Abstract
Chronic pain conditions are associated with abnormalities in brain structure and function. Moreover, some studies indicate that brain activity related to the subjective perception of chronic pain may be distinct from activity for acute pain. However, the latter are based on observations from cross-sectional studies. How brain activity reorganizes with transition from acute to chronic pain has remained unexplored. Here we study this transition by examining brain activity for rating fluctuations of back pain magnitude. First we compared back pain-related brain activity between subjects who have had the condition for ∼2 months with no prior history of back pain for 1 year (early, acute/subacute back pain group, n = 94), to subjects who have lived with back pain for >10 years (chronic back pain group, n = 59). In a subset of subacute back pain patients, we followed brain activity for back pain longitudinally over a 1-year period, and compared brain activity between those who recover (recovered acute/sub-acute back pain group, n = 19) and those in which the back pain persists (persistent acute/sub-acute back pain group, n = 20; based on a 20% decrease in intensity of back pain in 1 year). We report results in relation to meta-analytic probabilistic maps related to the terms pain, emotion, and reward (each map is based on >200 brain imaging studies, derived from neurosynth.org). We observed that brain activity for back pain in the early, acute/subacute back pain group is limited to regions involved in acute pain, whereas in the chronic back pain group, activity is confined to emotion-related circuitry. Reward circuitry was equally represented in both groups. In the recovered acute/subacute back pain group, brain activity diminished in time, whereas in the persistent acute/subacute back pain group, activity diminished in acute pain regions, increased in emotion-related circuitry, and remained unchanged in reward circuitry. The results demonstrate that brain representation for a constant percept, back pain, can undergo large-scale shifts in brain activity with the transition to chronic pain. These observations challenge long-standing theoretical concepts regarding brain and mind relationships, as well as provide important novel insights regarding definitions and mechanisms of chronic pain.
Keywords: chronic back pain, fMRI, longitudinal, emotion, reward
Introduction
Chronic pain imparts a large socioeconomic burden [Institute of Medicine of the National Academies (www.iom.edu) states that chronic pain affects at least 100 million American adults, costing up to $635 billion each year]. Extensive human and animal evidence shows that it is associated with PNS and CNS reorganization (Apkarian et al., 2009, 2011; Costigan et al., 2009; Tracey and Bushnell, 2009). Human brain imaging studies indicate that different chronic pain syndromes exhibit distinct brain activity and functional/morphological alterations (Geha et al., 2008a; May, 2008; Staud et al., 2008; Apkarian et al., 2009, 2011, Baliki et al., 2011a; Weissman-Fogel et al., 2011; Farmer et al., 2012) and that chronic pain also alters brain dynamics by changing brain resting state interactions between networks implicated in default states, attention, salience and reward (Baliki et al., 2008a, 2011b; Cauda et al., 2009; Malinen et al., 2010; Napadow et al., 2010; Tagliazucchi et al., 2010). Thus, it is evident that changes in brain function and structure correlate with chronic pain. Yet, these studies are cross-sectional or retrospective, and as a result the relationship between brain reorganization and the onset and maintenance of chronic pain has remained unknown.
The core clinical issue is that only a fraction of subjects who experience an acute painful injury develop chronic pain, and although many clinical studies have searched for parameters predicting pain chronification, no consistent behavioural, psychological or neurobiological factors have emerged (Chou and Shekelle, 2010). Human brain imaging studies have identified potential anatomical and functional biomarkers that differentiate chronic pain from healthy subjects (Apkarian et al., 2009, 2011; Farmer et al., 2012), yet definitive evidence required to inter-relate brain states and chronic pain requires repeated longitudinal observations of individuals throughout the period of transition from acute to chronic pain.
Cross-sectional functional MRI studies indicate preferential involvement of brain emotional and limbic circuitry in encoding fluctuations of ongoing pain for various chronic pain conditions (Baliki et al., 2006, 2010; Geha et al., 2007, 2008b; Farmer et al., 2011; Parks et al., 2011; Hashmi et al., 2012). Specifically in chronic back pain (CBP, back pain persisting >6 months) we have shown that rating of spontaneous pain primarily activates the medial prefrontal cortex (Baliki et al., 2006, 2010; Hashmi et al., 2012). In contrast, acute painful stimuli (mechanical or thermal) applied in healthy subjects gives rise to a consistent pattern of activity that engages, at least, multiple sensorimotor regions, bilateral insula, thalamus, basal ganglia, and dorsal anterior cingulate cortex (Apkarian et al., 2005). Moreover, in CBP we have shown a double-dissociation between brain regions activated for acute thermal painful stimuli and areas activated for rating spontaneous fluctuations of ongoing pain (Baliki et al., 2006, 2010).
Given these observations, we expected that when back pain is acute or subacute (SBP, back pain persisting for <3 months) the experienced pain is preferentially driven by acute/nociceptive mechanisms and related brain activity should be more similar to brain activity seen for acute pain in healthy subjects; in contrast, this activity should be dissimilar from brain activity for back pain in CBP. Additionally, we hypothesized that a spatiotemporal dynamical reorganization of brain activity accompanies the transition to chronic pain, during which the representation of back pain in time shifts away from sensory regions and gradually engages emotional and limbic structures.
To test these hypotheses, we conducted a combined cross-sectional and longitudinal anatomical and functional brain imaging study in a cohort of subjects with an episode of SBP (back pain persisting for at least 4 weeks, with no prior back pain experience for at least 1 year). SBP participants were followed over a period of 1 year as they either recovered or transitioned into chronic pain. We followed brain properties of back pain for 1 year, as (i) it is one of the most prevalent clinical conditions that becomes chronic; and (ii) functional and anatomical reorganization, as well as their partial reversal with adequate therapy, are best characterized in this condition (Apkarian et al., 2004; Baliki et al., 2006, 2011a; Seminowicz et al., 2011). Recently we reported on the anatomical and related functional connectivity changes with chronification from this longitudinal study (Baliki et al., 2012). Here, we compare brain activity for back pain between SBP with participants who have been suffering with CBP for >10 years. We also assessed longitudinal changes in back pain-related brain activity in SBP as participants either recovered or persisted to transition to chronic pain.
To directly test the assumption that early and later, or chronic, stages of back pain may be preferentially associated with acute pain versus emotion or reward/aversion circuitry, we used the Neurosynth framework (neurosynth.org), which combines text-mining, meta-analysis, and machine learning techniques to generate probabilistic maps for cognitive constructs (Yarkoni et al., 2011) based on forward or reverse inference statistics. We used maps derived from Neurosynth for the terms pain, emotion and reward, and we examined the overlap between these meta-analytic maps and back pain-related maps across the different groups, and at different times from inception of back pain.
Materials and methods
Participants
Data presented in this manuscript are part of an ongoing study in which we examine longitudinal changes in brain structure and function in patients with SBP as they transition into persistence or recovery. One hundred and twenty patients with SBP were recruited into the study where subjects were scanned over a period of 1 year, at four separate visits. At visit 1, 94 patients with SBP participated in the functional MRI scans (48 females; age: mean = 42.09, SEM = 1.15 years). At the time of this report, 47 patients with SBP had complete data for the four brain scans. Out of the 47 subjects, eight subjects were excluded due to missing data points or excessive head motion artefacts. Two subjects did not have functional scans for visit 2, one subject did not have functional scan for visit 3, two subjects did not have spontaneous pain ratings and three subjects had excessive head motion artefacts (head motion >10 mm). It is important to note that out of the 39 patients used in this study, 30 patients were from the same data set that were recently used to track anatomical and functional brain properties in pain chronification (Baliki et al., 2012). In addition, 31 patients with CBP were recruited for this study, and their data were combined with additional functional MRI data collected in patients with CBP (n = 38) from two of our earlier studies (Baliki et al., 2010; Hashmi et al., 2012). Healthy subjects were also recruited into the longitudinal study, but these data were not used in the current analysis. Overall, scans collected in patients with SBP at visit 1 (designated as early or early SBP; n = 94) were compared with scans collected in patients with CBP (back pain for >10 years, n = 59) in a cross-sectional analysis. In addition, scans from 39 patients with SBP who had completed all four scans were analysed for longitudinal changes.
The definition of chronic pain remains arbitrary and operational. For back pain, 0–7 days of pain is considered acute, 7 days to 3 months is classified as subacute, and >3 months is categorized as chronic pain (Frank, 1993). We recruited subjects with SBP who reported a single intense episode of back pain lasting 4–16 weeks and no prior back pain for at least 1 year, performed brain scans as soon as possible (mean ± SEM pain duration from injury at visit 1 = 9.14 ± 0.48 weeks) and followed their pain and mood parameters, as well as brain activity, over three additional visits for the next year (visit 2: 7.15 ± 0.26 weeks; visit 3: 29.20 ± 0.63 weeks; visit 4: 54.36 ± 2.14 weeks; from visit 1).
All participants were right-handed and gave informed consent to procedures approved by the Northwestern University Insistutional Review Board committee. Subjects were recruited by newspaper or internet advertisements in the Chicago city area. All patients were diagnosed by a clinician and fulfilled the International Association for the Study of Pain criteria for back pain. An additional list of criteria was imposed, including the following: for SBP, back pain intensity >40/100 on the Visual Analogue Scale and duration < 16 weeks; and for CBP, back pain intensity > 40/100 on the Visual Analogue Scale and duration > 6 months. Subjects were excluded if they reported other chronic painful conditions, systemic disease, history of head injury or coma, psychiatric diseases, or more than mild to moderate depression (Beck Depression Inventory score > 19). For demographics see Tables 1 and 3.
Table 1.
Demographics, pain and mood parameters for patients with CBP and early SBP
| CBP | Early SBP | CBP > early SBP, t-score (P-value) | |
|---|---|---|---|
| Number of subjects | 59 | 94 | |
| Age | 48.8 ± 1.2 | 42.1 ± 1.15 | 3.81 (P < 0.01) |
| Gender | 25 females (42.4%) | 48 females (51.1%) | – |
| Duration | 13.5 ± 1.3 years | 9.14 ± 0.48 weeks | 14.91 (P < 0.01) |
| VAS | 69.58 ± 2.61 | 58.25 ± 1.95 | 3.67 (P < 0.01) |
| MPQ sensory | 15.9 ± 0.78 | 11.2 ± 0.62 | 4.36 (P < 0.01) |
| MPQ affective | 5.29 ± 0.46 | 3.04 ± 0.41 | 3.4 (P < 0.01) |
| MPQ radiculopathy | 4.61 ± 0.31 | 4.90 ± 0.21 | −0.82 (P = 0.41) |
| BDI | 7.30 ± 0.61 | 6.53 ± 0.61 | 0.87 (P = 0.38) |
| NPS | 52.81 ± 2.22 | 40.32 ± 1.81 | −3.91 (P < 0.01) |
BDI = Beck Depression Index; MPQ = McGill Pain Questionnaire; NPS = Neuropathic Pain Scale; PANAS = Positive Affect Negative Affect Scale; VAS = Visual Analogue Scale.
*P < 0.05 **P < 0.01, unpaired t-test. Data presented as mean ± SEM.
Table 3.
Pain and mood parameters and differences between and within persisting SBP and recovering SBP over 1 year
| Visit 1 | Visit 4 | |||||
|---|---|---|---|---|---|---|
| Persisting SBP (mean ± SEM) | Recovering SBP (mean ± SEM) | Persisting SBP > recovering SBP (t-score) | Persisting SBP (mean ± SEM) | Recovering SBP (mean ± SEM) | Persisting SBP > recovering SBP (t-score) | |
| VAS (0–100) | 57.61 ± 4.12 | 52.93 ± 4.33 | 0.78 | 53.61 ± 6.13 | 27.77 ± 5.10 ↓ | 5.94** |
| MPQ sensory | 12.42 ± 1.59 | 9.66 ± 1.05 | 1.43 | 11.50 ± 1.42 | 5.65 ± 1.37 ↓ | 2.96** |
| MPQ affective | 3.05 ± 0.61 | 1.43 ± 0.51 | 1.44 | 3.20 ± 0.69 | 0.89 ± 0.42 ↓ | 3.43** |
| MPQ radiulopathy | 5.6 ± 0.49 | 4.30 ± 0.50 | 1.80 | 5.30 ± 0.90 | 3.50 ± 0.73 ↓ | 2.48* |
| NPS | 47.71 ± 4.25 | 34.4 ± 3.44 | 2.55* | 42.00 ± 4.78 | 16.53 ± 2.98 ↓ | 5.14** |
| BDI | 6.45 ± 1.01 | 6.55 ± 1.32 | −0.05 | 6.06 ± 1.46 | 3.54 ± 1.17 ↓ | 1.25 |
| PANAS positive | 33.05 ± 8.05 | 32.97 ± 1.89 | 0.03 | 29.73 ± 6.69 | 34.42 ± 1.90 | −1.9* |
| PANAS negative | 19.4 ± 1.80 | 16.63 ± 1.44 | 1.32 | 20.07 ± 1.54 | 15.26 ± 2.39 ↓ | 2.40* |
| Duration (weeks) | 12.4 ± 1.11 | 12.15 ± 1.08 | 0.15 | 65.93 ± 1.22 | 68.80 ± 1.10 | −0.70 |
| Mean pain variance | 10.8 ± 3.29 | 10.8 ± 4.57 | 0.01 | 6.2 ± 1.89 | 1.52 ± 0.49 ↓ | 2.30* |
| MQS | 1.91 ± 0.60 | 2.62 ± 0.68 | −0.71 | 3.71 ± 0.99 | 4.10 ± 0.99 | −0.25 |
| MAD | 0.53 ± 0.41 | 0.51 ± 0.58 | 0.10 | 0.67 ± 0.53 | 0.41 ± 0.42 | 1.7 |
Clinical pain and mood parameters for persisting SBP (n = 21) and recovering SBP (n = 18) at visit 1 (within weeks from entry into study) and visit 4 (1 year after entry into study). Significant changes between visit 1 and visit 4 (paired t-test, P < 0.01) are displayed as increases (↑), or decreases (↓).
BDI = Beck Depression Index; MAD = mean absolute displacement (motion); MPQ = McGill Pain Questionnaire; MQS = Medication Quantification Scale; NPS = Neuropathic Pain Scale; PANAS = Positive Affect Negative Affect Scale; VAS = Visual Analogue Scale.
*P < 0.05 **P < 0.01, unpaired t-test. Data presented as mean ± SEM.
Pain and mood parameters
For all visits, patients with SBP completed the short-form of the McGill Pain Questionnaire. The main components of the McGill Pain Questionnaire are 12 sensory and four affective descriptors, which are used to compute the sensory and affective scores, respectively. Radiculopathy scores were quantified from pain locations based on the body regions that patients had shaded in with pencil on the McGill Pain Questionnaire form. The McGill Pain Questionnaire form also includes a visual analogue scale (0 = no pain, 100 = maximum imaginable pain) and pain duration. In addition, patients with SBP completed the Positive Affect Negative Affect Score (PANAS), which includes 60 items and measures the two original higher order scales for positive and negative affect. Depression scores for all subjects were assessed using the Beck Depression Inventory. All questionnaires were given 1 h before brain scanning.
Thirty-four patients primarily used acetaminophen and non-steroidal anti-inflammatory drugs (ibuprofen, Motrin, Aleve, Naproxen, Tylenol). Six patients also used opiates (Vicodin or Precocet). One subject used epidural steroid shots (Tramadol), serotonin–norepinephrine reuptake inhibitors (Effexor and pregabalin) and muscle relaxants (cyclobenzaprine). Five patients received no treatment. Patients were subdivided into early (treatment commencement before visit 1) or late drug (treatment commencement post visit 1) groups. Drug consumption at each visit was quantified using the Medication Quantification Scale, which computes a scalar value representation of dosage and duration of drug use.
Experimental tasks
Participants were trained to perform two tasks using a finger-span device with which they provided continuous ratings. The device was composed of a potentiometer, the voltage of which was digitized and time-stamped in reference to functional MRI image acquisition and connected to a computer providing visual feedback of the ratings (Apkarian et al., 2001). For the first task, patients provided continuous ratings of fluctuations in spontaneously occurring back pain from 0–100 visual analogue scale for a period of 10 min during a functional MRI scan. The second functional MRI scan was acquired while subjects conducted a visual rating control task (Baliki et al., 2006), during which subjects rated the changes in the length of a visual analogue scale bar (0–100) projected on a screen for a 10 min period. The length of the bar varied over time to match the pain ratings obtained from the subject in the preceding scan. Thus this task serves as a control for task-related activations, such as visual inputs, motor performance, magnitude estimation, attention and anticipation.
Image preprocessing
Image analysis to reveal significant brain activity based on changes in blood oxygen level-dependent signal was performed on each patient’s data using Functional Magnetic Resonance Imaging of the Brain (FMRIB) Expert Analysis Tool [(FEAT; Smith et al., 2004; http://www.fmrib.ox.ac.uk/fsl)]. Preprocessing was conducted using the FSL 4.1 and MATLAB 7.9. The first four volumes were removed to compensate for scanner drifts, and slice-time correction, spatial smoothing with 5 mm kernel, intensity normalization, and high-pass filtering (150 s) were applied. Mean blood oxygen level-dependent signal from white matter, CSF, whole brain (after skull removal), six motion components, and motion outlier vectors were regarded as covariates of no interest and regressed out from the blood oxygen level-dependent signal. In addition, probabilistic Independent Component Analysis was then implemented in MELODIC (Multivariate Exploratory Linear Decomposition into Independent Components) to select artefact components, using an automated procedure that identified and removed edge components and signal dropout components. The functional MRI signal was then linearly modelled on a voxel-by-voxel basis using FMRIB’s Improved Linear Model (FILM) with local autocorrelation correction (Woolrich et al., 2001, 2004).
Scan parameters
For all participants and visits, MPRAGE type T1-anatomical brain images were acquired with a 3 T Siemens Trio whole-body scanner with echo-planar imaging (EPI) capability using the standard radio-frequency head coil with the following parameters: voxel size 1 × 1 × 1 mm; repetition time = 2500 ms; echo time = 3.36 ms; flip angle = 9°; in-plane matrix resolution, 256 × 256; slices, 160; field of view, 256 mm. Functional MRI images were acquired on the same day and scanner with the following parameters: multi-slice T2*-weighted echo-planar images with repetition time repetition time = 2.5 s, echo time = 30 ms, flip angle = 90°, number of volumes = 244, slice thickness = 3 mm, in-plane resolution = 64 × 64. The 36 slices covered the whole brain from the cerebellum to the vertex.
General linear model analysis
The patients with SBP with functional MRI scans at visit 1 were designated as the early SBP group and the relationship between their back pain-related brain activity was compared with patients with CBP in a cross-sectional analysis.
Brain functional activity in the early SBP and CBP groups was assessed for ratings of spontaneous pain and for visual control ratings. Ratings (Baliki et al., 2006) were convolved with a canonical haemodynamic response function [gamma function: lag, 6 s; standard deviation (SD), 3 s]. The significance of the model fit to each voxel time series was calculated, yielding statistical parametric maps for each subject and condition using the general linear modelling (GLM) procedure in FSL. After the co-registration of individual scans to standard space [152 subject average Montreal Neurological Institute (MNI) space, http://www.bic.mni.mcgill.ca/cgi/icbm_view/], group level analyses were carried out using Randomise in FSL. This technique uses permutation-based inference to allow for rigorous comparisons of significance within the framework of the general linear model with P < 0.05. Group differences were tested against 5000 random permutations, which exactly accounts for multiple comparisons. Significant clusters were identified using the threshold-free cluster enhancement method, and activity maps were corrected for multiple comparisons using family-wise error correction (P < 0.05).
Average group activity maps associated with the pain task were generated for the early SBP patients and for CBP patients. Furthermore, brain activation was contrasted between the early SBP and the CBP group using an unpaired t-test. All activity comparisons were corrected for confounds due to age, sex and pain intensity.
Perception-triggered regional activity
The objective of this analysis was to identify the relationship between pain ratings and regional brain activity for regions identified in the general linear model analyses. Functional regions of interest were determined from the spontaneous pain-related activation maps in patients with early SBP and those with CBP, and the contrast between these maps. The region of interest analysis should be considered post hoc and designed to identify changes in time course of regional activity. The regions were fixed size (5 × 5 × 5 voxel) masks centred at activation peak coordinates in standard MNI space. For each subject, individual brain space functional MRI was normalized to standard space, and then the blood oxygen level-dependent signal averaged for all voxels in the region was extracted and converted into per cent blood oxygen level-dependent change. These events were averaged for a fixed time window relative to a trigger, defined as pain perception crossing an arbitrary threshold (set to 1 SD). These responses were calculated in all patients (early SBP and CBP) for the spontaneous pain rating task. The list of regions of interest used for this analysis included the left medial prefrontal cortex (x = −4, y = 48, z = 0) and right amygdala (x = 24, y = −2, z = −18) that represented CBP-related regions, as well as the left insula (x = −38, y = 20, z = −4) and left thalamus (x = −14, y = −2, z = −18) that represented early SBP-related regions. Regional differences in peak blood oxygen level-dependent responses to spontaneous pain rating in CBP and early SBP were compared using an unpaired t-test at a single time point (15 s from the trigger), which corresponded to the mean peak pain rating for both groups. Similar region of interest analyses were performed for the visual rating task.
Back pain-related brain activity in relation to meta-analytic maps
We used the web tool Neurosynth to create (reverse inference) meta-analytic maps for the terms: pain, emotion and reward (Yarkoni et al., 2011) and generated masks to compare brain activity for different back pain groupings in relation to these signatures. It is of note that for the term ‘pain’, the obtained map is based on all identified papers in which the term is used, and thus the map does not distinguish between acute and chronic pain conditions. However, the large majority of publications used to generate the map were from studies for acute pain conditions (only 6 of 224 studies used included chronic pain data).
The extent of back pain activity relative to a given meta-analytic map was computed as the percentage of non-zero voxels activated in the mean general linear model-based contrast of parameter estimates for early SBP or CBP conditions encompassed within the meta-analytic maps. For instance, the amount of overlap between the early SBP map and the meta-analytic pain map is represented by the percentage of voxels activated in the early SBP mean map within the meta-analytic pain map, divided by the total number of voxels in the meta-analytic pain map. Identical procedures were used for computing per cent overlap with the emotion and reward maps.
The meta-analytic maps for pain, emotion and reward are not completely segregated from each other, and the extent of their overlap varied with threshold. Therefore, we calculated overlap with meta-analytic maps at two different thresholds, including the top 5% and 1% of voxels from the reverse inference meta-analytic statistical maps, which generated the 95th percentile and 99th percentile maps, respectively.
Longitudinal changes in back pain representation relative to meta-analytic maps
The 39 patients with SBP who completed the study (visits 1 to 4) were subdivided into recovering (recovering SBP, n = 19) and persisting (persisting SBP, n = 20), based on a self-reported 20% change in back pain intensity from first assessment to 1 year later (e.g. difference in pain between visits 1 and 4). To assess changes in pain representation between the persisting and the recovering SBP groups within the selected meta-analytic maps over time, first, the statistical parametric maps were generated using convolved spontaneous pain ratings using a general linear model procedure for all four visits. Next, we assessed the mean activation for each subject within the three, reverse inference, meta-analytic maps for pain, emotion and reward, thresholded at the 95th and 99th percentiles. To assess group (persisting SBP versus recovering SBP) by time (visits 1–4) interactions, we used a two-way repeated-measures ANOVA. Post hoc comparisons between groups were performed using repeated measures one-way ANOVA and Tukey’s test for pair-wise comparisons.
Results
To test the hypothesis that brain representation for back pain may be distinct in subjects who have lived with the condition for different durations of time, we examined brain activity for back pain in CBP and in early SBP (early SBP, first functional MRI scan in SBP subjects). In early SBP (n = 94), the back pain intensity was 58.25 ± 1.95 (mean ± SEM) and present for a duration of 9.14 ± 0.48 weeks. In contrast, in CBP (n = 59) back pain intensity was slightly, but significantly, higher 69.58 ± 2.61 and was present for a far longer duration of 13.5 ± 1.3 years, compared with early SBP.
Recent evidence shows that increasing the number of subjects in functional MRI can lead to the identification of more extensive brain activity (Gonzalez-Castillo et al., 2012). This may be especially true for the task used in the present study, because it entails rating subjective fluctuations of an ongoing perception, wherein the fidelity of ratings may differ between participants, and because the temporal variability of the perception can also differ between subjects. Thus, the brain activity we have reported in CBP in the past (Baliki et al., 2006, 2008b; Hashmi et al., 2012) may have underestimated the breadth of brain regions related to back pain. Therefore, the CBP data were pooled from multiple studies to make the number of subjects more similar to that of early SBP, and also to increase confidence (by improving detection power) in the brain areas activated for back pain. With these two groups (CBP and early SBP), we examined brain activity for back pain of approximately comparable intensity, between a large group of subjects who have lived with the condition either for a few months or for many years. Pain properties and demographics are presented in Table 1.
Cross-sectional analysis: differences between early subacute back pain and chronic back pain
Pain, mood and demographics
Important pain and mood parameters were matched between CBP and early SBP, yet there were some demographic and pain-related parameters that differed. The CBP and early SBP groups were not significantly different in levels of neuropathic pain (Neuropathic Pain Scale) or depression and had equivalent numbers of males and females. However, age, self-reported back pain intensity (based on Visual Analogue Scale), and the sensory and affective McGill Pain Questionnaire scores were significantly higher in the CBP group compared to early SBP (Table 1).
Task variability
Given that the brain activity for back pain was based on subjective ratings of spontaneous fluctuations, the extent of variability of these ratings is a critical parameter that can control functional MRI signal size. Two spontaneous pain properties were contrasted between the two groups: variance and number of trigger events (i.e. number of times in one scan a subject rates an increase in their pain larger than 1 SD compared with the mean pain rating, Fig. 1A and B). The ratings of spontaneous pain exhibited a mean variance of 14.7 ± 4.3 (on a 0–100 visual analogue scale) in patients with CBP (n = 59) and 12.2 ± 2.35 in patients with early SBP patients (n = 94), with no differences found between the two groups (t = 0.55, P = 0.58). Furthermore, both groups showed similar numbers of trigger events per scan (CBP: 3.07 ± 1.56 versus early SBP: 3.03 ± 1.55, t = 0.14, P = 0.88). These results indicated that CBP and early SBP exhibited similar spontaneous pain properties, and thus any differences observed in brain activation in relationship to spontaneous pain are independent from the dynamical properties of fluctuations of spontaneous pain.
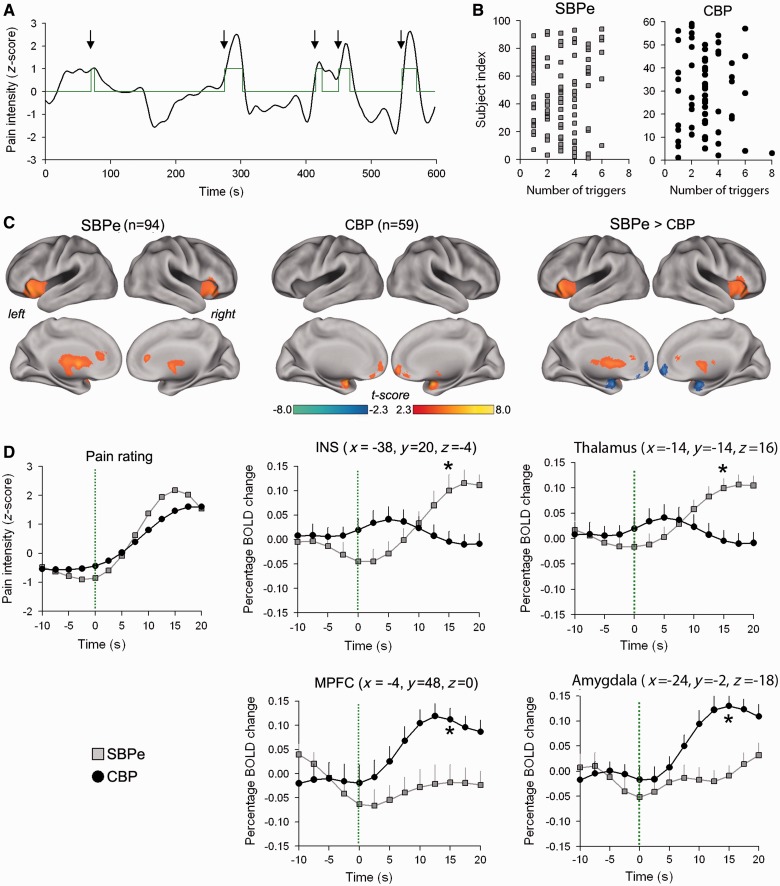
Figure 1.
Brain activity for rating back pain is distinct in early sub-acute back pain (SBPe) in comparison to CBP. (A) Individual subject examples of the trigger pulses generated from ratings of back pain, after convolving the rating with a canonical haemodynamic response in a subject with early sub-acute back pain. Arrows and green curve represent pain onset triggers and green curve represents example of durations for which subjects reported a greater than 1 SD increase in pain. (B) Shows the number of pain triggers in each subject within the scan in SBP and CBP patients. (C) Group-averaged brain activity for rating fluctuations of back pain in 94 subjects with early SBP (right), and in 59 subjects with CBP (middle). The contrast between the two groups (early SBP > CBP is shown in blue, and CBP > early SBP in red) (left). The contrasts largely reproduce corresponding group activity maps, indicating that early SBP and CBP engage separate brain regions. Results are thresholded at P < 0.05 (FWE corrected). (D) Trigger evoked blood oxygen level-dependent response in regions of interest. Pain ratings (left) and blood oxygen level-dependent signal (right) were extracted over a 30 s time window that spanned the pain onset (10 s before and 20 s after onset) for every event and averaged in each subject to construct a group early SBP and CBP average. Regions were selected based on peak activations in the General Linear Model based early SBP mean map [anterior cingulate cortex (ACC) and insula] and CBP mean map (medial; prefrontal cortex, amygdala). *P < 0.01, unpaired two-tailed t-test.
Brain activity
Within and across group comparisons
The spontaneous pain ratings activated a different set of brain regions in early SBP and CBP groups. The mean activation map in early SBP showed activity extending from the anterior to mid insula bilaterally with contiguous activations in the thalamus, striatum, and lateral aspects of the orbitofrontal and inferior cortex, as well as the dorsal parts of the anterior cingulate cortex. In contrast, CBP patients’ mean brain activity was localized bilaterally in the perigenual anterior cingulate cortex (Brodmann area 32) extending into the medial prefrontal cortex (Brodmann area 10) and parts of the amygdala. Contrasts between the two groups (CBP > early SBP and early SBP > CBP) essentially reproduced the corresponding group activity maps, indicating that early SBP and CBP back pain engage separate brain regions (Fig. 1C, Table 2). Note that all activity maps and contrasts were corrected for age, pain (visual analogue scale) and sex. Although depression (Beck Depression Inventory) was not different between the groups (Table 1), we tested correcting for Beck Depression Inventory, as well as for McGill Pain Questionnaire sensory and McGill Pain Questionnaire affective scores, and observed no differences in obtained contrast maps.
Table 2.
Coordinates of brain activity for rating spontaneous fluctuations of back pain in early SBP and CBP groups
| Brain region | Early SBP | CBP | Early SBP > CBP | |||
|---|---|---|---|---|---|---|
| Coordinates x, y, z | t-score | Coordinates x, y, z | t-score | Coordinates x, y, z | t-score | |
| Right INS | 44, 16, 0 | 5.26 | 44, 14, 4 | 4.53 | ||
| Left INS | −36, 22, −2 | 5.13 | −38, 20, −4 | 5.09 | ||
| Right caudate | 10, 14, 6 | 5.34 | 12, 14, 4 | 5.01 | ||
| Left caudate | −12,18, 6 | 5.30 | −10, 14, 4 | 5.53 | ||
| Left putamen | −24, 0, 10 | 6.98 | −28, 4, 2 | 2.84 | −20, 12, 8 | 5.31 |
| Right putamen | 24, 10, 6 | 5.93 | 24, 10, 0 | 3.82 | 26, 6, 6 | 4.47 |
| ACC | 2, 30, 16 | 5.88 | 0, 30, 16 | 5.62 | ||
| Right thalamus | 14, −12, 12 | 6.32 | 14, −12, 12 | 4.36 | ||
| Left thalamus | −14, −14, 16 | 5.51 | −14, −14, 16 | 5.32 | ||
| MPFC | −4, 48, 0 | 6.46 | −4, 48, 0 | −4.28 | ||
| OFC | −2, 42, −12 | 7.30 | −2, 40, −12 | −4.19 | ||
| Left amygdala | −24, −2, −18 | 8.51 | −24, −2, −18 | −6.14 | ||
| Right amygdala | 28, −2, −16 | 8.73 | 28, −2, −16 | −5.14 | ||
INS = insula; OFC=orbitofrontal cortex; ACC=anterior cingulate cortex; MPFC=medial prefrontal cortex.
As activity maps for back pain were focal and distinct in early SBP and CBP, we examined the time course of the blood oxygen level-dependent signal for regions of interest identified from peak activations of each map. The SBP group average (n = 94) showed greater peak responses (computed at 15 s post-trigger) than the CBP group (n = 59) in the thalamus (t = 4.00, P < 0.001) and insula (t = 4.32, P < 0.001), whereas the CBP group showed more pain-related activation in amygdala (t = 4.98, P < 0.001) and medial prefrontal cortex (t = 4.50, P < 0.001) (Fig. 1D). Given that all subjects also performed a rating task for a visual task (a control for task performance), we also extracted blood oxygen level-dependent signals for the corresponding regions from this visual control task. The comparison of blood oxygen level-dependent between the two conditions differentiates pain-related activity from that of task demands. For this contrast, we statistically compared trigger-evoked blood oxygen level-dependent responses in regions of interest between the spontaneous pain task and the matched visual task in the early SBP and CBP groups (Supplementary Fig. 1). Left insula showed greater blood oxygen level-dependent responses for pain but not for visual ratings in early SBP (t = 4.77, P < 0.01) but not in CBP (t = 0.88, P = 0.85). We observed the opposite pattern for medial prefrontal cortex, which showed larger blood oxygen level-dependent responses to the pain in CBP patients (t = 4.47, P < 0.001). These results indicate that the insula and medial prefrontal cortex reflect pain-specific responses in early SBP and CBP, respectively.
Overlap with meta-analysis maps
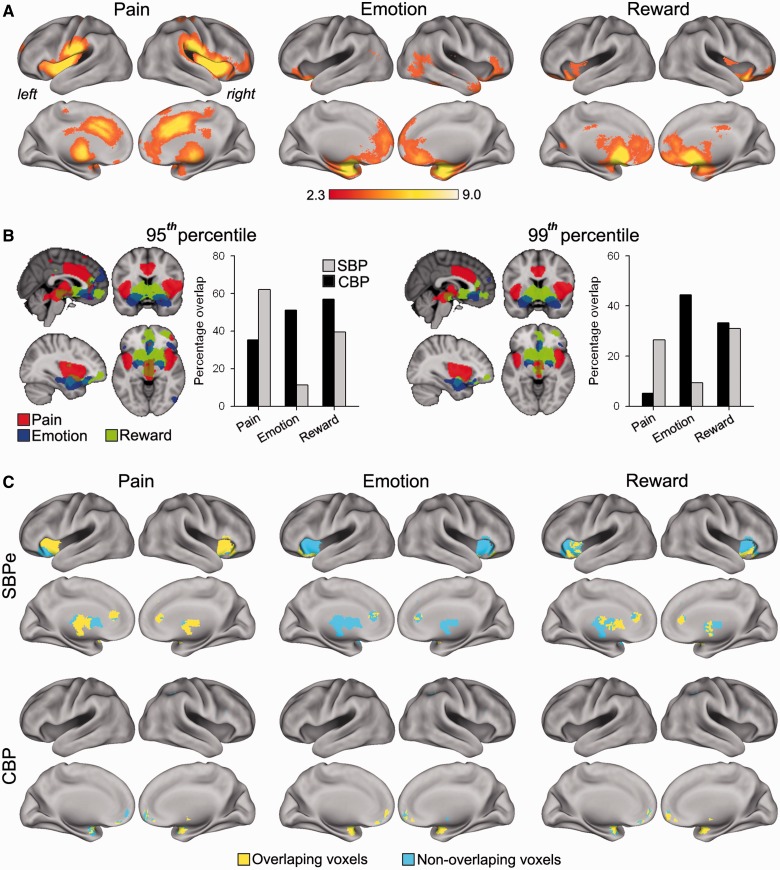
Visual inspection of activity maps for early SBP and CBP hint that the former includes brain regions commonly seen for acute pain, whereas the latter engages more emotional regions (Fig. 1A and B). To formally test this idea, we calculated the overlap of each map with meta-analysis maps generated for the words: pain (derived from 224 studies, identifying 14.5% voxels), emotion (derived from 324 studies, identifying 8.6% voxels), and reward (derived from 203 studies, identifying 5.7% voxels), generated from Neurosynth (Yarkoni et al., 2011). The meta-analytic map for pain showed peak activations in the insula, thalamus, mid-brain, anterior cingulate cortex and somatosensory area 1. In comparison, the emotion map showed greatest activations in amygdala, hippocampus, orbitofrontal cortices, and operculum and in dorsal, ventral and rostral regions of the medial prefrontal cortex. The reward map overlapped with emotion maps in the medial and orbitofrontal cortices, but showed high levels of activation in the basal ganglia and mid-brain, and showed relatively less activation in the amygdala and hippocampus in comparison to the emotion map (Fig. 2A).
Figure 2.
Early SBP (SBPe) and CBP activation maps correspond to distinct meta-analytic circuits. (A) Brain meta-analytic maps for the terms: pain, emotion and reward, from Neurosynth (Yarkoni et al., 2011). (B) Brain images represent masks derived from maps above at different thresholds (top five and one percentile voxels) for pain (red), reward (green) and emotion (blue) meta-analytic maps. Bar graphs represent the % overlap for CBP (black) and early SBP (grey) with the three meta-analytic maps at the 95th and 99th percentile thresholds. Overall SBP activity is more similar to the pain term related mask, whereas CBP activity is similar to emotion term related mask. Activity in both groups engage parts of the reward mask. (C) Brain images show the overlapping (yellow) and non-overlapping (blue) voxels for early SBP (top row) and CBP (bottom row) with the 95th percentile thresholded meta-analytical masks. Early SBP overlaps with pain mainly in bilateral insula, thalamus and anterior cingulate cortex (ACC), whereas CBP overlaps with emotion in bilateral amygdala and medial prefrontal cortex (mPFC).
The top 5% and 1% of voxels of meta-analysis z-score maps were identified and used to generate two, threshold-dependent (95th and 99th percentile) term-specific binarized maps (Supplementary Fig. 2). The emotion and reward maps exhibited the largest overlap with each other, and the overlap between any two meta-analytic maps decreased with increasing the threshold (Supplementary Fig. 2). The percentage overlap between the back pain maps and the term-specific maps, for both thresholds, are shown in Fig. 2B. The CBP map exhibited greater overlap with emotion compared to early SBP for both the 95th percentile threshold (CBP: 51.19 %; SBP: 11.12 %) and the 99th percentile threshold (CBP: 44.49 %; SBP: 9.36%). In contrast to CBP, the early SBP activation map showed the highest overlap with the pain meta-analytic map, and this held true at the 95th percentile threshold (CBP: 35.27%; SBP: 62.18%) and 99th percentile threshold (CBP: 5.09%; SBP: 24.71%). The reward map showed slightly higher overlap with CBP map at the 95th percentile threshold (CBP: 56.93%; SBP: 39.41%), but not at the 99th percentile threshold (CBP: 33.19 %; SBP: 30.94%). Overall, the pain map overlap was consistently higher for early SBP compared to CBP. This was mainly due to the unique activation of bilateral insular cortex in the early SBP. The emotion map overlap was consistently higher for CBP compared with early SBP, primarily because of the CBP-specific activation of the medial prefrontal cortex and bilateral amygdala. CBP and early SBP both showed similar overlap with the reward map, reflecting engagement of different parts of the basal ganglia. Finally, early SBP shared more overlap with the dorsal striatum, whereas CBP overlapped with the ventral striatum. The spatial distribution of overlap for early SBP and CBP activity across the pain, emotion and reward term maps are illustrated in Fig. 1C.
Longitudinal analysis
Of the 94 patients with early SBP, 39 completed the longitudinal part of the study and had complete functional MRI scans for back pain at four sessions over a 1 year period. In this subgroup of patients, we examined brain activity longitudinally as participants either recovered (recovering SBP) from back pain or persisted into chronification (persisting SBP).
Pain, mood and demographics
In the 39 patients with early SBP, brain scans for back pain were performed as soon as possible upon recruitment (mean ± SEM pain duration from injury at visit 1 = 12.28 ± 4.80 weeks) and we followed their pain and mood parameters, as well as brain activity, over three additional visits for the next year (visit 2: 7.15 ± 2.48 weeks; visit 3: 29.20 ± 6.06 weeks; visit 4: 54.36 ± 6.12 weeks; from visit 1) (Fig. 3A)
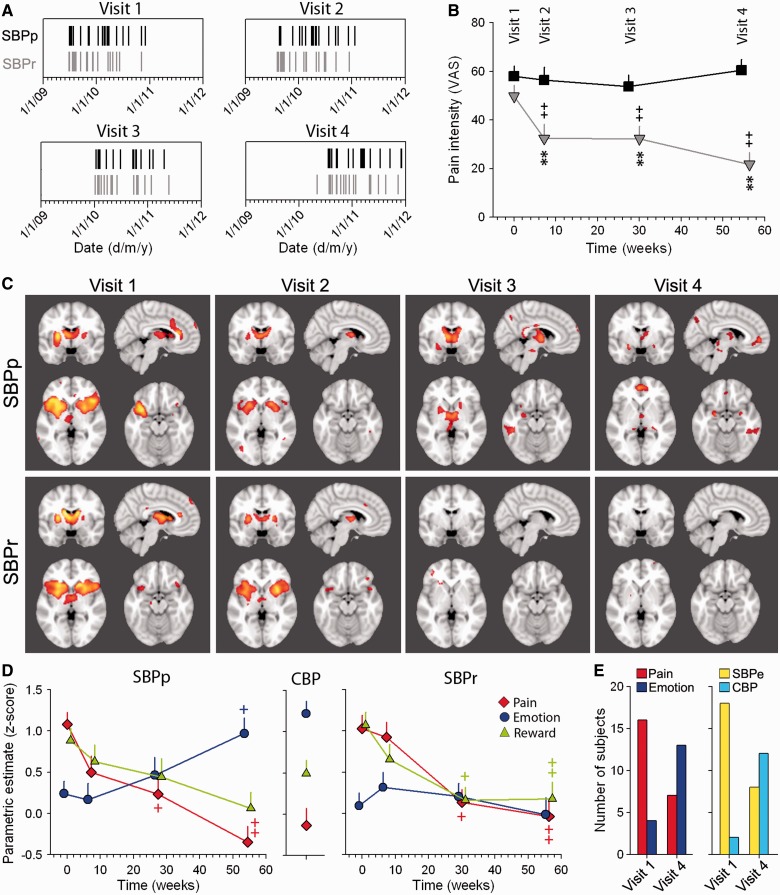
Figure 3.
Longitudinal changes in brain activity underlying spontaneous pain when patients transition from acute to chronic back pain state. (A) Plots show the scanning calendar dates of subjects with recovering SBP (SBPr) and persistent SBP (SBPp) for all four visits. Vertical marks represent individual persistent SBP (black) and recovering SBP (grey) subjects. Groups were scanned within the same time window (major ticks are years; minor ticks are months). (B) Recovering SBP in contrast to persistent SBP patients exhibited decreased pain in time. (C) Group average activation maps (P < 0.01 uncorrected) for recovering and persistent SBP groups at the four visits. Recovering and persistent SBP groups show activation within acute pain regions for visits 1 and 2 encompassing bilateral insula, thalamus and anterior cingulate cortex (ACC). Recovering SBP patients show no significant activity for visits 3 and 4, whereas persistent SBP shows increased activation in the medial prefrontal cortex and amygdala at visit 4. (D) Plots show the group average cope (normalized) for pain, emotion and reward masks, for each group (persistent SBP, CBP, recovering SBP), across all visits. Persistent SBP exhibited decreased presentation of their spontaneous pain within the pain mask. This decrease was coupled with an increased activity within the emotion mask. The middle panel shows CBP activity for all three masks. These values correspond to those we observe in persistent SBP at 1-year scans. In contrast to persistent SBP, the recovering SBP group exhibited decreased activity within all masks in time. (E) Classifier performance applied to individual persistent SBP activation maps for either pain/emotion or CBP/early SBP, at visits 1 and 4. Persistent SBP activity mainly classified as pain or early SBP at visit 1, and as emotion or CBP at visit 4. +P < 0.05, ++P < 0.01, within group comparison to visit 1; **P < 0.01 comparison between groups at a corresponding time.
We subdivided the group into recovering (recovering SBP, n = 19) and persisting (persisting SBP, n = 20), based on a self-reported 20% change in back pain intensity from first assessment to 1 year later (i.e. difference in pain between visits 1 and 4). These two groups diverged in back pain intensity at visits 2 and 3 (weeks 15 and 45 from onset of back pain, Fig. 3B) and exhibited no significant difference in pain duration at visit 1 (persisting SBP: 12.4 ± 1.12; recovering SBP: 12.16 ± 1.06; two-sided unpaired t-test: t = 0.16, P > 0.05). At visit 1, both groups reported similar pain and mood characteristics (Table 3). In addition, both groups had similar variance in spontaneous pain ratings of back pain, in the amount of head motion, and in medication use (Table 3, Supplementary Fig. 3). At visit 4, the recovering SBP group showed a decrease in mood impairment and pain parameters, indicating recovery from back pain.
Brain activity
The results of the cross-sectional analysis suggest that, with chronification of pain, the brain activity for back pain is spatially transformed from the pattern observed for the term ‘pain’ to that identified for the term ‘emotion.’ However, the latter does not provide information regarding the time-span within which such reorganization may occur. We directly tested this notion in the longitudinal group, expecting that, in time, the recovering group (recovering SBP) would show decreases in back pain-related brain activity, whereas those persisting to pain chronification (persisting SBP) would exhibit a shift of pattern towards the emotion term related map, as we observed for CBP cross-sectionally. Therefore, we tracked brain activity for persisting SBP and recovering SBP for rating fluctuations of back pain over time, in relation to the meta-analytic maps, and compared with masks generated from early SBP and CBP activity.
First, as observed in the scanning schedule in Fig. 3A, the persisting SBP and recovering SBP groups were scanned within the same time window and with a distribution of times, at all four visits, that were random across groups, thereby eliminating the potential bias of scan order as a factor in group differences in brain activity. As expected the patients with recovering SBP, in contrast to those with persisting SBP, exhibited decreased pain in time (Fig. 3B). Changes in pain intensity across groups and time were computed using a two-way repeated-measures ANOVA. Persisting SBP and recovering SBP showed significant group differences [F(1,37) = 16.09, P < 0.001], time effect [F(3,111) = 7.09, P < 0.001], and group × time interaction [F(3,111) = 6.92, P < 0.001]. Post hoc comparisons between pain scores were performed using Tukey’s test and indicated that patients with recovering SBP reported an immediate decrease in pain ratings at visit 2 as compared to baseline (visit 1), and in persisting SBP, the pain intensity was maintained for the duration of the study.
Pain-related brain activity for persisting SBP and recovering SBP for the four visits are shown in Fig. 3C. At visits 1 and 2, recovering SBP and persisting SBP exhibited similar brain activation patterns that included bilateral thalamus, insula, mid anterior cingulate cortex and basal ganglia. At visit 3, persisting SBP showed activity in the thalamus, basal ganglia and brainstem regions, whereas recovering SBP exhibited no significant activation in response to pain, which is consistent with the significant decreases in pain intensity reported by recovering SBP at visit 3 compared with visit 1. At visit 4, persisting SBP showed activity within the amygdala and medial prefrontal cortex, in addition to the basal ganglia. On the other hand, the recovering SBP did not show any significant activity. Therefore recovering SBP showed overall activity confined to pain-specific regions (mainly insula, anterior cingulate cortex and thalamus) for visits 1 and 2, which significantly decreased at visits 3 and 4. Persisting SBP showed similar activation patterns for the two visits, which shifted to a more emotion term-related representation 1 year later (visit 4), (Tables 4 and 5).
Table 4.
Coordinates of brain activity for rating spontaneous fluctuations of back pain in persistent SBP across visits
| Brain region | Visit 1 | Visit 2 | Visit 3 | Visit 4 | ||||
|---|---|---|---|---|---|---|---|---|
| Coordinates x, y, z | t-score | Coordinates x, y, z | t-score | Coordinates x, y, z | t-score | Coordinates x, y, z | t-score | |
| Right INS | 40, 12, −6 | 6.71 | 44, 14, −4 | 2.97 | ||||
| Left INS | 34, 12, 8 | 6.32 | −44, 24, −4 | 2.73 | ||||
| Right caudate | 16, 16, 10 | 6.25 | 12, 20, 4 | 3.05 | 16, 18, 8 | 2.94 | ||
| Left caudate | −14, 14, 10 | 6.20 | −16, 18, 6 | 4.74 | −18, 18, 6 | 3.41 | −16, 14, 18 | 2.47 |
| Right putamen | 26, 10, 4 | 5.63 | 24, 6, 2 | 3.42 | 26, 6, −4 | 2.99 | ||
| Left putamen | −24, 6, 8 | 5.89 | −28, 8, −2 | 4.07 | ||||
| ACC | 4, 28, 16 | 6.25 | ||||||
| Right thalamus | 8, −16, 10 | 4.05 | 10, −14, 6 | 2.80 | 8, −6, 6 | 5.12 | ||
| Left thalamus | −10, −10, 10 | 3.12 | −6, −8, 6 | 2.53 | −10, −12, 6 | 3.97 | ||
| Right S2 | 58, −24, 26 | 5.10 | 2, −34, 24 | 3.47 | ||||
| PCC | ||||||||
| MPFC | 0, 50, 2 | 4.51 | ||||||
| Right amygdala | 24, −4, −16 | 2.69 | 24, −2, −16 | 4.02 | ||||
| Left amygdala | −28, 0, −18 | 3.01 | ||||||
| Right ITG | −50, −36, −16 | 3.35 | ||||||
| Left ITG | −62, −30, −20 | 3.49 | ||||||
| Precuneus | 12, −70, 36 | 3.32 | ||||||
ACC = anterior cingulate cortex; INS = insula; ITG = inferior temporal gyrus; MPFC = medial prefrontal cortex; PCC = posterior cingulate cortex; S2 = secondary somatosensory cortex.
Table 5.
Coordinates of brain activity for rating spontaneous fluctuations of back pain in recovering SBP across visits
| Brain region | Visit 1 | Visit 2 | ||
|---|---|---|---|---|
| Coordinates x, y, z | t-score | Coordinates x, y, z | t-score | |
| Right INS | 42, 12, −6 | 6.90 | 36, 10, −2 | 5.40 |
| Left INS | 38, 12, 4 | 6.77 | 38, 14, 0 | 5.12 |
| Right caudate | 14, 16, 12 | 6.44 | 14, 14, 12 | 3.78 |
| Left caudate | −16, 14, 10 | 6.05 | −16, 12, 10 | 4.21 |
| Right putamen | 24, 10, 4 | 5.93 | 24, 10, 4 | 4.90 |
| Left putamen | −26, 6, 6 | 5.75 | −24, 6, 8 | 4.95 |
| ACC | −2, 32, 14 | 5.05 | −2, 32, 16 | 3.55 |
| Right thalamus | 8, −18, 14 | 5.62 | 12, −20, 10 | 4.47 |
| Left thalamus | −14, −10, 12 | 5.50 | −8, −10, 12 | 3.75 |
| Right IPS | 50, −52, 46 | 3.93 | 56, −52, 48 | 2.41 |
| Left IPS | −46, −52, 52 | 3.54 | ||
| Right DLPFC | 44, 26, 32 | 3.21 | ||
| Left DLPFC | 48, 30, 32 | 4.05 | −46, 40, 16 | 2.31 |
| Right S2 | 50, −42, 32 | 2.66 | ||
ACC = anterior cingulated cortex; DLPFC = dorsal prefrontal cortex; INS = insula; IPS = intraparietal sulcus; S2 = secondary somatosensory cortex. There were no significant activations for visits 3 and 4.
Longitudinal changes in back pain-related brain activity were determined using a region of interest analysis with the 99th percentile map masks for the terms pain, emotion and reward, and by calculating the extent of activity observed within these masks in the persisting SBP and recovering SBP groups, as a function of the time of brain scan. For any given subject, the fit for spontaneous pain with each mask was computed as the mean contrast of parametric estimate value of all voxels within each mask. These values were compared using two-way repeated-measures ANOVA to evaluate the variability of parametric estimate values across meta-analytic map types and time, separately for persisting SBP and recovering SBP. Persisting SBP exhibited decreased representation of back pain-related brain activity within the meta-analytic pain map (Fig. 3D). There was no significant effect of meta-analytic map type [F(2,56) = 0.43, P = 0.65], yet both time [F(3,111) = 3.99, P < 0.01] and mask type-by-time interaction [F(6,168) = 9.76, P < 0.001] were significant. Tukey’s post hoc indicated that the persisting SBP activity decreased in the pain mask at visits 3 and 4, compared with visit 1. This decrease was coupled with an increased activity within the emotion mask at visit 4 compared to visit 1. The persisting SBP activity within the reward mask did not show any changes in activity across all time points. Values for overlap for the three masks with CBP (shown for comparison in Fig. 3D) were similar to the persisting SBP values for overlap at visit 4. In contrast, recovering SBP exhibited decreased activity across all three meta-analytic masks with time (Fig. 3C). This finding is consistent with their pain reports, which also significantly decreased in time. Thus, there was a significant group effect [F(2,54) = 4.85, P < 0.05], time effect [F(6,162) = 11.83, P < 0.001] and group × time interaction [F(6,162) = 2.71, P < 0.05). Post hoc analyses showed that activity within both the reward and pain masks showed significant decreases at visits 3 and 4, as compared to visit 1.
In a second alternative approach, we used a classification technique to investigate the similarity of individual persisting SBP activation maps (for visits 1 and 4) to pain and emotion meta-analysis maps, and to early SBP and CBP maps (generated from the cross-sectional analysis). Individual persisting SBP brain activity was classified as either pain or emotion using a conjunction analysis. For each subject, the top 1% of activated voxels were identified and the ratio of overlap between the pain or emotion maps, or either early SBP or CBP maps, was determined. Subjects were then classified dependent on higher overlap with either map. Results are shown in Fig. 3E. Brain activity for patients with persisting SBP were more often classified as pain at visit 1 (n = 16 of 20), and as emotion at visit 4 (n = 13 of 20) (Fisher’s exact test, P < 0.004). Similar results were obtained when subjects were classified as either CBP or early SBP. At visit 1 the majority of patients with persisting SBP were classified as early SBP (n = 18 of 20), whereas at visit 4 they were classified as CBP (n = 12 of 20) (Fisher’s exact test, P < 0.001). Thus, with two separate approaches we show that persisting SBP brain activity shifts in time to become more similar to the emotion map, as well as to the CBP map.
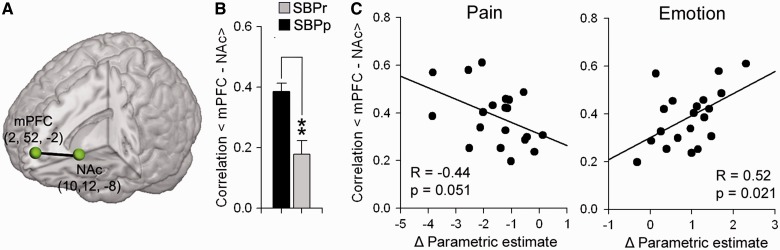
We have recently shown that early increased functional connectivity between nucleus accumbens and medial prefrontal cortex can highly predict whether back pain persists in patients with persisting SBP 1 year after the onset of pain (Baliki et al., 2012). Here we investigate the relationship between medial prefrontal cortex–nucleus accumbens connectivity and the observed change in brain activity underlying spontaneous pain in patients with persisting SBP. Similar to our previous report, medial prefrontal cortex–nucleus accumbens functional connectivity strength was significantly higher in persisting SBP (0.38 ± 0.03) compared with recovering SBP (0.17 ± 0.05) at visit 1 (t-score = 4.48, P < 0.01). More importantly, medial prefrontal cortex-nucleus accumbens connectivity at visit 1 was significantly correlated to change in brain activity underlying spontaneous pain (average cope at visit 4 − average cope at visit 1), for both pain (R = −0.44, P = 0.051) and emotion (R = 0.52, P = 0.021) term defined regions (Fig. 4).
Figure 4.
Medial prefrontal cortex–nucleus accumbens (mPFC-NAc) functional connectivity strength predicts extent of shift in brain activity underlying spontaneous pain in patients with persistent SBP from pain-related to emotion-related regions. (A) Brain image shows the location and coordinates of the medial prefrontal cortex and nucleus accumbens seeds used. (B) Bar graph shows the mean ± SEM for medial prefrontal cortex–nucleus accumbens functional connectivity in persistent SBP (black) and recovering SBP (grey) at visit 1. Persistent SBP exhibited higher medial prefrontal cortex–nucleus accumbens connectivity compared with recovering SBP. (C) Scatter plots show the relationship between medial prefrontal cortex–nucleus accumbens connectivity at visit 1 and change in brain activity (Δ Parametric estimate = average cope at visit 4 − average cope at visit 1) for pain (left) and emotion (right) term related masks. High medial prefrontal cortex–nucleus accumbens connectivity showed a strong relationship with decreased activation in pain regions and increased activiations in emotional regions in patients with persistent SBP over a 1-year period (visit 4 versus visit 1).
Discussion
To our knowledge this is the first study that examines a highly prevalent clinical pain condition, back pain, as its brain signature evolves in time, by following reorganization of underlying brain representation for the percept. Our main findings are:
Perception of back pain, as determined by continuous rating of spontaneous pain fluctuations, activates two separate non-overlapping brain circuits in patients in which the pain is present for only 2 months (early SBP), in comparison to those who have lived with the same condition for >10 years (CBP).
This cross-sectional result was closely replicated in the longitudinal analysis in a sub-group of early SBP for which pain and brain activity were followed over a 1-year time span. When the SBP group was subdivided into recovering and persisting SBP groups, we observed that in recovering SBP brain activity for rating back pain decreases over this 1-year period. In contrast in persisting SBP, the brain regions initially encoding back pain diminish in response as the back pain continues to persist, whereas activity in another set of brain regions emerges to encode the ongoing pain perception.
We adopted a novel approach of identifying brain functional circuits associated with specific terms, including pain, emotion and reward, to identify related brain activity derived from an automated meta-analysis of brain imaging literature (Yarkoni et al., 2011). We then used these maps to quantify the relationship between these term-related circuits and the reorganization of brain activity encoding back pain over time (in early SBP and CBP, as well as in persisting SBP and recovering SBP). This analysis indicates that acute/subacute back pain primarily engages pain and reward circuitry; in contrast, CBP and late persisting SBP (after 6–12 months of back pain persistence) show a shift away from the acute pain circuit and the gradual engagement of the emotion and reward circuits. Moreover, we could demonstrate that the extent of shift of activity in persisting SBP from pain to emotion related maps (over the 1-year period) was related to the strength of functional connectivity between medial prefrontal cortex and nucleus accumbens determined at the time of entry into the study. These results were obtained after correcting for pain intensity, sex, and age differences between groups. Therefore, we conclude that the representation of the percept of back pain is not a unitary construct; rather it engages distinct brain circuitry as a function of the persistence of back pain. As a result, we have identified the brain signature underlying back pain chronification. Furthermore, the demonstration that a unitary percept, back pain, can correspond to different brain circuitry configurations poses a fundamental challenge to the localization theory that posits a one-to-one correspondence between brain regional activity and the mind.
Brain activity for acute/subacute and chronic back pain is non-overlapping
One of the most notable findings of this study is the observation that the perception of back pain engages distinct brain activations across different groups of patients who suffer from back pain of comparable intensities. The observed CBP brain activity pattern replicates and further extends earlier observations. In the first study examining brain activity for rating spontaneous perception of back pain, we predominantly identified medial prefrontal cortex activity as a unique marker for CBP (Baliki et al., 2006). This result was replicated in two separate CBP cohorts, with functional MRI conducted on 1.5 and 3.0 T magnets. Compared with the current study, these earlier results were obtained in a smaller number of participants (Baliki et al., 2006, 2008b; Hashmi et al., 2012). After quadrupling the number of subjects, the only additional brain regions identified here included the bilateral amygdala and parts of the basal ganglia. Given that the meta-analytic map for emotion is mainly comprised of medial prefrontal cortex and amygdala, whereas the reward map encompasses these regions as well as large portions of the basal ganglia, we can conclude that perception of back pain in subjects living with the condition for >10 years mainly engages the emotion and reward circuitry.
For early SBP, in contrast to CBP, back pain was experienced for ∼2 months with no prior back pain history for at least a year, and brain regions that encode back pain perception are regions that have been repeatedly observed to be activated for acute pain (Price, 2000; Apkarian et al., 2005), as identified by the meta-analytic map for the term pain. When we contrasted brain activity between CBP and early SBP, we observed group-specific patterns of activity, with no brain regions showing activity of comparable magnitude in both groups. Given the large number of subjects we studied, it is highly unlikely that critical brain areas involved in back pain perception were missed. Furthermore, because the group contrasts were conducted with corrections for pain intensity, the SBP and CBP maps illustrate distinct brain circuits for perceiving back pain in the two groups.
Brain activity within the same group of subjects, and for a constant percept, undergoes large-scale spatial shift with chronification of back pain
In patients with SBP whose pain persisted over the observation period, persisting SBP, we observed that the same percept of back pain, characterized by stable intensity and no discernable changes in anxiety or depression, is associated with a brain activity pattern that, in time, continuously shifts away from the meta-analytic acute pain circuit and progressively activates the emotion (as well as reward) circuit. Therefore, these longitudinal results replicate the cross-sectional between-subjects comparisons and reinforce that, even within the same individual, brain activity for back pain can activate different brain circuits.
It is remarkable that the brain signature for CBP, once developed within the first year (as shown in persisting SBP), is then stabilized and seems to remain constant over 10 years (brain activity for persisting SBP at 1 year closely matches that for CBP). Thus within the first year, the brain carves a chronic pain state, implying that this first year can be viewed as a critical period for back pain chronification. Importantly, this time period closely matches the clinical definition of the transition to chronic pain (commonly assumed to be between 3–12 months; Frank, 1993), and as such, our results provide the first objective marker for the clinical transition to chronic pain. It is likely that the time required to reach a stable representation of chronic pain is variable across clinical pain conditions, given that the anatomical reorganization of cortical grey matter shows diverse time constants for different chronic pain conditions (Baliki et al., 2011a). Similarly, the brain activity signature for different chronic pain conditions may also be distinct, although in post-herpetic neuropathy and in knee osteoarthritis, but not in pelvic pain, the brain activity for rating spontaneous pain shows close similarities to CBP (Geha et al., 2007, 2008b; Farmer et al., 2011; Parks et al., 2011). In the past, we have interpreted the dissociation between acute and chronic pain as reflecting cognitive and attentional disengagement from sensory properties of the pain and the enhancement of self-referential emotional relevance of the condition (Apkarian et al., 2008, 2009). Given the common clinical observation that chronic pain conditions are comorbid with depression and anxiety (Rubin, 2007; Tunks et al., 2008), we had expected the shift in brain activity to be accompanied by, and dependent on, increases in depression and/or anxiety. Here, we see that the transitional shift in brain activity is, in fact, independent of the latter factors in persisting SBP, indicating that the early SBP reorganization is not necessarily predicated on psychological comorbidities. Rather, the properties that capture the qualitative subjective salience of the pain seem sufficient to shift its representation from acute pain to emotion circuitry.
Mechanistic considerations
In the 1-year period marking the transition to chronic pain in persisting SBP, but not in recovering SBP, the brain undergoes regional morphological changes, including decreased grey matter density in insula, S1/M1 and nucleus accumbens, and related alterations in functional connectivity, which collectively correlate with back pain intensity (Baliki et al., 2012). Within this same time frame, brain activity related to back pain shifts from the insula, anterior cingulate cortex, thalamus, and basal ganglia to medial prefrontal cortex, amygdala and basal ganglia. We surmise that all these processes are inter-related and define the transition to chronic pain. The specific mechanistic relationships remain to be identified. Yet, the baseline medial prefrontal cortex-nucleus accumbens functional connectivity strength, which is stable over time and predicts the development of CBP with 80% accuracy, suggests that enhanced mesolimbic circuitry drives brain reorganization. Here we tested this hypothesis directly in persisting SBP and demonstrated a direct relationship between extent of shift of back pain-related brain activity (from pain to emotion related regions) reorganization over 1 year and medial prefrontal cortex–nucleus accumbens functional connectivity strength at time of entry into the study. Therefore, we postulate that the predisposing characteristics of mesolimbic circuitry at time of pain inception initiates a cascade of emotionally-driven learning events that effectually reorganize the brain into a chronic pain state with distinct functional, anatomical and resting state properties (Fields, 2006; Apkarian, 2008). The precise details of underlying mechanisms remain to be unravelled, as well as the inter-relationship between cortico-mesolimbic reorganization with the peripheral and spinal cord reorganization that has been extensively documented in animal models (Julius and Basbaum, 2001; Costigan et al., 2009).
Theoretical implications: localization versus construction of the mind from the brain
Our results show that the perception of back pain is associated with two grossly distinct spatial patterns of brain activity, depending on the time lapsed from inception of the pain. We demonstrate this principle both within and across patient groups, for matching pain intensities and for matching levels of anxiety and depression (as specifically observed in persisting SBP). Therefore, we show for the first time that two distinct and stable cortical activity patterns, macroscopically located in distinct parts of the cortex, can encode a unitary perception of pain derived from verbal reports. The latter observation poses the challenge that either the reported pain is a poor verbal descriptor of subjectively subtly distinct states (the Wittgenstein position); or alternatively, the brain localization (or specificity) theory—defined as discrete perceptual categories consistently and specifically corresponding to distinct brain circuits [(Lindquist et al., 2012), perhaps first conceptualized by Descartes (Finger, 2001), later expounded into phrenology by Gall and Spurzheim 1835 (Gall, 1835), and in modern neuroscience commonly referred as ‘particular circuits’ (Kandel, 1992)]—is not tenable. The notion of subjectivity, and thus the verbal incommunicability, of personal pain was seminal in Wittgenstein’s abandonment of logic and his subsequent emphasis on language-based philosophical inquiry (Wittgenstein, 1953). To overcome these difficulties, modern pain researchers use long lists of questionnaires to interrogate the patient about the cognitive and emotional disturbances that may distinguish between pain states. However in the current study, the back pain, whether it lasts a few months or a year, is described with equivalent descriptive characteristics, and thus limitations inherent in verbal communication cannot explain dual brain representations of back pain. Instead, the results challenge localization theory by demonstrating large-scale changes in brain anatomical and functional connectivity with sustained back pain (Baliki et al., 2012), some of which may be reversed with successful therapy (Seminowicz et al., 2011). Therefore, the spatial shift in brain areas encoding back pain are accompanied by, and thus reflect, reorganized brain network properties. This is consistent with evidence that the acute pain circuit itself may not be specific for pain perception (Iannetti and Mouraux, 2010; Mouraux et al., 2011) [note, however, the recent strong evidence for a brain activity signature for acute pain (Wager et al., 2013)], with neurobiological theories (McIntosh, 2000; Sporns et al., 2004) that emphasize the importance of the interaction between brain regions in explaining brain function, and with accumulating evidence that distinct emotions cannot be spatially mapped to specific brain regions, and instead they must be emergent properties of interactive networks (Lindquist et al., 2012).
From a localization viewpoint of emotions, the shift of back pain-related activity from the acute pain circuit to medial prefrontal cortex/amygdala suggests that the perception is transformed from pain-oriented to a focus on the specific emotions of fear, anger and sadness (Murphy et al., 2003; Johansen et al., 2011). Yet, this seems to be a highly artificial conclusion, as it suggests that these patients are conflating fear and sadness with back pain. Instead, we interpret our results as complimentary to and consistent with recent ideas regarding the relationship between the brain and emotions (Kober et al., 2008; Hamann, 2012; Lindquist et al., 2012). Meta-analyses of brain imaging studies for emotions find little evidence that discrete emotion categories can be consistently localized to distinct brain regions. Rather, they hypothesize a psychological constructionist theory that posits that emotions of a certain type are constructed from more general brain modules whose function is not specific to that emotion, or to emotions at all, thereby emphasizing context and prior learning in shaping an emotional experience. Within this framework, the amygdala is implicated in the more general functions of orienting to motivationally relevant, or novel, or uncertain stimuli (Wilson and Rolls, 1993; Breiter et al., 1996; Holland and Gallagher, 1999; Herry et al., 2007; Blackford et al., 2010; Moriguchi et al., 2011), and that it preferentially responds to salient sensations (Lindquist et al., 2012). On the other hand, the medial prefrontal cortex is associated with the process of assigning meaning to sensory cues, based on stored memories of prior experiences (Vincent et al., 2006; Bar, 2009; Mitchell, 2009), and in linking episodic memory with affective qualities of sensory events, and as such playing a unique role in transducing concepts into affective behavioural and physiological responses (Roy et al., 2012). Given that the definition of pain is ‘an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage’ (Merskey and Bogduk, 1994), we interpret the observed spatial shift in back pain representation as evidence for the pain percept reflecting less its sensory properties and instead becoming a heightened and more complex emotional state, constructed from learning and resultant memory traces of the presence and persistence of the condition, which orient the motivational preferences of the subject toward ‘suffering’ with the condition.
Technical considerations
One can always argue that the functional MRI technique is not sensitive enough to detect the nociceptive-specific neurons that, in a fixed location in the cortex, encode back pain. Given that a single functional MRI voxel reflects average activity of ∼20 million neurons (Logothetis, 2008) and only a handful of nociceptive neurons have been described in the cortex (Kenshalo and Isensee, 1983; Peyron et al., 2002; Chen et al., 2009; Benison et al., 2011; Vierck et al., 2013), their presence may in fact not be detected with functional MRI. Yet, those neurons would still be correlated to the large-scale circuits we identify for back pain, and thus their identification would not resolve the issues we have discussed regarding the interface between brain representation and perception.
The meta-analytic maps provide a powerful tool for testing hypotheses related to brain structure and function relationships, as illustrated with the present results. Yet, the term-to-circuit correspondence should not be interpreted as a unique relationship. For example, the pain circuit, although observed in more than 200 studies, reflects activity related to acute pain, and is likely not specific to nociception (Baliki et al., 2009; Iannetti and Mouraux, 2010; Mouraux et al., 2011; Yarkoni et al., 2011). Similarly, the reward circuit, although best characterized for positive rewards (Kalivas et al., 2005; Taha and Fields, 2006), also encodes value for negative reinforcement for aversive conditions (Maeda and Mogenson, 1982; Schultz and Romo, 1987; Bassareo et al., 2002; Ungless et al., 2004; Badrinarayan et al., 2012), as well as for pain (Becerra et al., 2001; Seymour et al., 2005; Scott et al., 2006; Baliki et al., 2010; Navratilova et al., 2012), and is more generally involved in emotionally learned motivated behaviour (Reynolds and Berridge, 2002; Fields, 2006; Berridge, 2007; Fields et al., 2007). The emotion circuit is intimately linked with the reward circuit, as it modulates the properties of the latter and, as expounded above, should more generally be viewed as part of the salience and value-based circuitry controlling motivated behaviour. Note also that there are significant overlaps between these circuits, especially between reward and emotion. Thus, classification of brain circuitry using such meta-analytic maps should be interpreted with caution. We used the reverse inference maps of Neurosynth as they are the more stringent and specific brain activity-to-term relationships (Yarkoni et al., 2011). Regarding formal Bayesian inference, our results demonstrate a mismatch between forward and reverse inference for back pain and thus provide an example that, at least under some specific conditions, reverse inference can be incorrect, thereby adding to the list of pitfalls associated with the use of brain imaging data to identify mental states or processes (Poldrack, 2011).
Conclusion
Brain activity related to the perception of back pain shifts in location from regions involved in acute pain to engage emotion circuitry as the condition persists, thereby providing a percept-linked brain signature for the transition to chronic pain. We provide a spatial template and time window (6–12 months) for the stabilization of this signature, which identifies a specific functional biomarker for back pain chronification. Thus, these results have important clinical implications regarding the definition of chronic pain, its aetiology, and the optimal time window for treatments targeting its prevention. Additionally, these results challenge long standing theoretical constructs of brain-mind relationships.
Supplementary Material
Acknowledgements
We thank all participants and the Apkarian lab personnel for help in various aspects of the study and insightful discussions, especially Dr. M. Farmer for help in editing the manuscript. We also acknowledge Dr. Todd Parrish and personnel at the Northwestern University brain imaging core facility (Center for Translational Imaging) for help in data collection.
Glossary
Abbreviations
- CBP
chronic back pain
- SBP
subacute back pain
Funding
The study was funded by NIH NINDS R01 NS035115 and NIDCR R01 DE022746 (A.V.A.), and by an anonymous foundation (M.N.B.).
Supplementary material
Supplementary material is available at Brain online.
References
- Apkarian AV, Baliki MN, Geha PY. Towards a theory of chronic pain. Prog Neurobiol. 2009;87:81–97. doi: 10.1016/j.pneurobio.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Hashmi JA, Baliki MN. Pain and the brain: specificity and plasticity of the brain in clinical chronic pain. Pain. 2011;152:s49–64. doi: 10.1016/j.pain.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–84. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Sosa Y, Sonty S, Levy RE, Harden RN, Parrish TB, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004;24:10410–5. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Krauss BR, Fredrickson BE, Szeverenyi NM. Imaging the pain of low back pain: functional magnetic resonance imaging in combination with monitoring subjective pain perception allows the study of clinical pain states. Neurosci Lett. 2001;299:57–60. doi: 10.1016/s0304-3940(01)01504-x. [DOI] [PubMed] [Google Scholar]
- Apkarian AV. Pain perception in relation to emotional learning. Curr Opin Neurobiol. 2008;18:464–8. doi: 10.1016/j.conb.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Schnitzer TJ, Bauer WR, Apkarian AV. Brain morphological signatures for chronic pain. PloS One. 2011a;6:e26010. doi: 10.1371/journal.pone.0026010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Baria AT, Apkarian AV. The cortical rhythms of chronic back pain. J Neurosci. 2011b;31:13981–90. doi: 10.1523/JNEUROSCI.1984-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Geha PY, Apkarian AV, Chialvo DR. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J Neurosci. 2008a;28:1398–403. doi: 10.1523/JNEUROSCI.4123-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Chialvo DR, Geha PY, Levy RM, Harden RN, Parrish TB, et al. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci. 2006;26:12165–73. doi: 10.1523/JNEUROSCI.3576-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Geha PY, Fields HL, Apkarian AV. Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron. 2010;66:149–60. doi: 10.1016/j.neuron.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Petre B, Torbey S, Herrmann KM, Huang L, Schnitzer TJ, et al. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci. 2012;15:1117–9. doi: 10.1038/nn.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Geha PY, Jabakhanji R, Harden N, Schnitzer TJ, Apkarian AV. A preliminary fMRI study of analgesic treatment in chronic back pain and knee osteoarthritis. Mol Pain. 2008b;4:47. doi: 10.1186/1744-8069-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Geha PY, Apkarian AV. Parsing pain perception between nociceptive representation and magnitude estimation. J Neurophysiol. 2009;101:875–87. doi: 10.1152/jn.91100.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M. The proactive brain: memory for predictions. Philos Trans R Soc Lond B Biol Sci. 2009;364:1235–43. doi: 10.1098/rstb.2008.0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassareo V, De Luca MA, Di CG. Differential expression of motivational stimulus properties by dopamine in nucleus accumbens shell versus core and prefrontal cortex. J Neurosci. 2002;22:4709–19. doi: 10.1523/JNEUROSCI.22-11-04709.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badrinarayan A, Wescott SA, Vander Weele CM, Saunders BT, Couturier BE, Maren S, et al. Aversive stimuli differentially modulate real-time dopamine transmission dynamics within the nucleus accumbens core and shell. J Neurosci. 2012;32:15779–90. doi: 10.1523/JNEUROSCI.3557-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra L, Breiter HC, Wise R, Gonzalez RG, Borsook D. Reward circuitry activation by noxious thermal stimuli. Neuron. 2001;32:927–46. doi: 10.1016/s0896-6273(01)00533-5. [DOI] [PubMed] [Google Scholar]
- Benison AM, Chumachenko S, Harrison JA, Maier SF, Falci SP, Watkins LR, et al. Caudal granular insular cortex is sufficient and necessary for the long-term maintenance of allodynic behavior in the rat attributable to mononeuropathy. J Neurosci. 2011;31:6317–28. doi: 10.1523/JNEUROSCI.0076-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology. 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Blackford JU, Buckholtz JW, Avery SN, Zald DH. A unique role for the human amygdala in novelty detection. NeuroImage. 2010;50:1188–93. doi: 10.1016/j.neuroimage.2009.12.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, et al. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–87. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Cauda F, Sacco K, Duca S, Cocito D, D'Agata F, Geminiani GC, et al. Altered resting state in diabetic neuropathic pain. PloS One. 2009;4:e4542. doi: 10.1371/journal.pone.0004542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LM, Friedman RM, Roe AW. Area-specific representation of mechanical nociceptive stimuli within SI cortex of squirrel monkeys. Pain. 2009;141:258–68. doi: 10.1016/j.pain.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou R, Shekelle P. Will this patient develop persistent disabling low back pain? JAMA. 2010;303:1295–302. doi: 10.1001/jama.2010.344. [DOI] [PubMed] [Google Scholar]
- Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Ann Rev Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer MA, Baliki MN, Apkarian AV. A dynamic network perspective of chronic pain. Neurosci Lett. 2012;520:197–203. doi: 10.1016/j.neulet.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer MA, Chanda ML, Parks EL, Baliki MN, Apkarian AV, Schaeffer AJ. Brain functional and anatomical changes in chronic prostatitis/chronic pelvic pain syndrome. J Urol. 2011;186:117–24. doi: 10.1016/j.juro.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields HL. Proceedings of the 11th world congress on pain. Seattle, USA: IASP Press; 2006. A motivation-decision model of pain: the role of opioids; pp. 449–59. [Google Scholar]
- Fields HL, Hjelmstad GO, Margolis EB, Nicola SM. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annu Rev Neurosci. 2007;30:289–316. doi: 10.1146/annurev.neuro.30.051606.094341. [DOI] [PubMed] [Google Scholar]
- Finger S. Origins of neuroscience: a history of explorations into brain function. London: Oxford University Press; 2001. [Google Scholar]
- Frank A. Low back pain. BMJ. 1993;306:901–9. doi: 10.1136/bmj.306.6882.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall FJ. On the function of the brain and each of its parts: with observations on the possibility of determining the instincts, propensities and talents, or the moral and intellectual dispositions of men and animals, by the configuration of the brain and head. Boston: Marsh, Capen and Lyon; 1835. [Google Scholar]
- Geha PY, Baliki MN, Harden RN, Bauer WR, Parrish TB, Apkarian AV. The brain in chronic CRPS pain: abnormal gray-white matter interactions in emotional and autonomic regions. Neuron. 2008a;60:570–81. doi: 10.1016/j.neuron.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geha PY, Baliki MN, Chialvo DR, Harden RN, Paice JA, Apkarian AV. Brain activity for spontaneous pain of postherpetic neuralgia and its modulation by lidocaine patch therapy. Pain. 2007;128:88–100. doi: 10.1016/j.pain.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geha PY, Baliki MN, Wang X, Harden RN, Paice JA, Apkarian AV. Brain dynamics for perception of tactile allodynia (touch-induced pain) in postherpetic neuralgia. Pain. 2008b;138:641–56. doi: 10.1016/j.pain.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Castillo J, Saad ZS, Handwerker DA, Inati SJ, Brenowitz N, Bandettini PA. Whole-brain, time-locked activation with simple tasks revealed using massive averaging and model-free analysis. Proc Natl Acad Sci USA. 2012;109:5487–92. doi: 10.1073/pnas.1121049109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashmi JA, Baliki MN, Huang L, Parks EL, Chanda ML, Schnitzer T, et al. Lidocaine patch (5%) is no more potent than placebo in treating chronic back pain when tested in a randomised double blind placebo controlled brain imaging study. Mol Pain. 2012;8:29. doi: 10.1186/1744-8069-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann S. Mapping discrete and dimensional emotions onto the brain: controversies and consensus. Trends Cogn Sci. 2012;16:458–66. doi: 10.1016/j.tics.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Herry C, Bach DR, Esposito F, Di Salle F, Perrig WJ, Scheffler K, et al. Processing of temporal unpredictability in human and animal amygdala. J Neurosci. 2007;27:5958–66. doi: 10.1523/JNEUROSCI.5218-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala circuitry in attentional and representational processes. Trends Cogn Sci. 1999;3:65–73. doi: 10.1016/s1364-6613(98)01271-6. [DOI] [PubMed] [Google Scholar]
- Iannetti GD, Mouraux A. From the neuromatrix to the pain matrix (and back) Exp Brain Res. 2010;205:1–12. doi: 10.1007/s00221-010-2340-1. [DOI] [PubMed] [Google Scholar]
- Johansen JP, Cain CK, Ostroff LE, LeDoux JE. Molecular mechanisms of fear learning and memory. Cell. 2011;147:509–24. doi: 10.1016/j.cell.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–10. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- Kandel ER. Cognitive neurosciences, editorial overview. Curr Opin Neurobiol. 1992;2:143–5. [Google Scholar]
- Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–50. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Kenshalo DR, Jr, Isensee O. Responses of primate SI cortical neurons to noxious stimuli. J Neurophysiol. 1983;50:1479–96. doi: 10.1152/jn.1983.50.6.1479. [DOI] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. NeuroImage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF. The brain basis of emotion: a meta-analytic review. Behav Brain Sci. 2012;35:121–43. doi: 10.1017/S0140525X11000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453:869–78. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- Maeda H, Mogenson GJ. Effects of peripheral stimulation on the activity of neurons in the ventral tegmental area, substantia nigra and midbrain reticular formation of rats. Brain Res Bull. 1982;8:7–14. doi: 10.1016/0361-9230(82)90021-1. [DOI] [PubMed] [Google Scholar]
- Malinen S, Vartiainen N, Hlushchuk Y, Koskinen M, Ramkumar P, Forss N, et al. Aberrant temporal and spatial brain activity during rest in patients with chronic pain. Proc Natl Acad Sci USA. 2010;107:6493–7. doi: 10.1073/pnas.1001504107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May A. Chronic pain may change the structure of the brain. Pain. 2008;137:7–15. doi: 10.1016/j.pain.2008.02.034. [DOI] [PubMed] [Google Scholar]
- McIntosh AR. Towards a network theory of cognition. Neural Netw. 2000;13:861–70. doi: 10.1016/s0893-6080(00)00059-9. [DOI] [PubMed] [Google Scholar]
- Merskey H, Bogduk N. Classification of Chronic Pain. In: Merskey H, Bogduk N, editors. IASP Task Force on Taxonomy. 2nd edn. Seattle: IASP Press; 1994. [Google Scholar]
- Mitchell JP. Inferences about mental states. Philos Trans R Soc Lond Biol Sci. 2009;364:1309–16. doi: 10.1098/rstb.2008.0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouraux A, Diukova A, Lee MC, Wise RG, Iannetti GD. A multisensory investigation of the functional significance of the “pain matrix”. NeuroImage. 2011;54:2237–49. doi: 10.1016/j.neuroimage.2010.09.084. [DOI] [PubMed] [Google Scholar]
- Moriguchi Y, Negreira A, Weierich M, Dautoff R, Dickerson BC, Wright CI, et al. Differential hemodynamic response in affective circuitry with aging: an FMRI study of novelty, valence, and arousal. J Cogn Neurosci. 2011;23:1027–41. doi: 10.1162/jocn.2010.21527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotions: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:207–33. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- Napadow V, Lacount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 2010;62:2545–55. doi: 10.1002/art.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navratilova E, Xie JY, Okun A, Qu C, Eyde N, Ci S, et al. Pain relief produces negative reinforcement through activation of mesolimbic reward-valuation circuitry. Proc Natl Acad Sci USA. 2012;109:20709–13. doi: 10.1073/pnas.1214605109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks EL, Geha PY, Baliki MN, Katz J, Schnitzer TJ, Apkarian AV. Brain activity for chronic knee osteoarthritis: dissociating evoked pain from spontaneous pain. Eur J Pain. 2011;15:843, e1–14. doi: 10.1016/j.ejpain.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron R, Frot M, Schneider F, Garcia-Larrea L, Mertens P, Barral FG, et al. Role of operculoinsular cortices in human pain processing: converging evidence from PET, fMRI, dipole modeling, and intracerebral recordings of evoked potentials. Neuroimage. 2002;17:1336–46. doi: 10.1006/nimg.2002.1315. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. Inferring mental states from neuroimaging data: from reverse inference to large-scale decoding. Neuron. 2011;72:692–7. doi: 10.1016/j.neuron.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–72. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Positive and negative motivation in nucleus accumbens shell: bivalent rostrocaudal gradients for GABA-elicited eating, taste “liking”/“disliking” reactions, place preference/avoidance, and fear. J Neurosci. 2002;22:7308–20. doi: 10.1523/JNEUROSCI.22-16-07308.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DI. Epidemiology and risk factors for spine pain. Neurol Clin. 2007;25:353–71. doi: 10.1016/j.ncl.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Roy M, Shohamy D, Wager TD. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn Sci. 2012;16:147–56. doi: 10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminowicz DA, Wideman TH, Naso L, Hatami-Khoroushahi Z, Fallatah S, Ware MA, et al. Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. J Neurosci. 2011;31:7540–50. doi: 10.1523/JNEUROSCI.5280-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Romo R. Responses of nigrostriatal dopamine neurons to high-intensity somatosensory stimulation in the anesthetized monkey. J Neurophysiol. 1987;57:201–17. doi: 10.1152/jn.1987.57.1.201. [DOI] [PubMed] [Google Scholar]
- Seymour B, O'Doherty JP, Koltzenburg M, Wiech K, Frackowiak R, Friston K, et al. Opponent appetitive-aversive neural processes underlie predictive learning of pain relief. Nat Neurosci. 2005;8:1234–40. doi: 10.1038/nn1527. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Heitzeg MM, Koeppe RA, Stohler CS, Zubieta JK. Variations in the human pain stress experience mediated by ventral and dorsal basal ganglia dopamine activity. J Neurosci. 2006;26:10789–95. doi: 10.1523/JNEUROSCI.2577-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl 1):208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sporns O, Chialvo DR, Kaiser M, Hilgetag CC. Organization, development and function of complex brain networks. Trends Cogn Sci. 2004;8:418–25. doi: 10.1016/j.tics.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Staud R, Bovee CE, Robinson ME, Price DD. Cutaneous C-fiber pain abnormalities of fibromyalgia patients are specifically related to temporal summation. Pain. 2008;139:315–23. doi: 10.1016/j.pain.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliazucchi E, Balenzuela P, Fraiman D, Chialvo DR. Brain resting state is disrupted in chronic back pain patients. Neurosci Lett. 2010;485:26–31. doi: 10.1016/j.neulet.2010.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha SA, Fields HL. Inhibitions of nucleus accumbens neurons encode a gating signal for reward-directed behavior. J Neurosci. 2006;26:217–22. doi: 10.1523/JNEUROSCI.3227-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey I, Bushnell MC. How neuroimaging studies have challenged us to rethink: is chronic pain a disease? J Pain. 2009;10:1113–20. doi: 10.1016/j.jpain.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Tunks ER, Crook J, Weir R. Epidemiology of chronic pain with psychological comorbidity: prevalence, risk, course, and prognosis. Can J Psychiatry. 2008;53:224–34. doi: 10.1177/070674370805300403. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Magill PJ, Bolam JP. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science. 2004;303:2040–2. doi: 10.1126/science.1093360. [DOI] [PubMed] [Google Scholar]
- Vierck CJ, Whitsel BL, Favorov OV, Brown AW, Tommerdahl M. Role of primary somatosensory cortex in the coding of pain. Pain. 2013;154:334–44. doi: 10.1016/j.pain.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, et al. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J Neurophysiol. 2006;96:3517–31. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- Wager TD, Atlas LY, Lindquist MA, Roy M, Woo CW, Kross E. An fMRI-based neurologic signature of physical pain. N Engl J Med. 2013;368:1388–97. doi: 10.1056/NEJMoa1204471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman-Fogel I, Moayedi M, Tenenbaum HC, Goldberg MB, Freeman BV, Davis KD. Abnormal cortical activity in patients with temporomandibular disorder evoked by cognitive and emotional tasks. Pain. 2011;152:384–96. doi: 10.1016/j.pain.2010.10.046. [DOI] [PubMed] [Google Scholar]
- Wilson FA, Rolls ET. The effects of stimulus novelty and familiarity on neuronal activity in the amygdala of monkeys performing recognition memory tasks. Exp Brain Res. 1993;93:367–82. doi: 10.1007/BF00229353. [DOI] [PubMed] [Google Scholar]
- Wittgenstein L. Philosophical investigations [english translation. 2001. Oxford: Blackwell Publishing; 1953. [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 2004;21:1732–47. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14:1370–86. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8:665–70. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.