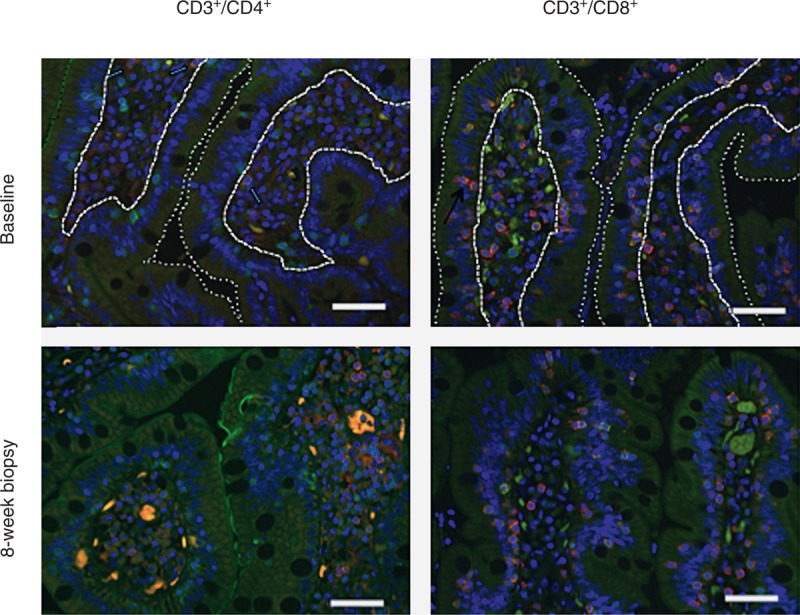
Representative examples from CD3+/CD4+ and CD3+/CD8+ staining before and after 8 weeks of bovine serum immunoglobulin (SBI) are shown. The dotted lines demark the intestinal lumen from the epithelial layer and the dashed lines demark the basement membrane of the epithelial layer from the lamina propria. Nuclei stained blue with 4’,6-diamidino-2-phenylindole (DAPI); background tissue pale green. Double-positive cells fluoresce a pale red-orange and are recorded as the number of positive cells per mm2 of lamina propria or epithelial zone. Slides were visualized with epifluorescent illumination using a Zeiss Axioplan 2 microscope (Carl Zeiss Inc., Thornwood, New York, USA) with a x40/0.75 plain neofluar objective (Carl Zeiss Inc.) and appropriate filters (Omega Optical, Brattleboro, Vermont, USA) at room temperature. Digital images were captured by using a QImaging Micropublisher 5.0 RTV camera (QImaging, Surrey, Canada) and Openlab software (Inprovision, Waltham, Massachusetts, USA). Five fields per section were randomly chosen. Color images were captured by only adjusting the exposure time without other modification. IFA, immunofluorescent antibody assay.
Measurement of markers of microbial translocation, inflammatory cytokines, and fibrosis/collagen kinetics
LPS was performed on serum obtained using pyrogen-free vacutainers as well as the standard serum separator and EDTA tubes for plasma with the Lonza LAL QCL-1000 kit (Lonza, Walkersville, Maryland, USA) according to the manufacturer's protocol in duplicate. Plasma LPS-binding protein (LBP) and bactericidal/permeability increasing protein (BPI; Uscn Life Science Inc., Missouri City, Texas, USA) were quantified by ELISA according to the manufacturer's protocol. Intestinal fatty acid binding protein (I-FABP; R&D Systems, Minneapolis, Minnesota, USA) was measured on serum diluted to 10% in sCD14 dilution buffer. Levels of proinflammatory (interleukin-1β [IL-1β], IL-6, tumor necrosis factor-α [TNF-α]), Th1 (interferon-γ [IFN-γ] and IL-12p70), Th2 (IL-4 and IL-10), and regulatory (IL-10) cytokines, chemokines (IL-8, and monocyte chemoattractant protein-1 [MCP-1], and biomarkers of collagen regulation (hyaluronic acid, transforming growth factor-β (TGF-β), matrix metalloproteinases-1 [MMP-1], MMP-9, tissue inhibitor of metalloproteinases-1 [TIMP-1], and TIMP-2) were all measured with multiplex enzyme-linked immunosorbent assay (ELISA)-based assays (MesoScale Discovery) except for hyaluronic acid (Corgenix, Broomfield, Colorado, USA).
Statistical approaches
Nonparametric statistical methods were used throughout. Wilcoxon matched-pairs signed rank test was used for assessing changes between baseline and postintervention results and Mann–Whitney test was used to compare participants with normal control samples. The Spearman rank correlation coefficient was used to study correlations between parameters. All values are represented as the median (interquartile ranges) unless otherwise stated. As a pilot project designed to explore hypotheses for a larger placebo-controlled trial, no adjustments for multiple comparisons were included [34]. Analyses were performed with GraphPad Prism Software V5.0 (GraphPad Software, Inc., La Jolla, California, USA).
Results
Participant demographics and clinical results
All eight participants were men (five white, three African–American) with a median age of 44.5 years (38.8–47.8) and a peripheral blood CD4+ T-cell count of 372 cells/μl (193–459). All had been on ART for more than 1 year, including four on protease inhibitor-based, three on nonnucleoside reverse transcriptase inhibitor-based, and one on integrase inhibitor-based regimens with consistently undetectable viral loads. Two participants had CD4+ T-cell counts below 200 cells/μl and were on chronic trimethoprim-sulfamethoxazole prophylaxis therapy. The specimens from controls were used from a study with similar procedures including duodenal biopsies and stool and blood collection reported previously [16].
All tolerated SBI well with excellent compliance initially, as determined by pill counts, which declined during the extension period. Ironically, the only ‘adverse event’ reported by participants was perceived constipation when stool frequency fell to once a day in two of the participants. Three were either lost to follow-up or did not have a rebound of symptoms after the washout period and five completed the 48 weeks of observation on SBI. Thus, EOT analysis is at week 48 for five participants and week 8 for three participants. All participants experienced a consistent improvement in symptoms after 2–3 weeks, and all five participants on chronic antimotility agents at baseline only used them sporadically at the most. Bowel movements/day decreased from 5.8 (5.0–8.4) to 2 (2–3.8; P = 0.008), stool consistency [1-formed to 6-watery] decreased from 5.3 (5–6) to 3 (2.2–3.8; P = 0.008), and the gastrointestinal questionnaire score decreased from 17 (15.6–21.8) to 8.0 (3.5–11.9; P = 0.008) at week 8 compared to baseline (Fig. 2). Participants who were experiencing urgency with episodes of soiling or night-time bowel movements reported complete resolution of such episodes. For those five who continued SBI to week 48, durable bowel movements/day, stool consistency, and questionnaire responses were reported at 2.0 (2.0–3.0), 2.5 (1.0–3.8), and 6.0 (2.0–12.8), respectively (all P = 0.062 compared to baseline).
Fig. 2.
Gastrointestinal questionnaire.
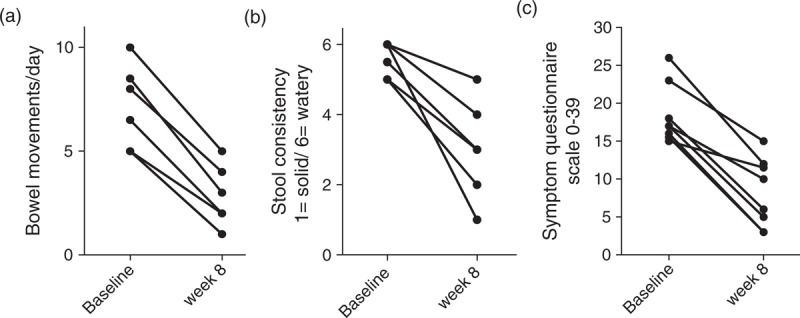
Participants responded to a validated questionnaire using a 1-week recall to record number of bowel movements/day (a), the typical – ‘most of the time’ – consistency of the stool using a pictorial scoring scale for solid [1] to watery [6] (b), and series of questions eliciting symptoms covering abdominal pain, urgency, incontinence, and nighttime bowel movements with a scoring scale that has a maximum of 39 points (c).
Intestinal permeability and absorptive function
Interestingly, as measured by the gold standard disaccharide absorption test, increased intestinal permeability was not observed in this cohort of participants. This is consistent with previous reports in HIV individuals without intestinal pathogens [30,35,36]. Gut permeability was normal before [0.024% (0.0–0.048)] and after [0.032% (0.0–0.047)] intervention (Fig. 3a). However, d-xylose urinary excretion, reflecting distal duodenal absorption function, increased in seven of eight participants with an overall increase from a median of 33.8 mg (28.7–38.2) at baseline to 40.9 mg (19.8–44.4) at week 7 (P = 0.19; Fig. 3b) [5].
Fig. 3.
Intestinal permeability and absorptive function.
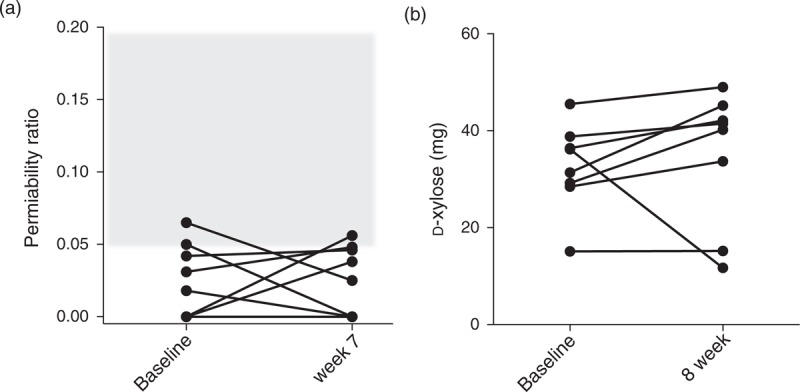
Disaccharide urine absorption assay was employed as the gold standard for intestinal permeability The gray area represents abnormal gut permeability. Except for two participants at the cut-off for normal values at each of the time points, results were unchanged before and after the intervention (a). d-Xylose absorption increased in seven of eight participants after 8 weeks of bovine serum immunoglobulin (SBI) (b).
Histopathology and immunologic responses
Hematoxylin and eosin stain demonstrated mild reduction of the villus to crypt ratio of 2.1 (normal ≥ 3.0) overall. Focal tip apoptosis and nonspecific chronic inflammation were noted in four of eight without a trend related to SBI administration. Peripheral CD4+ T-cell counts were unchanged at 339 cells/μl (210–468) after 8 weeks and 348 cells/μl (225–397) after 48 weeks. Both CD4+ and CD8+ T cells with the activated phenotype remained unchanged over the 8 weeks of the study: CD4+ T-cell activation – 6.6% (5.1–9.9) and 6.7% (4.2–12.9) and CD8+ T-cell activation – 20.1% (17.8–27.9) and 20.3% (17.8–29.2). By flow cytometry, duodenal tissue CD4+ T-cell percentage increased minimally in six of eight participants with a median increase of 1.2% (not significant) but remained unchanged overall at 15.9% (12.0–26.6) at baseline and 15.5% (13.9–30.2) at week 8. However, by IFA, lamina propria CD3+/CD4+ lymphocytes (CD4+ LPL) increased from 213 cells/mm2 (152–243) to 322 cells/mm2 (228–433; P = 0.016; Fig. 4a). This represented a rise of 139.5 cells/mm2 (66.1–216) after 8 weeks of SBI. Whereas the lamina propria CD3+/CD8+ (CD8+ LPL) density was unchanged [502 cells/mm2 (416–650) and 598 cells/mm2 (361–699)] at baseline and week 8, respectively, the lamina propria CD4+/CD8+ ratio increased from 0.41 (0.23–0.51) to 0.62 (0.34–0.99; P = 0.016; Fig. 4b). We previously reported the impact of 9 months of suppressive ART on CD4+ LPL density using identical methods wherein an increase from 100.0 cells/mm2 (72–145.5) pre-ART to 140.5 cells/mm2 (126.7–170.8) at month 9 was measured (P = 0.006) [16]. This represented an increase of 57 cells/mm2 (−9.0, 82) after 9 months of ART, which was significantly less than that observed in the participants receiving 8 weeks of SBI (Fig. 4c). To put these results in perspective, in a cohort of 12 control volunteers of similar demographics and lifestyle who have undergone identical procedures, we have observed CD4+ and CD8+ LPL densities of 748.8 cells/mm2 (510.6–998.5) and 270.8 cells/mm2 (226.7, 557.5), respectively [16].
Fig. 4.
Mucosal immune reconstitution.
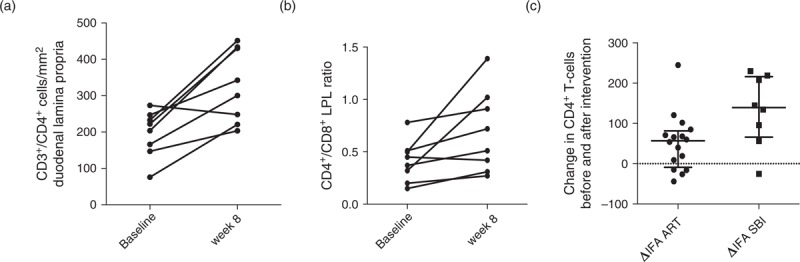
Immunofluorescent antibody assay (IFA) results for duodenal lamina propria CD4+ lymphocyte densities (a) and CD4+/CD8+ ratios (b) showed significant increases over the 8 weeks of bovine serum immunoglobulin (SBI) administration. Compared to a cohort of HIV individuals naive to antiretroviral therapy who were assessed for immune reconstitution in the duodenal lamina propria using identical methods, participants on SBI had a statistically significant more robust recovery of lamina propria lymphocytes. (c) Although these are clinically different cohorts of participants, the comparison provides a context to interpret the magnitude of the increase in mucosal CD4+ lymphocytes.
Biomarkers of inflammation, enterocyte damage, and collagen kinetics
Circulating LPS (irrespective of vacutainer type), IL-1β, IL-6, TNF-α, IFN-γ, IL-12p70, IL-4, IL-10, and IL-8 were similar to the levels of controls at baseline and were not changed at the EOT. Results from several parameters were available among those who continued for 48 weeks as described above. MCP-1 was higher in participants at baseline than controls [476.4 ng/ml (426.4–598.1) versus 336.6 ng/ml (307.9–400.8; P = 0.03)]. MCP-1 levels were unchanged at week 8, but decreased in five of five participants at week 48 [379.5 ng/ml (225–502) (P = 0.06); Fig. 5)].
Fig. 5.
Biomarkers of inflammation, enterocyte damage, and collagen kinetics.
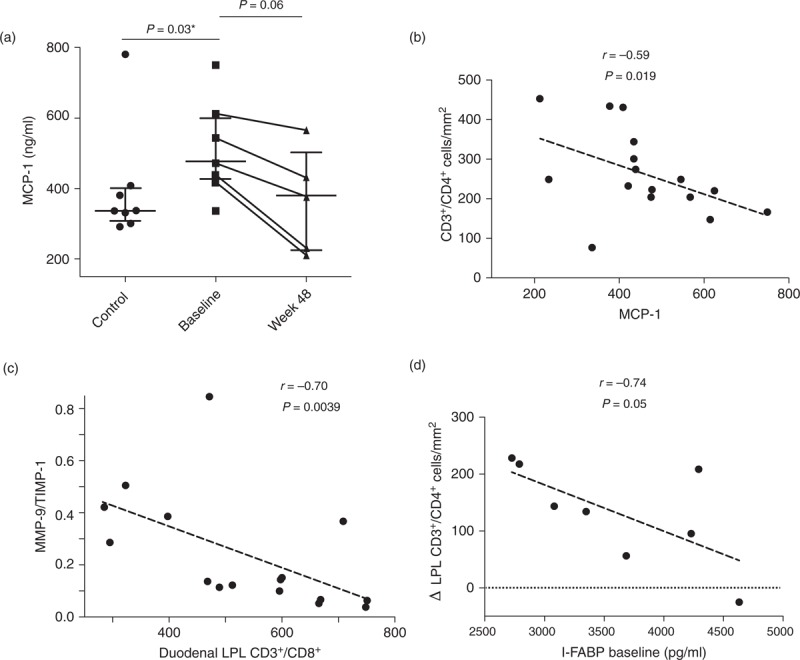
Monocyte chemoattractant protein-1 (MCP-1) was elevated at baseline in this cohort of chronically virologically suppressed and fell in the five participants who continued bovine serum immunoglobulin (SBI) for 48 weeks (a). MCP-1 levels negatively correlated with mucosal CD4+ lymphocyte density suggesting that common pathways influence systemic inflammation and mucosal immunity (b). Similarly, a negative correlation was observed between the biomarker of collagen kinetics, MMP-9/TIMP-1 ratios, and elevated concentrations of CD8+ lymphocytes in the duodenal lamina propria (c). Perhaps as expected, evidence for higher levels of epithelial/enterocyte damage at baseline correlated negatively with subsequent recovery of lamina propria CD4+ lymphocyte reconstitution following SBI administration (d).
We next examined I-FABP levels in serum. I-FABP reflects enterocyte damage, is elevated in the setting of HIV, and is negatively correlated with survival in recent HIV studies [37,38]. Unexpectedly, the values initially rose in seven of eight participants after 8 weeks from 3514 pg/ml (2858–4275) to 4042 pg/ml (3233–5613; P = 0.039) and then fell below baseline in four of the five who continued receiving SBI to 2442 pg/ml (1267–2875; P = 0.12 compared to baseline and 0.06 compared to week 8). Each of these timepoint results is significantly higher in comparison to HIV-negative controls [21.59 pg/ml (20.0–454.7) (P < 0.0001)].
Analysis of biomarkers of collagen kinetics provided potential mechanistic explanation for the observed increase in CD4 LPL. Although peripheral blood levels of TGF-β 1, 2, or 3, and hyaluronic acid were not different from those in the controls and did not decline following SBI administration, MMP-9/TIMP-1 ratios in participants were significantly lower than controls at baseline [0.13 (0.07–0.33) versus 0.42 (0.23–0.44) (P = 0.007)], respectively, and tended to increase at the EOT to 0.33 (0.13–0.73; P = 0.08). MMP-2/TIMP-2 levels were not different from those in controls at baseline and did not rise after SBI administration. MMP-2/TIMP-2 and MMP-9/TIMP-1 ratios regulate reabsorption/repair of collagen deposition and have been reported as abnormal in lymph nodes in HIV disease [39].
Finally, correlations were sought between lamina propria lymphocyte subset densities and these biomarkers of monocyte activation, enterocyte turnover, and collagen kinetics (Fig. 5). MCP-1 levels were negatively correlated to CD4+ LPL (r = −0.59, P = 0.019) with all time points examined together, suggesting that MCP-1 expression is associated with mucosal immunologic damage. Similarly, MMP-9/TIMP-1 ratios were negatively correlated with CD8+ LPL density (r = −0.70, P = 0.0039) suggesting that factors promoting CD8+ T-cell infiltration into the lamina propria correlate with impaired collagen kinetics. Finally, more severe baseline enterocyte damage blunted the magnitude of mucosal immunity recovery as baseline serum I-FABP levels were negatively correlated with subsequent rise in CD4+ LPL (r = −0.74, P = 0.046). These data support the hypothesis that HIV enteropathy presents in a spectrum of severity and that GALT CD4+ T-cell recovery is impaired in more severe enterocyte damage.
Discussion
Serum-derived bovine immunoglobulin was serendipitously discovered to reverse severe inflammatory diarrhea in animal husbandry and has been available as a medical food with GRAS FDA (generally regarded as safe – Food and Drug Administration) status since 2008 [40]. Extensive investigations have demonstrated that SBI has broad bacterial and viral antigen neutralizing capacity and reduces intestinal inflammation [23,25–27,41–46]. It has been tested in two clinical trials that confirmed its safety in humans [28,47]. This pilot, proof-of-concept clinical trial was designed to examine the impact of SBI on HIV enteropathy with the primary focus on gut immunologic and functional parameters. We observed a uniform improvement in stool frequency, consistency, and gastrointestinal related symptoms in all participants over the course of the 8-week intensive study with sustained improvements in those who entered the 48-week extension study. Notably, severe urgency, self-soiling, and nighttime bowel movements, indicative of high water-content stools, resolved during exposure to SBI and returned in a subset of participants during the 4-week ‘washout period’. The five participants who opted to continue the extension phase were those who reported return of symptoms (except for one who was subsequently lost to follow-up).
Unexpectedly, we did not observe increased intestinal permeability in this population of virologically suppressed patients. The urine disaccharide assays were performed in the same reference laboratory and methods as employed in the early reports of increased permeability in untreated AIDS patients and those with infectious diarrhea [30]. Sharpstone et al. reported lactulose/l-rhamnose mean ratios of 0.03 (SD ± 0.01) in their normal controls and 0.04 (SD ± 0.5) in their asymptomatic AIDS patients, which we reproduced in our cohort 0.03 (0.02), overall. However, they did report abnormal ratios (>0.05) in their AIDS patients with weight loss and pathogen-negative diarrhea (means of 0.14 and 0.15, respectively), which represent a very different population in the pre-HAART era than our cohort of chronically virologically suppressed patients [30]. Increased intestinal permeability is only one reason for increased bacterial translocation. Other conditions, such as bacterial overgrowth syndrome, demonstrate increased bacterial antigen translocation with normal intestinal permeability [48]. This report suggests that the mechanisms behind bacterial translocation in HIV patients are due to pathways other than increased macromolecule permeability.
The improved d-xylose absorption was modest but was observed in seven of eight participants after 7 weeks of SBI. Interestingly, the one participant who did not demonstrate increased absorptive function also did not show an increase in duodenal CD4+ T-cell density. Longer observation periods are needed to confirm the improved gut function with SBI administration.
This is the first intervention that demonstrates a substantial increase in duodenal GALT lymphocyte populations in HIV patients. Although the pathologic mechanisms underlying delayed CD4+ T-cell immune reconstitution in duodenal lamina propria are unknown, several authors have noted increased collagen staining in both intestinal and lymph node tissue [49–52]. The disrupted architecture may lead to disruption of critical cellular maturational interactions mediated by cytokine homeostasis [53]. In addition, an inverse correlation between cross-sectional area of collagen stained and CD4+ T-cell stained tissue in the small intestines has been observed [50]. The initiating events triggering the profibrotic pathways are believed to be a consequence of HIV infection itself and manifested by a broad range of cytokine-mediated immune activation including IL-6, TGF-β, and hyaluronic acid – all key contributors to collagen deposition [54–57]. Interestingly, in this cohort of virologically suppressed patients, we did not identify increased systemic levels of any of these or related inflammatory markers, including hsCRP or D-dimer (data not shown) when compared to a cohort of HIV-negative controls. However, the elevated levels of MCP-1 and depressed levels of MMP-9/TIMP-1 ratios describe an environment of monocyte inflammation and migration to the effector site of the lamina propria coupled with abnormal collagen kinetics. Wound repair, collagen deposition, and fibrosis are tightly regulated processes entailing multiple potential pathways with compensatory mechanisms for collagen reabsorption that is primarily performed by the matrix metalloproteinases and modulated by TIMP [58–60]. In addition, MCP-1 is one of several important regulators of fibrosis [61]. The persistence of abnormal levels of MCP-1 and MMP-9/TIMP-1 has been noted previously in HIV patients [39,62]. This persistence likely reflects events that are ongoing and uncontrolled in the setting of HIV suppressive therapy. Correlations between MCP-1 and CD4+ LPL and between MMP-9/TIMP-1 ratios and CD8+ LPL further suggest that these parameters are linked to sustained, uncontrolled antigenic stimulation at the gut mucosal interface. The observation that I-FABP increased in all participants acutely and then fell to below baseline in four of five participants suggests a biological explanation for the clinical improvements and increased absorption function. I-FABP is a marker for enterocyte damage that has been validated in coeliac disease response to a gluten-free diet. It is also related to repair and proliferation among other important cellular pathways [63,64]. The initial increase may reflect enterocyte repair and turnover following neutralization of damaging inflammatory bacterial antigen by SBI. The strong correlation between baseline I-FABP levels and subsequent recovery in CD4+ LPL following SBI administration is consistent with the hypothesis that neutralization of bacterial antigen promotes improved clinical, functional, and immunologic composition of the duodenal mucosal compartment. The absence of circulating CD4+ T-cell count increases likely reflects the complex architectural and functional abnormalities that will require a longer duration of treatment and observation to reverse. Longer duration studies are planned to test this hypothesis.
NexGen sequencing analysis of the gut microbiota is ongoing to demonstrate the impact of SBI on gut microbiota as well as gastrointestinal functional and immunologic parameters. Sustained reversal of ongoing profibrotic pathways and interruption of collagen repair in the setting of HIV disease are needed to restore the mucosal immune system to preinfection levels.
Acknowledgements
The authors would like to thank the team of scientists at EnteraHealth, Eric Weaver, PhD, and Gerald Klein, MD for their support and advice throughout the design, execution, and analysis of this project.
The authors are deeply indebted to the generosity and willingness of the volunteers, without whom this research would be impossible. They would like to thank the nurses and support personnel in the University of California CTSC Clinical Research Center, the Veterans Administration Hospital, and University of California Gastroenterology Suites for their dedication and assistance in this research effort.
They thank faculty and nursing staff of the Center for AIDS Research, Education and Services clinic for help in study participant recruitment.
D.M.A., T.H.K., T.Y., and E.T. contributed to study concept and design.
D.M.A., Z.-M.M., T.H.K., T.Y., T.H., T.T.W., C.J.M., and N.S. contributed to acquisition of data.
D.M.A., Z.-M.M., T.H.K., T.T.W., T.Y., N.M.F., E.T., D.D., and C.J.M. contributed to analysis and interpretation of data.
D.M.A., Z.-M.M., A.A., T.T.W., N.M.F., E.T., D.D., and C.J.M. contributed to drafting and critical revision of article.
D.M.A., T.Y., N.M.F., and E.T. contributed to study recruitment and supervision.
Clinical Trial Registry Number (Clinicaltrials.gov Identifier): NCT01313910.
This clinical trial was supported in part by a research grant from the Investigator-Initiated Studies Program of EnteraHealth, Inc. The opinions expressed in this paper are those of the authors and do not necessarily represent those of EnteraHealth, Inc.
This research was made possible by Grant Number UL1 RR024146 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health, and NIH Roadmap for Medical Research. The contents do not necessarily represent the views of the Department of Veterans Affairs or the United States Government.
Conflicts of interest
There are no conflicts of interest.
These data were presented in part at the 19th International AIDS Conference; 22–27 July, Washington DC, USA.
Correspondence to David M. Asmuth, MD, Professor of Medicine, Division of Infectious & Immunologic Diseases, UC Davis Medical Center, 4150 V Street, PSSB G500, Sacramento, CA 95817, USA. Tel: +1 916 734 8695; fax: +1 916 734 7766; e-mail: david.asmuth@ucdmc.ucdavis.edu
References
- 1.Jarry A, Cortez A, Rene E, Muzeau F, Brousse N. Infected cells and immune cells in the gastrointestinal tract of AIDS patients. An immunohistochemical study of 127 cases. Histopathology 1990; 16:133–140 [DOI] [PubMed] [Google Scholar]
- 2.Griffin GE. Malabsorption, malnutrition and HIV disease. Baillieres Clin Gastroenterol 1990; 4:361–373 [DOI] [PubMed] [Google Scholar]
- 3.Kotler DP, Gaetz HP, Lange M, Klein EB, Holt PR. Enteropathy associated with the acquired immunodeficiency syndrome. Ann Intern Med 1984; 101:421–428 [DOI] [PubMed] [Google Scholar]
- 4.Asmuth DM, Hammer SM, Wanke CA. Physiological effects of HIV infection on human intestinal epithelial cells: an in vitro model for HIV enteropathy. AIDS 1994; 8:205–211 [DOI] [PubMed] [Google Scholar]
- 5.Gillin JS, Shike M, Alcock N, Urmacher C, Krown S, Kurtz RC, et al. Malabsorption and mucosal abnormalities of the small intestine in the acquired immunodeficiency syndrome. Ann Intern Med 1985; 102:619–622 [DOI] [PubMed] [Google Scholar]
- 6.Palella FJ, Jr, Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr 2006; 43:27–34 [DOI] [PubMed] [Google Scholar]
- 7.Siddiqui U, Bini EJ, Chandarana K, Leong J, Ramsetty S, Schiliro D, et al. Prevalence and impact of diarrhea on health-related quality of life in HIV-infected patients in the era of highly active antiretroviral therapy. J Clin Gastroenterol 2007; 41:484–490 [DOI] [PubMed] [Google Scholar]
- 8.MacArthur RD, DuPont HL. Etiology and pharmacologic management of noninfectious diarrhea in HIV-infected individuals in the highly active antiretroviral therapy era. Clin Infect Dis 2012; 55:860–867 [DOI] [PubMed] [Google Scholar]
- 9.Veazey RS, Lackner AA. HIV swiftly guts the immune system. Nat Med 2005; 11:469–470 [DOI] [PubMed] [Google Scholar]
- 10.Mehandru S, Poles MA, Tenner-Racz K, Horowitz A, Hurley A, Hogan C, et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med 2004; 200:761–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 1998; 280:427–431 [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Rasmussen T, Pahar B, Poonia B, Alvarez X, Lackner AA, et al. Massive infection and loss of CD4+ T cells occurs in the intestinal tract of neonatal rhesus macaques in acute SIV infection. Blood 2007; 109:1174–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehandru S, Poles MA, Tenner-Racz K, Manuelli V, Jean-Pierre P, Lopez P, et al. Mechanisms of gastrointestinal CD4+ T-cell depletion during acute and early human immunodeficiency virus type 1 infection. J Virol 2007; 81:599–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehandru S, Poles MA, Tenner-Racz K, Jean-Pierre P, Manuelli V, Lopez P, et al. Lack of mucosal immune reconstitution during prolonged treatment of acute and early HIV-1 infection. PLoS Med 2006; 3:e484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guadalupe M, Reay E, Sankaran S, Prindiville T, Flamm J, McNeil A, et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol 2003; 77:11708–11717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asmuth DM, Ma ZM, Mann S, Knight TH, Yotter T, Albanese A, et al. Gastrointestinal-associated lymphoid tissue immune reconstitution in a randomized clinical trial of raltegravir versus nonnucleoside reverse transcriptase inhibitor-based regimens. AIDS 2012; 26:1625–1634 [DOI] [PubMed] [Google Scholar]
- 17.Gautreaux MD, Deitch EA, Berg RD. T lymphocytes in host defense against bacterial translocation from the gastrointestinal tract. Infect Immun 1994; 62:2874–2884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gautreaux MD, Deitch EA, Berg RD. Bacterial translocation from the gastrointestinal tract to various segments of the mesenteric lymph node complex. Infect Immun 1994; 62:2132–2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006; 12:1365–1371 [DOI] [PubMed] [Google Scholar]
- 20.Jiang W, Lederman MM, Hunt P, Sieg SF, Haley K, Rodriguez B, et al. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis 2009; 199:1177–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellis CL, Ma ZM, Mann SK, Li CS, Wu J, Knight TH, et al. Molecular characterization of stool microbiota in HIV-infected subjects by panbacterial and order-level 16S ribosomal DNA (rDNA) quantification and correlations with immune activation. J Acquir Immune Defic Syndr 2011; 57:363–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nofrarias M, Manzanilla EG, Pujols J, Gibert X, Majo N, Segales J, et al. Effects of spray-dried porcine plasma and plant extracts on intestinal morphology and on leukocyte cell subsets of weaned pigs. J Anim Sci 2006; 84:2735–2742 [DOI] [PubMed] [Google Scholar]
- 23.Pierce JL, Cromwell GL, Lindemann MD, Russell LE, Weaver EM. Effects of spray-dried animal plasma and immunoglobulins on performance of early weaned pigs. J Anim Sci 2005; 83:2876–2885 [DOI] [PubMed] [Google Scholar]
- 24.Moreto M, Perez-Bosque A. Dietary plasma proteins, the intestinal immune system, and the barrier functions of the intestinal mucosa. J Anim Sci 2009; 87:E92–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perez-Bosque A, Miro L, Polo J, Russell L, Campbell J, Weaver E, et al. Dietary plasma proteins modulate the immune response of diffuse gut-associated lymphoid tissue in rats challenged with Staphylococcus aureus enterotoxin B. J Nutr 2008; 138:533–537 [DOI] [PubMed] [Google Scholar]
- 26.Perez-Bosque A, Miro L, Polo J, Russell L, Campbell J, Weaver E, et al. Dietary plasma protein supplements prevent the release of mucosal proinflammatory mediators in intestinal inflammation in rats. J Nutr 2010; 140:25–30 [DOI] [PubMed] [Google Scholar]
- 27.Perez-Bosque A, Moreto M. A rat model of mild intestinal inflammation induced by Staphylococcus aureus enterotoxin B. Proc Nutr Soc 2010; 69:447–453 [DOI] [PubMed] [Google Scholar]
- 28.Begin F, Santizo MC, Peerson JM, Torun B, Brown KH. Effects of bovine serum concentrate, with or without supplemental micronutrients, on the growth, morbidity, and micronutrient status of young children in a low-income, peri-urban Guatemalan community. Eur J Clin Nutr 2008; 62:39–50 [DOI] [PubMed] [Google Scholar]
- 29.Bjarnason I, MacPherson A, Hollander D. Intestinal permeability: an overview. Gastroenterology 1995; 108:1566–1581 [DOI] [PubMed] [Google Scholar]
- 30.Sharpstone D, Neild P, Crane R, Taylor C, Hodgson C, Sherwood R, et al. Small intestinal transit, absorption, and permeability in patients with AIDS with and without diarrhoea. Gut 1999; 45:70–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shacklett BL, Yang O, Hausner MA, Elliott J, Hultin L, Price C, et al. Optimization of methods to assess human mucosal T-cell responses to HIV infection. J Immunol Methods 2003; 279:17–31 [DOI] [PubMed] [Google Scholar]
- 32.Li Q, Duan L, Estes JD, Ma ZM, Rourke T, Wang Y, et al. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 2005; 434:1148–1152 [DOI] [PubMed] [Google Scholar]
- 33.Vaccari M, Boasso A, Ma ZM, Cecchinato V, Venzon D, Doster MN, et al. CD4+ T-cell loss and delayed expression of modulators of immune responses at mucosal sites of vaccinated macaques following SIV(mac251) infection. Mucosal Immunol 2008; 1:497–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990; 1:43–46 [PubMed] [Google Scholar]
- 35.Bjarnason I, Sharpstone DR, Francis N, Marker A, Taylor C, Barrett M, et al. Intestinal inflammation, ileal structure and function in HIV. AIDS 1996; 10:1385–1391 [DOI] [PubMed] [Google Scholar]
- 36.Keating J, Bjarnason I, Somasundaram S, Macpherson A, Francis N, Price AB, et al. Intestinal absorptive capacity, intestinal permeability and jejunal histology in HIV and their relation to diarrhoea. Gut 1995; 37:623–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 2011; 203:780–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nowak P, Vesterbacka J, Barqasho B, Funaoka H, Kanda T, Gisslen M, et al. Persistent enterocyte damage despite decreased microbial translocation in patients on effective antiretroviral therapy. J Int AIDS Soc 2012; 15:18142 [Google Scholar]
- 39.Diaz A, Garcia F, Mozos A, Caballero M, Leon A, Martinez A, et al. Lymphoid tissue collagen deposition in HIV-infected patients correlates with the imbalance between matrix metalloproteinases and their inhibitors. J Infect Dis 2011; 203:810–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.FDA Bovine Globulin -Agency Response Letter GRAS Notice No. GRN 000255; 2008. http://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/ucm154991.htm [accessed 13 June 2013] [Google Scholar]
- 41.Peace RM, Campbell J, Polo J, Crenshaw J, Russell L, Moeser A. Spray-dried porcine plasma influences intestinal barrier function, inflammation, and diarrhea in weaned pigs. J Nutr 2011; 141:1312–1317 [DOI] [PubMed] [Google Scholar]
- 42.Perez-Bosque A, Amat C, Polo J, Campbell JM, Crenshaw J, Russell L, et al. Spray-dried animal plasma prevents the effects of Staphylococcus aureus enterotoxin B on intestinal barrier function in weaned rats. J Nutr 2006; 136:2838–2843 [DOI] [PubMed] [Google Scholar]
- 43.Perez-Bosque A, Pelegri C, Vicario M, Castell M, Russell L, Campbell JM, et al. Dietary plasma protein affects the immune response of weaned rats challenged with S. aureus Superantigen B. J Nutr 2004; 134:2667–2672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Campbell JM, Russell LE, Crenshaw JD, Weaver EM, Godden S, Quigley JD, et al. Impact of irradiation and immunoglobulin G concentration on absorption of protein and immunoglobulin G in calves fed colostrum replacer. J Dairy Sci 2007; 90:5726–5731 [DOI] [PubMed] [Google Scholar]
- 45.Corl BA, Harrell RJ, Moon HK, Phillips O, Weaver EM, Campbell JM, et al. Effect of animal plasma proteins on intestinal damage and recovery of neonatal pigs infected with rotavirus. J Nutr Biochem 2007; 18:778–784 [DOI] [PubMed] [Google Scholar]
- 46.Jiang R, Chang X, Stoll B, Fan MZ, Arthington J, Weaver E, et al. Dietary plasma protein reduces small intestinal growth and lamina propria cell density in early weaned pigs. J Nutr 2000; 130:21–26 [DOI] [PubMed] [Google Scholar]
- 47.Earnest CP, Jordan AN, Safir M, Weaver E, Church TS. Cholesterol-lowering effects of bovine serum immunoglobulin in participants with mild hypercholesterolemia. Am J Clin Nutr 2005; 81:792–798 [DOI] [PubMed] [Google Scholar]
- 48.Wiest R, Rath HC. Gastrointestinal disorders of the critically ill. Bacterial translocation in the gut. Best Pract Res Clin Gastroenterol 2003; 17:397–425 [DOI] [PubMed] [Google Scholar]
- 49.Diaz A, Alos L, Leon A, Mozos A, Caballero M, Martinez A, et al. Factors associated with collagen deposition in lymphoid tissue in long-term treated HIV-infected patients. AIDS 2010; 24:2029–2039 [DOI] [PubMed] [Google Scholar]
- 50.Estes J, Baker JV, Brenchley JM, Khoruts A, Barthold JL, Bantle A, et al. Collagen deposition limits immune reconstitution in the gut. J Infect Dis 2008; 198:456–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schacker TW, Nguyen PL, Beilman GJ, Wolinsky S, Larson M, Reilly C, et al. Collagen deposition in HIV-1 infected lymphatic tissues and T cell homeostasis. J Clin Invest 2002; 110:1133–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schacker TW, Reilly C, Beilman GJ, Taylor J, Skarda D, Krason D, et al. Amount of lymphatic tissue fibrosis in HIV infection predicts magnitude of HAART-associated change in peripheral CD4 cell count. AIDS 2005; 19:2169–2171 [DOI] [PubMed] [Google Scholar]
- 53.Schacker TW, Brenchley JM, Beilman GJ, Reilly C, Pambuccian SE, Taylor J, et al. Lymphatic tissue fibrosis is associated with reduced numbers of naive CD4+ T cells in human immunodeficiency virus type 1 infection. Clin Vaccine Immunol 2006; 13:556–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Estes JD, Wietgrefe S, Schacker T, Southern P, Beilman G, Reilly C, et al. Simian immunodeficiency virus-induced lymphatic tissue fibrosis is mediated by transforming growth factor beta 1-positive regulatory T cells and begins in early infection. J Infect Dis 2007; 195:551–561 [DOI] [PubMed] [Google Scholar]
- 55.Petitjean G, Chevalier MF, Tibaoui F, Didier C, Manea ME, Liovat AS, et al. Level of double negative T cells, which produce TGF-beta and IL-10, predicts CD8 T-cell activation in primary HIV-1 infection. AIDS 2012; 26:139–148 [DOI] [PubMed] [Google Scholar]
- 56.Wiercinska-Drapalo A, Flisiak R, Jaroszewicz J, Prokopowicz D. Increased plasma transforming growth factor-beta1 is associated with disease progression in HIV-1-infected patients. Viral Immunol 2004; 17:109–113 [DOI] [PubMed] [Google Scholar]
- 57.Zeng M, Smith AJ, Wietgrefe SW, Southern PJ, Schacker TW, Reilly CS, et al. Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. J Clin Invest 2011; 121:998–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med 1994; 331:1286–1292 [DOI] [PubMed] [Google Scholar]
- 59.Consolo M, Amoroso A, Spandidos DA, Mazzarino MC. Matrix metalloproteinases and their inhibitors as markers of inflammation and fibrosis in chronic liver disease (review). Int J Mol Med 2009; 24:143–152 [DOI] [PubMed] [Google Scholar]
- 60.Gieling RG, Burt AD, Mann DA. Fibrosis and cirrhosis reversibility: molecular mechanisms. Clin Liver Dis 2008; 12:xi915–xi937 [DOI] [PubMed] [Google Scholar]
- 61.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol 2008; 214:199–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sozzani S, Introna M, Bernasconi S, Polentarutti N, Cinque P, Poli G, et al. MCP-1 and CCR2 in HIV infection: regulation of agonist and receptor expression. J Leukoc Biol 1997; 62:30–33 [DOI] [PubMed] [Google Scholar]
- 63.Adriaanse MP, Tack GJ, Passos VL, Damoiseaux JG, Schreurs MW, van Wijck K, et al. Serum I-FABP as marker for enterocyte damage in coeliac disease and its relation to villous atrophy and circulating autoantibodies. Aliment Pharmacol Ther 2013; 37:482–490 [DOI] [PubMed] [Google Scholar]
- 64.Glatz JF, van der Vusse GJ. Cellular fatty acid-binding proteins: their function and physiological significance. Prog Lipid Res 1996; 35:243–282 [DOI] [PubMed] [Google Scholar]


