Abstract
Genetic regulation of the cell fate transition from lateral plate mesoderm to the specification of cardiomyocytes requires suppression of Wnt/β-catenin signaling, but the mechanism for this is not well understood. By analyzing gene expression and chromatin dynamics during directed differentiation of human embryonic stem cells (hESCs), we identified a suppressor of Wnt/β-catenin signaling, transmembrane protein 88 (TMEM88), as a potential regulator of cardiovascular progenitor cell (CVP) specification. During the transition from mesoderm to the CVP, TMEM88 has a chromatin signature of genes that mediate cell fate decisions, and its expression is highly upregulated in advance of key cardiac transcription factors in vitro and in vivo. In early zebrafish embryos, tmem88a is expressed broadly in the lateral plate mesoderm, including the bilateral heart fields. Short hairpin RNA targeting of TMEM88 during hESC cardiac differentiation increases Wnt/β-catenin signaling, confirming its role as a suppressor of this pathway. TMEM88 knockdown has no effect on NKX2.5 or GATA4 expression, but 80% of genes most highly induced during CVP development have reduced expression, suggesting adoption of a new cell fate. In support of this, analysis of later stage cell differentiation showed that TMEM88 knockdown inhibits cardiomyocyte differentiation and promotes endothelial differentiation. Taken together, TMEM88 is crucial for heart development and acts downstream of GATA factors in the pre-cardiac mesoderm to specify lineage commitment of cardiomyocyte development through inhibition of Wnt/β-catenin signaling.
Keywords: Mesoderm, TMEM88, Gene, Wnt signaling, Chromatin, Zebrafish, Human embryonic stem cell, Heart development
INTRODUCTION
Studies in heart development have elucidated many crucial factors involved in mediating cell fate decisions. These include diffusible factors, such as Wnts, activin/nodal, BMPs and Noggin, that influence the establishment of cell fate gradients in the developing embryo. The heart, blood and vasculature are coordinately specified from a common origin: the lateral plate mesoderm (Kattman et al., 2006). Mounting evidence suggests that persistent Wnt/β-catenin signaling, together with other factors, such as vascular endothelial growth factor (VEGF), subsequently directs mesoderm into a hemangioblast fate that gives rise to blood lineages and endothelium of the posterior lateral plate (Wang et al., 2007). By contrast, downregulation of Wnt/β-catenin signaling is required to guide mesoderm into the cardiovascular lineages that form as bilateral heart fields in the ventrolateral mesoderm (Ueno et al., 2007; Paige et al., 2010).
Post-gastrulation, the pre-cardiac mesoderm gives rise to the primordial heart in domains derived bilaterally along the anterior ventral lateral mesoderm. These populations can give rise to all three lineages of the heart, including cardiomyocytes, endothelial cells and smooth muscle cells. Molecules such as Nkx2.5, Mef2c, and the GATA factors converge to specify heart development whereas lineage-restricted transcription factors such as Tbx5 (first heart field) and Isl1 (second heart field) mediate later stages of cardiac subtype specification (Yutzey and Benson, 2003; Brown et al., 2004; Qyang et al., 2007).
Several genes have also been identified as being crucial mediators of the lineage commitment of hemato-endothelial versus heart, including the ETS family of transcription factors (e.g. ER71; also known as ETV2) (Liu et al., 2012), and the well-known master transcription factor for blood and endothelial development SCL (TAL1) (Gering et al., 1998). Recent work by Van Handel et al., for example, showed that the absence of SCL results in ectopic heart development, mediated at least in part by increased expression of canonical Wnt/β-catenin inhibitors (Van Handel et al., 2012). Their data indicate that the expression of SCL not only establishes the hemogenic-endothelial lineage but also is simultaneously required to suppress the cardiogenic potential of those cells.
Although it is possible that the spatial pattern of pre-cardiogenic cells might be specified in large part by the overlapping patterns of soluble gradients of agonists or antagonists, we wondered whether cell-autonomous regulators of the Wnt/β-catenin pathway might exert fine control over the spatial domain of cardiogenic cells. This idea has precedent, as others have shown that temporal, spatial or cell type-dependent regulators of Wnt/β-catenin are active in embryonic development (Martin and Kimelman, 2012). We therefore sought cell-autonomous regulators of this pathway to investigate whether we could identify new context-dependent regulators of Wnt/β-catenin signaling that participate in cardiogenesis.
Human embryonic stem cell differentiation has been shown to recapitulate in vivo heart development (Murry and Keller, 2008), and recent work from our group has revealed an epigenetic signature for novel regulators of this process (Paige et al., 2012). Parallel work has also provided genome-wide transcriptional and epigenetic analyses in mouse embryonic stem cell (ESC) cardiac differentiation (Wamstad et al., 2012). Studies on genetic and epigenetic changes in heart development by our laboratory revealed a novel negative regulator of Wnt/β-catenin signaling, designated as transmembrane protein 88 (TMEM88), that is highly expressed during the specification of the cardiovascular progenitor cell. Aside from its known role as a negative regulator of Wnt signaling (Lee et al., 2010), currently there is little known about the biological function of TMEM88 in development or adult physiology. By studying hESCs in vitro and zebrafish development in vivo, we show here that TMEM88 is necessary for cardiomyocyte specification during the early stages of heart development.
MATERIALS AND METHODS
Cell culture
RUES2 human ESCs were maintained as previously described (Paige et al., 2012). In brief, cells were plated on Matrigel (BD Biosciences)-coated plates and maintained in an undifferentiated state with mouse embryonic fibroblast (MEF)-conditioned media containing 5 ng/ml human basic fibroblast growth factor (hbFGF) (Peprotech, 100-18B).
Lentiviral transduction
Lentivirus transduction was performed in cell suspension in the presence of 10 μM Y-27632 (Sigma). Lentiviruses were generated to express short hairpin (sh)RNA sequences targeting various domains of the TMEM88 transcript in a PLK0.1 backbone containing a puromycin resistance cassette (Sigma, TRCN0000178754, NM_203411.1-692s1c1; TRCN0000179147, NM_203411.1-94s1c1; TRC0000180154, NM_203411.1-677s1c1; TRCN0000180661, NM_203411.1-691s1c1). A lentivirus expressing a non-targeting shRNA was used as a control (Sigma). To assess Wnt/β-catenin signaling activity, cells were transduced with the β-catenin-activated reporter lentivirus driving either luciferase or venus.
Human ESC directed differentiation
Cardiac differentiation was performed as previously described (Gantz et al., 2012). In a subset of experiments, 1 μM XAV-939 (Tocris, 3748) was added at day 3 of differentiation.
Hematopoietic differentiation on OP9 cells
Cells were cultured until day 5, at which point media was changed to endothelial basal medium (EBM) media containing EGM BulletKit (Lonza CC-3156) for an additional 7-9 days. OP9 feeder cells were seeded in 24-well plates 1 day prior to co-culture. hESC-derived cells differentiated for about 14 days were seeded onto OP9 cells at 2-8×104 cells per well in α-MEM (Invitrogen) with 10% fetal bovine serum (FBS) (Hyclone), penicillin/streptomycin (Invitrogen) and recombinant cytokines (from R&D Systems unless otherwise indicated): hSCF (50 ng/ml), hTPO (20 ng/ml), hIL-6 (20 ng/ml), hIL-3 (20 ng/ml), hFLT3L (20 ng/ml), hIL-11 (10 ng/ml), hEPO (2 U/ml), hBMP4 (10 ng/ml), hGM-CSF (100 ng/ml), mVEGF (10 ng/ml; Peprotech). Following 5-6 days of co-culture, non-adherent/loosely adherent cells were removed by pipetting and were passed through a 35-μm cell strainer (BD Falcon). Cells were stained with various combinations of the following monoclonal antibodies for cell surface analysis: APC-conjugated anti-human CD43 (Clone 1G10, BD Pharmingen), PE-conjugated anti-human CD235a (Clone GA-R2, BD Pharmingen), PECy7-conjugated anti-human CD45 (Clone H130, Biolegend) and PECy7-conjugated anti-human CD41 (Clone HIP8, Biolegend), or corresponding isotype control antibodies. DAPI was used to exclude dead cells. Flow cytometry was performed on a Becton Dickinson Canto 2 and data were analyzed using FlowJo 7.6 Software.
Analysis of chromatin dynamics and gene expression during cardiac differentiation
Data generated previously by Paige et al. (Paige et al., 2012) are deposited in the NCBI Gene Expression Omnibus (GEO) database under accession numbers GSE35583 for epigenetics data and GSE19090 for gene expression data.
PCR
For quantitative PCR, total RNA was isolated using the RNeasy Miniprep Kit (Qiagen). First-strand cDNA was synthesized using the Superscript III enzyme kit (Invitrogen). Quantitative PCR was performed using Sensimix SYBR PCR Kit (Bioline) on a 7900HT Fast-Real-Time PCR System (Applied Biosystems). The copy number for each transcript is expressed relative to that of HPRT. Quantitative PCR results are displayed as normalized to control. Primers used for quantitative PCR are listed in supplementary material Table S1. For standard PCR, cDNA transcripts were amplified using GoTaq Flexi polymerase (Promega, M8291). Amplification of TMEM88 isoforms was performed using the following primers: Forward primer 1: GCAGCCGCGGCAAATCC; Forward primer 2: CCCGCAGCCGCGGCAAATCC; Reverse primer 3: GATGCTAGGACTCCGCTCAGGC.
Phylogenetic analysis of TMEM88 protein orthologs
Sequences were extracted by searching the NCBI protein database (supplementary material Table S2). Protein and nucleotide sequences were aligned by ClustalW analysis as originally described by Thompson et al. (Thompson et al., 1994). Multiple alignment parameters were as follows: gap penalty=15; gap length penalty=6.66; protein weight matrix: Gonnet series. Pairwise analysis of percent identity between sequences was used to define the relationship between proteins or within proteins across designated species. The values computed are the mean number of differences per site and fall between 0 and 1. Zero represents complete identity and 1 no identity. Scale of the phylogenetic tree indicates the number of nucleotide substitutions per 100 residues. Sequence ID and divergence values were calculated such that divergence (i,j) is calculated as {100[distance (i,j)]/total distance}, where (i,j) is the sum of the branch lengths between two sequences and total distance is the sum of all branch lengths.
Gene expression analysis by array
Total RNA integrity was checked using an Agilent 2200 TapeStation (Agilent Technologies, Santa Clara, CA, USA) and quantified using a Trinean DropSense96 spectrophotometer (Caliper Life Sciences, Hopkinton, MA, USA). High quality RNA samples were converted to cDNA and biotin-labeled for microarray analysis using Ambion’s Illumina TotalPrep RNA Amplification Kit (Life Technologies, Grand Island, NY, USA). Labeled cRNAs were processed on a HumanHT-12 v4 Expression BeadChip (Illumina, San Diego, CA, USA) and imaged using an Illumina iScan system.
Microarray data analysis
The complete set of microarray data was assessed for quality, followed by quantile normalization using the Bioconductor package lumi (Du et al., 2008). The dataset was filtered initially by flagging probes that were below a signal ‘noise floor’, which was established using the 75th percentile of the negative control probe signals within each array. We subsequently filtered the dataset by employing a variance filter using the ‘shorth’ function of the Bioconductor package genefilter. Pair-wise statistical analyses were performed using the Bioconductor package limma (Smyth, 2005), and a false discovery rate (FDR) method was used to correct for multiple testing (Reiner et al., 2003). Differential gene expression was defined as |log2 (ratio)| ≥1 (±twofold) with the FDR set to 5%. Heat maps were generated with ‘R’ using normalized values. Differentially expressed genes were classified according to gene ontology, using Bioconductor packages and online tools (DAVID/EASE, http://david.abcc.ncifcrf.gov/). Microarray data have been deposited in the NCBI GEO database under accession number GSE43805.
Flow cytometry
Cells were labeled for flow cytometry using the following antibodies: human anti-PDGFRα APC (R&D Systems) and human anti-VEGF R2/KDR PE (R&D Systems). Cells were analyzed using a BD FACSCANTO II or sorted on a BD FACSARIA II (Beckton Dickinson, San Jose, CA, USA) with FACSDiva software (BD Biosciences). Cells were analyzed or sorted using a 488-nm argon laser plotting PE (585/42 filter) and a 633-nm argon laser plotting APC (660/20). Instrument settings were adjusted to avoid spectral overlap. Data analysis was performed using FlowJo (Tree Star, Ashland, OR, USA).
Luciferase reporter assays
Cells were lysed in vitro. After one freeze/thaw cycle, cell lysate was mixed with luciferase assay reagent (Promega) and analyzed by bioluminometry using an Envision plate reader.
Immunofluorescence
Cells were fixed in 2% paraformaldehyde, permeabilized in PBS containing 0.025% Triton X-100, and blocked in PBS containing 1.5% normal goat serum. Cells were stained with anti-von Willibrand Factor (vWF) (1:500, Dako) followed by secondary staining with Alexa Fluor 488 (1:200, Molecular Probes). Nuclei were counterstained with DAPI.
Zebrafish in situ hybridization
The AB wild-type strain (Zebrafish International Resource Center, Eugene, OR, USA) was used for all fish experiments, maintained using standard procedures and used in accordance with Institutional Animal Care and Use Committee-approved protocols. Brightfield in situ hybridizations for nkx2.5 and tmem88a were performed using digoxigenin-UTP labeled probes as previously described (Thisse and Thisse, 2008) with the exception that maleic acid buffer (MAB) was used in place of PBS in all washes between hybridization and labeling. The nkx2.5 probe was synthesized from published constructs (Lee et al., 1996; Yelon et al., 1999). For the tmem88a probe, full-length mRNA sequence was PCR amplified from ATCC clone 10330432 and subcloned into the pGEM-T Easy vector (Promega). Double fluorescent in situ hybridizations were carried out as previously described (Welten et al., 2006), using a fluorescein-UTP-labeled tmem88a and a digoxigenin-UTP-labeled nkx2.5 probe. Images were taken with a Nikon SMZ1500 microscope using a Nikon Digital Sight DS-Ri1 camera.
Statistics
Single variable analysis between two samples was carried out using Student’s t-test. Single and multivariable assays were analyzed by one- or two-way ANOVA. Results are presented as mean±s.e.m. For all statistically significant results, *P<0.05.
RESULTS
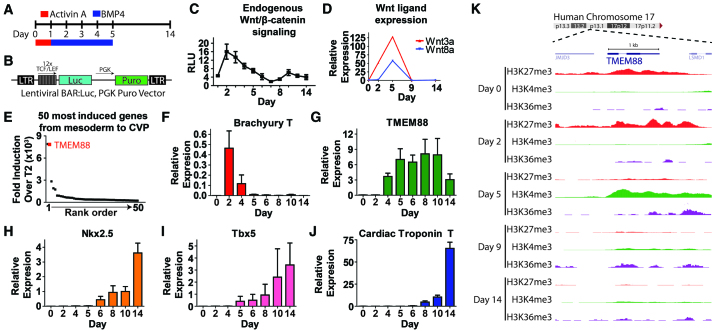
The cardiovascular progenitor cell population provides a potential source of cells useful for regenerating the injured heart owing to its capacity to give rise to multiple cardiovascular cell types, including endothelial cells, smooth muscle cells and cardiomyocytes. Work from our group and others has identified a biphasic role for Wnt/β-catenin signaling during cardiovascular development, with Wnt signals being required for mesoderm induction and their suppression being required for emergence of cardiomyocytes (Ueno et al., 2007; Paige et al., 2010). In the context of high-density monolayer cardiac directed differentiation (Fig. 1A), a Wnt/β-catenin-activated reporter (Fig. 1A) expressed in hESCs was used to assay endogenous Wnt/β-catenin pathway activity during cardiomyocyte development. These results show that Wnt/β-catenin signaling activity peaks at 2 days (mesoderm) and declines significantly by day 5 when the cardiovascular progenitor is prominent (Fig. 1C). Interestingly, the mRNA for multiple Wnt ligands capable of activating this pathway persisted at high levels between days 2 and 5 (Fig. 1D), suggesting that ligand availability could not explain this decline in activity.
Fig. 1.
TMEM88 is strongly activated during development of the cardiac progenitor cell when Wnt signaling is downregulated. (A) Schematic of high-density monolayer cardiac directed differentiation of hESCs. (B) Schematic of lentiviral vector expressing β-catenin activated reporter (BAR). (C) BAR-luciferase measurements during cardiac differentiation of hESCs. RLU, relative light units. (D) mRNA expression of canonical Wnt ligands during cardiac directed differentiation of hESCs. (E) Rank order of the top 50 genes most highly induced from mesoderm (day 2) to development of the CVP (day 5). (F-J) Expression profiles by qPCR of brachury T (F), TMEM88 (G), NKX2.5 (H), TBX5 (I) and cardiac troponin T (J) during cardiac directed differentiation of human ESCs (RUES2). (K) Chromatin immunoprecipitation/deep sequencing analysis of epigenetic histone modifications, including H3K27me3 (red), H3K4me3 (green) and H3K36me3 (purple) around the TMEM88 locus during different cell state transitions of hESC (H7) cardiac directed differentiation. Error bars indicate s.e.m.
To gain more insight into this developmental process, gene expression arrays and chromatin profiles recently generated by Paige et al. (Paige et al., 2012) were analyzed to identify potential negative regulators of Wnt/β-catenin signaling that were highly induced during the transition of hESC derivatives from mesoderm to the cardiac progenitor cell. The top 50 most induced genes were collated (Fig. 1E). Transmembrane protein 88 (TMEM88) was identified as a protein reported to negatively regulate Wnt/β-catenin in a cell-autonomous manner and was highly induced during fate specification of the CVP (Lee et al., 2010). We selected this gene for further study to test the working hypothesis that TMEM88 regulates Wnt/β-catenin signaling during cardiogenesis.
There are two TMEM88 isoforms (TMEM88 CRA_a: GI 119610530; and TMEM88 CRA_b: GI 119610531), which are expressed from the same genomic position on human chromosome 17 (supplementary material Fig. S1A). Note that TMEM88 CRA_b is not yet annotated and has no sequence relation to the annotated TMEM88B gene (GI 226442743) located on human chromosome 1. TMEM88 CRA_a contains the c-terminal PDZ binding motif. TMEM88 CRA_b has the same transcript as TMEM88 CRA_a with the exception of a second intron starting precisely upstream of the Val-Trp-Val PDZ binding motif and after 68 bp initiates a third exon. As a consequence, TMEM88 CRA_b has a distinct C-terminus and does not have the crucial PDZ binding motif known to regulate Wnt/β-catenin signaling. To determine which isoform is expressed during hESC cardiac differentiation, transcripts from day 5 cardiovascular progenitor cells were analyzed (supplementary material Fig. S1B). RT-PCR was used to amplify the domain encompassing the transcript difference between TMEM88 CRA_a and TMEM88 CRA_b with the difference detected by variations in nucleotide length. These data show that TMEM88 CRA_a, which contains the PDZ binding motif, is the dominant isoform expressed during hESC cardiac differentiation. TMEM88 CRA_a is hereafter referred to as TMEM88.
Gene expression analysis by array as well as epigenetic chromatin dynamics revealed a tightly regulated temporal activation profile for TMEM88 during cardiac differentiation. By qPCR, TMEM88 is activated shortly after the mesoderm maker brachyury (T) (Fig. 1F) and increases during the lineage specification of the cardiovascular progenitor cell at day 5 (Fig. 1G). TMEM88 activates prior to key transcription factors involved in heart development, such as NKX2.5 (Fig. 1H) and TBX5 (Fig. 1I), and well in advance of myofilament genes, such as cardiac troponin T (Fig. 1J). This places the TMEM88 transcript temporally at a point at which it could affect the fate of cardiovascular progenitors.
Chromatin dynamics during different stages of cardiac directed differentiation of hESCs were acquired by chromatin immunoprecipitation followed by deep sequencing (Paige et al., 2012). Epigenetic analysis was performed for lysine 27 trimethylation on histone 3 (H3K27me3), which is a repressive chromatin mark, as well as for marks of actively transcribed chromatin as measured by lysine 4 trimethylation on histone 3 (H3K4me3) and lysine 36 trimethylation on histone 3 (H3K36me3, a surrogate for RNA polymerase II activity) (Fig. 1K). The TMEM88 locus at human chromosome 17p13.1 is marked by repressive H3K27 trimethylation in the undifferentiated state (day 0) and during the transition through mesoderm lineage specification (day 2). Consistent with gene expression array data, the activating H3K4me3 and H3K36me3 marks remain low during pluripotency and mesoderm specification. However, as cell fate restricts to the cardiovascular progenitor at day 5, the repressive H3K27me3 decreases markedly, and the activating H3K4me3 state expands (Fig. 1K). Thus, TMEM88 has a large dynamic range of RNA expression, associated with tight regulation by repressive and activating waves of chromatin.
In order to obtain anatomical information about the expression pattern of TMEM88 during embryogenesis, we studied the zebrafish ortholog tmem88a during development. In situ hybridization of zebrafish embryos shows no expression prior to the two-somite stage. As the lateral plate mesoderm emerges at 10.5 hours post-fertilization (hpf) (two-somite stage), tmem88a is expressed in bilateral ‘stripes’ ahead of other cardiac markers, such as nkx2.5 (Fig. 2A,A′,F,F′). At 11.5 hpf (five-somite stage), nkx2.5 emerges in a subset of the domain outlined by tmem88a expression (Fig. 2B,B′,G,G′). By 13 hpf (eight-somite stage), tmem88a marks a broad swath of lateral plate mesoderm anterior to posterior, extending beyond the cardiogenic mesoderm marked by nkx2.5 (Fig. 2C,C′,H,H′). Whole-mount analyses at 19 and 24 hpf revealed that tmem88a is expressed within the primordial heart field but seems to be more expansively expressed in the emerging vasculature (Fig. 2D,E,I,J), consistent with the findings of Gomez et al. (Gomez et al., 2012).
Fig. 2.
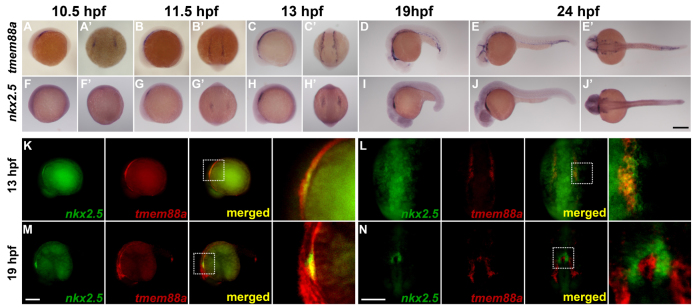
In situ hybridization of tmem88a and nkx2.5 in developing zebrafish embryos. (A-J′) tmem88a and nkx2.5 expression during early development in zebrafish. Whole-mount in situ hybridizations for tmem88a (A-E′) and nkx2.5 (F-J′) were performed on embryos ranging from 10.5 hpf to 24 hpf. (K-N) Double fluorescent in situ hybridizations for nkx2.5 (green) and tmem88a (red) at 13 hpf (eight-somite stage; K,L) and 19 hpf (M,N). The white dotted box in merged panels (K-M) outlines the region shown at 4× magnification to the right. A-J, K and M are lateral views with anterior to the left. A′-J′, L and N are dorsal views with anterior to the bottom. Scale bars: in J′, 200 μm for A-J′; in M, 200 μm for K,M; in N, 200 μm for L,N.
To gain more clarity about the localization of tmem88a in the developing heart, double in situ hybridization was performed for tmem88a and nkx2.5 (Fig. 2K-N). At 13 hpf, lateral and dorsal views show that tmem88a and nkx2.5 have a clear overlap in the emerging heart field, although tmem88a extends both anteriorly and posteriorly from nkx2.5 (Fig. 2K,L). At 19 hpf, tmem88a is expressed in the developing vasculature but is not expressed in coordination with nkx2.5 in the heart (Fig. 2M,N). These data indicate that tmem88a is expressed at a time and place where it could regulate pre-cardiac mesoderm specification but is downregulated at later stages of development, even as expression increases in the developing vasculature.
Because TMEM88 is potentially a crucial regulator of CVP differentiation, we wanted to determine whether this protein has been conserved throughout evolution. Amino acid sequences were compared in multiple species using known or predicted sequences for TMEM88. Interestingly, sequences were found within the chordate ancestry but were restricted to vertebrate species (supplementary material Fig. S2). No orthologs were identified for TMEM88 within any invertebrate chordates, nor were orthologs found in insects such as Drosophila melanogaster. Sequence percent identity and phylogenetic analysis of protein sequences shows bird and amphibian TMEM88 diverging markedly from fish, reptiles and mammals. Although various regions and residues showed high sequence similarity, the C-terminal PDZ binding motif valine-tryptophan-valine (VWV) showed high homology among all species and perfect homology among all species for the last two WV residues (supplementary material Fig. S2). Thus, TMEM88 protein appears as vertebrate chordates diverge from their invertebrate counterparts, and the PDZ domain is a particularly conserved region.
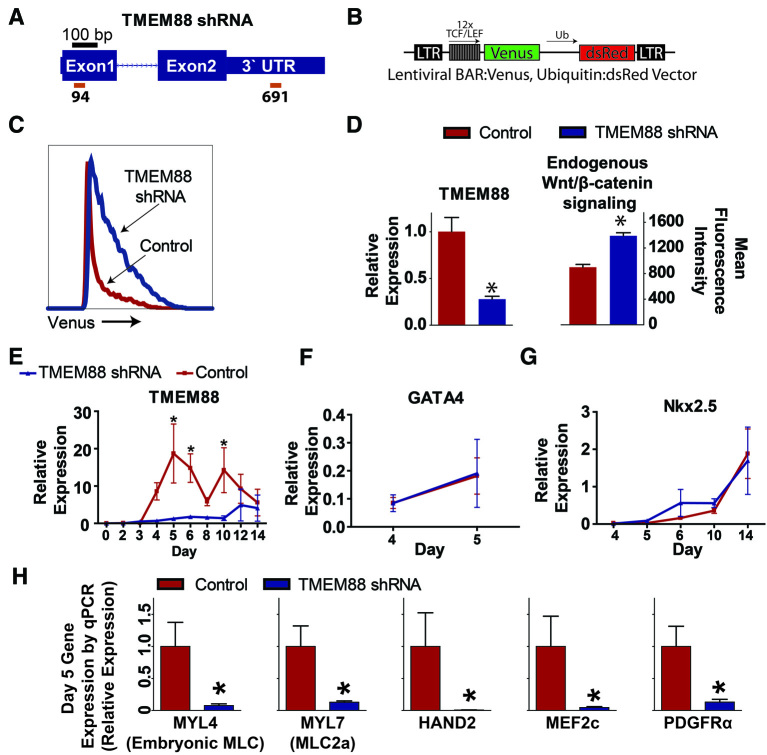
To determine whether TMEM88 is functionally required for cardiac differentiation, two different lentiviruses were used that express short hairpin RNA (shRNA) sequences targeting the first exon (shRNA 94) and the 3′ UTR (shRNA 691) of the TMEM88 transcript (Fig. 3A). We first sought to determine whether TMEM88 regulates Wnt pathway activity during hESC cardiovascular differentiation. Using a BAR-venus lentiviral vector (Davidson et al., 2012), endogenous Wnt pathway activity was analyzed to determine an effect on Wnt/β-catenin signaling in TMEM88 knockdown (KD) cells (Fig. 3B). At day 6 of differentiation, TMEM88 KD cells had significantly higher Wnt pathway activity compared with controls, based on measurements of mean venus fluorescence intensity (Fig. 3C,D). Thus, TMEM88 functions as a negative regulator of β-catenin signaling during cardiac differentiation.
Fig. 3.
Knockdown of TMEM88 increases Wnt/β-catenin signaling and attenuates cardiomyocyte differentiation in vitro. (A) Schematic of shRNA binding sites (red bars) on the TMEM88 transcript. (B) Schematic of BAR-venus vector. (C) Representative histogram of venus activity in control versus TMEM88 shRNA populations. (D) TMEM88 expression by qPCR (left) and mean fluorescence intensity of BAR-venus activity (right) in control versus TMEM88 shRNA samples. (E) Gene expression analysis by qRT-PCR of TMEM88 comparing TMEM88 shRNA KD with control shRNA during cardiac directed differentiation. (F,G) Expression of GATA4 (F) and NKX2.5 (G) in control versus TMEM88 shRNA samples by qRT-PCR during the time course of cardiac differentiation. (H) Validation of key cardiac genes identified by array using qRT-PCR analysis showing significant downregulation of the cardiac gene program at day 5. TMEM88 shRNA runs n=5 from two different shRNA vectors, control shRNA runs n=7. Error bars represent s.e.m. *P<0.05.
Cells transduced with TMEM88 or control shRNAs were induced for cardiac differentiation and samples were isolated for RNA analysis at various time points. By quantitative RT-PCR, TMEM88 transcripts were significantly diminished throughout differentiation in the shRNA TMEM88 cohort compared with the control shRNA cells (Fig. 3E). Day 2 brachyury expression was not different between control and knockdown cohorts, indicating that TMEM88 KD did not alter cell fate commitment into the mesoderm lineage (brachyury expression with control shRNA: 1.6±0.69; versus TMEM88 shRNA: 3.0±1.4; P=0.36). Expression of GATA4 from day 4 to 5 was not altered by knockdown of TMEM88 (Fig. 3F), consistent with findings by Novikov and Evans indicating that TMEM88 acts downstream of GATA factors during cardiac development (Novikov and Evans, 2013). Furthermore, expression of NKX2.5 did not show a significant difference during the time course of cardiac differentiation between TMEM88 shRNA and control samples (Fig. 3G).
To determine the genome-wide implications for TMEM88 KD in cardiovascular progenitor cells, RNA from day 5 of differentiation was assayed by gene expression array. We found 298 genes that were greater than twofold differentially expressed between control and TMEM88 shRNA samples at day 5 (supplementary material Table S3). Many genes with known functions in cardiovascular development or function were significantly downregulated by TMEM88 KD (supplementary material Table S4). DAVID bioinformatics analysis of gene ontologies downregulated in TMEM88 shRNA samples showed a significant enrichment for genes involved in processes of solid tissue development, such as tissue morphogenesis and extracellular matrix, as well as a wide range of ontologies crucial for cardiac development and function (supplementary material Table S5). Quantitative PCR of key genes involved in heart development analyzed at day 5 confirmed the array observations across all TMEM88 and control shRNA samples (Fig. 3H). Analysis of array data from control versus TMEM88 KD also revealed that downregulation of TMEM88 resulted in reduced expression of multiple other Wnt/β-catenin pathway regulators, including WNT5A, DKK1 and SFRP5 (supplementary material Table S4).
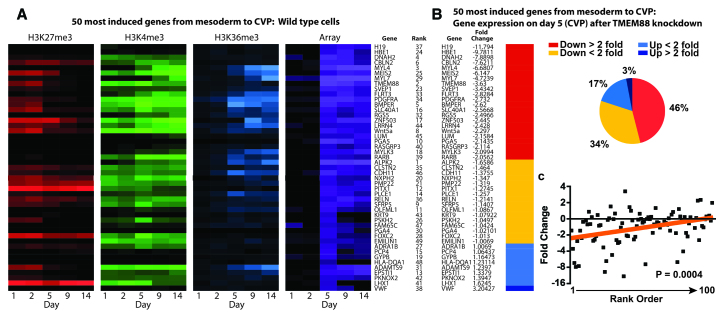
Next, we analyzed the effect of TMEM88 KD on the genes induced to the greatest extent during the lineage transition from mesoderm to the cardiovascular progenitor cell population. Gene expression array data at day 5 were normalized to the expression at day 2 to define the fold induction for each gene during the transition from mesoderm to the cardiovascular progenitor cell. The top 50 most induced genes were then analyzed by k-means clustering to generate a heat map of chromatin and transcript expression for each gene (Fig. 4A). This is an unbiased approach, as no pre-existing knowledge about gene function is required to determine the rank order. As expected, transcript expression for all genes is highly induced at day 5, with many genes showing early repressive chromatin, followed at later stages by activating chromatin (e.g. TMEM88), an epigenetic signature indicative of genes involved in mediating cell fate transitions (Fig. 4A). We consider this collection of genes to be a molecular signature of the cardiovascular progenitor cell, because it defines in an unbiased fashion those genes most highly induced during the development of the CVP.
Fig. 4.
TMEM88 KD results in global downregulation of genes that signify development of the CVP. (A) Chromatin profile and gene expression from day 0 (undifferentiated state) to day 14 (mature cardiomyocyte) in wild-type cells for the top 50 most highly induced genes during development of the CVP. (B) Array data were used to assess the impact of TMEM88 KD for all genes identified in the CVP signature. Genes were grouped by fold change relative to control shRNA. (C) Linear regression (red line) was performed comparing the top 100 most induced genes in the CVP ranked sequentially and analyzed against the fold change for each gene in the TMEM88 KD.
In the presence of TMEM88 shRNA, 46% of these highly induced genes are downregulated greater than twofold, and an additional 34% are downregulated by less than twofold (Fig. 4B). The remaining 20% of genes had some degree of upregulation. Linear regression analysis shows a significant (P=0.0004) correlation between the degree of downregulation after TMEM88 KD relative to the induction level of that gene during the transition from mesoderm to the CVP (Fig. 4C). Taken together, these data show that TMEM88 KD results in a global downregulation of genes that define the development of cardiovascular progenitor cells.
Because TMEM88 KD has a marked impact on cardiovascular progenitor cell development, cells were analyzed at the day 14 time point to determine the impact on cardiac fate. At day 14, when beating cardiomyocytes are present in control cultures, the TMEM88 KD cells showed a marked downregulation of markers of definitive cardiomyocytes, including reduced expression of myofilament and calcium handling proteins (Fig. 5A). To determine whether the deficiency in cardiac differentiation resulting from TMEM88 KD could be recovered by introducing an exogenous Wnt inhibitor, XAV-939 was added at day 3 of differentiation. XAV-939 is a tankyrase inhibitor that causes increased steady state levels of axin, thus blocking β-catenin signaling at a molecular level expected to be downstream of TMEM88. Addition of 1 μM XAV-939 had no effect on TMEM88 transcript levels (Fig. 5B) but partially recovered the cardiac differentiation of cells with TMEM88 KD based on flow cytometry analysis of cardiac troponin T (cTnT)-positive cells at day 14 of differentiation (Fig. 5C).
Fig. 5.
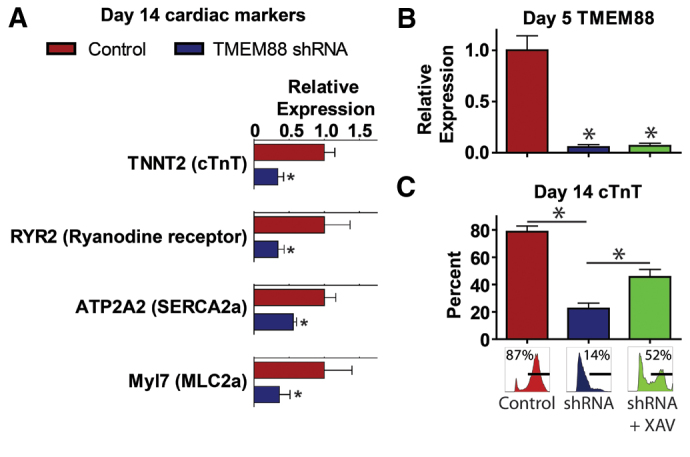
TMEM88 KD attenuates cardiac maturation, which can be rescued by exogenous Wnt inhibition. (A) Quantitative PCR of cardiac genes at the day 14 time point comparing TMEM88 shRNA versus control shRNA cohorts. (B,C) TMEM88 expression by qPCR at day 5 of differentiation (B) and percentage cTnT by flow cytometry at day 14 of differentiation (C) in control versus TMEM88 shRNA versus TMEM88 shRNA + 1 μM XAV-939. shRNA runs ± XAV-939 n=3-5 from two different TMEM88 shRNA vectors; control shRNA runs n=5-7. Error bars represent s.e.m. *P<0.05.
We also explored whether knockdown of TMEM88 promoted alternative lineages at the expense of cardiovascular progenitors. Bioinformatics analysis of gene ontology show that gene clusters greater than twofold upregulated in the TMEM88 KD cells included the Notch signaling pathway (Kim et al., 2013), EGF signaling (Doan et al., 2013), regulation of cell proliferation, mesenchymal and mesodermal developmental, and, most interestingly, genes involved in hematopoiesis and blood vessel development (supplementary material Table S6). The endothelial potential of these cells was validated by qPCR of specific genes involved in development of hemato-endothelial lineages (Fig. 6A). In addition to upregulation of HHEX (a hemato-endothelial specific homeobox gene) and OTX1 (a homeobox gene involved in hematopoiesis and neural development), we found that SCL (TAL1), encoding a master transcription factor that drives hemato-endothelial specification and represses cardiomyogenesis (Gering et al., 1998; Van Handel et al., 2012; Kim et al., 2013), was significantly increased in TMEM88 KD cohorts. SOX17, also greater than twofold upregulated in TMEM88 KD cells, has recently been shown to be expressed in hemogenic endothelium and emerging hematopoietic stem cells (HSCs) and is functionally required for HSC development (Clarke et al., 2013). Other genes with known functions in blood and endothelial development that were twofold upregulated in TMEM88 KD cells are listed in supplementary material Table S7.
Fig. 6.
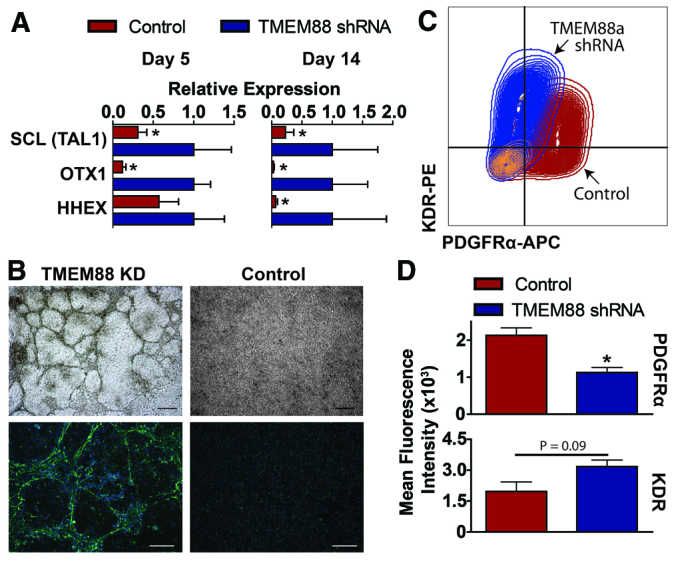
Genes upregulated in TMEM88 KD cells suggest activation of endothelial lineages. (A) Quantitative PCR analysis at day 5 and day 14 of selected genes involved in mediating endothelial cell development and differentiation. (B) Brightfield images (top) and immunofluorescence for vWF (bottom) of TMEM88 shRNA KD cells compared with control at day 5. vWF, green; DAPI, blue. Scale bars: 100 μm (brightfield); 200 μm (immunofluorescence). (C,D) Representative flow cytometry plot (C) and quantification (D) of mean fluorescence intensity of KDR and PDGFRα at day 5 for control versus TMEM88 KD populations. n=3-7 per group. Error bars represent s.e.m. *P<0.05.
TMEM88 KD induced striking morphological changes as well. Whereas the cardiovascular progenitors grew as flattened monolayers, the TMEM88 KD cells grew as networks of cell cords/tubes, reminiscent of endothelial cell cultures on Matrigel (Fig. 6B). Immunostaining for the endothelial marker von Willebrand factor showed that the cellular networks were composed almost entirely of endothelial cells (growing atop non-endothelial cells), whereas endothelial cells were rare in the control population (Fig. 6B). These data suggest that knockdown of TMEM88 re-directs pre-cardiac mesodermal derivatives into an endothelial pathway instead of the cardiomyocyte progenitor population.
Previous studies have shown that cardiac progenitors are derived from a KDRlow, PDGFRα+ population, whereas endothelium transitions through a KDRhigh, PDGFRα- population (Shalaby et al., 1997; Kattman et al., 2011). We therefore sought to determine whether endothelial cells observed in TMEM88 KD cells have a similar phenotype. We analyzed KDR and PDGFRα expression by flow cytometry at day 5 of cardiac differentiation and found that control cells were KDRlow, PDGFRα+ (Fig. 6C,D). However, TMEM88 KD cells were shifted towards KDRhigh expression with a significant downregulation of PDGFRα, consistent with an endothelial phenotype (Fig. 6C,D).
During in vivo development as well as ESC differentiation in vitro, populations of endothelial cells are known to have hemogenic potential, and this has recently been shown to be true of cardiovascular endothelium (Van Handel et al., 2012; Nakano et al., 2013; Rafii et al., 2013). We therefore determined whether endothelial cells derived from TMEM88 KD could generate blood lineages. At day 5 of differentiation, cultures were transitioned to EGM media to facilitate endothelial development (supplementary material Fig. S3A). After 2 weeks of differentiation, hESC-derived cells were harvested and plated in co-culture with OP9 cells with hematopoietic cytokines (supplementary material Fig. S3A). Following 5 to 6 days of co-culture, non-adherent cells were removed from co-culture and stained with the hematopoietic-specific markers CD43 (SPN), CD235a (GYPA) and CD45 (PTPRC). Flow cytometric analysis showed no significant expression of these markers in control cultures. However, TMEM88 KD cultures contained cells co-expressing CD43 and CD235a, a phenotype consistent with primitive erythropoiesis (supplementary material Fig. S3B,C) (Vodyanik et al., 2006; Rafii et al., 2013). Some evidence of non-erythroid blood was observed in TMEM88 KD cultures based on CD45 expression, although not consistently (data not shown). The predominance of the erythroid phenotype in the TMEM88 KD cultures perhaps reflects the culture conditions used or, alternatively, the known role in Wnt/β-catenin signaling in promoting primitive erythropoiesis (Cheng et al., 2008).
DISCUSSION
Using functional assays in vitro and in vivo, this study shows that TMEM88 is required for development of cardiomyocytes from the multilineage cardiovascular progenitor cells. A previous study by Lee et al. has shown that TMEM88 is a negative regulator of Wnt/β-catenin signaling (Lee et al., 2010), and we verified enhanced β-catenin activity after TMEM88 KD in our hESC differentiation system. Array and epigenetic analysis of TMEM88 shows that this gene is highly induced (∼7500-fold) during the transition from mesoderm to the CVP and has the chromatin signature emblematic of genes involved in mediating cell fate decisions. During zebrafish heart development, tmem88a marks the bilateral heart fields in the ventrolateral mesoderm. Knockdown of TMEM88 blocks development of the CVP in vitro at least in part because of increased Wnt/β-catenin signaling. Furthermore, in the absence of TMEM88, many of the cells acquire a functional endothelial phenotype in vitro. Cumulatively, these data suggest that TMEM88 mediates a cell fate decision in the pre-cardiac mesoderm that defines the onset of cardiomyocyte specification through inhibition of Wnt signaling.
In a parallel study using a genetic screen to identify genes de-regulated after depletion of Gata5 and Gata6 in zebrafish, Novikov and Evans (Novikov and Evans, 2013) also identified Tmem88 as a key regulatory molecule during heart development. Using transgenic reporter fish analyzed at 51 hpf, they showed that the total number of atrial and ventricular cardiomyocytes was significantly reduced in Tmem88 morpholino-treated zebrafish compared with controls. A failure to generate sufficient cardiomyocytes ultimately results in subsequent morphological issues, such as looping defects, in vivo.
In the current study, TMEM88 is identified as a protein required for development of the cardiovascular progenitor cell and fits the model surrounding proteins that mediate the specification of cell fates. We have previously (Paige et al., 2012) identified a chromatin and gene expression signature that identifies genes involved in mediating cell fate choices, which is of particular utility for those genes that do not fit under the paradigm of transcription factors mediating key cell fate transitions. TMEM88, a transmembrane protein, exhibits this signature because the locus shows repressive chromatin (H3K27me3) during stages at which expression of the gene would be deleterious to the state or fate of the cell, e.g. by blocking mesoderm formation. During specification of the CVP, the repressive chromatin diminishes and the euchromatic H3K4me3 mark increases, resulting in strong activation of the gene precisely during the period when it functions to mediate restriction into the cardiovascular lineage.
Lee et al. showed that the C-terminal PDZ binding motif of TMEM88 binds Disheveled (Dvl) (Lee et al., 2010). In the absence of TMEM88, binding of Wnt ligands to Frizzled family receptors results in the recruitment of axin by Dvl to the membrane. This dissociates the destruction complex and allows β-catenin to translocate to the nucleus and activate Wnt target genes. If Dvl is competitively recruited by TMEM88, axin does not translocate to the membrane, and canonical Wnt signaling is blocked. Consistent with this hypothesis, our functional assays showed that knockdown of TMEM88 increases Wnt pathway activity, and addition of the exogenous Wnt inhibitor XAV-939 partially rescues cardiac differentiation. Novikov and Evans (Novikov and Evans, 2013) similarly found that increased Wnt activation exacerbated the cardiac developmental defects in TMEM88 morpholino-treated zebrafish, but inhibiting Wnt signaling using heat shock-inducible Dkk1 rescued the knockdown phenotype.
Downregulation of Wnt signaling has long been known to be crucial for cardiovascular development (Ueno et al., 2007), and inclusion of inhibitors (such as XAV-939, IWP or KY02111) in the post-gastrulation phase of protocols for ESC cardiac differentiation have become commonplace (Willems et al., 2011; Lian et al., 2012; Minami et al., 2012). Despite this, the specific mechanisms mediating endogenous inhibition of Wnt signaling have not been fully elucidated. We propose that TMEM88, a known negative regulator of Wnt signaling (Lee et al., 2010), is integral to this process.
Functionally, a failure to express TMEM88 in the pre-cardiac mesoderm shifts cell fate towards endothelial development. In vitro data presented here cumulatively show that knockdown of TMEM88 has no effect on GATA4 or NKX2.5 expression. This suggests that TMEM88 functions downstream of GATA factors at the bifurcation of lineages from the pre-cardiac mesoderm to specify cardiomyocyte development. Recent studies have shown the requirement for Nkx2.5 in endothelial lineages by fate mapping (Ma et al., 2008) and functionally in the hemogenic capacity of pre-cardiac mesoderm (Ferdous et al., 2009) and endocardial-endothelial derivatives (Nakano et al., 2013). Furthermore, studies have shown that sustained Wnt/β-catenin signaling mediates the development of primitive erythroid lineage (Cheng et al., 2008), which is predominantly what we observe in TMEM88 KD cells in co-culture with OP9 cells. Further studies will be required to delineate whether the hemogenic potential observed from TMEM88 KD results from endocardial-endothelial derivatives or reflects a role of TMEM88 in modulating Wnt/β-catenin signaling during earlier stages of hemato-endothelial development.
Interestingly, results from Gomez et al. (Gomez et al., 2012) show that Tmem88 is expressed in the posterior mesoderm in the developing vasculature of the zebrafish. In situ hybridization data shown here also indicate that Tmem88 is expressed in the developing vasculature. Expression of Tmem88 in the anterior to posterior poles of the lateral plate mesoderm indicate that Tmem88 might be involved in early stages of hemogenic-endothelial development and potentially other mesodermal derivatives. Expression of Tmem88 clearly increases in the developing vasculature at stages of development when it is markedly diminished in heart development. This may indicate that the endothelium in our hESC system is different from that of the zebrafish, e.g. resembling endocardial endothelium more than that of the peripheral vasculature (Nakano et al., 2013). Further studies are required to clarify this point.
Taken together, we propose a model for TMEM88 in heart development (Fig. 7). The data presented here suggest that TMEM88 is expressed in the pre-cardiac mesoderm after GATA factors in NKX2.5 derivatives to specify cardiomyocyte development through downregulation of canonical Wnt/β-catenin signaling. Endothelial development occurs in cells depleted of TMEM88 in which sustained Wnt pathway activity is required for activation of endothelial gene programs. Cumulatively, this study provides evidence that TMEM88 is indispensable for cardiomyocyte development through its role in modulating Wnt signaling in the pre-cardiac mesoderm.
Fig. 7.
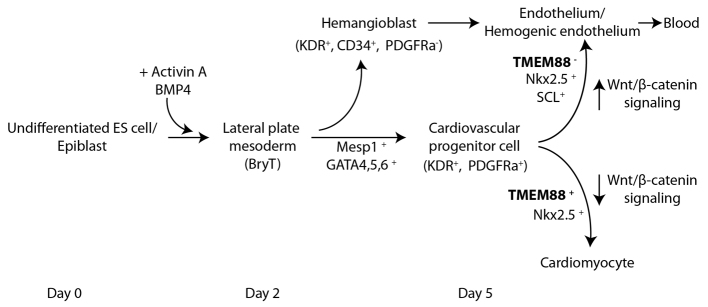
Model for TMEM88 function in cardiovascular development. During heart development, cells transition through lateral plate mesoderm before specifying the cardiovascular lineage. The data described in this study suggest that TMEM88 acts late in cardiovascular development after the cardiovascular progenitor has been specified. TMEM88 inhibits Wnt signaling in order to drive development of the cardiomyocyte from the multilineage CVP. Cells lacking TMEM88 differentiate to an endothelial fate, which develops as a consequence of sustained high Wnt signaling activity.
Acknowledgments
We thank the Genomics Resource Center at the Fred Hutchinson Cancer Research Center; Lisa Maves for technical help with the double fluorescent in situ hybridization; the Ellison stem cell core; Mark Saiget (bioinformatics assistance); and Stanley Kim and Jerry Ament (zebrafish maintenance).
Footnotes
Funding
This work was supported by grants from the National Institutes of Health (NIH) [P01 HL094374, P01 GM081719, U01 HL100405, R01 HL084642 and U01 HL100395; F30 HL095343 to S.P.; T32 HL007312 to N.J.P.]. R.T.M. is an investigator of the HHMI. Deposited in PMC for release after 6 months.
Competing interests statement
The authors declare no competing financial interests.
Author contributions
N.J.P. conceived and performed experiments, and wrote the manuscript; L.P. conceived experiments; J.S.R. conceived and performed experiments; B.K.H. performed experiments; C.L.S.-C. performed important preliminary studies in support of this study; S.L.P. performed important preliminary studies in support of this study; I.D.B. supervised work by B.K.H.; R.T.M supervised work by C.L.S.-C. and J.S.R., and contributed important intellectual insight to the formation of the study; and C.E.M. supervised work by N.J.P. and S.L.P., contributed important intellectual insight to the formation of the study, obtained funding for the research, and wrote the manuscript.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.094789/-/DC1
References
- Brown C. O., 3rd, Chi X., Garcia-Gras E., Shirai M., Feng X. H., Schwartz R. J. (2004). The cardiac determination factor, Nkx2-5, is activated by mutual cofactors GATA-4 and Smad1/4 via a novel upstream enhancer. J. Biol. Chem. 279, 10659–10669 [DOI] [PubMed] [Google Scholar]
- Cheng X., Huber T. L., Chen V. C., Gadue P., Keller G. M. (2008). Numb mediates the interaction between Wnt and Notch to modulate primitive erythropoietic specification from the hemangioblast. Development 135, 3447–3458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke R. L., Yzaguirre A. D., Yashiro-Ohtani Y., Bondue A., Blanpain C., Pear W. S., Speck N. A., Keller G. (2013). The expression of Sox17 identifies and regulates haemogenic endothelium. Nat. Cell Biol. 15, 502–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson K. C., Adams A. M., Goodson J. M., McDonald C. E., Potter J. C., Berndt J. D., Biechele T. L., Taylor R. J., Moon R. T. (2012). Wnt/β-catenin signaling promotes differentiation, not self-renewal, of human embryonic stem cells and is repressed by Oct4. Proc. Natl. Acad. Sci. USA 109, 4485–4490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doan P. L., Himburg H. A., Helms K., Russell J. L., Fixsen E., Quarmyne M., Harris J. R., Deoliviera D., Sullivan J. M., Chao N. J., et al. (2013). Epidermal growth factor regulates hematopoietic regeneration after radiation injury. Nat. Med. 19, 295–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du P., Kibbe W. A., Lin S. M. (2008). lumi: a pipeline for processing Illumina microarray. Bioinformatics 24, 1547–1548 [DOI] [PubMed] [Google Scholar]
- Ferdous A., Caprioli A., Iacovino M., Martin C. M., Morris J., Richardson J. A., Latif S., Hammer R. E., Harvey R. P., Olson E. N., et al. (2009). Nkx2-5 transactivates the Ets-related protein 71 gene and specifies an endothelial/endocardial fate in the developing embryo. Proc. Natl. Acad. Sci. USA 106, 814–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantz J. A., Palpant N. J., Welikson R. E., Hauschka S. D., Murry C. E., Laflamme M. A. (2012). Targeted genomic integration of a selectable floxed dual fluorescence reporter in human embryonic stem cells. PLoS ONE 7, e46971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gering M., Rodaway A. R., Göttgens B., Patient R. K., Green A. R. (1998). The SCL gene specifies haemangioblast development from early mesoderm. EMBO J. 17, 4029–4045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez G., Lee J. H., Veldman M. B., Lu J., Xiao X., Lin S. (2012). Identification of vascular and hematopoietic genes downstream of etsrp by deep sequencing in zebrafish. PLoS ONE 7, e31658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattman S. J., Huber T. L., Keller G. M. (2006). Multipotent flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev. Cell 11, 723–732 [DOI] [PubMed] [Google Scholar]
- Kattman S. J., Witty A. D., Gagliardi M., Dubois N. C., Niapour M., Hotta A., Ellis J., Keller G. (2011). Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell 8, 228–240 [DOI] [PubMed] [Google Scholar]
- Kim P. G., Albacker C. E., Lu Y. F., Jang I. H., Lim Y., Heffner G. C., Arora N., Bowman T. V., Lin M. I., Lensch M. W., et al. (2013). Signaling axis involving Hedgehog, Notch, and Scl promotes the embryonic endothelial-to-hematopoietic transition. Proc. Natl. Acad. Sci. USA 110, E141–E150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. H., Xu Q., Breitbart R. E. (1996). A new tinman-related gene, nkx2.7, anticipates the expression of nkx2.5 and nkx2.3 in zebrafish heart and pharyngeal endoderm. Dev. Biol. 180, 722–731 [DOI] [PubMed] [Google Scholar]
- Lee H. J., Finkelstein D., Li X., Wu D., Shi D. L., Zheng J. J. (2010). Identification of transmembrane protein 88 (TMEM88) as a dishevelled-binding protein. J. Biol. Chem. 285, 41549–41556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian X., Hsiao C., Wilson G., Zhu K., Hazeltine L. B., Azarin S. M., Raval K. K., Zhang J., Kamp T. J., Palecek S. P. (2012). Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc. Natl. Acad. Sci. USA 109, E1848–E1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Kang I., Park C., Chang L. W., Wang W., Lee D., Lim D. S., Vittet D., Nerbonne J. M., Choi K. (2012). ER71 specifies Flk-1+ hemangiogenic mesoderm by inhibiting cardiac mesoderm and Wnt signaling. Blood 119, 3295–3305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q., Zhou B., Pu W. T. (2008). Reassessment of Isl1 and Nkx2-5 cardiac fate maps using a Gata4-based reporter of Cre activity. Dev. Biol. 323, 98–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B. L., Kimelman D. (2012). Canonical Wnt signaling dynamically controls multiple stem cell fate decisions during vertebrate body formation. Dev. Cell 22, 223–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami I., Yamada K., Otsuji T. G., Yamamoto T., Shen Y., Otsuka S., Kadota S., Morone N., Barve M., Asai Y., et al. (2012). A small molecule that promotes cardiac differentiation of human pluripotent stem cells under defined, cytokine- and xeno-free conditions. Cell Rep 2, 1448–1460 [DOI] [PubMed] [Google Scholar]
- Murry C. E., Keller G. (2008). Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell 132, 661–680 [DOI] [PubMed] [Google Scholar]
- Nakano H., Liu X., Arshi A., Nakashima Y., van Handel B., Sasidharan R., Harmon A. W., Shin J. H., Schwartz R. J., Conway S. J., et al. (2013). Haemogenic endocardium contributes to transient definitive haematopoiesis. Nat Commun 4, 1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikov N., Evans T. (2013). Tmem88a Mediates GATA-dependent specification of cardiomyocyte progenitors by restricting canonical WNT signaling. Development 140, 3787–3798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paige S. L., Osugi T., Afanasiev O. K., Pabon L., Reinecke H., Murry C. E. (2010). Endogenous Wnt/beta-catenin signaling is required for cardiac differentiation in human embryonic stem cells. PLoS ONE 5, e11134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paige S. L., Thomas S., Stoick-Cooper C. L., Wang H., Maves L., Sandstrom R., Pabon L., Reinecke H., Pratt G., Keller G., et al. (2012). A temporal chromatin signature in human embryonic stem cells identifies regulators of cardiac development. Cell 151, 221–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qyang Y., Martin-Puig S., Chiravuri M., Chen S., Xu H., Bu L., Jiang X., Lin L., Granger A., Moretti A., et al. (2007). The renewal and differentiation of Isl1+ cardiovascular progenitors are controlled by a Wnt/beta-catenin pathway. Cell Stem Cell 1, 165–179 [DOI] [PubMed] [Google Scholar]
- Rafii S., Kloss C. C., Butler J. M., Ginsberg M., Gars E., Lis R., Zhan Q., Josipovic P., Ding B. S., Xiang J., et al. (2013). Human ESC-derived hemogenic endothelial cells undergo distinct waves of endothelial to hematopoietic transition. Blood 121, 770–780 [DOI] [PubMed] [Google Scholar]
- Reiner A., Yekutieli D., Benjamini Y. (2003). Identifying differentially expressed genes using false discovery rate controlling procedures. Bioinformatics 19, 368–375 [DOI] [PubMed] [Google Scholar]
- Shalaby F., Ho J., Stanford W. L., Fischer K. D., Schuh A. C., Schwartz L., Bernstein A., Rossant J. (1997). A requirement for Flk1 in primitive and definitive hematopoiesis and vasculogenesis. Cell 89, 981–990 [DOI] [PubMed] [Google Scholar]
- Smyth G. K. (2005) Limma: linear models for microarray data. In Bioinformatics and Computational Biology Solutions Using R and Bioconductor. (ed. Dudoit S., Gentleman V. C. R., Irizarry R., Huber W.). New York, NY: Springer; [Google Scholar]
- Thisse C., Thisse B. (2008). High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat. Protoc. 3, 59–69 [DOI] [PubMed] [Google Scholar]
- Thompson J. D., Higgins D. G., Gibson T. J. (1994). CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno S., Weidinger G., Osugi T., Kohn A. D., Golob J. L., Pabon L., Reinecke H., Moon R. T., Murry C. E. (2007). Biphasic role for Wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc. Natl. Acad. Sci. USA 104, 9685–9690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Handel B., Montel-Hagen A., Sasidharan R., Nakano H., Ferrari R., Boogerd C. J., Schredelseker J., Wang Y., Hunter S., Org T., et al. (2012). Scl represses cardiomyogenesis in prospective hemogenic endothelium and endocardium. Cell 150, 590–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodyanik M. A., Thomson J. A., Slukvin I. I. (2006). Leukosialin (CD43) defines hematopoietic progenitors in human embryonic stem cell differentiation cultures. Blood 108, 2095–2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamstad J. A., Alexander J. M., Truty R. M., Shrikumar A., Li F., Eilertson K. E., Ding H., Wylie J. N., Pico A. R., Capra J. A., et al. (2012). Dynamic and coordinated epigenetic regulation of developmental transitions in the cardiac lineage. Cell 151, 206–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Gilner J. B., Bautch V. L., Wang D. Z., Wainwright B. J., Kirby S. L., Patterson C. (2007). Wnt2 coordinates the commitment of mesoderm to hematopoietic, endothelial, and cardiac lineages in embryoid bodies. J. Biol. Chem. 282, 782–791 [DOI] [PubMed] [Google Scholar]
- Welten M. C., de Haan S. B., van den Boogert N., Noordermeer J. N., Lamers G. E., Spaink H. P., Meijer A. H., Verbeek F. J. (2006). ZebraFISH: fluorescent in situ hybridization protocol and three-dimensional imaging of gene expression patterns. Zebrafish 3, 465–476 [DOI] [PubMed] [Google Scholar]
- Willems E., Spiering S., Davidovics H., Lanier M., Xia Z., Dawson M., Cashman J., Mercola M. (2011). Small-molecule inhibitors of the Wnt pathway potently promote cardiomyocytes from human embryonic stem cell-derived mesoderm. Circ. Res. 109, 360–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelon D., Horne S. A., Stainier D. Y. (1999). Restricted expression of cardiac myosin genes reveals regulated aspects of heart tube assembly in zebrafish. Dev. Biol. 214, 23–37 [DOI] [PubMed] [Google Scholar]
- Yutzey K. E., Benson D. W. (2003). TBX5: a developmental key that fits many locks. J. Mol. Cell. Cardiol. 35, 1175–1177 [DOI] [PubMed] [Google Scholar]