Abstract
Objective
Interleukin-6 (IL-6) is a pro-inflammatory cytokine that is associated with the production of C-reactive protein, the acute phase response, and chronic low-grade inflammation. In this report, we describe and validate a highly sensitive enzyme-linked immunosorbent assay (ELISA) for quantifying IL-6 at low concentrations in samples of capillary whole blood collected from a simple finger stick and dried on filter paper (dried blood spots, DBS).
Methods
A commercially available ELISA for IL-6 was modified to develop a protocol for the quantification of IL-6 in DBS samples. Procedures for sample elution and incubation were optimized for precision, reliability, accuracy, and lower limit of detection using small volumes of DBS. A set of 46 matched serum/DBS samples were used to evaluate agreement between serum and DBS results.
Results
The protocol demonstrated acceptable levels of precision, reliability, accuracy and agreement with serum-based results. The lower limit of detection was sufficiently low to measure levels of IL-6 associated with both chronic, low-grade inflammation and acute increases in inflammatory activity.
Conclusions
This protocol adds to the growing panel of analytes validated for quantification in DBS samples and should facilitate future research on the causes and consequences of inflammation in diverse non-clinical settings.
Keywords: interleukin-6, inflammation, immunoassay, laboratory methods
Interleukin-6 (IL-6) is a pro-inflammatory cytokine associated with the acute phase response and the differentiation of T- and B-cells. It is produced by antigen-presenting cells, endothelial cells, mast cells, and adipocytes, and is involved in the production of C-reactive protein (CRP), a generalized biomarker of acute and chronic inflammation (Esposito et al., 2003, Maachi et al., 2004, Yudkin et al., 2002). Previous research has established considerable variation in IL-6 across populations (McDade et al., 2011), and because of its relationship to chronic degenerative diseases (Bermudez et al., 2002, Ridker et al., 2000), IL-6 is of considerable interest to researchers investigating the physiological pathways linking social and physical environments, inflammation, and disease.
IL-6 is typically measured in serum using venipuncture-based blood collection that requires a phlebotomist, prompt processing of samples, and storage under temperature-controlled conditions; all impediments to research in community-based settings. Dried blood spots are a convenient, low-cost, and reliable method for collecting blood in non-clinical settings (McDade et al., 2007; Worthman and Stallings, 1997) The measurement of IL-6 poses an additional challenge: acute elevations in IL-6 are relatively easy to detect with existing immunoassays, but concentrations of IL-6 in healthy adults often range below 1 pg/mL (McDade et al. 2011). This level is at or below the lower limit of detection of most IL-6 immunoassays.
In this paper we present results of our validation of a highly sensitive immunoassay for the measurement of IL-6 in DBS samples. A method for quantifying IL-6 in DBS has been previously reported (Skogstrand et al., 2005), but the method has two limitations. First, it is based on a particle-based multiplex immunoassay platform which requires equipment that is very costly, not widely available, and less effective than other immunoassay platforms for detecting IL-6 (Thompson et al., 2012). Second, the lower limit of detection is reported to be 24 pg/mL, more than an order of magnitude higher than the level of detection necessary to quantify low levels of IL-6 activity of relevance to chronic disease processes. Our immunoassay method improves on these limitations to facilitate research on inflammation in diverse field-based settings.
PROTOCOL
Samples of capillary whole blood are collected by applying a sterile, single-use lancet to a finger cleaned with isopropyl alcohol. Up to five drops of blood are absorbed onto filter paper (Whatman #903, GE Healthcare, Piscataway, NJ) via capillary action, allowed to dry for at least 4 hours, and stored in air-tight plastic bags with desiccant (61161, VWR, Radnor, PA). Samples should be stored frozen (−20°C or lower) prior to analysis.
The method is based on a commercially available assay kit: Quantikine HS ELISA Human IL-6 (HS600B, R&D Systems, Minneapolis, MN), and assay reagents were prepared according to kit specifications. Calibration material specific to the DBS assay was made to account for the complex sample matrix of DBS samples. Serum calibrators were reconstituted with calibrator diluent and serially diluted in concentrations ranging from 50 pg/mL to 0 pg/mL. Each calibrator was combined in a 1:1 ratio with washed erythrocytes (McDade et al., 2004), gently mixed by hand or with a laboratory rotator, and 50 μL spotted on each card for final calibrator concentrations of 25, 10, 5, 2.5, 1.25, 0.625, 0.312, and 0 pg/mL.
The day before the assay, calibrators, controls, and unknown samples were eluted in a Millipore MultiScreen HTS Filter Plate (Millipore, MSHVN4510). Four 3.2-mm disks were removed from each spot using a hole punch and placed in each well, in duplicate, for a total of 8 disks. Samples were eluted by adding 100 μL in-house assay buffer (phosphate buffered saline, 0.1% Tween-20) to each well, making sure each disk was submerged. Plates were covered, placed on a non-wicking surface, and incubated overnight at 4°C.
The following day, the filter plate was stacked on top of the pre-coated anti-IL-6 assay plate provided with the kit and centrifuged for 2 minutes at 2,100 × g in a centrifuge equipped with a microtiter plate rotor (Eppendorf 5804, A-2-DWP), allowing eluate to pass through the filter into the assay plate. This elution protocol maximizes the recovery of sample since all material flows through the filter plate directly into the assay plate; no material is wasted due to pipetting, and filter paper discs are efficiently removed from the sample.
After incubation with shaking for 2 hours, the assay plate was washed 6 times with wash buffer. Wells were incubated with anti-IL-6 antibody conjugated to alkaline phosphatase, shaking at 550 rpm for two hours before washing 6 times. Samples were incubated with 50 μL NADPH substrate without shaking for one hour; 50 μL enzyme amplifier was added and incubated for 30 minutes for color development before the addition of stop solution. The plate was read at 450 nm (BioTek Elx 808); unknown samples were calculated from the 4-parameter best fit standard curve generated by the calibration materials (KC Junior, BioTek).
VALIDATION OF ASSAY PERFORMANCE
The lower limit of detection of the assay is the lowest concentration of IL-6 that can be distinguished from 0 with confidence. It was obtained by determining the IL-6 concentration that corresponded with the optical density value 2 SD above the mean value of 10 duplicates of the 0.00 pg/mL calibrator. The lower limit of detection of this assay is 0.67 pg/mL.
Linearity of dilution was assessed by eluting a sample with high concentrations of IL-6 and serially diluting in elution buffer (1:2, 1:4, 1:8, 1:16). The R2 for the serial dilution was 0.995. The ratio of observed values to expected values ranged from 0.80 to 1.25, with a mean ratio of 1.05.
The precision of the assay was calculated by the percent coefficient of variation (CV; SD/mean × 100) for 10 replicates each of samples with high, medium, and low IL-6 concentrations, all run within one assay. The within-assay CVs were 6.3%, 8.9%, and 14.6%, respectively. Reliability was assessed by running the same control samples across seven assays, each performed on a separate day. Between assay CVs were 9.4%, 9.5%, and 15.4%, respectively.
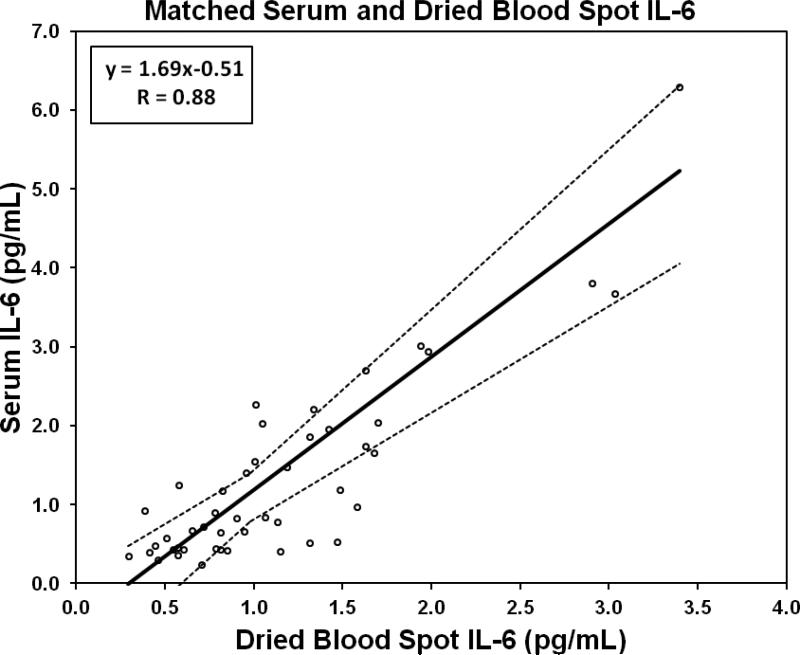
We compared IL-6 concentrations in a set of matched serum/DBS samples, collected from 46 individuals for the purposes of assay validation. Serum samples were analyzed by following the protocol provided with the commercial kit. The relationship between concentrations derived from serum and DBS samples was linear across the measurement range, with a moderate correlation using least-squares parametric regression (Pearson R = 0.88). We used Passing-Bablok regression (Passing & Bablok, 1983) to model the relationship between serum and DBS IL-6 concentrations (Figure 1).
Figure 1.
Passing-Bablok regression of matched serum and dried blood spot samples assayed for IL-6 (n = 46). Dotted lines represent 95% confidence intervals.
We generated two Bland-Altman plots to assess consistency between the serum and dried blood spot methods. Typically, Bland-Altman plots are derived by plotting the difference between matched measurements against their mean, and inspecting the mean difference and 95% limits of agreement for evidence of bias or inconsistent variability across the range of measurement (Bland and Altman, 1986). We converted DBS values to serum-equivalent values using the equation generated by the Passing-Bablok regression before calculating means and differences. For this plot, there was a consistent pattern of variation across the range of mean values, a bias (mean difference, serum equivalent - serum) of 0.10, and the 95% limits of agreement were −1.04 to 1.23. Three values fell outside the 95% limits of agreement with no consistent pattern to the distribution of these outliers. For the second Bland-Altman plot we plotted the ratio of serum values to DBS values against their mean and generated the 95% limits of agreement of the ratios. This plot showed a consistent pattern of variation across the mean value range, a bias (mean ratio, serum:DBS) of 1.098, and the 95% limits of agreement were 0.09 and 2.1. The bias is higher in this plot, as expected, because dried blood spots were not converted to serum equivalents before plotting; serum values are expected to be higher than unadjusted DBS values since erythrocytes are removed from serum samples following centrifugation. Three values fell outside of the 95% CI at the low end of the assay range (< 2 pg/mL), where there is less precision and reliability.
DISCUSSION
In this report, we have described and validated a method to assay IL-6 in DBS samples based on modifications to a commercially available ELISA kit. While this protocol uses relatively large amounts of sample relative to other DBS protocols (e.g. McDade et al., 2004), this amount is optimal for detecting very low levels of IL-6; initial evaluations (not reported) indicated that the addition of more sample did not significantly improve the lower limit of detection. The potential for calculated values to fall below the lower limit of detection is common in cytokine assays due to their low concentrations in circulation, and the use of the high sensitivity IL-6 assay kit maximizes our ability to detect IL-6 in DBS. The linear relationship between IL-6 in the matched serum and DBS samples is influenced by the lower reliability and precision at the bottom of the assay range, potentially reflecting limitations in current immunoassay technology. Previously, we developed and validated a method for quantifying CRP in DBS (McDade et al., 2004), and we hope that additional methods for quantifying inflammatory cytokines in DBS will promote further field-based research on the regulation of inflammation.
REFERENCES
- Bermudez EA, Rifai N, Buring J, Manson JE, Ridker PM. Interrelationships among circulating interleukin-6, C-reactive protein, and traditional cardiovascular risk factors in women. Arterioscler Thromb Vasc Biol. 2002;22(10):1668–1673. doi: 10.1161/01.atv.0000029781.31325.66. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310. [PubMed] [Google Scholar]
- Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A, Giugliano D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans. Circulation. 2002;106(16):2067–2072. doi: 10.1161/01.cir.0000034509.14906.ae. [DOI] [PubMed] [Google Scholar]
- Maachi M, Pieroni L, Bruckert E, Jardel C, Fellahi S, Hainque B, Capeau J, Bastard JP. Systemic low-grade inflammation is related to both circulating and adipose tissue TNF-α, leptin and IL-6 levels in obese women. Int J Obes. 2004;28(8):993–997. doi: 10.1038/sj.ijo.0802718. [DOI] [PubMed] [Google Scholar]
- McDade TW, Burhop J, Dohnal J. High-sensitivity enzyme immunoassay for C-reactive protein in dried blood spots. Clin Chem. 2004;50(3):652–654. doi: 10.1373/clinchem.2003.029488. [DOI] [PubMed] [Google Scholar]
- McDade TW, Williams S, Snodgrass JJ. What a drop can do: Dried blood spots as a minimally invasive method for integrating biomarkers into population-based research. Demography. 2007;44(4):899–925. doi: 10.1353/dem.2007.0038. [DOI] [PubMed] [Google Scholar]
- McDade TW, Tallman PS, Adair LS, Borja J, Kuzawa CW. Comparative insights into the regulation of inflammation: Levels and predictors of interleukin-6 and interleukin-10 in young adults in the Philippines. Am J Phys Anth. 2011;146(3):373–384. doi: 10.1002/ajpa.21586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passing H, Bablok W. A new biometrical procedure for testing the equality of measurements from two different analytical methods. Application of linear regression procedures for method comparison studies in clinical chemistry, Part I. J Clin Chem Clin Biochem. 1983;21:709–720. doi: 10.1515/cclm.1983.21.11.709. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342(12):836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- Skogstrand K, Thorsen P, Nørgaard-Pedersen B, Schendel DE, Sørensen LC, Hougaard DM. Simultaneous measurement of 25 inflammatory markers and neurotrophins in neonatal dried blood spots by immunoassay with xMAP technology. Clin Chem. 2005;51(10):1854–1866. doi: 10.1373/clinchem.2005.052241. [DOI] [PubMed] [Google Scholar]
- Thompson DK, Huffman KM, Kraus WE, Kraus VB. Critical appraisal of four IL-6 immunoassays. PLoS ONE. 2012;7(2):e30659. doi: 10.1371/journal.pone.0030659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthman CM, Stallings JF. Hormone measures in finger-prick blood spot samples: new field methods for reproductive endocrinology. American journal of physical anthropology. 1997;104(1):1–21. doi: 10.1002/(SICI)1096-8644(199709)104:1<1::AID-AJPA1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Yudkin JS, Stehouwer CDA, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: Associations with obesity, insulin resistance, and endothelial dysfunction: A potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19(4):972–978. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]