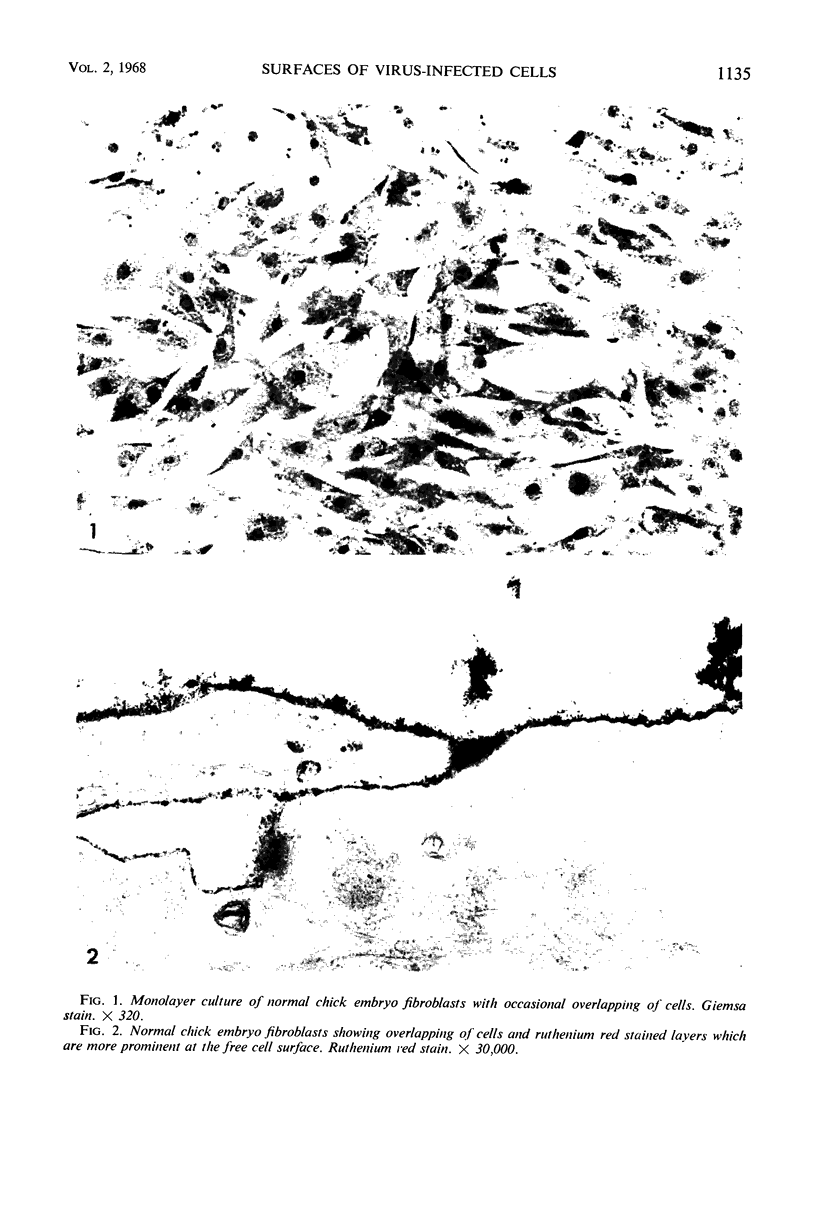
Abstract
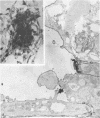
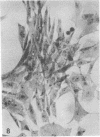
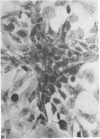
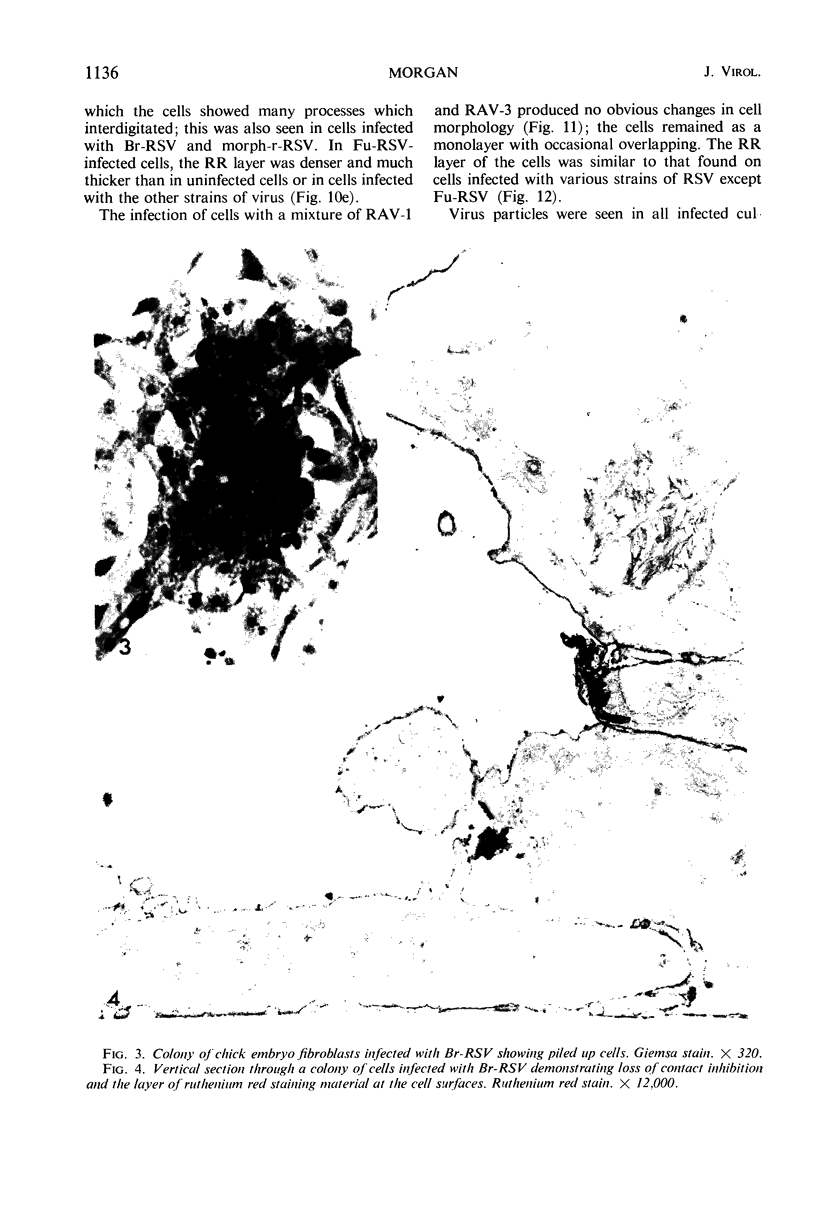
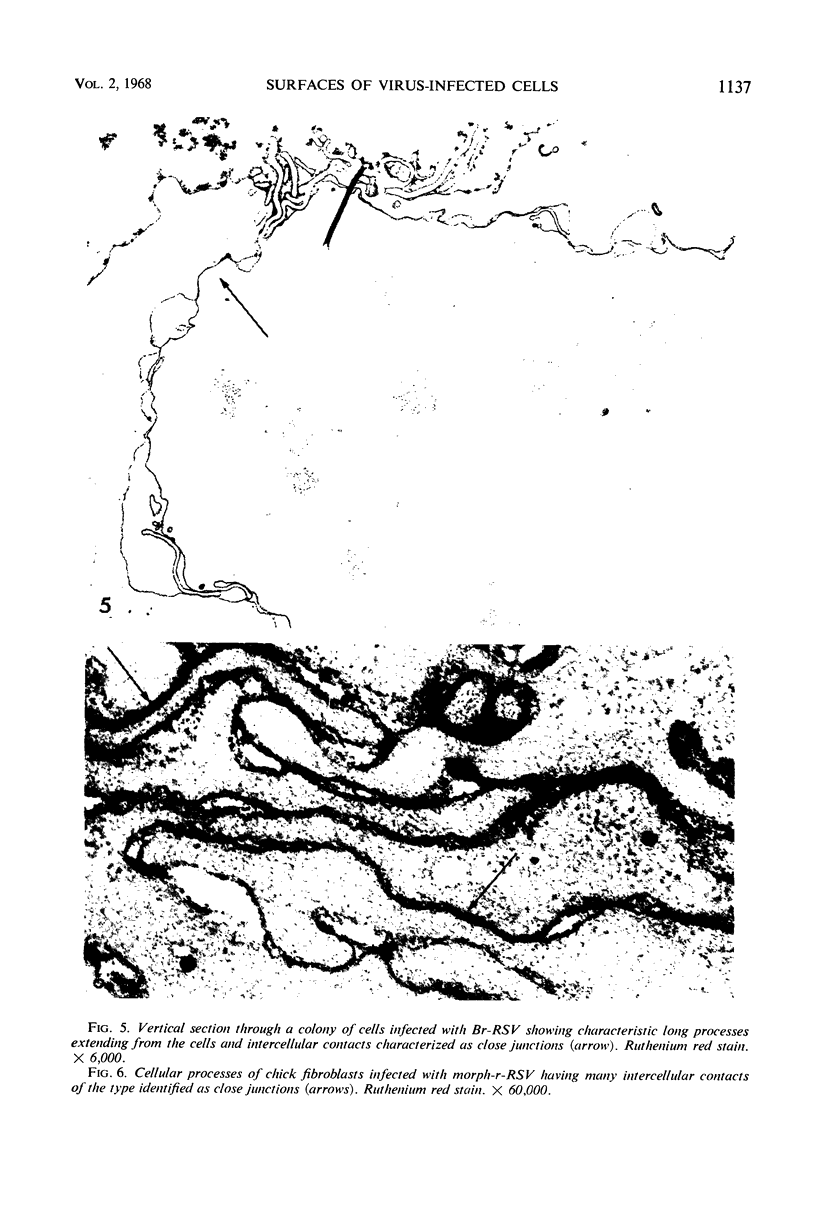
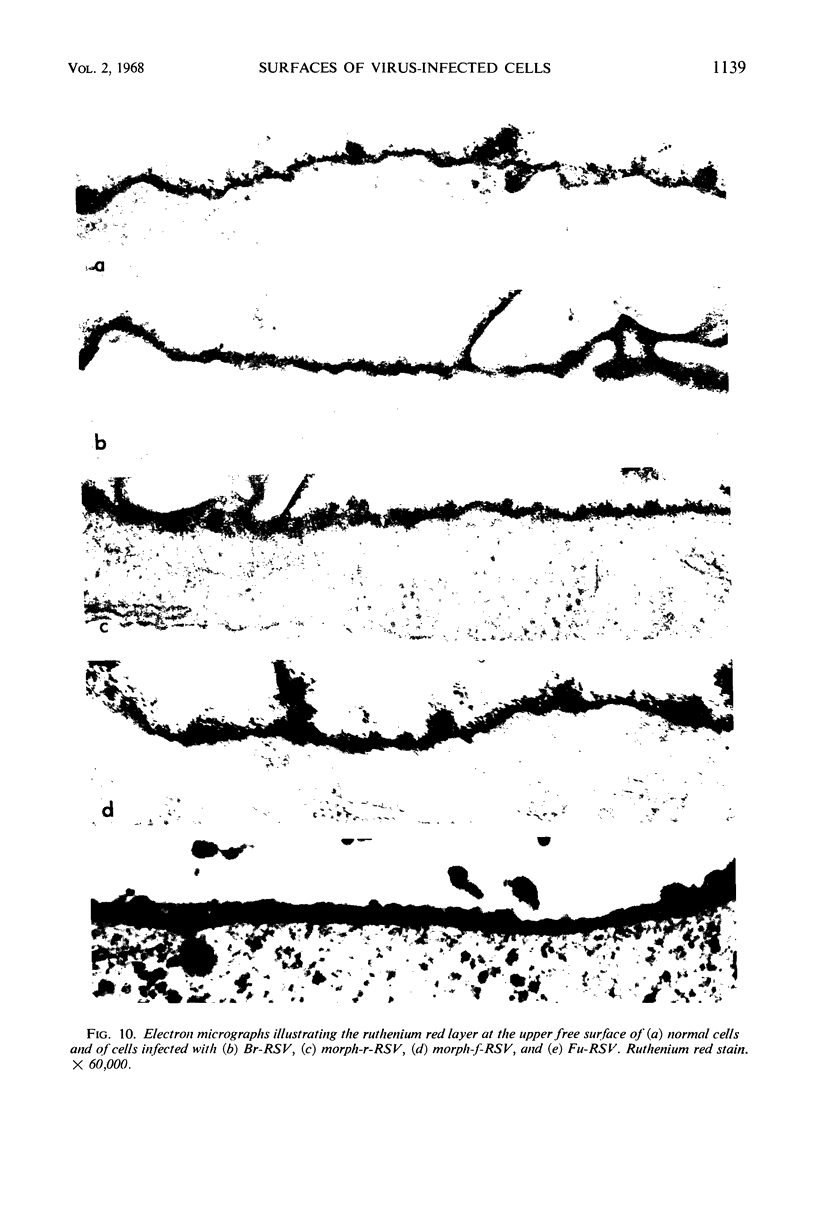
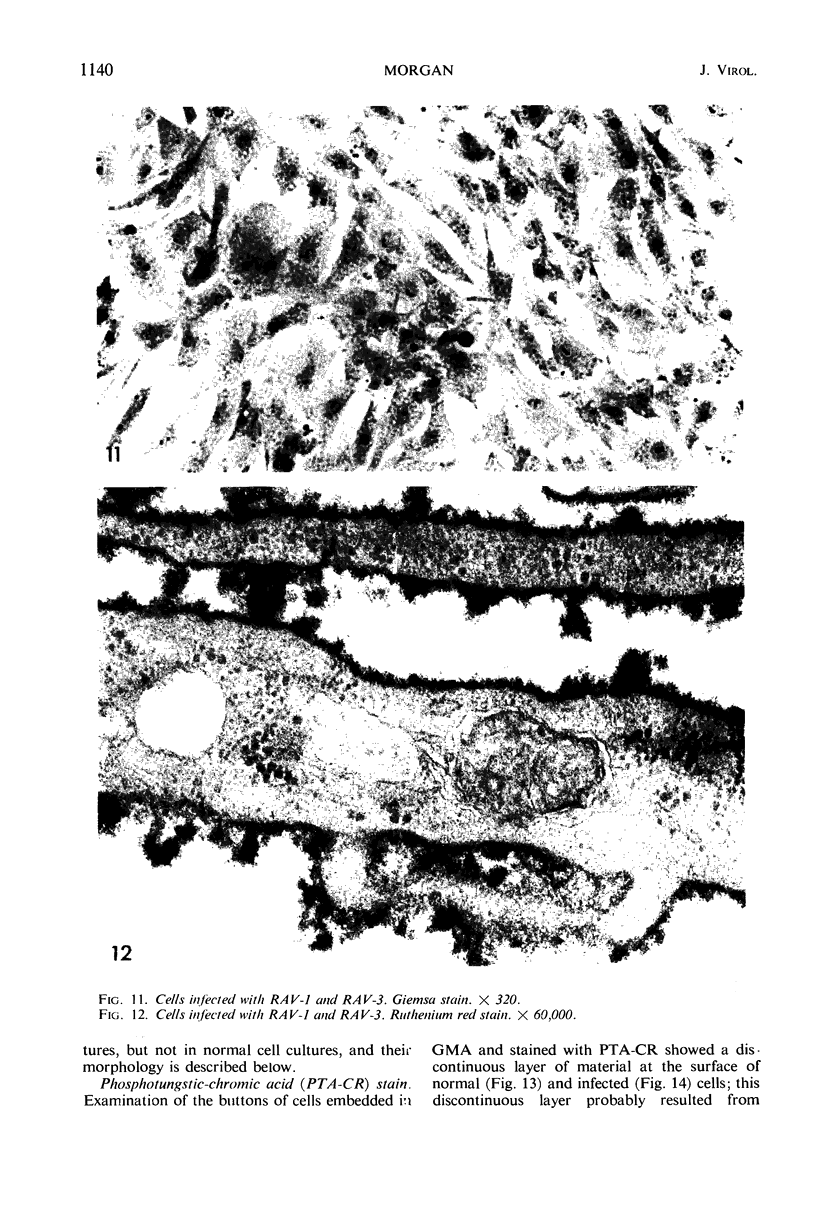
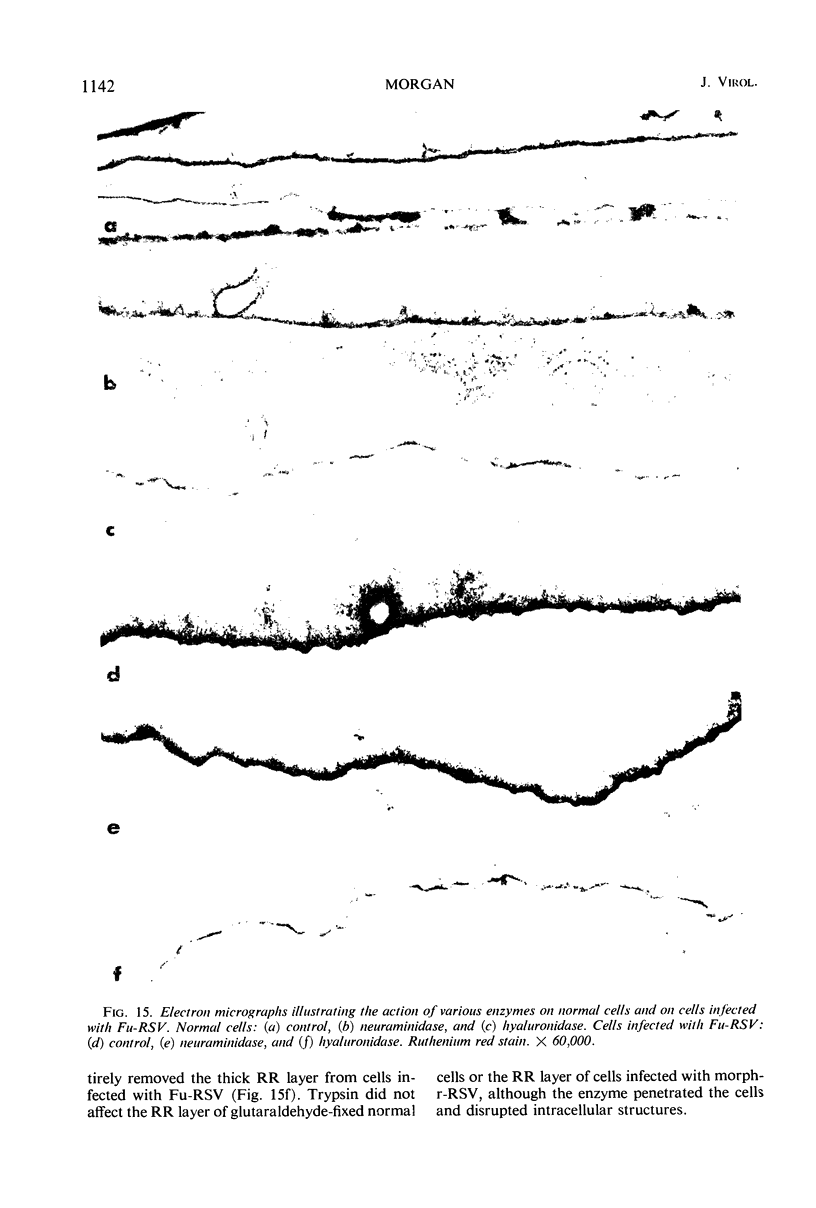
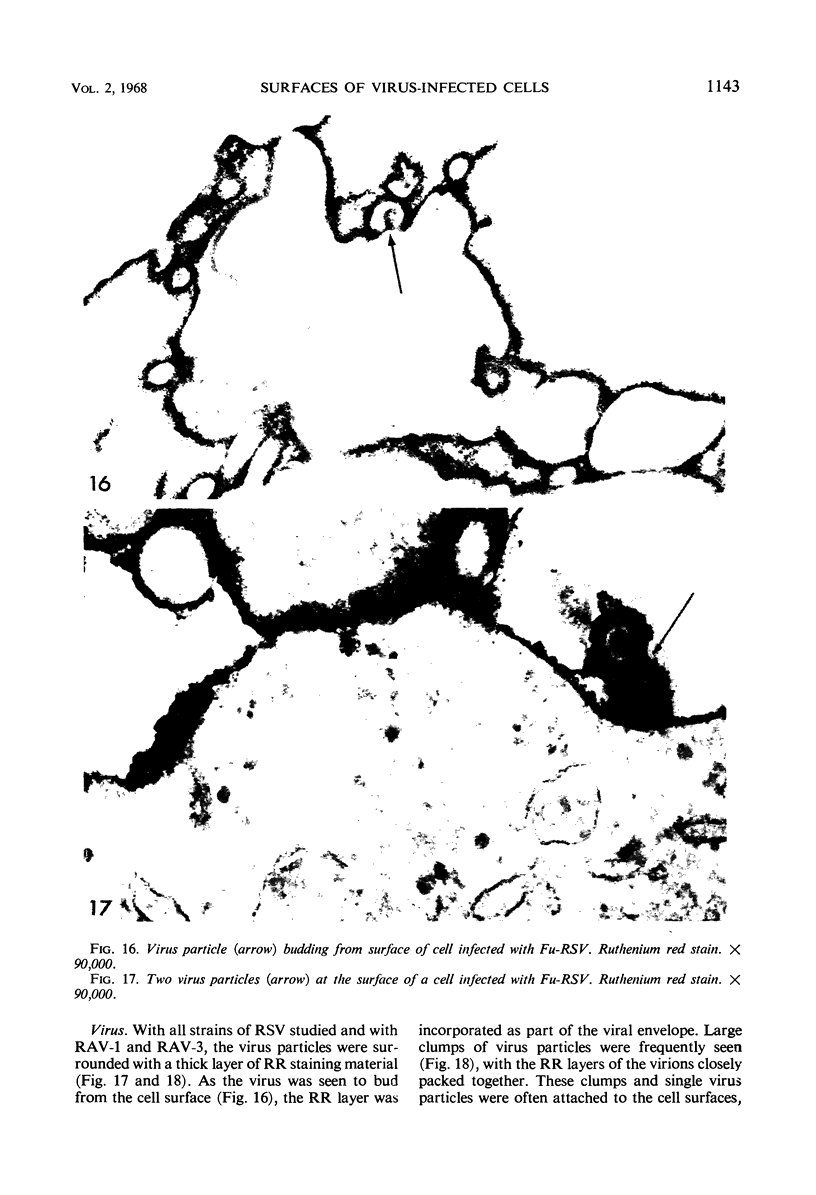
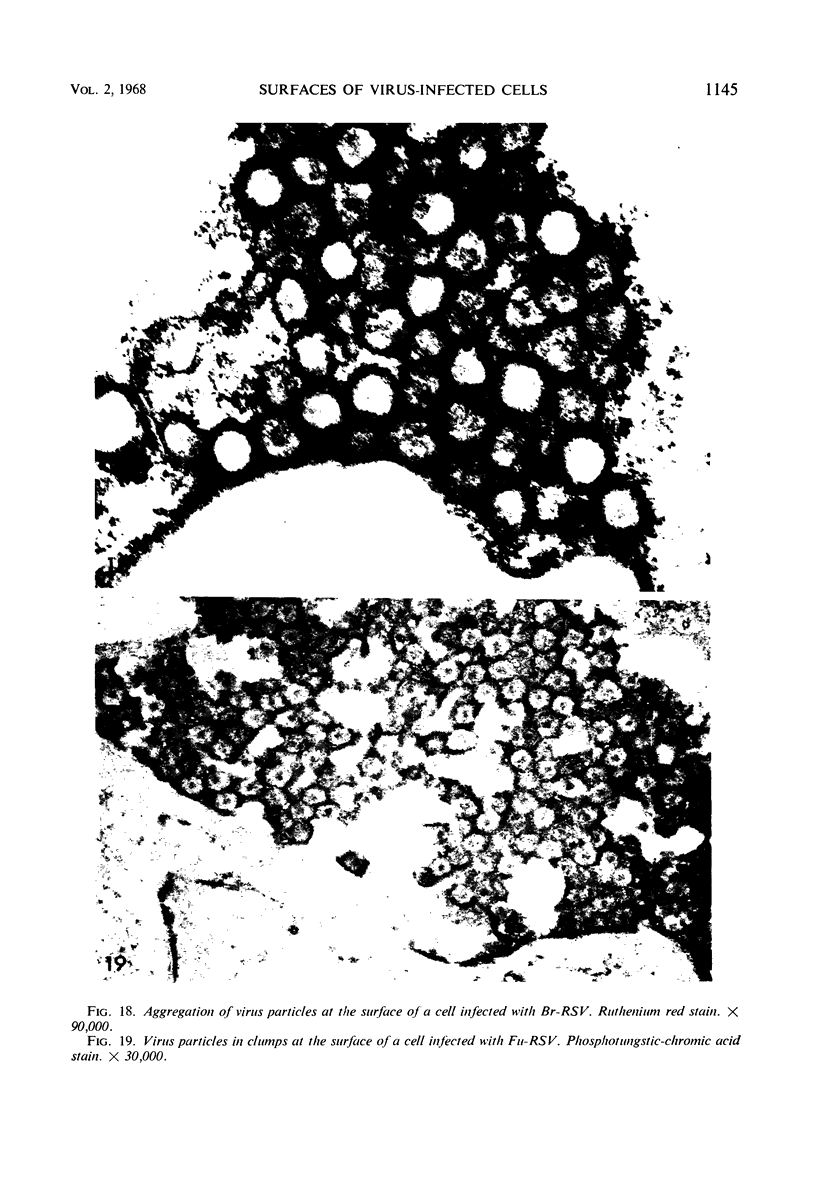
When stained with ruthenium red (RR), chick embryo cells infected with various strains of Rous sarcoma virus (RSV) and with avian leukosis viruses RAV-1 and RAV-3 showed an increase in the layer of acid mucopolysaccharides (AMPS) at their surfaces as compared with uninfected cells. This increase was most prominent in cells infected with the Fujinami strain of RSV. The layer was resistant to digestion with neuraminidase or trypsin but was readily removed by exposure to hyaluronidase. The thickness of this AMPS layer was not correlated with the varying degree of loss of contact inhibition exhibited by cells infected with the different strains of virus. The staining of the cell envelope with a solution of phosphotungstic and chromic acids (PTA-CR) suggested the presence of glycoproteins. The outer surface of the virions showed the same staining as the cell surface with RR and PTA-CR, and the budding virus particle was seen to incorporate the RR layer of the cell into its structure. The RR layers of cells and virions appeared to fuse, as did those between virus particles, suggesting that these layers play a role in the aggregation of virus particles and in their adherence to the surface of the cell.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABERCROMBIE M., AMBROSE E. J. The surface properties of cancer cells: a review. Cancer Res. 1962 Jun;22:525–548. [PubMed] [Google Scholar]
- DEFENDI V., GASIC G. SURFACE MUCOPOLYSACCHARIDES OF POLYOMA VIRUS TRANSFORMED CELLS. J Cell Physiol. 1963 Aug;62:23–32. doi: 10.1002/jcp.1030620105. [DOI] [PubMed] [Google Scholar]
- HEINE U., DE THE G., ISHIGURO H., BEARD J. W. Morphologic aspects of Rous sarcoma virus elaboration. J Natl Cancer Inst. 1962 Jul;29:211–223. [PubMed] [Google Scholar]
- Ishimoto N., Temin H. M., Strominger J. L. Studies of carcinogenesis by avian sarcoma viruses. II. Virus-induced increase in hyaluronic acid synthetase in chicken fibroblasts. J Biol Chem. 1966 May 10;241(9):2052–2057. [PubMed] [Google Scholar]
- KIMOTO E., SHETLAR M. R. PROPERTIES OF ROUS SARCOMA HYALURONIC ACID. Proc Soc Exp Biol Med. 1965 Mar;118:627–631. doi: 10.3181/00379727-118-29923. [DOI] [PubMed] [Google Scholar]
- Leduc E. H., Bernhard W. Recent modifications of the glycol methacrylate embedding procedure. J Ultrastruct Res. 1967 Jul;19(1):196–199. doi: 10.1016/s0022-5320(67)80068-6. [DOI] [PubMed] [Google Scholar]
- Luft J. H. Fine structures of capillary and endocapillary layer as revealed by ruthenium red. Fed Proc. 1966 Nov-Dec;25(6):1773–1783. [PubMed] [Google Scholar]
- Martinez-Palomo A., Brailovski C. Surface layer in tumor cells transformed by adeno-12 and SV40 viruses. Virology. 1968 Feb;34(2):379–382. doi: 10.1016/0042-6822(68)90255-9. [DOI] [PubMed] [Google Scholar]
- Morgan H. R. Antibodies for Rous sarcoma virus in fowl, animal, and human populations of East Africa. I. Antibodies in domestic chickens and wildfowl. J Natl Cancer Inst. 1965 Dec;35(6):1043–1045. [PubMed] [Google Scholar]
- Temin H. M. The mechanism of carcinogenesis by avian sarcoma viruses. 1. Cell multiplication and differentiation. J Natl Cancer Inst. 1965 Oct;35(4):679–693. [PubMed] [Google Scholar]