There is a large, poorly regulated international market of putative stem cell products, and demand for these products is growing. Very few stem cell biologics have passed regulatory scrutiny, and authorities in many countries have begun to step up their enforcement activities to protect patients and the integrity of health care markets.
Summary
There is a large, poorly regulated international market of putative stem cell products, including transplants of processed autologous stem cells from various tissues, cell processing devices, cosmetics, and nutritional supplements. Despite the absence of rigorous scientific research in the form of randomized clinical trials to support the routine use of such products, the market appears to be growing and diversifying. Very few stem cell biologics have passed regulatory scrutiny, and authorities in many countries, including the United States, have begun to step up their enforcement activities to protect patients and the integrity of health care markets.
The Industry Landscape
Charlatans are drawn to frontiers. The scientific frontier of stem cell biology has attracted an unusual amount of dubious business activity, despite the paucity of credible evidence for the efficacy of these cells in the treatment of indications for which they are marketed. The first company to sell stem cell treatments in the United States opened in 2002, just 4 years after the derivation of human embryonic stem cells triggered a wave of optimism and hyperbole surrounding this nascent field of research. In the decade that followed, an industry encompassing hundreds of private clinics advertising stem cells (derived from bone marrow, umbilical cord blood and other perinatal tissue, fat, fetal tissue, etc.) for the treatment of medical conditions too numerous to list sprang up in countries around the world. If such companies' claims are to be believed, many tens of thousands of patients have paid many hundreds of millions of dollars pursuing stem cell dreams unsupported by rigorous scientific research. It is a vast medical experiment, uncontrolled, unsupervised, unreported, and conducted on a for-profit basis.
This industry has, however, seen its share of innovation in the form of developing business plans that avoid premarket testing for safety and efficacy. Clinical outfits have proliferated and diversified to cultivate demand for stem cell remedies in neurology, orthopedics, cosmetic surgery, general rejuvenation, even pediatric conditions. (Worryingly, studies have shown that nearly half of the patients whose treatments are described in social media are children [1].) A niche industry of companies providing ancillary services ranging from patient recruitment and travel support to physician training has also emerged, and numerous “AstroTurf” organizations (meaning groups that seek to portray their sponsored activities as driven by grassroots initiatives), such as industry-operated foundations and professional societies, have been formed to lobby for weaker regulations and rally members of the public against government intervention. Those efforts notwithstanding, the sector of the industry that markets stem cell transplants, injections, and infusions has also received significant regulatory attention. In numerous smaller countries, national medical councils and ministries of health have taken actions such as issuing public warnings on the risks of unapproved stem cell interventions or restricting the activities of clinics operating within their borders. The European Medicines Agency, the competent regulatory authority in the European Union, has also been active in its development of a regulatory framework for advanced therapeutic medicinal products, which includes many forms of human cell biologics, and placing limits on the ability of commercial operators to exploit the “hospital exemption” that allows for nonroutine experimental care for individual patients. The U.S. federal government has been especially active in this regard over the past 2 years, as outlined below.
Some companies follow other, less rigorous pathways to the market for their putative therapeutics. Makers of point-of-care cell processing devices, for example, have sought and, in some cases, obtained 510(k) clearance from the U.S. Food and Drug Administration (FDA), a form of market authorization that does not require advance testing for safety and efficacy for devices deemed to have substantially equivalent predicates already on the market, whereas individual physicians frequently use marketed devices not approved for stem cell applications for harvesting, minimal processing, and retransplantation of autologous cells as part of the same surgical procedure, thereby bypassing the more stringent federal standards over stem cell biologics. A review of the 510(k) clearance process by the Institute of Medicine in 2011 recommended that the system be scrapped, on the basis that it does not require premarket testing of safety and efficacy for potentially risky devices [2], and the FDA rejected applications for two cell processing devices submitted for 510(k) clearance [3], which may have implications for the future of this pathway to the market.
Regulatory shopping remains a commonplace strategy for circumventing inconvenient laws, with companies based in countries with stringent regulatory oversight setting up shop in border towns and sending patients to clinics in less strictly policed neighboring countries, often on a same-day basis. This form of arbitrage also pads the bottom line, as operating costs in many of the destination countries are a fraction of what they would be in the country of origin. More importantly, this practice externalizes a great deal of liability to patients, who not only assume the risks of undergoing invasive procedures outside the standard of care, but also effectively abandon many of the legal protections they would enjoy in more closely supervised settings.
Even in cases in which patients do not travel outside their home country, significant ethical considerations surround any commercial activity in which products or procedures are marketed as potentially therapeutic without having been validated scientifically. Although it appears that most businesses selling stem cells seek to indemnify themselves by indicating the “experimental” nature of their interventions on websites and informed consent forms, it seems fair to observe that such waivers of responsibility are weighted in favor of the provider, rather than the patient. Also concerning is that many such companies target vulnerable populations, such as patients with neurological conditions and parents of children with developmental and other diseases, with stem cell interventions that lack not only rigorous evidence but often a plausible basis in science.
Other firms have accessed consumer markets by developing nutritional supplements (nutraceuticals) purported to increase the number of endogenous stem cells in circulation or otherwise boost stem cell activity. In the United States, such products are regulated under the porous and ineffectual Dietary Supplement Health and Education Act, which creates a third category of product that is neither food nor drug and over which the FDA has only limited regulatory authority [4]. One of the first such product lines to use “stem cell” in its advertising was introduced via a multilevel marketing scheme; the company (Stemtech Health Sciences) claimed to have netted more than $1,000,000 in its first month [5]. This is symptomatic of the larger movement to portray stem cells as a component of alternative or complementary medicine, rather than a nascent and unproven form of orthodox biomedicine. A cursory survey of online advertisements reveals stem cells offered as adjuncts to or therapeutic targets of acupuncture, homeopathy, ayurvedic medicine, and phytotherapy, among others [6].
The cosmetics industry has also caught the stem cell fever. Numerous makers have introduced lines of so-called cosmeceuticals, such as skin creams, lotions, hair products, and even sunscreens, that claim to contain either stem cell extracts or bioactive compounds that stimulate stem cells within the skin or hair follicles, ostensibly promoting the regeneration of surface tissue. A growing subcategory of these products invoke the rejuvenating power of plant stem cells extracted from Swiss apple, argan, bilberry, or edelweiss, to name a few. As the FDA has shown generally limited ability to challenge cosmetics manufacturers on efficacy claims [7], the industry remains very much governed by a caveat emptor ethic, although there have been signs of increased scrutiny in the past year, as described in the following section.
Of greater concern than stem cell cosmetics, a significant number of aesthetic plastic surgeons have embraced a range of expensive and invasive procedures using autologous adipose-derived stem cells (or stromal vascular fraction) in breast and buttocks augmentation and so-called stem cell facelifts, with only sketchy evidence to support their routine use. To their credit, the American Society of Plastic Surgeons and American Society for Aesthetic Plastic Surgery issued a joint statement asserting that “the marketing and promotion of stem cell procedures in aesthetic surgery is not adequately supported by clinical evidence at this time” [8].
Regulators Take Action
Regulators have begun to respond to these alarming developments. In the United States, the FDA has inspected sites and issued untitled or formal warning letters to companies in violation of the relevant section of the Code of Federal Regulations (CFR 1271) [9]. The first federal action against a self-styled stem cell clinic was initiated against BioMark International Inc. in 2005 (the proprietors subsequently fled the country and resumed business using a new corporate identity) [10]. In 2008, the FDA sent an untitled letter to Regenerative Sciences Inc., triggering a protracted legal battle that centered on the question of whether processed autologous stem cells should be classified as a biologic drug, and therefore federally regulated, or subsumed within the practice of medicine, which is not under federal jurisdiction. The case was resolved in July 2012, with the court upholding the FDA's authority and issuing a permanent injunction against the company [11]. The FDA took no public action against other domestic stem cell companies in 2009 or 2010, a hiatus that may have been a consequence of the uncertainties introduced by the ongoing litigation. But beginning in 2011, the FDA began to signal its renewed interest reining in unapproved stem cell products (or perhaps greater confidence that the courts would affirm its authority in the area), and in a 1-year period issued multiple warning letters and injunctions to companies and individual physicians (Table 1).
Table 1.
U.S. Food and Drug Administration warning letters relating to stem cell companies
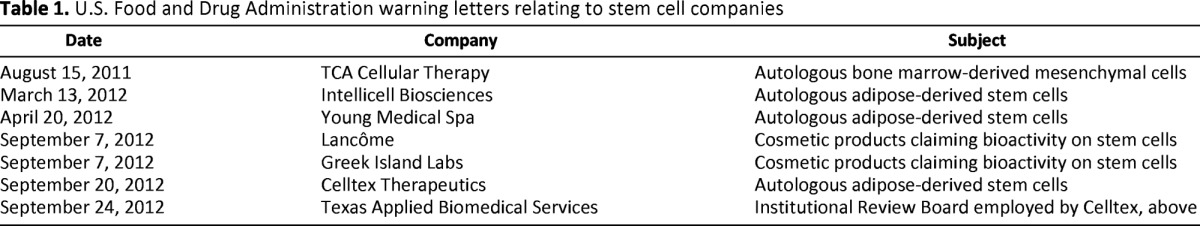
The nature of the businesses that triggered regulatory responses suggests that the authorities were selective in their targeting. Four of the seven companies that were issued Warning Letters had been engaged in the sale of autologous stem cell products that the agency determined to be more than minimally manipulated and/or intended for nonhomologous use, either of which triggers classification as a biologic drug under CFR 1271. Two others had made unsupported marketing claims about the activity of their cosmetic products on endogenous skin stem cells. The last was a private institutional review board that had provided services to one of the companies (Celltex Therapeutics) involved in unauthorized clinical use of autologous adipose-derived cells. This was a particularly pointed exercise of federal authority in this area, as only a few months prior to these actions the state medical board in Texas (where both Celltex and its institutional review board [IRB] are located) published new rules that appeared to allow investigational uses of stem cell products to be approved by private IRBs without FDA authorization [12]. Celltex has since announced that it will begin offering stem cell interventions to patients across the border in Mexico [13].
State medical boards and law enforcement agencies have also taken action against individuals making commercial stem cell claims. Proprietors of purported stem cell businesses have been arrested in Nevada, Texas, and California for making fraudulent claims regarding their products and services; convictions were returned in all cases [14–16]. In Florida, the state board of medicine first restricted and then suspended the license of a local doctor who had begun offering injections of autologous stem cells to patients within the state [17]. His attorney has sought to portray this activity as off-label use within the ordinary practice of medicine or, alternatively, as alternative medicine [18]. Seemingly undeterred, the physician launched a new stem cell business even as his administrative hearing proceeded [19].
Conclusion
Despite these numerous enforcement actions, dozens of companies and private medical practices within the United States continue to openly market stem cell interventions directly to consumers. Although some of these companies send patients to Mexico, Central America, or the Caribbean, a surprising number appear to deliver their unlicensed interventions domestically. A researcher at the University of Minnesota has identified 20 such clinics in the state of Texas alone [20]. It appears for now that the considerable profit incentive outweighs the perceived risks inherent in violating the law. Presumably this situation will prevail until either the federal authorities take greater action against individuals and firms that persist in the unapproved marketing of stem cell interventions or fully validated safe and effective stem cell biologics receive market authorization and are covered by health care insurance, which would allow them to outcompete the current range of stem cell nostrums on their own terms.
Author Contributions
D.S.: manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The author indicates no potential conflicts of interest.
References
- 1.Zarzeczny A, Caulfield T. Stem cell tourism and doctors' duties to minors: A view from Canada. Am J Bioeth. 2010;10:3–15. doi: 10.1080/15265161003702865. [DOI] [PubMed] [Google Scholar]
- 2.Institute of Medicine of the National Academies. Consensus report. Washington, D.C.: Institute of Medicine of the National Academies; 2011. Medical Devices and the Public's Health: The FDA 510(k) Clearance Process at 35 Years. [Google Scholar]
- 3. Cytori Therapeutics Inc. v. FDA, D.C. Cir., No. 11-1268.
- 4.Cohen PJ. Science, politics, and the regulation of dietary supplements: It's time to repeal DSHEA. Am J Law Med. 2005;31:175–214. doi: 10.1177/009885880503100203. [DOI] [PubMed] [Google Scholar]
- 5.Stemtech Health Sciences. Everything Stemtech. [Accessed March 4, 2013]. Available at http://rsvp4life.net.
- 6.Sipp D. Stem cell stratagems in alternative medicine. Regen Med. 2011;6:407–414. doi: 10.2217/rme.11.13. [DOI] [PubMed] [Google Scholar]
- 7.Liang BA, Hartman KM. It's only skin deep: FDA regulation of skin care cosmetics claims. Cornell J Law Public Policy. 1999;8:249–280. [PubMed] [Google Scholar]
- 8.Eaves FF, 3rd, Haeck PC, Rohrich RJ. ASAPS/ASPS position statement on stem cells and fat grafting. Plast Reconstr Surg. 2012;129:285–287. doi: 10.1097/PRS.0b013e3182362caf. [DOI] [PubMed] [Google Scholar]
- 9. [Accessed March 4, 2013]. U.S. Code of Federal Regulations, Title 21, Part 1271 (2012). Available at http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=1271.
- 10.Casewatch. Stem cell swindler charged with fraud. [Accessed March 4, 2013]. Available at http://www.casewatch.org/doj/biomark/indictment.html.
- 11.United States of America v. Regenerative Sciences, LLC et al. Civil Action No. 2010-1327 (RMC) U.S. District Court DC (2012) [Accessed March 4, 2013]. https://ecf.dcd.uscourts.gov/cgi-bin/show_public_doc?2010cv1327-47.
- 12.Kaiser J. Stem cells: Texas Medical Board approves rules for controversial treatment. Science. 2012;336:284. doi: 10.1126/science.336.6079.284. [DOI] [PubMed] [Google Scholar]
- 13.Cyranoski D. Stem cells in Texas: Cowboy culture. Nature. 2013;494:166–168. doi: 10.1038/494166a. [DOI] [PubMed] [Google Scholar]
- 14.Preventive therapy [Editorial] Nature. 2013;494:147–148. doi: 10.1038/494147b. [DOI] [PubMed] [Google Scholar]
- 15.U.S. District Attorney's Office, Southern District of Texas. Convictions entered in two separate Texas cases involving stem cells. [Accessed March 4, 2013]. Available at http://www.justice.gov/usao/txs/1News/Releases/2012%20September/120907%20Morales%20and%20Stowe.html.
- 16.CBS News. Encinitas woman pleads guilty to treating patients without a license. [Accessed March 4, 2013]. Available at http://www.cbs8.com/story/19867994/encinitas-woman-pleads-guilty-to-treating-patients-without-a-license.
- 17. Florida Dept. of Health v. Zannos G. Grekos. DOAH Case No. 11-4240PL.
- 18.Freeman L. Bonita Springs stem-cell doctor asks judge to dismiss state's case against him. [Accessed March 4, 2013];Naples News. 2013 Feb 9; Available at http://www.naplesnews.com/news/2013/feb/09/bonita-springs-stem-cell-doctor-grekos-license/ [Google Scholar]
- 19.Intercellular Sciences website. [Accessed March 4, 2013]. Available at http://www.intercellularsciences.com.
- 20.Turner L. Adult stem cell banks and clinics marketing stem cell procedures in Texas. [Accessed March 4, 2013];Health in the Global Village blog. 2012 Sep 17; Available at http://www.healthintheglobalvillage.com/2012/09/17/adult-stem-cell-banks-and-clinics-marketing-stem-cell-procedures-in-texas/ [Google Scholar]


