Abstract
Prospective studies of infants at risk for autism spectrum disorder have provided important clues about the early behavioural symptoms of autism spectrum disorder. Diagnosis of autism spectrum disorder, however, is not currently made until at least 18 months of age. There is substantially less research on potential brain-based differences in the period between 6 and 12 months of age. Our objective in the current study was to use magnetic resonance imaging to identify any consistently observable brain anomalies in 6–9 month old infants who would later develop autism spectrum disorder. We conducted a prospective infant sibling study with longitudinal magnetic resonance imaging scans at three time points (6–9, 12–15, and 18–24 months of age), in conjunction with intensive behavioural assessments. Fifty-five infants (33 ‘high-risk’ infants having an older sibling with autism spectrum disorder and 22 ‘low-risk’ infants having no relatives with autism spectrum disorder) were imaged at 6–9 months; 43 of these (27 high-risk and 16 low-risk) were imaged at 12–15 months; and 42 (26 high-risk and 16 low-risk) were imaged again at 18–24 months. Infants were classified as meeting criteria for autism spectrum disorder, other developmental delays, or typical development at 24 months or later (mean age at outcome: 32.5 months). Compared with the other two groups, infants who developed autism spectrum disorder (n = 10) had significantly greater extra-axial fluid at 6–9 months, which persisted and remained elevated at 12–15 and 18–24 months. Extra-axial fluid is characterized by excessive cerebrospinal fluid in the subarachnoid space, particularly over the frontal lobes. The amount of extra-axial fluid detected as early as 6 months was predictive of more severe autism spectrum disorder symptoms at the time of outcome. Infants who developed autism spectrum disorder also had significantly larger total cerebral volumes at both 12–15 and 18–24 months of age. This is the first magnetic resonance imaging study to prospectively evaluate brain growth trajectories from infancy in children who develop autism spectrum disorder. The presence of excessive extra-axial fluid detected as early as 6 months and the lack of resolution by 24 months is a hitherto unreported brain anomaly in infants who later develop autism spectrum disorder. This is also the first magnetic resonance imaging evidence of brain enlargement in autism before age 2. These findings raise the potential for the use of structural magnetic resonance imaging to aid in the early detection of children at risk for autism spectrum disorder or other neurodevelopmental disorders.
Keywords: autism, magnetic resonance imaging, infant brain development, cerebrospinal fluid, external hydrocephalus
Introduction
Autism spectrum disorder (ASD) affects 1 in 88 children in the USA (CDC, 2012). Diagnosis is made on average at ∼4 years of age (CDC, 2012) and is based on behaviourally defined impairments in social interaction and communication, along with the occurrence of restricted interests and repetitive behaviours (APA, 2000). Early detection is critical because early intervention is effective in decreasing impairments (Dawson et al., 2010) and may result in more positive long-term outcomes for the child (Rogers and Vismara, 2008).
Efforts to identify early risk markers for ASD have benefitted from prospective longitudinal assessments of infant siblings of children with ASD. The recurrence rate of ASD in siblings is 18.7% (Ozonoff et al., 2011), a nearly 20-fold greater risk than in the general population. An additional 20–30% of infant siblings of children with ASD will have other developmental delays (Gamliel et al., 2007; Constantino et al., 2010). Thus, infant sibling studies provide an efficient strategy for identifying early signs of ASD. To date, prospective studies of infant siblings have not identified behavioural differences at 6 months in infants who are ultimately diagnosed with ASD (Rogers, 2009; Ozonoff et al., 2010; Landa et al., 2012). Symptoms more fully diagnostic of ASD appear to develop gradually between 12 and 24 months of age (Zwaigenbaum et al., 2005; Ozonoff et al., 2010; Landa et al., 2012).
Currently, there is no definitive early biological marker for ASD risk. There is some indication of early biological differences in young children who will later be diagnosed with ASD, including larger head size by 4–6 months of age (Lainhart et al., 1997; Courchesne et al., 2003; Dawson et al., 2007; Nordahl et al., 2011). There is also evidence for alterations in event-related potentials (Elsabbagh et al., 2012) and in the microstructural organization of white matter tracts (Wolff et al., 2012) in infants later diagnosed with ASD. It remains unclear, however, whether MRI analyses could detect clinically significant structural anomalies in infants at risk for ASD and whether these would be associated with developing ASD. We conducted a longitudinal MRI study of infant siblings starting at 6 months of age, which was carried out in conjunction with behavioural assessments leading to an outcome classification at 24 months or later (mean age: 32.5 months). Infants were imaged at three time points: 6–9 months, 12–15 months, and 18–24 months of age. Our objective was to identify any consistently observable MRI findings as early as 6 months that differentiate infants who develop ASD from those with typical development or other delays.
Materials and methods
Participants
Participants were recruited from a larger, ongoing longitudinal behavioural study (Ozonoff et al., 2010). From September 2009 to February 2011, all participants recruited to the behavioural study were also invited to take part in the MRI study. During the initial phone screening to determine eligibility in the behavioural study, each parent was asked if they would be interested in the MRI component. Parents who responded affirmatively were described the MRI process and safety screened for any MRI contraindications. This resulted in the recruitment of 64 infant participants into the MRI study, 41 of whom were ‘high-risk’ infants (68% male, n = 28) having at least one older biological sibling with ASD and 23 of whom were ‘low-risk’ infants (65% male, n = 15) having no biological relatives with ASD (to third-degree relatives). Infant participants underwent their first MRI scan at either 6 months (n = 36) or 9 months of age (n = 28), a second MRI scan at 12–15 months, and a third MRI scan at 18–24 months. This study was approved by the UC Davis Institutional Review Board.
Clinical procedures and measures
Assessment visits
Participants were seen in the laboratory for an assessment that measured cognitive, social, communication and motor functioning, along with other experimental measures and parent questionnaires. Infants were scheduled for assessment visits at 6, 9, 12, 15, 18, 24 and 36 months. Participants were classified with a clinical outcome at their 24 and 36 month visits. The following testing was carried out.
The Mullen Scales of Early Learning (Mullen, 1995) is a standardized developmental assessment appropriate for use between birth and 68 months of age. It provides scores in four cognitive areas (expressive language, receptive language, visual reception and fine motor) as well as gross motor functioning. Developmental quotient scores were used, rather than standard scores, in order to avoid truncating the scores of very low performing subjects. Developmental quotient scores for each subtest were calculated using the age-equivalents provided by the Mullen norms, divided by chronological age at the time of testing, and multiplied by 50 (in order to be scaled similarly to the subtest standard scores). The overall developmental quotient score was calculated as the average of the age-equivalent scores on the four cognitive subtests, divided by the child’s chronological age, and multiplied by 100 (in order to be scaled similarly to the Mullen Early Learning Composite).
The Autism Diagnostic Observation Schedule (ADOS; Lord et al., 2002) is a standardized assessment comprised of structured interactions that elicit behaviours diagnostic of ASD. Module 1 of the ADOS (designed for non-verbal children or children with only single words) or Module 2 (children with consistent phrase speech) was administered starting at 18 months. The communication and social interaction total score served as an index of symptom severity (ranging from 0–24). The ADOS yields empirically derived cut-offs for ASD (7 or higher on Module 1, and 8 or higher on Module 2).
Physical measures including head circumference, weight and height were measured at each assessment visit.
Outcome classification
Infants were classified into the following outcomes at their 24- and 36-month visits: ASD, other developmental delays and typical development. To be classified as ASD, the infant was diagnosed by a licensed clinician using DSM-IV criteria (APA, 2000), ADOS scores and clinical observation. To be classified as having typical development, the infant had ADOS and Mullen scores in the normal range, defined as having: (i) an ADOS score <4 on Module 1 or <5 on Module 2; (ii) an overall Mullen developmental quotient score of at least 85 [i.e. 1.5 standard deviation (SD) below the mean of 100]; (iii) at most one Mullen subtest score <35 (i.e. 1.5 SD below the mean of 50); and (iv) no single Mullen subtest score <30 (i.e. 2 SD below the mean of 50). Infants not meeting criteria for either typical development or ASD were classified as having other developmental delays. For statistical comparisons, the typical development classification was further subdivided by risk status, which yielded four outcome groups: (i) ASD; (ii) other developmental delays; (iii) high-risk typical development; and (iv) low-risk typical development.
Imaging procedures
Infants were scanned during natural sleep (Nordahl et al., 2008) at the UC Davis Imaging Research Centre on a 3 Tesla Siemens TIM Trio MRI system using an eight-channel head coil. T1-weighted 3D MP-RAGE structural scans were acquired first (1 mm3 voxels; repetition time = 3200 ms; echo time = 5.08 ms; field of view = 176 mm; 192 sagittal slices). T2-weighted scans were also acquired for radiological evaluation. Success rate in acquiring the MP-RAGE was 78%. A calibration phantom (Phantom Laboratory, Inc.) was scanned at the end of each MRI session in order to produce a 3D image distortion map (Image Owl) and distortion correction was implemented as previously described (Nordahl et al., 2012).
Imaging analysis
Radiologist evaluation
A board-certified paediatric radiologist (S.L.W-G.), unaware of risk group, evaluated all scans for incidental findings (i.e. unexpected asymptomatic brain abnormalities).
Total cerebral volume
Distortion-corrected images were preprocessed by removing non-brain tissue (Smith, 2002) and correcting inhomogeneity (Sled et al., 1998). Each participant’s image was warped to a paediatric template (Nordahl et al., 2011) and a mask of the cerebrum (Schumann et al., 2004) was applied to each image. The cerebrum excludes the brainstem and cerebellum, which yields the total cerebral volume as our main measurement for brain size. The resulting total cerebral volume image was transformed back to native space and cleaned by removing any remaining non-brain voxels (Analyze Software; Robb et al., 1989). Inter-rater reliability checks of the protocol produced an intraclass correlation coefficient of 0.99.
Quantification of extra-axial fluid volume
While reviewing the radiologist evaluations, we found one incidental finding that seemed to be associated with high-risk infants, the presence of ‘prominent extra-axial fluid.’ Extra-axial fluid is characterized by excessive cerebrospinal fluid (CSF) in the subarachnoid space (Figs 1 and 2). This prompted us to develop a quantitative protocol to objectively measure the volume of extra-axial fluid in each participant’s scan. Experimenters unaware of risk group or outcome status manually traced the dura on successive coronal slices throughout the rostrocaudal extent of the brain. The sum of the area contained within the dura yielded the intracranial volume (Fig. 3A). Subtracting the total cerebral volume (Fig. 3B) from the intracranial volume resulted in the volume of extra-axial fluid (Fig. 3C). Since extra-axial fluid was most prominent in the subarachnoid space over the dorsal convexity of the brain, we defined a ventral boundary of our region of interest as a horizontal slice through the anterior commissure. Defining this boundary had the added benefit of avoiding the complication of interpreting extra-axial fluid in ventral regions where identifying the dura is difficult and where there are sinuses and vasculature that should not be classified as CSF. Reliability checks of the protocol produced an intraclass correlation coefficient of 0.99.
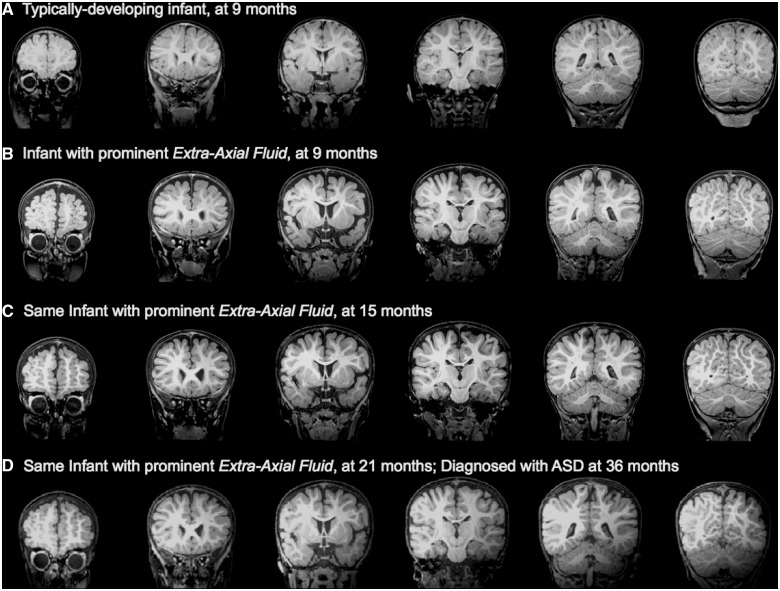
Figure 1.
(A) Low-risk infant with normal MRI at 9 months, confirmed as having typical development at 36 months. (B) High-risk infant with excessive extra-axial fluid at 9 months. (C) The same high-risk infant with excessive extra-axial fluid still present at 15 months, and (D) at 21 months; infant was diagnosed with ASD at 36 months.
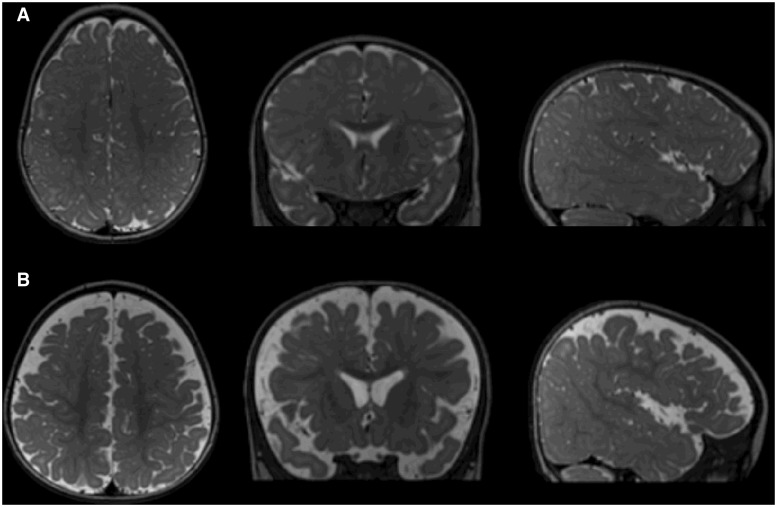
Figure 2.
(A) T2-weighted image of a low-risk infant with normal MRI at 9 months, confirmed as having typical development at 36 months. CSF is indicated as brighter regions in these images. Images are of a horizontal section (left) coronal section (middle) and sagittal section (right) through the brain. (B) Similar T2-weighted images of a high-risk infant with excessive extra-axial fluid at 9 months, diagnosed with ASD at 36 months.
Figure 3.
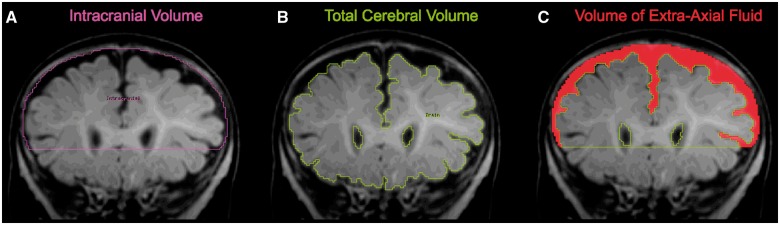
Quantification of extra-axial fluid. (A) Manual tracing of dura on successive coronal slices are summed to yield intracranial volume; (B) semi-automated tissue segmentation with manual intervention yields total cerebral volume; (C) resulting space between dura and cerebral surface yields extra-axial fluid volume.
Lateral ventricle volume
In addition to quantifying the amount of CSF in the subarachnoid space, we quantified the amount of CSF in the ventricular system by measuring the volume of the lateral ventricles. Distortion-corrected images were preprocessed by removing non-brain tissue (Smith, 2002), correcting inhomogeneity (Sled et al., 1998) and segmenting CSF from brain tissue (Zhang et al., 2001). An experimenter unaware of risk or outcome status defined the lateral ventricles from the resulting CSF image on successive coronal slices. Intraclass correlation coefficient was 0.99.
Analytical approach
We used a linear mixed model for repeated measures approach to the analysis of the data, with MRI time point as the within subjects factor. We tested whether outcome group (i.e. ASD, other developmental delays, high-risk typical development, low-risk typical development) significantly explained the individual variability of each MRI measure—total cerebral volume, extra-axial fluid and lateral ventricle volume—above and beyond covariates of age, weight and gender. A model for each MRI measure examined covariates and outcome group in succession, testing the unique effect of each subsequent variable as a chi-square test for the improvement of model fit. Aside from age, variables that did not uniquely contribute to the MRI variable (i.e. a significant main effect was not found to improve model fit) were not retained in the model. To examine the effect of outcome group, the ASD group was designated as the reference group for a priori comparisons between groups. All analyses were performed using SAS JMP software (SAS Institute).
Results
Sample description
Table 1 shows the sample characteristics by risk group. Of the original 64 infants in the study, nine infants underwent their first MRI but did not return for their 24 or 36-month outcome visits, and thus were not included in the analysis. There were no differences in developmental quotient between the nine infants who were dropped from the study and the remaining sample (n = 55). The groups comprised of the remaining 33 high-risk and 22 low-risk infants did not differ significantly on gender, age at Time 1 MRI, interval between MRI time points, or age at outcome assessment (Table 1). Analyses included these 55 infants who were successfully scanned at Time 1 (6–9 months). Of these infants, 43 were scanned again at Time 2 (12–15 months), and 42 were scanned again at Time 3 (18–24 months).
Table 1.
Sample characteristics by autism risk group
| Autism risk group, mean (SD) |
||
|---|---|---|
| Variable | High-risk | Low-risk |
| (n = 33) | (n = 22) | |
| Gender (% male) | 66.7% (n = 22) | 68.2% (n = 15) |
| Age at Time 1, months | 7.3 (1.5) | 8.0 (1.6) |
| Time 1 to Time 2 interval, months | 6.1 (0.6) | 6.2 (0.5) |
| Time 2 to Time 3 interval, months | 6.5 (1.1) | 7.1 (1.4) |
| Age at outcome, months | 33.3 (5.3) | 31.2 (6.4) |
| Mullen overall developmental quotient at outcome** | 93.1 (16.4) | 105.9 (10.4) |
| ADOS algorithm score at outcome*** | 7.1 (4.9) | 2.2 (1.5) |
| ASD diagnosis† | 30.3% (n = 10) | 0.0% (n = 0) |
| Other developmental delays† | 24.2% (n = 8) | 13.6% (n = 3) |
| Typical development | 45.5% (n = 15) | 86.4% (n = 19) |
**P < 0.005; ***P < 0.0001 difference between high-risk versus low-risk groups on outcome scores.
†High-risk infants were significantly more likely than low-risk infants (P < 0.005) to be classified as ASD or other developmental delays.
Data is reported as mean (SD), unless otherwise noted as % of group (n = number of subjects).
Outcome classifications
All infants in the study (n = 55) received an outcome classification at 24 months or later; a majority of these infants (38 of 55) have reached 36 months, and their outcome classification was also determined at this visit. Ten infants (eight male, two female; all high-risk; 30.3% of high-risk group) received an ASD classification at 24 months. Eight of the 10 infants have reached 36 months and have had their ASD diagnosis confirmed at this visit. None of the ASD diagnoses made at 24 months were changed at 36 months. There were 24.2% of the high-risk group (8 of 33) and 13.6% of the low-risk group (3 of 22) who were classified as having other developmental delays. There were 45.5% of the high-risk group (15 of 33) and 86.4% of the low-risk group (19 of 22) who were determined to have typical development. Table 2 shows behavioural measures at outcome for each group.
Table 2.
Descriptive statistics by outcome group
| Outcome group, mean (SD) |
||||
|---|---|---|---|---|
| Variable | ASD | Other delays | High-risk typical | Low-risk typical |
| Number of subjects | 10 | 11 | 15 | 19 |
| Age at outcome, months | 33.7 (5.3) | 31.9 (5.9) | 33.2 (5.5) | 31.7 (6.4) |
| Mullen overall developmental quotient** | 77.37 (13.77) | 93.37 (13.79) | 104.38 (9.87) | 107.11 (8.83) |
| Expressive language*** | 34.24 (8.62) | 48.00 (5.26) | 51.01 (4.58) | 51.54 (8.43) |
| Receptive language** | 33.99 (11.85) | 46.47 (12.54) | 51.59 (8.83) | 56.81 (6.05) |
| Fine motor* | 41.38 (5.36) | 47.38 (9.69) | 48.03 (4.22) | 48.57 (4.64) |
| Visual reception | 45.12 (10.18) | 44.88 (6.57) | 58.15 (10.43) | 57.29 (9.35) |
| Gross motor* | 39.98 (3.79) | 47.33 (2.04) | 52.45 (5.32) | 51.17 (4.87) |
| ADOS communication + social*** | 13.30 (2.67) | 5.73 (2.72) | 3.20 (2.04) | 2.00 (1.45) |
*P < 0.05; **P < 0.005; ***P < 0.0001 ASD versus all other comparison groups.
Mullen subtest scores have standardized mean = 50, SD = 10.
Data is reported as mean (SD), unless otherwise noted as number of subjects.
Total cerebral volume
Age, gender and weight all had significant effects on total cerebral volume. Total cerebral volume increased with age between the three time points (χ2 = 54.19, df = 1, P < 0.0001), was higher in males than females (χ2 = 19.26, df = 1, P < 0.0001), and was higher for children who weighed more (χ2 = 22.93, df = 1, P < 0.0001). Above and beyond these variables, there was a significant outcome group × age interaction (χ2 = 10.93, df = 3, P = 0.01) and significant main effect of outcome group (χ2 = 26.94, df = 3, P < 0.0001), which indicates that the ASD group had a significantly faster growth trajectory of total cerebral volume than the other groups, and by 12–15 months, had significantly larger total cerebral volume. Figure 4 shows the estimated marginal means for total cerebral volume at each MRI time point. Infants who developed ASD had 7% larger total cerebral volume than low-risk typical infants by 12–15 months (P < 0.05) and 8% larger total cerebral volume at 18–24 months (P < 0.01). There were no interactions with gender.
Figure 4.
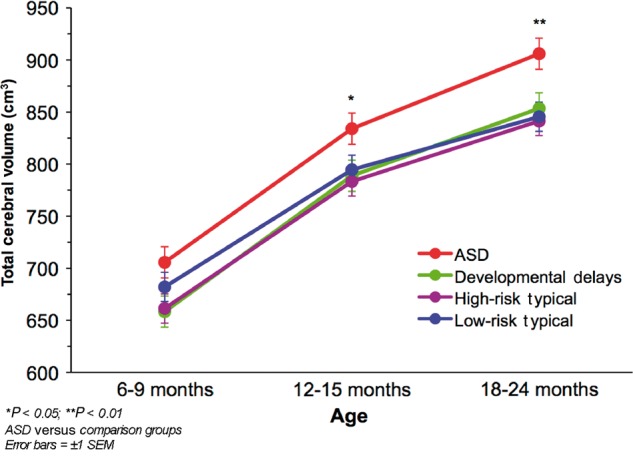
Mean total cerebral volume by outcome group at each MRI time point.
Quantified volume of extra-axial fluid
Outcome group was a significant predictor of extra-axial fluid (χ2 = 28.35, df = 3, P < 0.0001) after controlling for differences in age and total cerebral volume. The ASD group had significantly greater extra-axial fluid than all other groups (Figs 1 and 2). Post hoc comparisons revealed no significant differences between the three remaining comparison groups. There was no interaction between age and outcome group, indicating that extra-axial fluid was elevated in the ASD group at all ages (6–9, 12–15 and 18–24 months of age). There was no significant effect or interactions of gender, suggesting that the elevated extra-axial fluid in the ASD group was present in both girls and boys. Figure 5 shows the estimated marginal means for extra-axial fluid at each MRI time point. Infants who developed ASD had 20% greater extra-axial fluid than low-risk typical infants at 6–9 months (P < 0.01), 33% greater fluid at 12–15 months (P < 0.005), and 22% greater fluid at 18–24 months (P < 0.005).
Figure 5.
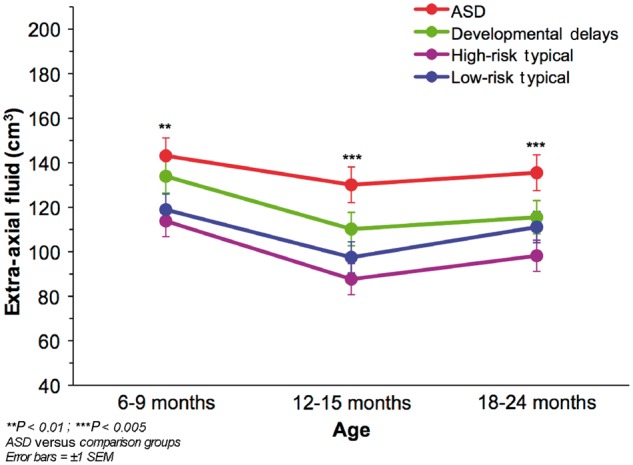
Mean extra-axial fluid volume by outcome group at each MRI time point.
Associations between extra-axial fluid and later autism symptoms
We examined whether the amount of extra-axial fluid in early infancy was associated with cognitive function and autism severity at the time of outcome. We tested Mullen overall developmental quotient and then ADOS scores in succession, while controlling for differences in age and total cerebral volume. Extra-axial fluid had a trend association with Mullen overall developmental quotient at the time of outcome (χ2 = 3.13, df = 1, P = 0.08). Then, after controlling for differences in overall developmental quotient across all subjects and outcome groups, we found that elevated extra-axial fluid during infancy was predictive of later autism severity at 24–36 months (i.e. main effect of ADOS communication + social interaction total score, χ2 = 7.87, df = 1, P < 0.01). Thus, the increased extra-axial fluid detected as early as 6 months of age was predictive of later ASD symptoms, above and beyond levels of overall cognitive functioning. Furthermore, there was a significant interaction between age and the ADOS Communication subscore (χ2 = 3.83, df = 1, P = 0.05), which suggests that the longer extra-axial fluid remained elevated, the worse the communicative symptoms of ASD at the time of outcome.
Lateral ventricles
After controlling for age and total cerebral volume, there was a significant effect of gender on lateral ventricle volume, with male infants having larger lateral ventricles than females (χ2 = 5.80, df = 1, P < 0.05). There was also a marginally significant effect of outcome group, above and beyond age, total cerebral volume, and gender, with the ASD group having slightly larger lateral ventricles compared to all other groups (χ2 = 6.75, df = 3, P = 0.08).
Head circumference
Table 3 shows that head circumference is correlated with body size, total cerebral volume, and extra-axial fluid. We examined physical and MRI measures in succession to evaluate how each measure uniquely contributes to head circumference. Both age (χ2 = 94.01, df = 1, P < 0.0001) and weight (χ2 = 23.87, df = 1, P < 0.0001) had significant effects on head circumference. Total cerebral volume was a highly significant predictor of head circumference (χ2 = 43.35, df = 1, P < 0.0001), above and beyond differences in age and body weight. Adding extra-axial fluid was also found to have an independent and unique effect on head circumference (χ2 = 7.03, df = 1, P < 0.01). This indicates that both total cerebral volume and extra-axial fluid contribute to individual variability in head circumference. Furthermore, there was a significant effect of outcome group on head circumference, and no interaction with age, which indicates that the ASD group had significantly larger head circumference across all ages (χ2 = 10.66, df = 3, P < 0.05).
Table 3.
Correlations between Time 1 MRI and physical measures
| Total cerebral volume | Fluid | Lateral ventricles | Height | Weight | |
|---|---|---|---|---|---|
| Total cerebral volume | |||||
| Extra-axial fluid | 0.41** | ||||
| Lateral ventricle volume | 0.18 | 0.36** | |||
| Height | 0.45** | 0.33** | 0.07 | ||
| Weight | 0.52** | 0.35** | −0.03 | 0.67** | |
| Head circumference | 0.69** | 0.52** | 0.14 | 0.49** | 0.60** |
*P < 0.05; **P < 0.01.
Discussion
Extra-axial fluid is characterized by an excess of CSF in the subarachnoid space, particularly along the convexities of the frontal lobe (Barlow, 1984; Maytal et al., 1987; Odita, 1992; Hellbusch, 2007). It is known by many synonyms (e.g. benign extra-axial fluid of infancy, benign external hydrocephalus, communicating hydrocephalus, subarachnoid fluid collections; reviewed in Zahl et al., 2011), which may reflect how little is understood about its pathophysiology and long-term outcome. The presenting symptom is usually rapid head growth in the first year of life, with the greatest increase in head circumference around 6 months (Sahar, 1978; Barlow, 1984; Alvarez et al., 1986). The lateral ventricles of these children are either normal or mildly enlarged (Sahar, 1978; Alvarez et al., 1986; Odita, 1992), and intracranial pressure is normal or slightly elevated (Sahar, 1978; Chazal et al., 1985). Boys comprise about two-thirds of the infants detected with extra-axial fluid (Sahar, 1978; Nickel and Gallenstein 1987; Hellbusch, 2007). The presence of extra-axial fluid in infancy has been considered ‘benign’ because it is not currently associated with a clinical syndrome or gross anatomical abnormality (stroke, trauma, tumour) and because excess fluid typically resolves without intervention later in infancy (Maytal et al., 1987; Odita, 1992). However, several reports have associated extra-axial fluid with a co-occurrence of seizures (Sahar, 1978; Chazal et al., 1985; Hellbusch, 2007) and motor delays (Sahar, 1978; Nickel and Gallenstein, 1987; Lorch et al., 2004; Hellbusch, 2007), although the long-term outcomes are poorly understood because there is typically no longitudinal follow-up with imaging and behavioural assessments.
The clinical presentation of extra-axial fluid shares common characteristics with ASD, including enlarged head circumference early in life, higher rate in boys than girls, and a co-occurrence with seizures, but the presence of extra-axial fluid has not previously been associated with infants who develop ASD. There are some previous findings of increased CSF in ASD, but these reports of older children and adults did not focus on the subarachnoid space (McAlonan et al., 2005; Hallahan et al., 2009). We conducted a longitudinal MRI study of infants at risk for ASD and found that, relative to typically developing infants and those with other developmental delays, infants who developed ASD had significantly greater extra-axial fluid by 6–9 months of age. This finding persisted with extra-axial fluid remaining significantly elevated at 12–15 and 18–24 months of age. The ASD group had mildly enlarged lateral ventricles, which is consistent with reports that increased extra-axial fluid is usually found in the absence of severe ventricular enlargement (Sahar, 1978; Alvarez et al., 1986; Odita, 1992). These results lead us to the provisional conclusion, pending replication, that the presence of excessive extra-axial fluid that does not resolve by the second year of life may be a hitherto unappreciated characteristic of infants who develop ASD.
The quantification of extra-axial fluid was key to characterizing the nature of the pathology and its relation to autism symptoms. Our finding that elevated extra-axial fluid during early infancy was associated with both a diagnosis of ASD and more severe autism symptoms calls into question the ‘benign’ nature of this finding. We found that the amount of extra-axial fluid was associated with later ASD symptoms, above and beyond levels of overall cognitive functioning. This suggests that elevated fluid detected at 6 months and still elevated through 24 months may be a predictive marker specific to ASD symptoms, and not merely of general developmental delays. Furthermore, the longer extra-axial fluid remained elevated, the worse the ASD communication symptoms were.
In typical brain development, on the other hand, the subarachnoid space increases from birth to 7 months, declines between 12–24 months, and is minimal by 24 months (Kleinman et al., 1983; Lam et al., 2001). Our finding that extra-axial fluid is elevated and persists until at least 24 months suggests that the normal mechanisms for absorption of CSF may be aberrant in infants who develop ASD. There are two primary mechanisms that are responsible for CSF outflow. In the mature brain, return of CSF to the venous circulation takes place through re-absorption into arachnoid granulations that are located along the superior sagittal sinus (Kapoor et al., 2008). However, arachnoid granulations are not present at birth and only mature over the first 18 months of life (Le Gros Clark, 1920; Gómez et al., 1981). The immaturity of arachnoid granulations in infancy may cause CSF to accumulate in the subarachnoid space, leading to elevated extra-axial fluid (Barlow, 1984; Maytal et al., 1987). Beyond the arachnoid granulations, there is evidence that CSF can exit the brain in other ways. Human and animal studies demonstrate that CSF absorption occurs through lymphatic pathways, primarily through the cribriform plate and ultimately into the extracranial lymphatics located in the paranasal region (Johnston et al., 2004). In fact, lymphatic drainage may be the primary pathway for CSF absorption during the neonatal period (Johnston, 2003). There is a marked increase in CSF production in the first year of life (Yasuda et al., 2002), which may not be a problem for typical infants whose CSF production is balanced by proper absorption through the mechanisms discussed above. However, there may be an imbalance between CSF production and CSF drainage in infants with excessive extra-axial fluid.
MRI studies have used injected isotopes or contrast agents to measure the flow of CSF in infants with extra-axial fluid and have demonstrated that CSF is stagnant or has limited flow in the subarachnoid space over the convexities of the brain (Sahar, 1978; Briner and Bodensteiner, 1981; Chazal et al., 1985; Nickel and Gallenstein, 1987). How could the accumulation of stagnant CSF over the superficial surface of the brain have a deleterious affect on brain development? CSF has many important functions, including the removal of potentially harmful byproducts of brain metabolism (Johanson et al., 2008). Stagnation of CSF could lead to the accumulation of waste byproducts and neuromodulators in brain tissue that may alter the extracellular environment of neurons and impact their function (Del Bigio, 2010). CSF also serves as a means of transporting important cytokines, growth factors, and other signalling molecules that are required for the normal development of the neocortex (Johanson et al., 2008). An imbalance of CSF production and absorption alters the concentration of these factors and could lead to serious consequences on cortical development (Mashayekhi et al., 2002). For example, stagnation of CSF flow in animal models leads to an alteration of neurogenesis and premature migration of progenitor cells (Mashayekhi et al., 2002). Indeed, the composition of CSF drawn from the subarachnoid space in infants with extra-axial fluid has a markedly higher protein concentration than CSF drawn from the ventricles or from lumbar puncture (Chazal et al., 1985), and also compared to CSF in normal infants (Briner and Bodensteiner, 1981). CSF is recycled at a much slower rate in early life (Johanson et al., 2008), and coupled with a higher ratio of CSF to brain volume (Yasuda et al., 2002), these factors give the developing brain less ability to eliminate harmful metabolites and toxins, thus making it more vulnerable to damage (Johanson et al., 2008). In fact, it is possible that the accumulation of extra-axial fluid begins before birth, since prenatal MRI has shown that infants with extra-axial fluid had prominent CSF-filled subarachnoid spaces during the foetal period (Girard and Raybaud, 2001).
The findings reported here may have a bearing on studies that report electrophysiological indices of children at risk for ASD. Nelson and colleagues, for example, have used EEG signals to accurately classify infants to either the high-risk or typical group (Bosl et al., 2011) and have found resting EEG differences between high- and low-risk infants (Tierney et al., 2012). However, these studies did not conduct parallel MRI analyses. Therefore, if a subset of the high-risk children had increased extra-axial fluid, this may explain, in part, the EEG differences since CSF is known to alter conductivity of the EEG signal (Baumann et al., 1997).
The longitudinal trajectories of brain growth acquired through this study may enhance our understanding of early brain development in ASD. One of the most often cited and replicated findings of the neuropathology of ASD is that individuals with ASD are born with normal head size, but that head growth accelerates, compared to typically developing children, sometime during the first year of life (Lainhart et al., 1997; Courchesne et al., 2003). These findings are largely based on retrospective head circumference studies, and the general assumption has always been that head circumference is tightly coupled to brain volume. Brain volume, however, can only be directly studied using MRI. We found that both brain and extra-axial fluid volumes contribute to infant head circumference. In addition to elevated extra-axial fluid, we found that infants who later developed ASD had enlarged brain size as early as 12 months, consistent with reports in toddlers (Courchesne et al., 2001; Hazlett et al., 2005; Schumann et al., 2010; Nordahl et al., 2011). Thus, both brain volume and extra-axial fluid volume are important aspects of brain development that should be considered as potential predictive factors in infants at risk for ASD. This is the first MRI study to prospectively evaluate brain growth trajectories from infancy in children who develop ASD, and it is the first direct MRI evidence of brain enlargement in these children before 2 years of age.
Although we believe that the findings reported here are intriguing and should be put on record, there are also important caveats associated with this study. The number of infants with an ASD outcome (n = 10) was relatively small. We welcome attempts at replication in larger, ongoing imaging studies of infants at risk for autism. A necessary future direction to evaluate the use of this as a potential biomarker is to gain more information about how often it is associated with ASD versus other atypical developmental outcomes or typical development, since some infants with extra-axial fluid did not have ASD outcomes. Furthermore, it will be necessary to develop thresholds for extra-axial fluid that best predict risk and then determine the sensitivity and specificity of such cut-offs. Figure 6 illustrates how this could be done, using the current study sample as an example. The objective was to determine the threshold at which extra-axial fluid is disproportionate to brain size, and that could best predict ASD risk. The ratio of extra-axial fluid to total cerebral volume (fluid:brain) was calculated for each infant. At each level of fluid:brain, the sensitivity (% of ASD cases above the threshold, i.e. true positive rate) and specificity (% of non-ASD cases below the threshold, i.e. true negative rate) was determined. This was done for each MRI time point. The level of fluid:brain was chosen that maximized the sensitivity and specificity of the threshold. The dotted line in Fig. 6 represents the fluid:brain ratio of 0.14 that yields 78% sensitivity and 79% specificity in predicting ASD cases at 12–15 months. By comparison, parental concerns at 12 months have 82% sensitivity and 60% specificity in predicting ASD diagnosis (Ozonoff et al., 2009). Although our example is based purely on a single MRI measure, this strategy could be used in conjunction with behavioural and clinical profiles to improve its predictive utility.
Figure 6.
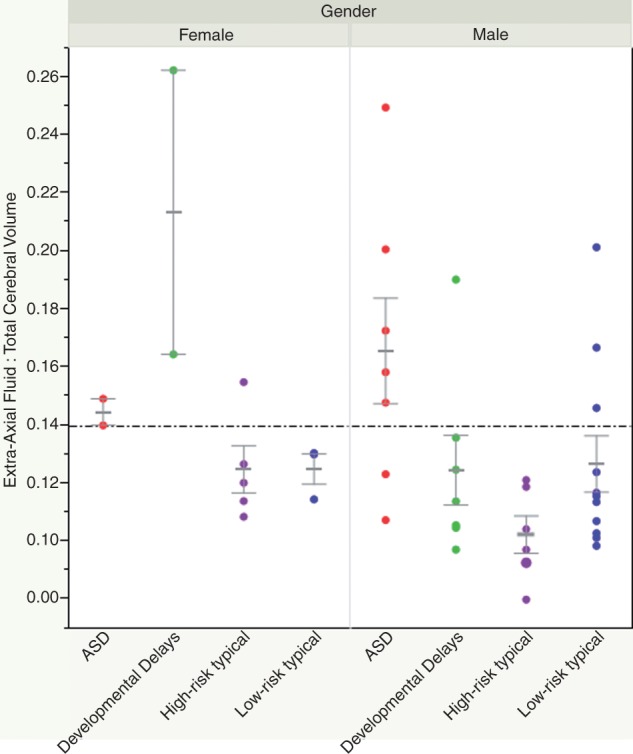
Predicting ASD cases using an extra-axial fluid threshold. The dotted line represents a level of fluid-to-brain volume that yields 78% sensitivity (percentage of ASD cases above the threshold) and 79% specificity (percentage of non-ASD cases below the threshold) in predicting ASD cases at 12–15 months (i.e. ratio of extra-axial fluid to total cerebral volume = 0.14).
If our findings are replicated in a larger cohort, we believe that the presence of increased extra-axial fluid at 6–9 months of age in siblings of children with ASD, particularly if it persists at 12-24 months, may be a useful biomarker for early detection of ASD risk. This will increase justification for renewed discussions as to whether monitoring structural MRIs should become standard of practice for children at high risk for ASD. The scans in the current study were carried out during natural sleep (Nordahl et al., 2008) and present a potentially safe and informative index of early abnormal brain growth. If these findings are replicated, it would also raise the question of whether the increased extra-axial accumulation of CSF may directly contribute to abnormal brain development, particularly of the frontal lobe where the accumulation is most apparent, and could underlie some of the behavioural alterations associated with ASD.
Acknowledgements
We are especially appreciative of the families and children who participated in this research. The authors acknowledge M. DiNino, M. Hill, I. Kyle, D. Li, and S. Ram for valuable technical assistance and data preparation; R.T. Johnson, J. Martinez, T.J. Simon, S. Subramanian, and L. Wessel for assistance in acquiring MRI data; M. Buonocore and C. Tanase for technical expertise in developing the MRI protocol; A. Belding and A. Hill for coordination of behavioural visits; and E. Hanzel, M. Miller, S.J. Rogers, A.J. Schwichtenberg, M.B. Steinfield, and C. Zierhut for expert behavioural assessment and data collection. We are very grateful for useful advice provided by James P. (Pat) McAllister, Ph.D. and Deborah Grzybowski, Ph.D.
Glossary
Abbreviations
- ADOS
Autism Diagnostic Observation Schedule
- ASD
autism spectrum disorder
Funding
This work was supported by the National Institutes of Health [R01MH068398, 1K99MH085099] and by the UC Davis MIND Institute.
References
- Alvarez LA, Maytal J, Shinnar S. Idiopathic external hydrocephalus: natural history and relationship to benign familial macrocephaly. Pediatrics. 1986;77:901–7. [PubMed] [Google Scholar]
- American Psychiatric Association (APA) Diagnostic and statistical manual of mental disorders (text revision) 4th edn. Washington, DC: APA; 2000. [Google Scholar]
- Barlow CF. CSF dynamics in hydrocephalus—with special attention to external hydrocephalus. Brain Dev. 1984;6:119–27. doi: 10.1016/s0387-7604(84)80060-1. [DOI] [PubMed] [Google Scholar]
- Baumann SB, Wozny DR, Kelly SK, Meno FM. The electrical conductivity of human cerebrospinal fluid at body temperature. IEEE Trans Biomed Eng. 1997;44:220–3. doi: 10.1109/10.554770. [DOI] [PubMed] [Google Scholar]
- Bosl W, Tierney A, Tager-Flusberg H, Nelson C. EEG complexity as a biomarker for autism spectrum disorder risk. BMC Med. 2011;9:18. doi: 10.1186/1741-7015-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briner S, Bodensteiner J. Benign subdural collections of infancy. Pediatrics. 1981;67:802–4. [PubMed] [Google Scholar]
- Bryson SE, Zwaigenbaum L, Brian J, Roberts W, Szatmari P, Rombough V, et al. A prospective case series of high-risk infants who developed autism. J Autism Dev Disord. 2007;37:12–24. doi: 10.1007/s10803-006-0328-2. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Prevalence of autism spectrum disorders–autism and developmental disabilities monitoring network, 14 sites, United States, 2008. MMWR Surveill Summ. 2012;61:1–19. [PubMed] [Google Scholar]
- Chazal J, Tanguy A, Irthum B, Janny P, Vanneuville G. Dilatation of the subarachnoid pericerebral space and absorption of cerebrospinal fluid in the infant. Anat Clin. 1985;7:61–6. doi: 10.1007/BF01654631. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Zhang Y, Frazier T, Abbacchi AM, Law P. Sibling recurrence and the genetic epidemiology of autism. Am J Psychiatry. 2010;167:1349–56. doi: 10.1176/appi.ajp.2010.09101470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Carper R, Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. JAMA. 2003;290:337–44. doi: 10.1001/jama.290.3.337. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, et al. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57:245–54. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- Dawson G, Munson J, Webb SJ, Nalty T, Abbott R, Toth K. Rate of head growth decelerates and symptoms worsen in the second year of life in autism. Biol Psychiatry. 2007;61:458–64. doi: 10.1016/j.biopsych.2006.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Rogers S, Munson J, Smith M, Winter J, Greenson J, et al. Randomized, controlled trial of an intervention for toddlers with autism: the early start Denver model. Pediatrics. 2010;125:e17–23. doi: 10.1542/peds.2009-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bigio MR. Neuropathology and structural changes in hydrocephalus. Dev Disabil Res Rev. 2010;16:16–22. doi: 10.1002/ddrr.94. [DOI] [PubMed] [Google Scholar]
- Elsabbagh M, Mercure E, Hudry K, Chandler S, Pasco G, Charman T, et al. Infant neural sensitivity to dynamic eye gaze is associated with later emerging autism. Curr Biol. 2012;22:338–42. doi: 10.1016/j.cub.2011.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamliel I, Yirmiya N, Sigman M. The development of young siblings of children with autism from 4 to 54 months. J Autism Dev Disord. 2007;37:171–83. doi: 10.1007/s10803-006-0341-5. [DOI] [PubMed] [Google Scholar]
- Girard NJ, Raybaud CA. Ventriculomegaly and pericerebral CSF collection in the fetus: early stage of benign external hydrocephalus? Childs Nerv Syst. 2001;17:239–45. doi: 10.1007/pl00013727. [DOI] [PubMed] [Google Scholar]
- Gómez DG, DiBenedetto AT, Pavese AM, Firpo A, Hershan DB, Potts DG. Development of arachnoid villi and granulations in man. Acta Anatomica. 1981;111:247–58. doi: 10.1159/000145473. [DOI] [PubMed] [Google Scholar]
- Gupta SN, Belay B. Intracranial incidental findings on brain MR images in a pediatric neurology practice: a retrospective study. J Neurol Sci. 2008;264:34–7. doi: 10.1016/j.jns.2007.06.055. [DOI] [PubMed] [Google Scholar]
- Hallahan B, Daly EM, McAlonan G, Loth E, Toal F, O’Brien F, et al. Brain morphometry volume in autistic spectrum disorder: a magnetic resonance imaging study of adults. Psychol Med. 2009;39:337–46. doi: 10.1017/S0033291708003383. [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Poe M, Gerig G, Smith RG, Provenzale J, Ross A, et al. Magnetic resonance imaging and head circumference study of brain size in autism: birth through age 2 years. Arch Gen Psychiatry. 2005;62:1366–76. doi: 10.1001/archpsyc.62.12.1366. [DOI] [PubMed] [Google Scholar]
- Hellbusch LC. Benign extracerebral fluid collections in infancy: clinical presentation and long-term follow-up. J Neurosurg. 2007;107:119–25. doi: 10.3171/PED-07/08/119. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Duncan JA, Klinge PM, Brinker T, Stopa EG, Silverberg GD. Multiplicity of cerebrospinal fluid functions: new challenges in health and disease. Cerebrospinal Fluid Res. 2008;5:1–32. doi: 10.1186/1743-8454-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston M. The importance of lymphatics in cerebrospinal fluid transport. Lymphat Res Biol. 2003;1:41–5. doi: 10.1089/15396850360495682. [DOI] [PubMed] [Google Scholar]
- Johnston M, Zakharov A, Papaiconomou C, Salmasi G, Armstrong D. Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, non-human primates and other mammalian species. Cerebrospinal Fluid Res. 2004;1:1–13. doi: 10.1186/1743-8454-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor KG, Katz SE, Grzybowski DM, Lubow M. Cerebrospinal fluid outflow: an evolving perspective. Brain Res Bull. 2008;77:327–34. doi: 10.1016/j.brainresbull.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Kleinman PK, Zito JL, Davidson RI, Raptopoulos V. The subarachnoid spaces in children: normal variations in size. Radiology. 1983;147:455–7. doi: 10.1148/radiology.147.2.6601281. [DOI] [PubMed] [Google Scholar]
- Lainhart JE, Piven J, Wzorek M, Landa R, Santangelo SL, Coon H, et al. Macrocephaly in children and adults with autism. J Am Acad Child Adolesc Psychiatry. 1997;36:282–90. doi: 10.1097/00004583-199702000-00019. [DOI] [PubMed] [Google Scholar]
- Lam WW, Ai VH, Wong V, Leong LL. Ultrasonographic measurement of subarachnoid space in normal infants and children. Pediatr Neurol. 2001;25:380–4. doi: 10.1016/s0887-8994(01)00349-6. [DOI] [PubMed] [Google Scholar]
- Landa RJ, Gross AL, Stuart EA, Faherty A. Developmental trajectories in children with and without autism spectrum disorders: the first 3 years. Child Dev. 2012;84:e1–13. doi: 10.1111/j.1467-8624.2012.01870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gros Clark WE. On the pacchionian bodies. J Anat. 1920;55:40–8. [PMC free article] [PubMed] [Google Scholar]
- Lorch SA, D'Agostino JA, Zimmerman R, Bernbaum J. “Benign” extra-axial fluid in survivors of neonatal intensive care. Arch Pediatr Adolesc Med. 2004;158:178–82. doi: 10.1001/archpedi.158.2.178. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. Autism diagnostic observation schedule: generic. Los Angeles: Western Psychological Services; 2002. [Google Scholar]
- Mashayekhi F, Draper CE, Bannister CM, Pourghasem M, Owen-Lynch PJ, Miyan JA. Deficient cortical development in the hydrocephalic texas (h-tx) rat: a role for CSF. Brain. 2002;125:1859–74. doi: 10.1093/brain/awf182. [DOI] [PubMed] [Google Scholar]
- Maytal J, Alvarez LA, Elkin CM, Shinnar S. External hydrocephalus: radiologic spectrum and differentiation from cerebral atrophy. Am J Roentgenol. 1987;148:1223–30. doi: 10.2214/ajr.148.6.1223. [DOI] [PubMed] [Google Scholar]
- McAlonan GM, Cheung V, Cheung C, Suckling J, Lam GY, Tai KS, et al. Mapping the brain in autism. A voxel-based MRI study of volumetric differences and intercorrelations in autism. Brain. 2005;128:268–76. doi: 10.1093/brain/awh332. [DOI] [PubMed] [Google Scholar]
- Mullen EM. Mullen scales of early learning. Circle Pines, MN: American Guidance Service; 1995. [Google Scholar]
- Nickel RE, Gallenstein JS. Developmental prognosis for infants with benign enlargement of the subarachnoid spaces. Dev Med Child Neurol. 1987;29:181–6. doi: 10.1111/j.1469-8749.1987.tb02133.x. [DOI] [PubMed] [Google Scholar]
- Nordahl CW, Simon TJ, Zierhut C, Solomon M, Rogers SJ, Amaral DG. Brief report: methods for acquiring structural MRI data in very young children with autism without the use of sedation. J Autism Dev Disord. 2008;38:1581–90. doi: 10.1007/s10803-007-0514-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordahl CW, Lange N, Li DD, Barnett LA, Lee A, Buonocore MH, et al. Brain enlargement is associated with regression in preschool-age boys with autism spectrum disorders. Proc Natl Acad Sci USA. 2011;108:20195–200. doi: 10.1073/pnas.1107560108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordahl CW, Scholz R, Yang X, Buonocore MH, Simon T, Rogers S, et al. Increased rate of amygdala growth in children aged 2 to 4 years with autism spectrum disorders: a longitudinal study. Arch Gen Psychiatry. 2012;69:53–61. doi: 10.1001/archgenpsychiatry.2011.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odita JC. The widened frontal subarachnoid space. A CT comparative study between macrocephalic, microcephalic, and normocephalic infants and children. Childs Nerv Syst. 1992;8:36–9. doi: 10.1007/BF00316560. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Steinfeld MB, Hill MM, Cook I, Hutman T, et al. How early do parent concerns predict later autism diagnosis? J Dev Behav Pediatr. 2009;30:367–75. doi: 10.1097/dbp.0b013e3181ba0fcf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Iosif AM, Baguio F, Cook IC, Hill MM, Hutman T, et al. A prospective study of the emergence of early behavioral signs of autism. J Am Acad Child Adolesc Psychiatry. 2010;49:256–66. [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Carter S, Messinger D, Yirmiya N, Zwaigenbaum L, et al. Recurrence risk for autism spectrum disorders: a baby siblings research consortium study. Pediatrics. 2011;128:e1–6. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb RA, Hanson DP, Karwoski RA, Larson AG, Workman EL, Stacy MC. Analyze: a comprehensive, operator-interactive software package for multidimensional medical image display and analysis. Comput Med Imaging Graph. 1989;13:433–54. doi: 10.1016/0895-6111(89)90285-1. [DOI] [PubMed] [Google Scholar]
- Rogers SJ. What are infant siblings teaching us about autism in infancy? Autism Res. 2009;2:125–37. doi: 10.1002/aur.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJ, Vismara LA. Evidence-based comprehensive treatments for early autism. J Clin Child Adolesc Psychol. 2008;37:8–38. doi: 10.1080/15374410701817808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahar A. Pseudohydrocephalus–megalocephaly, increased intracranial pressure and widened subarachnoid space. Neuropadiatrie. 1978;9:131–9. doi: 10.1055/s-0028-1085418. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. JMP® 10 Modeling and Multivariate Methods. Cary, NC: SAS Institute Inc; 2012. [Google Scholar]
- Schumann CM, Hamstra J, Goodlin-Jones BL, Lotspeich LJ, Kwon H, Buonocore MH, et al. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. J Neurosci. 2004;24:6392–401. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann CM, Bloss CS, Barnes CC, Wideman GM, Carper RA, Akshoomoff N, et al. Longitudinal magnetic resonance imaging study of cortical development through early childhood in autism. J Neurosci. 2010;30:4419–27. doi: 10.1523/JNEUROSCI.5714-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–55. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney AL, Gabard-Durnam L, Vogel-Farley V, Tager-Flusberg H, Nelson CA. Developmental trajectories of resting EEG power: an endophenotype of autism spectrum disorder. PLoS One. 2012;7:e1–10. doi: 10.1371/journal.pone.0039127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JJ, Gu H, Gerig G, Elison JT, Styner M, Gouttard S, et al. Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. Am J Psychiatry. 2012;169:589–600. doi: 10.1176/appi.ajp.2011.11091447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda T, Tomita T, McLone DG, Donovan M. Measurement of cerebrospinal fluid output through external ventricular drainage in one hundred infants and children: correlation with cerebrospinal fluid production. Pediatr Neurosurg. 2002;36:22–8. doi: 10.1159/000048344. [DOI] [PubMed] [Google Scholar]
- Zahl SM, Egge A, Helseth E, Wester K. Benign external hydrocephalus: a review, with emphasis on management. Neurosurg Rev. 2011;34:417–32. doi: 10.1007/s10143-011-0327-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. Int J Dev Neurosci. 2005;23:143–52. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]