Abstract
The Global Programme to Eliminate Lymphatic Filariasis has an urgent need for rapid assays to detect ongoing transmission of lymphatic filariasis (LF) following multiple rounds of mass drug administration (MDA). Current WHO guidelines support using the antigen card immunochromatographic test (ICT), which detects active filarial infection but does not detect early exposure to LF. Recent studies found that antibody-based assays better serve this function. In the present study, two tests, a rapid IgG4 enzyme-linked immunosorbent assay (ELISA) and a lateral-flow strip immunoassay, were developed based on the highly sensitive and specific Wuchereria bancrofti antigen Wb123. A comparison of W. bancrofti-infected and -uninfected patients (with or without other helminth infections) demonstrated that both tests had high sensitivities and specificities (93 and 97% [ELISA] and 92 and 96% [strips], respectively). When the W. bancrofti-uninfected group was separated into those with other filarial/helminth infections (i.e., onchocerciasis, loiasis, and strongyloidiasis) and those who were parasite uninfected, the specificities of the assays varied between 91 and 100%. In addition, the geometric mean response by ELISA of W. bancrofti-infected patients was significantly higher than the response of those without W. bancrofti infection (P < 0.0001). Furthermore, the Wb123 ELISA and the lateral-flow strips had high positive and negative predictive values, giving valuable information on the size of survey population needed to be reasonably certain whether or not transmission is ongoing. These highly sensitive and specific IgG4 tests to the W. bancrofti Wb123 protein give every indication that they will serve as useful tools for post-MDA monitoring.
INTRODUCTION
The Global Programme to Eliminate Lymphatic Filariasis (GPELF), begun in 2000, has led to 952 million treated individuals, a number that reflects 3.9 billion doses of albendazole plus either ivermectin or diethylcarbamazine administered in the first 11 years of GPELF's existence (1). Within this time frame, 53 of the 73 countries where lymphatic filariasis (LF) is endemic began implementation of mass drug administration (MDA), with several having completed or nearly completed the recommended 5 to 6 annual rounds of MDA. This rapid and unprecedented expansion of the GPELF has greatly increased the need for surveillance tools to assess interruption of transmission.
The current guidelines established by the WHO for post-MDA surveillance includes conducting transmission assessment surveys (TAS) that rely on the detection of adult-specific circulating filarial antigen (CAg) by use of a lateral-flow immunochromatographic test (ICT) (2). While extremely useful in detecting active Wuchereria bancrofti infection, the limitation of the ICT is in its inability to detect infection prior to the development of adult parasites, a process that may take up to 18 months following exposure to infective stage larvae. Thus, it has been suggested that a third-stage larva (L3)-specific antibody-based test might be better suited for this purpose, given that these antibodies may be detectable significantly earlier (months at least) than would the presence of circulating Ag, particularly as children 6 to 7 years old are targeted to be the sentinel population for TAS and surveillance (2–4). To this end, several serological tools based on antibody to recombinant filarial antigens have been tested. These antigens have included BmR1 and BmSXP (5), WbSXP-1 (6), and Bm33 (7). Perhaps the most widely used antigen in LF surveillance has been Bm14 (8), an antigen that is homologous/identical to BmSXP and WbSXP-1, which has been extensively studied in an enzyme-linked immunosorbent assay (ELISA) format (4, 8–11). More recently, this antigen and several of the others have been compared using a multiplex bead assay (3, 12).
Although the sensitivity of the assays based on some of these recombinant antigens has been excellent in regions of filarial endemicity in which only Wuchereria and/or Brugia are endemic, there remains the unresolved problem of cross-reactivity in regions of co-endemicity for other filarial infections, including Loa loa, Onchocerca volvulus, and Mansonella spp. (e.g., Africa and parts of Central and South America). In a multicenter trial, it was demonstrated that both Bm14 and WbSXP were cross-reactive in patients with either O. volvulus or L. Loa infection, and while BmR1 was not shown to be cross-reactive, its sensitivity in bancrofti-infected patients is <60% (10). In addition, while antibody to Bm33 appears earlier than antibodies to the other recombinants tested, it is a highly immunodominant antigen in the filaria and is likely to be very cross-reactive (12). To address the problem of specificity, a recent diagnostic method based on antibodies to the Wuchereria bancrofti antigen Wb123 was developed using a luciferase immunoprecipitation system (LIPS) (13) that has since been further tested in 2 populations in which W. bancrofti is endemic (12, 14) and has been shown to precede the appearance of antigenemia. While the LIPS format showed a very high degree of sensitivity and specificity to Wb123, the expense of new equipment needed for this assay (and some of the nonstandard reagent costs) remains a deterrent to its widespread use.
The purpose of this study was, therefore, to develop rapid, cost-effective formats for the detection of antibodies to the highly sensitive and specific antigen Wb123 for use in the early detection of filarial transmission following cessation of MDA. First, we developed a rapid immunoassay (ELISA) that can be made available immediately to any of the centralized laboratories charged with LF surveillance. However, since the ELISA still requires the accessibility of a laboratory, Wb123 has also been adapted to a lateral-flow strip test that is rapid and can be performed at the point of care.
MATERIALS AND METHODS
Ethics statement.
Patient serum samples were collected under several protocols approved by the Institutional Review Board of NIAID, with the majority collected under NCT00001230, NCT00342576, or 92-I-0155 (inactive). Sample collections as part of larger international field projects were also approved by the respective governments. Written informed consent was obtained from all subjects.
Expression and purification of the Wb123-glutathione S-transferase protein.
Wb123 was originally identified using Brugia malayi and W. bancrofti L3 expressed sequence tags (ESTs) (13) and has a length of 372 amino acids. It most likely belongs to the serpin family of proteins, known to be serine protease inhibitors that are secreted by filaria and highly immunogenic in humans. Expression and purification of Wb123-glutathione S-transferase (GST) was performed by the Protein Expression Laboratory under contract to the National Cancer Institute (FCRF, SAIC, Frederick, MD). Full-length Wb123 was cloned into an expression construct suitable for baculovirus expression. This construct, termed GST-tev-Wb123, was a 6.5-kb ampicillin-resistant baculovirus expression clone, containing a polyhedrin promoter and an N-terminal GST tag. For expression and purification, 1 liter of Hi5 cells was infected with baculovirus containing GST-tev-Wb123 at a multiplicity of infection of 3 and incubated at 21°C for 72 h. Cells were pelleted and then assessed for viability and cell size. The pellet was resuspended in 100 ml of extraction buffer (20 mM HEPES, 300 mM NaCl, and 2 mM β-mercaptoethanol) supplemented with 1.0 ml of complete protease inhibitor (Sigma-Aldrich, St. Louis, MO) and lysed under high pressure. After microscopic examination of the disrupted cells, 2 U benzonase/ml was added for 20 min on ice and the mixture was clarified by centrifugation. The sample was filtered and then applied to a 5-ml GSTrap column (Amersham, Pittsburgh, PA). After washing, the protein was eluted from the column over a 20-column volume gradient to 20 mM glutathione in extraction buffer. The product was dialyzed against 1× phosphate-buffered saline (PBS) and analyzed by SDS-PAGE. For SDS-PAGE, samples were prepared in 1× lithium dodecyl sulfate (LDS) buffer (Bio-Rad, Hercules, CA) with or without 25 mM TCEP [tris(2-carboxyethyl)phosphine] reducing agent (Bio-Rad), heated to 95°C, and then loaded onto a 10.5 to 14% precast Tris-glycine gel (Bio-Rad). Proteins were visualized with Coomassie brilliant blue. The final product appeared to be ∼90% pure and migrated near its predicted molecular mass of 70.4 kDa (Fig. 1).
Fig 1.
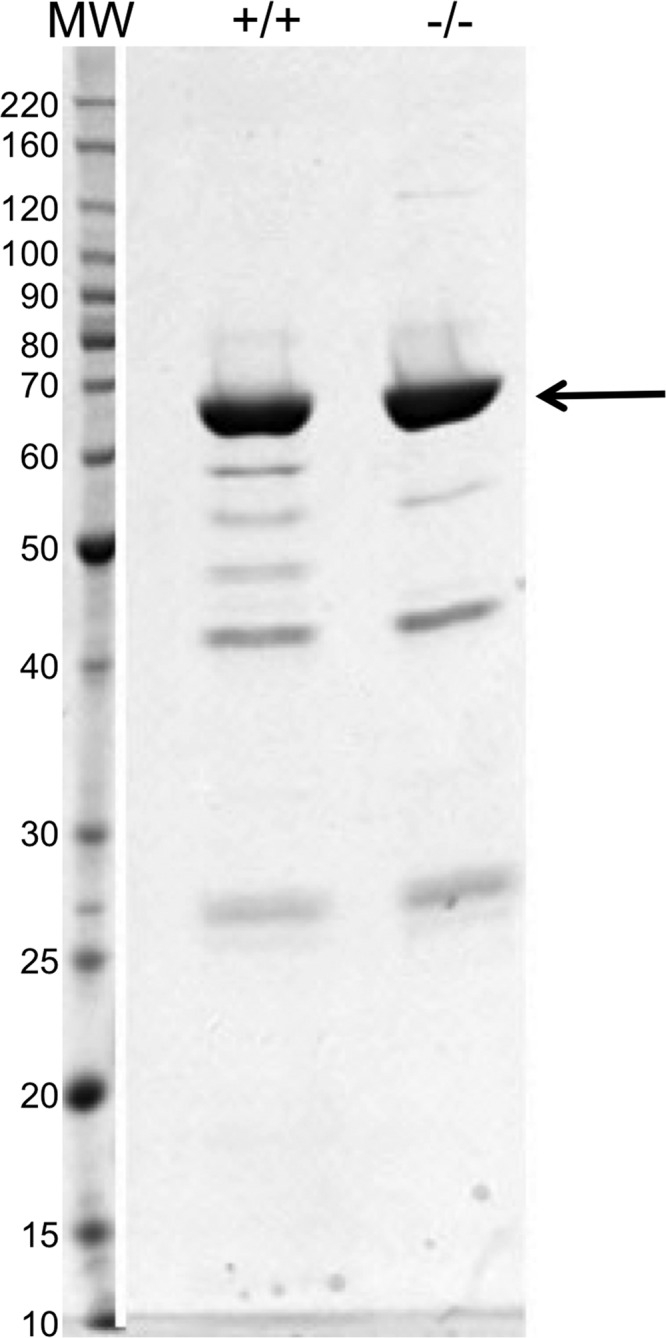
SDS-PAGE of GST-tev-Wb123. Final analysis of the Wb123-GST protein showing migration of the principal protein band (arrow). Samples were prepared with (+/+) or without (−/−) 25 mM TCEP (reducing agent), heated to 95°C, and loaded onto a 10.5 to 14% precast Tris-glycine gel. Proteins were visualized with Coomassie brilliant blue stain. MW, molecular mass in kDa.
Patient samples.
Serum samples were chosen from well-defined helminth-infected patients and from uninfected patients based on their country of origin and/or exposure to Wuchereria bancrofti (Table 1).The majority of samples were collected as part of studies conducted by the NIAID; samples from Haiti were kindly provided by Patrick Lammie. For analysis, a total of 95 patients with W. bancrofti infection were compared with subjects without W. bancrofti infection (n = 289 for ELISA analysis; n = 279 for lateral-flow strip analysis), who either had infections with other single filaria (Onchocerca volvulus [n = 116] or Loa loa [n = 40]) or with soil-transmitted helminths (Strongyloides stercoralis [n = 40]) or were helminth-uninfected normal subjects from both regions of filarial endemicity (EN) (n = 71 [ELISA] and n = 61 [lateral-flow strips]) and regions of nonendemicity (NEN) (n = 22). Patients with other filarial/helminth infections were diagnosed by skin-snip positivity or by identification of adult worms in nodules of O. volvulus, positive blood microfilariae or eye worm for L. Loa, or positive stool examination for S. stercoralis. Parasite-uninfected patients from countries of endemicity were either from a region free of helminth infection or were excluded based on negative results for both filarial CAg and by examination for other helminths as discussed above. Samples from patients from regions of nonendemicity were obtained from normal North American blood bank donors and other nonexposed individuals.
Table 1.
Patient populations
| Patient infection status and country of origin | No. (n = 384) |
|---|---|
| Wuchereria bancrofti positive | 95 |
| Cook Islands | 59 |
| India | 18 |
| Haiti | 16 |
| Guyana | 2 |
| Onchocerca volvulus positive | 116 |
| Ecuador | 46 |
| Guatemala | 41 |
| Ghana | 3 |
| Cameroon | 3 |
| Nigeria | 1 |
| Expatriates | 22 |
| Loa loa positive | 40 |
| Cameroon | 1 |
| Expatriates (Cameroon, CAR, DRC, Benin, Gabon) | 39 |
| Strongyloides stercoralis positive | 40 |
| Jamaica | 1 |
| Central America | 12 |
| West Africa | 1 |
| Southeast Asia | 18 |
| Middle East | 1 |
| Expatriates (Southeast Asia, Central and South America) | 7 |
| Non-helminth infected from country where LF is endemic | 71a |
| Ecuador (Quito) | 20 |
| Guatemala | 17 |
| Haiti (nonexposed) | 16 |
| Mali (Bamako) | 18 |
| Noninfected from country of W. bancrofti nonendemicity | |
| North America | 22 |
n = 384 represents the number of patients tested by ELISA. For strip testing, n = 374 (Haiti nonexposed, n = 6 rather than 16).
IgG4 ELISA to Wb123 antigen.
To develop a rapid ELISA for use in surveillance post-MDA, conditions were optimized for Wb123-GST-coating concentration, serum dilution, temperature, timing of the incubation periods, antibody conjugation and concentration, and development methodology. The assay was thus finalized as follows. Flat-bottomed 96-well plates (Immulon 4; Thermo Scientific, Milford, MA) were coated with 10 μg/ml of Wb123-GST in 50 μl/well of PBS overnight at 4°C. All subsequent incubations were done at room temperature. Plates were washed 6 times in PBS-Tween 20 and then blocked for 30 min with 200 μl/well of blocking buffer consisting of 1× PBS–0.05% Tween 20–5% bovine serum albumin (BSA) (Sigma-Aldrich). Plates were again washed and serum samples were added at 50 μl/well in duplicate at a 1:50 dilution in a diluent of PBS–1% BSA–0.05% Tween 20 for 30 min. Added to each plate was also a positive W. bancrofti serum pool sample (diluted to provide low and high positive controls), a negative serum pool, and ELISA diluent for plate blanks. Following washing, a 1:5,000 dilution of an alkaline phosphatase-conjugated mouse anti-human IgG4 (clone 6025; Southern Biotech) antibody was added at 50 μl/well in ELISA diluent for 30 min. Plates were washed and subsequently developed with 50 μl/well of alkaline phosphate yellow liquid substrate (pNPP) (Sigma-Aldrich) for 20 min. The reaction was stopped with 25 μl/well of 3 N NaOH, and plates were read at 405 nm using a microplate reader. It should be noted that this assay also works well using the horseradish peroxidase (HRP)-conjugated version of the antibody and an HRP developer such as ABTS [2,2′-azinobis(3-ethylbenzthiazoline sulfonic acid)]; however, all the results in this study are based on the alkaline phosphatase assay.
To simplify the process further and shorten the assay time for investigators doing surveillance of large populations, Immulon 4 plates were coated and blocked as above followed by a final wash. Plates were tapped to remove excess liquid and then allowed to air dry for several hours at room temperature. When dry, plates were sealed with an absorbent silica gel pack in a sealer pouch (Sorbent Systems, Los Angeles, CA) and stored at 4°C. Sealed plates have thus far been tested after storage for 6 weeks and proved comparable to plates freshly coated.
Comparison of Wb123 ELISA with other assays.
To compare the new Wb123-GST IgG4 ELISA results with those of antibody assays used in previous studies, either using Wb123 or whole filarial antigen, correlations were made for a subset of patients between the ELISA results from this study with the results from 3 other platforms as described below.
Luciferase immunoprecipitation system.
The LIPS assay for detection of both IgG and IgG4 antibodies to Wb123-GST has been described in detail previously (13). Very briefly, sera were diluted 1:10 and added to a Ruc-Wb123 enzyme reporter. Following a short incubation of 5 min, a suspension of either protein A/G beads (for IgG analysis) or anti-IgG4 beads was added. After a second 5-min incubation, plates were washed and processed on a Berthold LB 960 Centro microplate luminometer using a coelenterazine substrate mix (Promega, Madison, WI); data are expressed in luminometer units.
Suspension array technology for IgG4.
The Luminex suspension array technology assay has been described in detail elsewhere (15). For this analysis, 160 μg of Wb123-GST was coupled to 16 × 106 activated SeroMAP beads (Luminex Corporation, Austin, TX). Coupled beads were added to a 1:1,000 dilution of patient sera at a concentration of 5,000 beads/well in a prewetted filter plate (EMD Millipore, Billerica, MA). Following a 2-h incubation on a plate shaker in the dark, plates were washed through a vacuum manifold. Biotinylated mouse anti-human IgG4 (clone 6025; Hybridoma Reagent Laboratory, Baltimore, MD) was then added at a 1:1,000 dilution for 1 h with shaking (in the dark) after which streptavidin-conjugated phycoerythrin (PE) (Jackson ImmunoResearch, West Grove, PA) was added at 2 μg/ml for an additional 30 min. Plates were washed and samples were read using the Bio-Plex System (Luminex); data are expressed as mean fluorescence intensities (MFI).
ELISA for IgG4 to adult filarial antigen.
IgG4 antibody to a saline extract of Brugia malayi adult antigen (BmA) was measured as previously described (16).
Wb123 lateral-flow strip immunoassay.
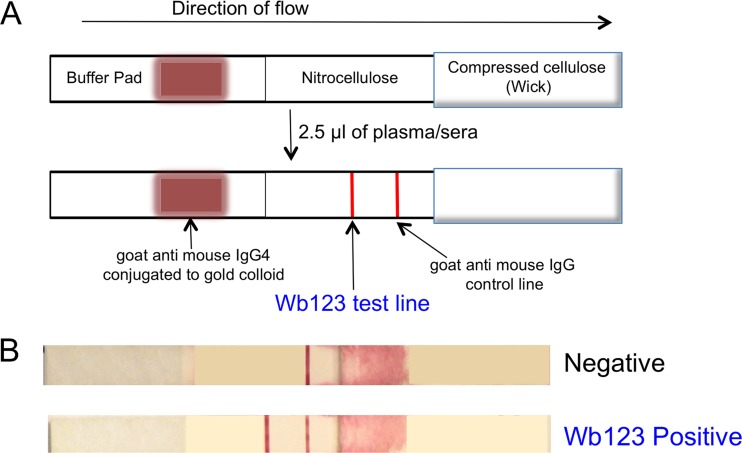
Lateral-flow strip materials were assembled on Lohmann diagnostic strip backing (300 by 75 mm) in a card format, using 25-mm-wide nitrocellulose membrane, 22-mm-wide conjugate pad material, and 32-mm-wide absorbent wick material (Fig. 2) and cut into 4-mm-wide strips. A BioDot XYZ reagent dispenser was used to apply Wb123 antigen and control antibody (goat anti-mouse; Jackson ImmunoResearch) stripes onto the nitrocellulose membrane and to spray anti-human IgG4 antibody (Hybridoma Reagent Laboratory) conjugated to gold colloid onto the conjugate pad material. For detection of the Wb123 antibody, 2.5 μl of patient serum or plasma was added to the center of the test strip approximately 4 mm from the conjugate pad (Fig. 2). The conjugate end of the strip was subsequently placed in a well of a flat-bottomed 96-well plate with 100 μl of lateral-flow test running buffer. Strips were read at 20 min, removed from the well, and then read again when dry.
Fig 2.
Representation of Wb123 lateral-flow strips. (A) Diagrammatic illustration of the lateral-flow strips showing the placement of the antibody conjugates and the appearance of the detection lines before (top) and following (bottom) the addition of patient sera. (B) Negative (top) and positive (bottom) results from representative patient serum samples.
Statistical analyses.
For calculation of the IgG4 antibody response to Wb123-GST by ELISA, optical densities (ODs) of duplicate patient samples were averaged and then divided by the OD of the corresponding plate blank. Determination of the positive threshold to Wb123 was accomplished using receiver operator characteristic (ROC) curves (GraphPad Prism version 6.0) to compare the ratios of W. bancrofti-infected patients to all patients not infected with W. bancrofti, including both helminth-infected and parasite-uninfected patients from regions of W. bancrofti endemicity and nonendemicity. To detect differences between the ratios of patients with and those without W. bancrofti infection, the Mann-Whitney test was used. Correlations between the Wb123 IgG4 ELISA and other immunological assays were accomplished using the Spearman rank test (GraphPad Prism). A sensitivity and specificity calculator was utilized to determine positive and negative predictive values for both the ELISA and the lateral-flow strips.
RESULTS
ELISA results.
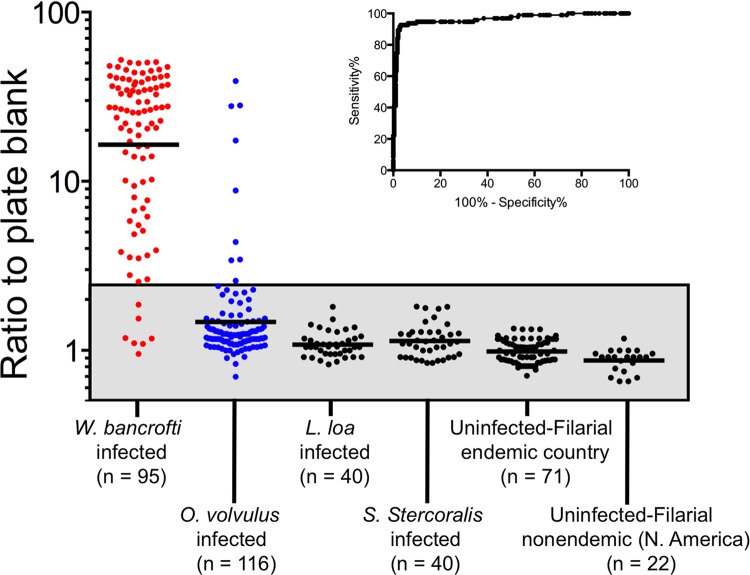
The ratios of the ODs of patient samples to the plate blanks were calculated for both patients with W. bancrofti infection and for each group of patients without W. bancrofti infection (Fig. 3). The ratios obtained using serum samples from patients with W. bancrofti infection were significantly higher than those obtained using serum samples from the entire group of individuals without W. bancrofti infection (P < 0.0001), with striking differences in the geometric means (GM) between the individual groups compared with W. bancrofti-infected patients (GM of those with W. bancrofti infection, 16.44; with O. volvulus, 1.48; with L. Loa, 1.08; with S. stercoralis, 1.14; in EN, 0.99; in NEN, 0.87).
Fig 3.
Determination of the appropriate cutoffs for the Wb123 ELISA-based immunoassay. The ODs of the duplicate patient samples were averaged and then divided by the corresponding plate blanks. Each dot represents an individual patient sample from patients with W. bancrofti (shown in red), O. volvulus (in blue), and other infections (in black). Shown also are parasite-uninfected controls from regions of filaria endemicity and nonendemicity (also in black). The geometric means (GM) for each group are illustrated by the individual horizontal lines and the normal range is indicated by the gray box. The inset shows the ROC curve for specificity/sensitivity based on the sample/plate blank ratios of 95 W. bancrofti-infected patients compared to 289 non-W. bancrofti-infected patients.
To determine the positive threshold response to Wb123, the ratios of W. bancrofti-infected patients were compared to all non-W. bancrofti-infected patients in a ROC analysis (Fig. 3 inset). By defining the optimal balance between sensitivity and specificity, we determined the positive threshold cutoff to be a sample/plate blank ratio of 2.5, giving a sensitivity of 92 to 93% and a specificity of 96 to 97%. Using this threshold, 7 patients had false-negative results (GM response, 1.24) and 9 had false-positive results (GM, 9.48) (Fig. 3). Interestingly, all of the false positives were from O. volvulus-infected patients from Guatemala (n = 6) or Ecuador (n = 3), regions where bancroftian filariasis is not endemic. The 7 patients with false-negative results (all of whom were microfilaria positive) were distributed among India (n = 2), the Cook Islands (n = 1), Guyana (n = 1), and Haiti (n = 3).
To further test the performance of the Wb123 IgG4 ELISA, the positive and negative predictive values for the assay were determined for each clinical group and for all W. bancrofti-negative patients (including those with other non-W. bancrofti helminth infections as well as parasite-uninfected individuals) compared to W. bancrofti-positive patients (Table 2). The sensitivity of the assay as established by these calculations was 92.6%, with specificity ranging from 92.2% (for O. volvulus patients) to 100% for “all parasite uninfected” and for patients with either loiasis or strongyloidiasis. Overall, based on calculations using the W. bancrofti-negative group, the specificity of the test was determined to be nearly 97%. The ELISA also gave high positive (ranging from 90.7 to 100%) and negative (ranging from 85.1 to 97.6%) predictive values. The lower negative predictive values for L. Loa and S. stercoralis patients were most likely due to the smaller numbers of samples in these patient groups (n = 40 for each).
Table 2.
Sensitivities and specificities of Wb123 IgG4 ELISA and lateral-flow strips
| W. bancrofti infected vs indicated infection | IgG4 strip results (%) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgG4 ELISA results (%) |
(20-min read) |
Dry |
||||||||||
| Sensitivity | Specificity | Positive predictive value | Negative predictive value | Sensitivity | Specificity | Positive predictive value | Negative predictive value | Sensitivity | Specificity | Positive predictive value | Negative predictive value | |
| Parasite uninfected | 92.6 | 100 | 100 | 93.0 | 91.6 | 98.8 | 98.9 | 91.1 | 92.6 | 97.6 | 97.8 | 92.1 |
| O. volvulus | 92.6 | 92.2 | 90.7 | 93.9 | 91.6 | 92.2 | 90.6 | 93.0 | 92.6 | 90.5 | 88.9 | 93.8 |
| L. loa | 92.6 | 100 | 100 | 85.1 | 91.6 | 100 | 100 | 83.3 | 92.6 | 100 | 100 | 85.1 |
| S. stercoralis | 92.6 | 100 | 100 | 85.1 | 91.6 | 100 | 100 | 83.3 | 92.6 | 100 | 100 | 85.1 |
| All non-W. bancrofti infected | 92.6 | 96.9 | 90.7 | 97.6 | 91.6 | 96.4 | 89.7 | 97.1 | 92.6 | 95.3 | 87.1 | 97.4 |
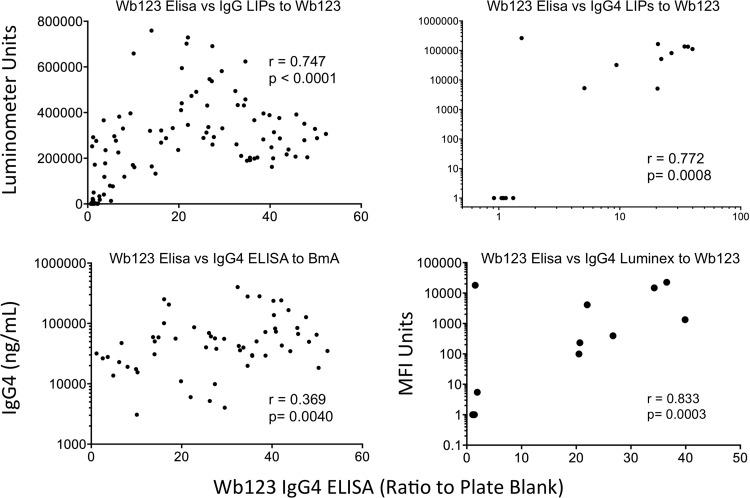
To determine how the Wb123 IgG4 rapid ELISA compares with platforms used in other studies (12–14, 17), subsets of samples previously tested by these methods in this laboratory were compared with the current ELISA results. The primary comparison was to the recently described Wb123-GST LIPS assay (13). Approximately 35% (134/384) of the patients used for the ELISA-based test had previously been tested by the IgG-based LIPS assay. Despite the differences in the isotypes measured between the LIPS and the ELISA, the tests were strongly correlated (r = 0.747, P < 0.0001) (Fig. 4). In addition, a much smaller subset (n = 16) was compared to the LIPS IgG4 assay, also showing a strong positive relationship (n = 0.772, P = 0.0008).
Fig 4.
Correlations between the Wb123 ELISA and other immunoassay formats. In a subset of patients, the ratios of patient samples to the corresponding plate blanks in the Wb123 IgG4 ELISA were compared to the Wb123 assays by LIPS (IgG [upper left], IgG4 [upper right]), the IgG4 Luminex assay (lower right), and the IgG4 ELISA antibody response to adult filarial antigen (BmA; lower left). The Spearman rank correlation analysis was used to generate r and P values.
Prior to the use of recombinant proteins, an IgG4-based ELISA to the adult filarial antigen BmA was frequently used for diagnosis (16). Interestingly, while Wb123 is primarily expressed in L3s, the IgG4 antibody levels to Wb123 were positively correlated (r = 0.369, P = 0.0040) to the levels of BmA-specific IgG4 for the subset of patients tested (n = 59), though understandably the relationship was not as strong as for the LIPS assay to Wb123. IgG4 antibody levels to Wb123 were not, however, correlated to the antibody levels to a Brugia microfilarial antigen preparation (data not shown). Finally, because a multiplex bead analysis had recently been used to compare several different recombinant filarial proteins (12), IgG4 responses in a small group of 15 patients were compared using both the Wb123 ELISA and Wb123 Luminex platforms. As shown for the LIPS assay, the results of the ELISA and Luminex formats were strongly correlated (r = 0.833, P = 0.0003) (Fig. 4).
Lateral-flow strip immunoassay.
Lateral-flow strips were read both at 20 min and after drying. Importantly, strips displayed great stability between the initial reading and that done subsequently, with only 4/374 (1.1%) changing from negative to positive status following drying. The sensitivity and specificity calculations were comparable to those derived from the ELISA (Table 2). Strips read at 20 min showed a sensitivity of 91.6% with specificity ranging from 92.2% (when patients with onchocerciasis were used as the comparator) to 100% (for Loa and Strongyloides patients) with an overall test specificity of 96.4%. The dry strips showed slightly greater sensitivity (92.6%) but also marginally lower specificity (95.3% overall, with a range for individual patient groups of 90.5 to 100%). The positive and negative predictive values were also similar to those obtained for the ELISA, with overall values of 89.7% and 87.1% (positive predictive values) and 97.1% and 97.4% (negative predictive values) for the 20-min and dry reads, respectively. Overall, there was quite good consistency between the ELISA results and those of the lateral-flow strips for patient status. In an examination of just the results that differed in outcome between the 20 min strips and the ELISA, 14/374 (3.7%) of sera tested gave a discordant result (O. volvulus, strips, 5 false positives [FP] and ELISA, 5 FP; W. bancrofti, strips, 2 false negatives [FN] and ELISA, 1 FN; EN, strips, 1 FP). Comparison between the ELISA and the dry strips showed a 4% (15/374) discordance (O. volvulus, strips, 6 FP, and ELISA, 4 FP; W. bancrofti, strips, 2 FN, and ELISA, 2 FN; EN, strips, 1 FP).
DISCUSSION
One of the immediate needs in the post-MDA world is the requirement for a highly sensitive and specific test for the assessment of potential ongoing transmission of the parasites that cause LF. In addition, such a test should be fairly rapid and relatively inexpensive to perform on large numbers of samples in centralized laboratories or ideally at the point of care. Based on WHO guidelines, the current tool for assessment of Wuchereria bancrofti transmission is the ICT circulating antigen card test (2). However, the focus on surveillance tools has shifted toward the use of antibody-based assays that can detect exposure to L3s, allowing for early warning signals of potential W. bancrofti transmission. Many such antibody tests have already been developed (5–8) but the requirement for specificity in regions of coendemicity for other filarial infections (e.g., Africa, parts of the Americas) has not been met. The purpose of this study was to adapt a recombinant W. bancrofti antigen, Wb123, already proven to be sensitive and specific in LIPS assays (13), to more standard immunoassay formatting, including those that could be used at the point of care. As found in the previous LIPS assay based on Wb123 (13), both the IgG4 ELISA and the lateral-flow strips demonstrated striking specificity and sensitivity when comparing patients infected with W. bancrofti to those infected with O. volvulus, L. loa, or S. stercoralis, parasites with highly homologous genomes and with potential antigenic cross-reactivity. As expected, individuals without helminth infection living in regions of either filarial endemicity or nonendemicity showed no IgG4 reactivity to Wb123 for the ELISA and only very minimal cross-reactivity by strip analysis.
The use of the Wb123 LIPS test for surveillance, particularly in children, was recently demonstrated in a South Pacific population that had undergone a single island-wide treatment with diethylcarbamazine (14). However, this study was based on a population where LF transmission had decreased but had not been eliminated. Nevertheless, when the current ELISA platform was used with dried blood spots, no reactivity was seen in individuals (n = 292, age ≤ 7 years; n = 45, age > 7 years) from 6 Malian villages that had undergone 6 rounds of MDA (data not shown). In addition to post-MDA surveillance, these rapid diagnostic assays will be highly useful in detecting new infections following an influx of immigrants from a region of filarial endemicity to either a region of endemicity or to one cleared of infection following MDA.
That the Wb123 ELISA and the lateral-flow strips have high positive and negative predictive values will be beneficial in the analysis of large posttreatment populations, giving needed information on the necessary sampling size to adequately survey a population. Moreover, these tests should be of benefit in mapping areas in which the W. bancrofti status is unknown (e.g., parts of Cameroon), given their relatively high throughput, reduced cost, and rapid turnaround time.
Additionally, for the ELISA, the results correlated well with other assay formats (Fig. 4), suggesting the consistency of this platform for immediate expanded use. Furthermore, we have also tested the use of Wb123 precoated and blocked plates and found them quite comparable to freshly coated plates (data not shown), further simplifying the transfer of this assay to regions of the world where needed. Since most regional and even district laboratories have the ability to perform ELISAs, we developed a methodology that requires no other equipment (such as incubators or automated washers) and works well at ambient temperatures.
The ultimate goal of any diagnostic is to be as rapid and accurate as possible. The highly sensitive and specific IgG4-based ELISA is one step in that direction. Indeed, the ongoing development and finalization of Wb123 in a lateral-strip format are the next logical steps in making a field-accessible, point-of-care test. Thus far, these strips have shown remarkable consistency with the ELISA and comparable sensitivity and specificity in positive and negative antibody determination. In addition, a similar prototype of this lateral-flow format has already been successfully tested with whole blood rather than sera for detection of antibody to the onchocerciasis antigen Ov16 (A. Golden, submitted for publication). For these strips (n = 99), there were only 3 results that were discordant between the strips assayed against sera or plasma (1 false positive) and those with whole blood (1 false negative and 1 false positive). However, until this rapid point-of-care test is fully developed and commercialized for Wb123, the IgG4 ELISA presented in this study is both ready to use and inexpensive (on a per sample basis) and should serve as a highly valuable tool for populations undergoing postcontrol surveillance currently and in the very near future.
ACKNOWLEDGMENTS
We thank Dominic Esposito, William Gillette, and other members of the SAIC Protein Expression Laboratory for the expression and purification of Wb123-GST.
This research was supported by the Intramural Research Program of the NIH, NIAID.
Footnotes
Published ahead of print 5 June 2013
REFERENCES
- 1. World Health Organization 2012. Global programme to eliminate lymphatic filariasis: progress report, 2011. Weekly epidemiological record, no. 37, 87, 346–356 World Health Organization, Geneva, Switzerland: http://www.who.int/wer/2012/wer8737.pdf [PubMed] [Google Scholar]
- 2. World Health Organization 2011. Global programme to eliminate lymphatic filariasis: monitoring and epidemiological assessment of mass drug administration. World Health Organization, Geneva, Switzerland [Google Scholar]
- 3. Moss DM, Priest JW, Boyd A, Weinkopff T, Kucerova Z, Beach MJ, Lammie PJ. 2011. Multiplex bead assay for serum samples from children in Haiti enrolled in a drug study for the treatment of lymphatic filariasis. Am. J. Trop. Med. Hyg. 85:229–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ramzy RM, El Setouhy M, Helmy H, Ahmed ES, Abd Elaziz KM, Farid HA, Shannon WD, Weil GJ. 2006. Effect of yearly mass drug administration with diethylcarbamazine and albendazole on bancroftian filariasis in Egypt: a comprehensive assessment. Lancet 367:992–999 [DOI] [PubMed] [Google Scholar]
- 5. Abdul Rahman R, Hwen-Yee C, Noordin R. 2007. Pan LF-ELISA using BmR1 and BmSXP recombinant antigens for detection of lymphatic filariasis. Filaria J. 6:10. 10.1186/1475-2883-6-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pandiaraja P, Arunkumar C, Hoti SL, Rao DN, Kaliraj P. 2010. Evaluation of synthetic peptides of WbSXP-1 for the diagnosis of human lymphatic filariasis. Diagn. Microbiol. Infect. Dis. 68:410–415 [DOI] [PubMed] [Google Scholar]
- 7. Krushna NS, Shiny C, Dharanya S, Sindhu A, Aishwarya S, Narayanan RB. 2009. Immunolocalization and serum antibody responses to Brugia malayi pepsin inhibitor homolog (Bm-33). Microbiol. Immunol. 53:173–183 [DOI] [PubMed] [Google Scholar]
- 8. Weil GJ, Curtis KC, Fischer PU, Won KY, Lammie PJ, Joseph H, Melrose WD, Brattig NW. 2010. A multicenter evaluation of a new antibody test kit for lymphatic filariasis employing recombinant Brugia malayi antigen Bm-14. Acta Trop. 120:S19–S22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Helmy H, Weil GJ, Ellethy AS, Ahmed ES, Setouhy ME, Ramzy RM. 2006. Bancroftian filariasis: effect of repeated treatment with diethylcarbamazine and albendazole on microfilaraemia, antigenaemia and antifilarial antibodies. Trans. R. Soc. Trop. Med. Hyg. 100:656–662 [DOI] [PubMed] [Google Scholar]
- 10. Lammie PJ, Weil G, Noordin R, Kaliraj P, Steel C, Goodman D, Lakshmikanthan VB, Ottesen E. 2004. Recombinant antigen-based antibody assays for the diagnosis and surveillance of lymphatic filariasis—a multicenter trial. Filaria J. 3:9. 10.1186/1475-2883-3-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weil GJ, Kastens W, Susapu M, Laney SJ, Williams SA, King CL, Kazura JW, Bockarie MJ. 2008. The impact of repeated rounds of mass drug administration with diethylcarbamazine plus albendazole on bancroftian filariasis in Papua New Guinea. PLoS Negl. Trop. Dis. 2:e344. 10.1371/journal.pntd.0000344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hamlin KL, Moss DM, Priest JW, Roberts J, Kubofcik J, Gass K, Streit TG, Nutman TB, Eberhard ML, Lammie PJ. 2012. Longitudinal monitoring of the development of antifilarial antibodies and acquisition of Wuchereria bancrofti in a highly endemic area of Haiti. PLoS Negl. Trop. Dis. 6:e1941. 10.1371/journal.pntd.0001941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kubofcik J, Fink DL, Nutman TB. 2012. Identification of Wb123 as an early and specific marker of Wuchereria bancrofti infection. PLoS Negl. Trop. Dis. 6:e1930. 10.1371/journal.pntd.0001930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Steel C, Kubofcik J, Ottesen EA, Nutman TB. 2012. Antibody to the filarial antigen Wb123 reflects reduced transmission and decreased exposure in children born following single mass drug administration (MDA). PLoS Negl. Trop. Dis. 6:e1940. 10.1371/journal.pntd.0001940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fouda GG, Leke RF, Long C, Druilhe P, Zhou A, Taylor DW, Johnson AH. 2006. Multiplex assay for simultaneous measurement of antibodies to multiple Plasmodium falciparum antigens. Clin. Vaccine Immunol. 13:1307–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lal RB, Ottesen EA. 1988. Enhanced diagnostic specificity in human filariasis by IgG4 antibody assessment. J. Infect. Dis. 158:1034–1037 [DOI] [PubMed] [Google Scholar]
- 17. Steel C, Ottesen EA, Weller PF, Nutman TB. 2001. Worm burden and host responsiveness in Wuchereria bancrofti infection: use of antigen detection to refine linear assessments from the South Pacific. Am. J. Trop. Med. Hyg. 65:498–503 [DOI] [PubMed] [Google Scholar]