Abstract
Participation of adult cancer patients in US based clinical trials has remained near 3% for decades. Traditional research methodology reaches a small fraction of the target population with a fixed number of predetermined sites. Solutions are needed to ethically increase patient participation and accelerate cancer trial completion. We compared enrollment outcomes of traditional and patient focused research methodologies.
A patient prioritized method (Just-In-Time, JIT) was implemented in parallel with traditionally managed sites in three cancer trials. JIT research sites were initiated after candidate patients presented, while traditional sites were initiated in advance. JIT sites enrolled with mean rates no less than, and up to 2.75 fold greater than, traditional sites. Mean patients enrolled per site was comparable (JIT-1.82, traditional-1.78). There were fewer non-enrolling JIT sites (2/28, 7%) compared to traditional sites 19/52, 37%). This retrospective analysis supports JIT as a prospective solution to increase cancer clinical trial enrollment and the efficiency of clinical trial administrative activities.
Introduction
Therapeutic R&D and Cancer Patients: Why the Disconnect?
Today more than 11 million Americans are living with cancer or as cancer survivors.1 It is estimated that in 2020 there will be 18 million patients who have experienced cancer, at an annual cost of $158 billion.2–3 Progress in the treatment of cancer has been exceedingly slow, but the new paradigm of molecular targeted therapeutics has the potential to be game changing in medical oncology. Currently there are over 800 oncology drugs in development,4 yet only 3% of adult cancer patients participate in clinical trials.5–6 Cancer biology is moving rapidly forward, but the rate-limiting step to our ability to test clinical hypotheses is our inability to enroll clinical trials quickly.
When cancer patients were asked about their experiences participating in clinical trials, over 90 percent responded positively.7 However, the vast majority of patients are never offered clinical trial treatment opportunities. Realization of the medical and social potential of precision cancer medicine demands greater participation of physicians and patients. The outdated stigma of the patient as “guinea pig” needs to be eliminated.8–11 Clinical trial based treatment should be considered by each physician and patient as a balanced option in the care continuum, particularly when standard treatment has low curative probability. We describe an enrollment strategy for oncology clinical trials, designed to facilitate participation by both patients and physicians.
Challenges of Modernizing Clinical Trials in the US
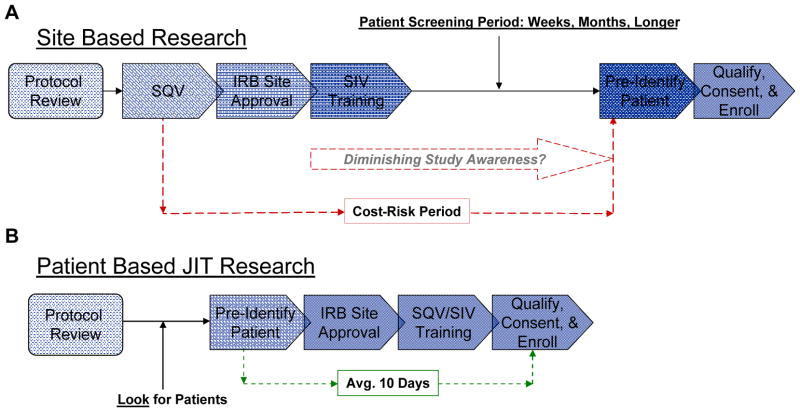
The molecular understanding of cancer is advancing rapidly, and a new generation of more effective, targeted cancer drugs are taking center stage in cancer care.4 Yet, our system for clinical testing of new agents has not kept pace with the revolution in cancer biology. Clinical research methodology has traditionally been a ‘one size fits all’ proposition, regardless of whether the indication being studied is common or rare. A fixed number of cancer research sites are selected, based on estimated enrollment potential, and undergo an extensive process of initiation prior to authorization to enroll subjects in the trial. The initial focus is administrative, prioritizing site documentation, with the focus on regulatory and procedural compliance (Figure 1A). With this approach, the medical and scientific research intents come into play only in select locations and only after ‘regulatory’ formalities are satisfied.12–13
Figure 1. Comparison of Clinical Trial Management Methodologies.
A – The traditional site based approach to clinical trials prioritizes site selection, typically through a pre-study site qualification visit (SQV), registration, institutional review board (IRB) and regulatory site approval, study specific training and site initiation visit (SIV). These administrative activities precede the goal of identifying and enrolling patients. B – The Just-In-Time methodology prioritizes patient identification, by a large group of capable investigators. Research administrative activities are delivered on an accelerated basis after a prospective research patient is identified and pre-qualified through standard of care assessments.
The administrative process takes 2–12 months per site,12–13 depending on the type of institution, at an estimated cost of $50,000 each,14 with no guarantee that patients will ever be enrolled at a given site.15 The process is not objective but interpretative, and procedural variability between trial sponsor, combined with duplication of effort across studies is highly inefficient for investigators.13 The site-based approach is not designed for efficient enrollment of subjects in a highly dispersed and segmented cancer population. Challenges of the traditional model are compounded by competition between trials in the same indications, low referral rates between practices, and procedural reimbursement between different levels of patient insurance and various payors.16–17 In cancer clinical trials, it is common that 20–30% of study centers never enroll a patient on a given study.13 Major advances in cancer care require greater efficiency in the way we approach clinical trials.
Patient Focused Solutions for Cancer Clinical Trials: A Landscape of Haystacks and Needles
Approximately 15% of cancer patients are treated at major institutions and tertiary referral centers. The research focus in institutions is on phase I and II trials, which tend to treat patients with advanced disease and fewer remaining clinical options. The remaining 85% of cancer patients are treated in thousands of private oncology practices and community or government hospitals.18 The US cancer population is highly fragmented, and most patients are not offered a clinical trial opportunity at any point in care. Up to 50% of private oncology practices conduct no trials.19 In contrast to other parts of the world, where adult cancer patient participation in clinical trials are higher,20 participation in the US has remained stagnant at 3% for over two decades.5–6 Despite much attention to the problem, 12,21 cancer trials proceed slowly in general and many are either not completed or become medically obsolete while in underway.22–23 The challenges mount when we consider the uniqueness of cancer as a research entity and the recent advances in cancer medicine.
Stratification by genotypic and phenotypic abnormalities further divides histological cancers into a myriad of clinically distinct diseases.24–25 Even common cancers, such as lung carcinoma, become arrays of rare subtypes when viewed through research selection criteria and molecular treatment criteria (Table 1). Rare cancers (< 40,000 annual cases) are further segmented into treatment indications of a few thousand patients.26 As the intent to treat population and predictability of patient presentation decrease, clinical trial enrollment becomes more difficult by traditional methods. A breakout solution is needed in clinical research methodology, to create a functioning, efficient means of developing new cancer drugs in the US.
Table 1.
Clinical Trials of Targeted Cancer Therapy Makes Many Cancers Rare
| Selection Criteria | Adjusted Study Population | Estd. Patients (US) |
|---|---|---|
| Non-Small Cell Lung Cancer | LC Incidence 222,000 Deaths 157,000 (12) NSCLC – 80% |
125,600 |
| Histological/Stage | Non squamous – 60% IIIb/IV = terminal First line – 80% (IIIb becomes IV) Measurable Disease -80% |
48,200 |
| Biomarker Specificity | EGFR over expression (non-squamous) (13) – 20% Kras mutation (14) – 30% |
2,900 |
| Risk Exclusions | Central or cavitory lesions – 70% Hemoptysis – 90% CNS metastases – 80% |
1,450 |
| General Selections | Organ function Performance status, etc. – 80% |
1,200 |
| Pancreatic Cancer | Incidence – 43,000 Deaths – 36,800 (12) |
36,800 |
| Histological/Stage | Inoperable, advanced or metastatic – 80% First line – 100% |
29,500 |
| Biomarker Specificity | SPARC – 80% (15) | 23,500 |
| Risk Exclusions | History of coagulopathy – 90% (first line) Anticoagulants – 50% |
10,600 |
| General Selections | Organ function Performance status, etc. – 60% |
6,400 |
Prioritizing Patients in Cancer Research: Aligning Form with Function
An efficient cancer research system requires: 1) the ability to identify rare patients within the care system and connect the right patients to the right clinical trials as a part of medical care; 2) research methods that appropriately support patient participation; and 3) maintaining research quality and proper oversight. By making trials more accessible and utilizing technology wisely, it should be possible to match cancer patients to appropriate clinical trials in real time and bring research opportunities into consideration for best clinical care. Cancer trials must align with the time limits of therapeutic selection, a matter of 2–4 weeks versus 3–6 months. With any solution, patient protection and full regulatory compliance are non-negotiable.
We report on a research methodology, developed to accelerate patient enrollment in cancer clinical trials involving community based practice.27–28 The method, called Just-InTime (JIT), is designed to leverage the collective patient population of many research ready practices and to identify individual patients who are appropriate candidates for specific clinical trials. Based on standard of care qualification review and patient interest, sites rapidly complete trial registration and initiation in time to formally consent and screen individual patients. JIT research is possible for research practices with the administrative flexibility to register quickly and use a central IRB. We hypothesize the JIT system may accelerate cancer trial enrollment rates and increase the probability that study sites enroll one or more patients on study. Retrospective data are presented from three clinical trial case studies in which the JIT system was implemented, and results are compared to traditionally managed sites in the same studies. The feasibility of implementing a more widespread Just-In-Time research system is considered.
Methods
A retrospective evaluation of three separate clinical trials which utilized a Just-In-Time (JIT) research methodology was conducted. The studies included industry sponsored IND trials for patients with pancreatic cancer, chronic myelogenous leukemia, and mantle cell lymphoma. JIT enrollment and site performance outcomes were compared to traditionally administered sites, which had been independently selected, activated, and managed by the respective study sponsors. As such, comparisons were not prospectively controlled for all potential factors affecting enrollment outcomes.
Traditional Site Management
Traditionally managed sites were selected and registered in the trials by the respective study sponsors, according to their standard operating procedures (SOP) (Figure 1A). Selected sites completed credentialing and documentation, contracting, and institutional review board (IRB) review according to sponsor standard operating procedure (SOP). Upon regulatory approval, traditional sites were supplied and initiated to begin enrolling patients. Ongoing site management, IRB approval and reporting, and site monitoring were the responsibilities of the respective study sponsors.
Just-In-Time Study Sites
Just-In-Time (JIT) sites were selected from research practices of the Pharmatech Oncology Research Network, based on scientific interest in the clinical trial and in the treatment option for prospective patients. Sites received advance training in conducting trials with the JIT methodology and support to pre-identify qualified patients using standard of care procedures (Figure 1B). A centrally supported research administration system was utilized to reduce site administrative workload and complete site registration, approvals and initiation, with a target of 10 business days from notice of a physician-patient interest to full site initiation to enroll. The investigator IRB review and approval time were shortened to 48 hours. No study related procedures were performed prior to site initiation and patient consent. Initiated JIT study centers functioned identically to traditional sites with regard to sponsor SOP and subsequent patient enrollment.
Data Analysis
Enrollment performance was determined on a site basis from the number of qualified patients enrolled by each study center initiated and the number of months that the site was authorized to enroll patients into the clinical trial. These data and total enrollment are reported as absolute counts. Non enrolling sites were included in the enrollment rate analysis and considered separately. Mean enrollment rates were determined as:
| Formula 1 |
Results
Study 1 – Pancreatic Cancer
NCT00116389 was an IND phase II clinical trial for patients with stage IV, chemo-naive pancreatic adenocarcinoma. Target enrollment was 60 patients. Based on entry criteria, the study population in the US was estimated to be 10,800, indicating that the study population is a rare cancer. The study sponsor selected 16 study centers and, at 13 months into the study, elected to open additional sites using the Just-In-Time (JIT) model as a prospective strategy to complete enrollment. A candidate patient identification group of 38 JIT capable sites was established from which 8 sites were initiated as study centers. The study was fully enrolled in 20 months (Table 2).
Table 2.
Clinical Trial Enrollment Performance - Pancreatic Cancer
| Parameter | JIT Sites | Traditional Sites |
|---|---|---|
| Patients enrolled | 20 | 42 |
| Months of enrollment | 7 | 20 |
| Sites Initiated | 8 | 16 |
| Non-enrolling sites | 0 | 2 |
| Patient accrual rate (pts/site*mo) | 0.357 | 0.131 |
Traditional sites enrolled 42 patients over a period of 20 months. JIT sites enrolled 20 patients in 7 months. The overall mean enrollment rates (patients/site-month) were 0.131 by traditional and 0.357 by JIT sites. The total patient enrollment by traditional sites was 2.1 times greater than JIT sites; however, the comparative enrollment rate of JIT sites was 2.72 times that of traditional sites. Mean total patients enrolled per site was identical, with 2.5 per traditional and 2.5 per JIT site. Twelve percent of traditional sites (2/16) failed to enroll one patient, while 0% of JIT sites (0/8) initiated failed to enroll.
Case Study 2 – Chronic Myelogenous Leukemia
NCT00574873 was an IND phase III clinical trial for patients with newly diagnosed, chronic phase, Philadelphia chromosome positive chronic myelogenous leukemia (CML). Target enrollment was 502 patients globally. Based on entry criteria, the study population in the US was estimated to be 3,250 annually, indicating a very rare cancer. The study sponsor selected 14 primarily institutional study centers, and 6 months into the study, opted for JIT as a means to enroll a proportion study subject from the community practice setting. A candidate patient identification group of 35 JIT ready sites was established, from which 14 were initiated. The study was fully enrolled in 23 months (Table 3).
Table 3.
Clinical Trial Enrollment Performance - Chronic Myelogenous Leukemia
| Parameter | JIT Sites | Traditional Sites |
|---|---|---|
| Patients enrolled | 23 | 17 |
| Months of enrollment | 17 | 23 |
| Sites Initiated | 14 | 14 |
| Non-enrolling sites | 0 | 4 |
| Patient accrual rate (pts/mo*site) | 0.097 | 0.053 |
Traditional sites enrolled 17 patients over a period of 23 months. JIT sites enrolled 23 patients in 17 months. Mean enrollment rates (patients/site-month) were 0.052 by traditional and 0.097 by JIT sites. The overall patient enrollment and comparative enrollment rates of JIT sites were 1.43 and 1.83 times greater than traditional sites respectively. Mean total patients enrolled per site was comparable (traditional = 1.21, JIT = 1.64). Twenty eight percent (4/14) of traditional sites failed to enroll one patient, while 0% (0/14) of JIT sites initiated failed to enroll.
Case Study 3 – Mantle Cell Lymphoma
NCT00891839 was a IND phase II clinical trial for patients with relapsed or treatment refractory mantle cell non-Hodgkins lymphoma (ongoing at submission), with a target enrollment of 52 patients. Based on entry criteria, the study population in the US was estimated to be approximately 5,000 annually. The study sponsor initiated 22 traditional sites, both institutional and private practice, and they undertook the JIT methodology as a means toward added enrollment. A candidate patient identification group of 27 JIT ready sites was established from which 6 sites were initiated. The study is ongoing and data are presented to date. (Table 4).
Table 4.
Clinical Trial Enrollment Performance - Mantle Cell Lymphoma
| Parameter | JIT Sites | Traditional Sites |
|---|---|---|
| Patients enrolled | 8 | 33 |
| Months of enrollment | 13 | 15 |
| Sites Initiated | 6 | 22 |
| Non-enrolling sites | 2 | 13 |
| Patient accrual rate (pts/mo*site) | 0.103 | 0.100 |
Traditional sites enrolled 33 patients over a period of 14.5 months. JIT sites enrolled 8 patients in 13 months. The overall enrollment of traditional sites was 4·1 times greater than JIT sites, in proportional to sites numbers by type; however the mean enrollment rates (patients/site-month) were 0.10 by traditional and 0.103 by JIT sites. Mean total patients enrolled per site was comparable (traditional = 1.5, JIT = 1.3). Fifty nine percent (13/22) of traditional sites failed to enroll one patient, while 33% (2/6) of JIT sites failed to enroll.
Discussion
Cancer incidence is projected to increase in the US 45% by 2030 and may double globally in the same time frame.29 Despite the clear need to accelerate the development of cancer medicines, the US struggles with very low patient participation in cancer trials and a systematic inefficiency in our cancer research infrastructure. As the molecular understanding of cancer and the clinical availability of precision therapeutics increasingly guide treatment selection, our capacity to readily evaluate new targeted drug candidates, particularly in combination, will depend on the ability to connect cancer patients to appropriate clinical trials studying highly selective molecular indications within specific clinical contexts. Research solutions are needed to increase patient access to clinical trials within the context of patients’ locally based cancer care.
Such solutions should encompass several key needs: 1) establish greater connectivity between cancer patients and clinical trials as component of care; 2) reduce the need for patients to travel to receive investigational treatments; 3) reduce the administrative burden associated with cancer trials; 4) align administrative tasks with patient care; and 5) accelerate trial enrollment with no compromise in the protection of research patients. A fundamental change in the cancer research system requires more participation of the 85% of patients in the community setting and systematic adoption of investigational treatment as a component of patient care. Setting a higher bar for administrative efficiency is required to meet the demands of patient focused clinical research and is expected to reduce the costs of conducting trials.
We report preliminary results obtained in three clinical trials that employed a patient directed research methodology known as Just-In-Time (JIT). The JIT system prioritizes identification of appropriate patients across a larger number of sites, versus administrative management of a fixed number of sites (Figure 1). This should result in a broader detection system, tuned for patient identification across a network of research sites and should enable the identification of many more candidate patients with a research indication than is possible using a fixed-site model. By placing patient identification foremost, the research challenge shifts to on-time delivery of clinical trial logistics to support individual patient care. The rate limiting issue is the amount of time that is acceptable for clinical staging and selection of therapy. Patients may not be subjected to administrative treatment delay in order to have access to a clinical trial. In the three studies evaluated, the activities associated with individual sponsor SOP for site selection, regulatory approval, and site initiation required a high level of collaboration between sponsor, CRO, IRB, and investigator30, but site initiation was consistently delivered within the 10 day target period. IRB review of research oversight and patient protection responsibilities and procedures has determined that the JIT methodology is fully compliant with CRF 21 Parts 50 and 56.31
The case studies presented here compare enrollment performance of the JIT methodology to traditionally managed cancer research sites. In each clinical trial, JIT sites enrolled with mean rates at least equivalent to, and up to 2.75 fold higher than, traditional sites. The aggregated enrollment rate for all three studies was 30% higher in favor of JIT (JIT 0.16, traditional 0.12). A potential economic concern expressed by study sponsors has been that JIT sites may fail to enroll the initial patient or enroll only one patient on study. We cannot rule this out, but aggregated results in these three trials demonstrate that JIT sites enrolled at least as many patients per site as traditional sites did (JIT 1.82, traditional 1.78). Only 7.1% of JIT sites (2 of 28 total), failed to enroll a patient in these three trials, in contrast to traditional sites, which had an aggregated non-performance rate 36.5% (19 of 52 total).
Financial analysis was not performed; however, the results suggest an efficiency of research resource utilization in favor of the JIT methodology. Prospective comparison of traditional and ‘pure’ JIT methodologies under ideal conditions was not possible here. In each trial, JIT implementation involved compromises in traditional sponsor SOP to accommodate JIT and modifications of JIT to meet sponsor SOP. The present results support the concept that patient focused clinical research methodologies such as JIT are clinically feasible and capable of accelerating clinical trial enrollment in studies of rare cancers, where subjects are challenging to recruit.
Conclusions
The JIT methodology is presently more applicable in community based practice than institutional research centers. This is primarily due to the institutional administrative process and the time required for local IRB review, which together often take 3–6 months to register for a trial. This time frame is simply not practical in the context of patient care. Our results suggest that the higher the potential for non-enrolling traditional sites, the higher the value of the JIT system. Conversely, in indications where there are many prospective patients and traditional sites enroll patients consistently, the JIT methodology may offer less value. The economic potential of JIT for administrative efficiency and putative cost savings for site participation need to be further explored. More thorough analyses of comparative enrollment performance, administrative efficiency, economic impact, and patient safety verification on study are ongoing. A major goal for development of the JIT research concept must be to increase patient access to cancer clinical trials as a treatment option in all stages of clinical care. The Just-In-Time methodology is proposed as one solution whereby investigators in community practice can offer their patients more investigational treatment options, by combining with many practices to identify rare cancer patients, connect them with appropriate clinical trials, and enabling cancer patients to access research based treatment while remaining in the community.
Acknowledgments
The project described was supported by Award Number R43CA144362 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Presented at DIA
Clinical Trials in the Age of Personalized Cancer Medicine: The Evolution of a More Efficient, Patient Focused Clinical Research System. DIA Annual Meeting; Philadelphia; 2010; June: 30691.
A Patient Focused Solution for Clinical Trial Enrollment
References
- 1.American Cancer Society. Cancer Facts & Figures 2011. Atlanta GA: American Cancer Society; 2011. [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, et al., editors. SEER Cancer statistics review, 1975–2008. Bethesda, MD: National Cancer Institute; http://seer.cancer.gov/csr/1975_2008/, based on November 2010 SEER data submission, posted to the SEER web site 2011. [Google Scholar]
- 3.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Nat Can Inst. 2011 Jan 19;103(2):117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schilsky RL, Allen J, Benner J, Sigal E, McClellan M. Commentary: Tackling the challenges of developing targeted therapies for cancer. Oncologist. 2010;15(5):484–7. doi: 10.1634/theoncologist.2010-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tejeda HA, Green SB, Trimble EL, et al. Representation of african americans, hispanics, and whites in National Cancer Institute cancer treatment trials. J Natl Cancer Inst. 1996;88:812–816. doi: 10.1093/jnci/88.12.812. [DOI] [PubMed] [Google Scholar]
- 6.Lara PN, Paterniti DA, Chiechi C, et al. Evaluation of factors affecting awareness of and willingness to participate in cancer clinical trials. J Clin Oncol. 2005;23:9282–9289. doi: 10.1200/JCO.2005.02.6245. [DOI] [PubMed] [Google Scholar]
- 7.Brief Summary of Harris Interactive Survey (2000) findings. Coalition of Cancer Cooperative Groups Publications; 2011. http://www.cancertrialshelp.org/CTHpdf/1226-9.pdf. [Google Scholar]
- 8.Friedman A, Cain DF. National Cancer Institute-sponsored cooperative clinical trials. Cancer. 1990;65(suppl 10):2376–2382. doi: 10.1002/1097-0142(19900515)65:10+<2376::aid-cncr2820651504>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 9.Go RS, Frisby KA, Lee JA, et al. Clinical trial accrual among new cancer patients at a community based cancer center. Cancer. 2006;106:426–433. doi: 10.1002/cncr.21597. [DOI] [PubMed] [Google Scholar]
- 10.Klabunde CN, Springer BC, Butler B, White MS, Atkins J. Factors influencing enrollment in clinical trials for cancer treatment. South Med J. 1999;92:1189–1193. doi: 10.1097/00007611-199912000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Comis RL, Colaizzi D, Miller JD. Cancer clinical trials (CCT) awareness and attitudes in cancer survivors (Ca surv). J Clin Oncol; 2006 ASCO Annual Meeting Proceedings (Post-Meeting Edition); p. 6061. [Google Scholar]
- 12.Dilts DM, Sandler AB. Invisible barriers to clinical trials: the impact of structural, infrastructural, and procedural barriers to opening oncology clinical trials. J Clin Oncol. 2006;24:4545–4552. doi: 10.1200/JCO.2005.05.0104. [DOI] [PubMed] [Google Scholar]
- 13.Transforming clinical research in the United States: Challenges and opportunities: workshop summary. Vol. 3. Washington (DC): Institute of Medicine (US) Forum on drug discovery, development, and translation, National Academies Press (US); 2010. http://www.ncbi.nlm.nih.gov/books/NBK50888/2011. [PubMed] [Google Scholar]
- 14.Handelsman D. Optimizing Clinical Research Operations with Business Analytics. 2011 SAS Global Forum Proceedings. 2011:204. [Google Scholar]
- 15.Durivage HJ, Bridges KD. Clinical trial metrics: Protocol performance and resource utilization from 14 cancer centers. J Clin Oncol. 2009;27(15s):6557. [Google Scholar]
- 16.Fleming R. Barriers to clinical trials. Part I: Reimbursement problems. Cancer. 1994;74:2662–2665. doi: 10.1002/1097-0142(19941101)74:9+<2662::aid-cncr2820741813>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 17.National Cancer Institute, Advisors BoS. Report of the National Cancer Institute Clinical Trials Program Review Group. Bethesda (MD): National Cancer Institute; 1997. Aug, [Google Scholar]
- 18.Cohen GI. Cancer clinical trials; A primer for participation of community physicians. Am Soc Clin Oncol. 2002:283–289. [Google Scholar]
- 19.Klabunde CN, Keating NL, Potosky AL, et al. A population-based assessment of specialty physician involvement in cancer clinical trials. J Natl Cancer Inst. 2011;103:1–14. doi: 10.1093/jnci/djq549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cameron D, Cooper M, Haward B, et al. Four-fold increase in recruitment of cancer patients to NCRN portfolio studies between 2001 and 2010: A tale of investment bringing returns. National Cancer Research Institute Cancer Conference. 2010 [Google Scholar]
- 21.Dilts DM, Sandler AB, Baker M, et al. Processes to activate phase III clinical trials in a cooperative oncology group: The case of Cancer and Leukemia Group B. J Clin Oncol. 2006;24:4553–4557. doi: 10.1200/JCO.2006.06.7819. [DOI] [PubMed] [Google Scholar]
- 22.Markman M. The clinical trials cooperative group program: Time for a painful reality check. Clin Oncol News. 2011;06(05) http://www.clinicaloncology.com/ViewArticle.aspx?d=Clinical%2BTrials&d_id=165&i=May%2B2011&i_id=728&a_id=17089&ses=ogst2011. [Google Scholar]
- 23.Cheng SK, Dietrich MS, Dilts DM. A sense of urgency: Evaluating the link between clinical trial development time and the accrual performance of cancer therapy evaluation program (NCI-CTEP) sponsored studies. Clin Cancer Res. 2010;16:5557–5563. doi: 10.1158/1078-0432.CCR-10-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stewart DJ, Kurzrock R. Cancer: The road to Amiens. J Clin Oncol. 2009;27 (3):328–333. doi: 10.1200/JCO.2008.18.9621. [DOI] [PubMed] [Google Scholar]
- 25.Braiteh F, Kurkrock R. Uncommon tumors and exceptional therapies: Paradox or paradigm? Mol Cancer Ther. 2007;6:1175–1179. doi: 10.1158/1535-7163.MCT-06-0674. [DOI] [PubMed] [Google Scholar]
- 26.Mikhail IS. 2nd NCI Epidemiology Leadership workshop: Understudied Rare Cancers. 2005. Sep, Design issues in the study of rare cancers. [Google Scholar]
- 27.Wiener MB, Newman HM, Spradley EA. Revolutionizing oncology patient enrollment in clinical trials: Just-in-time approach. Journal of Clinical Oncology 2007 ASCO Annual Meeting Proceedings Part I. 2007;25(18S):6577. [Google Scholar]
- 28.Lynam EB. Clinical Trials in the Age of Personalized Cancer Medicine: The Evolution of a More Efficient, Patient Focused Clinical Research System. DIA Annual Meeting Proceedings; 2010; June; p. 30691. [Google Scholar]
- 29.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: Burdens upon an aging, changing nation. J Clin Oncol. 2009;27(17):2758–2765. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 30.Patterson F, Gevorkian S. Challenging Oncology Indication and the Just-In-Time (JIT) Approach: A Case Study of Collaboration Between SMO and Sponsor. 3rd Annual Patient Recruitment in Clinical Trials Symposium; 2010; Mar. [Google Scholar]
- 31.Baker M. Protecting Patient Rights and Regulatory Compliance in a Patient-directed Clinical Research. DIA Annual Meeting Proceedings; 2010; June; p. 30691. [Google Scholar]