Abstract
Hypersensitivity pneumonitis (HP) is an immunoallergic disease characterized by a prominent interstitial infiltrate composed predominantly of lymphocytes secreting inflammatory cytokines. Dendritic cells (DCs) are known to play a pivotal role in the lymphocytic response. However, their cross talk with microorganisms that cause HP has yet to be elucidated. This study aimed to investigate the initial interactions between human monocyte-derived DCs (MoDCs) and four microorganisms that are different in nature (Saccharopolyspora rectivirgula [actinomycetes], Mycobacterium immunogenum [mycobacteria], and Wallemia sebi and Eurotium amstelodami [filamentous fungi]) and are involved in HP. Our objectives were to determine the cross talk between MoDCs and HP-causative agents and to determine whether the resulting immune response varied according to the microbial extract tested. The phenotypic activation of MoDCs was measured by the increased expression of costimulatory molecules and levels of cytokines in supernatants. The functional activation of MoDCs was measured by the ability of MoDCs to induce lymphocytic proliferation and differentiation in a mixed lymphocytic reaction (MLR). E. amstelodami-exposed (EA) MoDCs expressed higher percentages of costimulatory molecules than did W. sebi-exposed (WS), S. rectivirgula-exposed (SR), or M. immunogenum-exposed (MI) MoDCs (P < 0.05, Wilcoxon signed-rank test). EA-MoDCs, WS-MoDCs, SR-MoDCs, and MI-MoDCs induced CD4+ T cell proliferation and a Th1-polarized immune response. The present study provides evidence that, although differences were initially observed between MoDCs exposed to filamentous fungi and MoDCs exposed to bacteria, a Th1 response was ultimately promoted by DCs regardless of the microbial extract tested.
INTRODUCTION
Hypersensitivity pneumonitis (HP), also known as extrinsic allergic alveolitis, is an inflammatory lung disease associated with lymphocytic alveolitis that is caused by an exacerbated immune response to repeated inhalation of antigens. A wide variety of causative antigens and environmental settings have been described. The most commonly reported form is called farmer's lung disease (FLD) and is associated with farming (handling of moldy grain or hay) (1). A novel form of HP has recently emerged among machinists exposed to contaminated metalworking fluids (MWFs) (i.e., MWF-HP) (2–4).
Evidence supporting the pathogenic role in HP of two types of bacteria, namely, Saccharopolyspora rectivirgula thermoactinomycetes in FLD and Mycobacterium immunogenum mycobacteria in MWF-HP, was obtained using an established experimental model of HP involving repeated intranasal or intratracheal instillation of microorganisms into C57BL/6 mice (5–9). In contrast, filamentous fungi such as Eurotium amstelodami and Wallemia sebi are considered to be the main causes of FLD, on the basis of epidemiological and serological data (10, 11), but their pathogenic roles have not yet been confirmed by in vitro or in vivo experiments.
Experimental mouse models of HP contributed to the elucidation of the pathophysiology of HP by demonstrating an inflammatory response characterized by lymphocytic granulomatous lesions and diffuse interstitial mononuclear infiltrates (7, 12, 13). However, the exact mechanisms through which innate and adaptive immune cells are activated and recruited during HP remain to be elucidated.
Dendritic cells (DCs) are professional antigen-presenting cells that play a crucial role in initiation of the adaptive immune system. They act as sentinels that quickly respond to foreign antigens; when activated, DCs transfer important information about the invading pathogens and the innate response in the periphery to T cells, thus inducing an appropriate adaptive immune response (14). Mature DCs also trigger NK cell effector functions, which promote Th1 polarization (15, 16). On the basis of their ability to take up antigens, present the antigens to naive T cells, and activate those T cells, DCs constitute useful tools for assessing lymphocytic induction and polarization after exposure to microorganisms involved in HP.
Our study aimed to investigate the initial interactions between human monocyte-derived DCs (MoDCs) and four microorganisms that are involved in HP and are different in nature (actinomycetes, mycobacteria, and filamentous fungi), in order (i) to determine the cross talk between MoDCs and HP causative agents and (ii) to determine whether the resulting immune responses differ according to the microbial extract tested. Human MoDCs were exposed to microbial extracts of three main microorganisms involved in FLD, i.e., S. rectivirgula, E. amstelodami, and W. sebi, and to a microbial extract of M. immunogenum, the main causative agent of MWF-HP. The phenotypic activation of MoDCs was evaluated by measuring costimulatory molecule levels and cytokine/chemokine levels in supernatants. The functional activation of MoDCs was measured by assessment of their ability to induce lymphocytic proliferation and determination of the lymphocytic polarization.
MATERIALS AND METHODS
Strains.
We used Lacey's strain of S. rectivirgula (DSMZ 43113), two fungal strains isolated from FLD patient hay from Franche-Comté (a region in eastern France), namely, E. amstelodami (BCCM/IHEM 16286) and W. sebi (BCCM/IHEM 16284), and a strain of M. immunogenum (DSMZ 45496) isolated in MWF taken from a factory where cases of MWF-HP were diagnosed (17). All of the strains were cultured for 1 week under the following conditions: at 44°C on R8 medium for S. rectivirgula, at 20°C on Sabouraud agar (Becton, Dickinson and Company, Le Pont de Claix, France) for W. sebi, at 30°C on DG18 agar (Oxoid Unipath, Basingstoke, England) for E. amstelodami, and at 30°C on Muller-Hinton culture medium for M. immunogenum. Temperatures and media were chosen to reflect the optimal growth conditions for each species.
Preparation of total extracts.
For each species, 4 culture plates were gently brushed with a swab and the fungal structures were harvested in 2 ml of sterile water, resulting in milk-like suspensions (McFarland Scale values of >7). Each suspension was frozen at −20°C overnight. The following day, lyophilization was carried out in a Labconco lyophilizer (Labconco, Kansas City, MO). The microbial extracts were weighed and then suspended in sterile water at 1 mg/ml. The presence of endotoxins was measured in the total extracts using the Chromo-LAL method (Biogenic, Perols, France).
Generation of MoDCs.
Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation, using Ficoll-Paque Premium (Dutscher, Brumath, France), from healthy donor blood obtained from the French Blood Institute of the Bourgogne and Franche-Comté regions. A total of 1.1 × 108 PBMCs were incubated in a 75-cm2 culture flask (Fischer Scientific, Illkirch, France) containing culture medium, i.e., RPMI 1640 (Dutscher) supplemented with 10% fetal calf serum (Dutscher) and 5% penicillin-streptomycin (Dutscher). Two hours later, nonadherent cells (peripheral blood lymphocytes [PBLs]) were removed; adherent cells were gently washed three times with phosphate-buffered saline (Dutscher). In order to generate MoDCs, adherent cells were cultured for 5 days in culture medium supplemented with interleukin 4 (IL-4) (1,000 IU/ml; R&D Systems, Lille, France) and granulocyte-macrophage colony-stimulating factor (GM-CSF) (1,000 IU/ml; R&D Systems). Incubations were conducted in a humidified 5% CO2 atmosphere at 37°C for all experiments. No cytokines and no fresh medium were added during the 5 days of differentiation.
Exposure of MoDCs to microbial extracts.
The cocultivation of MoDCs (5 × 105 cells) and each of the HP causative agents tested (S. rectivirgula, E. amstelodami, W. sebi, and M. immunogenum), at 10 μg/ml, was carried out for 24 h. Lipopolysaccharide (LPS) (Sigma-Aldrich) was used as a positive control (also at 10 μg/ml). The exposure concentration of 10 μg/ml was selected after concentration assays using 0.1, 1, 10, and 100 μg/ml for each of the microbial extracts. The quantity of IL-8 secreted in the supernatants by MoDCs after 24 h of exposure and their expression of the costimulatory molecule CD40 were used to select the concentration of 10 μg/ml for further assays (see Fig. S1 in the supplemental material).
Phenotypic activation of MoDCs. (i) Costimulatory molecule expression.
Immature MoDCs (iMoDCs) (unexposed) and MoDCs 24 h after exposure were stained with labeled antibodies (BD Biosciences Pharmingen, Pont de Claix, France) against CD11c (phycoerythrin [PE]-labeled), CD80 (fluorescein isothiocyanate [FITC]-labeled), CD86 (PE- and Cy7-labeled), and CD40 (allophycocyanin [APC]-labeled). MoDCs were analyzed by flow cytometry (BD Canto II) using FACSDiva software (BD Biosciences).
(ii) Cytokine/chemokine production.
The secretion of IL-10, IL-12, IL-23, and IL-8 was analyzed by enzyme-linked immunosorbent assays (ELISAs) (Diaclone, Besançon, France) according to the manufacturer's instructions (detection thresholds of the ELISAs were 29 pg/ml for IL-8, 20 pg/ml for IL-23, 5 pg/ml for IL-10, and 2.2 pg/ml for IL-12).
Functional activation of MoDCs. (i) Mixed lymphocytic reaction with total allogeneic PBLs.
The ability of exposed MoDCs to induce the proliferation of both CD4+ and CD8+ T lymphocytes was investigated using total allogeneic PBLs. MoDCs (5 × 104) that had been previously exposed for 24 h to each of the microbial extracts tested were incubated for 5 days with 1 × 105 PBLs in round-bottomed wells (Sarstedt, Marnay, France). T cell proliferation was assessed by flow cytometry with carboxyfluorescein succinimidyl ester (CFSE) labeling, as recommended by the manufacturer (eBioscience, Paris, France).
(ii) Mixed lymphocytic reaction with purified naive CD4+ T lymphocytes. (a) Cells.
The lymphocytic CD4+ polarization was investigated using naive CD4+ T lymphocytes (CD3+ CD4+ CD45RA+ CD25−) that had been purified from PBMCs by immunomagnetic depletion using the Naive CD4+ T Cell Isolation Kit II (Miltenyi Biotec, Paris, France), according to the manufacturer's instructions. MoDCs (5 × 104) that had been previously exposed to each of the microbial extracts tested were incubated for 5 days with 1 × 105 CD4+ T lymphocytes in round-bottomed wells (Sarstedt). A MoDC/T cell ratio of 1:2 was selected due to the results of mixed lymphocytic reaction (MLR) testing with different MoDC/T cell ratios (1:2, 1:5, 1:10, and 1:100), using MoDCs that had been previously exposed to LPS for 24 h (see Fig. S2 in the supplemental material).
(b) Transcription factors.
After 3 days of cocultivation, the RNA of naive CD4+ T lymphocytes was extracted using an RNeasy minikit and an RNase-free DNase set (Qiagen, Courtaboeuf, France), according to the manufacturer's instructions. Reverse transcription was carried out in a final volume of 40 μl, as follows: 20 μl RNA was added to 8 μl of RNA-to-cDNA Master Mix (Applied Biosystems, Courtaboeuf, France). Real-time PCR was carried out in a LightCycler LC 480 system (Roche Diagnostics, Meylan, France), in a 20-μl final volume containing 10 μl TaqMan Universal PCR Master Mix (Applied Biosystems), 1 μl TaqMan Gene Expression Assays-on-Demand primer solution (Applied Biosystems), and 2 μl cDNA. The expression of four transcription factors (target genes), each representative of a polarization pathway, i.e., T-bet (Th1), Gata-3 (Th2), FoxP3 (Treg), and RORc (Th17), was measured. Values for each sample were normalized on the basis of the contents in comparison with a reference gene, GAPDH. The results were expressed as the N-fold difference in target gene expression, relative to GAPDH gene expression (termed Ntarget), and were obtained using the following formula: Ntarget = 2ΔCq,sample.
(c) Intracellular markers.
After 5 days of coculture, CD4+ T lymphocytes were stimulated for 4 h with phorbol myristate acetate (PMA) (50 μg/ml; Sigma) and ionomycin (500 ng/ml; Sigma). T cells were fixed and permeabilized with a BD Cytofix/Cytoperm Plus kit with GolgiPlug (BD Biosciences) and then were incubated with labeled antibodies (BD Biosciences) against IL-17 (PE-labeled), IL-4 (FITC-labeled), tumor necrosis factor alpha (TNF-α) (PE- and Cy7-labeled), and gamma interferon (IFN-γ) (APC-labeled). Cells were washed, and cytokine profiles were analyzed.
(d) Cytokine/chemokine levels.
After 5 days of cocultivation, secreted chemokines and cytokines were quantified using Luminex multiplex bead technology. Human cytokine/chemokine panel I (MPXHCYTO-60K, Milliplex Map; Millipore, Billerica, MA) was used to quantify IL-6, IL-8, IFN-γ, IL-17, IL-4, IL-10, IL-12, and TNF-α (assay sensitivity of 0.4 pg/ml for all). Each sample was measured in duplicate, and the assay was performed following the manufacturer's instructions.
Th1, Th2, Treg, and Th17 polarization controls.
Naive CD4+ T lymphocytes were incubated in round-bottomed wells with Dynabeads Human T-Activator CD3/CD28 (Life Technologies, Saint Aubin, France) plus IL-12 (50 ng/ml) and anti-IL-4 (10 μg/ml) for Th1 polarization; IL-4 (50 ng/ml) and anti-IFN-γ (10 μg/ml) for Th2 polarization; transforming growth factor β1 (TGF-β1) (50 ng/ml), anti-IFN-γ (10 μg/ml), and anti-IL-4 (10 μg/ml) for Treg polarization; or TGF-β1 (5 ng/ml), IL-6 (50 ng/ml), IL-23 (25 ng/ml), IL-1β (25 ng/ml), anti-IFN-γ (10 μg/ml), and anti-IL-4 (10 μg/ml) for Th17 polarization.
Transcription factor expression, intracellular marker levels, and cytokine secretion were measured as described above. In the case of the Treg polarization control, Human Regulatory T Cell Staining Kit 2 (eBioscience, San Diego, CA) was used to measure the intracellular markers FoxP3 and CD25.
Statistical analysis.
Data are presented as means ± standard deviations (SD) from at least six separate experiments. For comparisons of continuous paired data (related samples), the Friedman test and the Wilcoxon signed-rank test were performed using the software package Stata v10 (StataCorp LP, College Station, TX). All tests were two-tailed, and P values of <0.05 were considered statistically significant.
RESULTS
Phenotypic activation of MoDCs. (i) Costimulatory molecule expression.
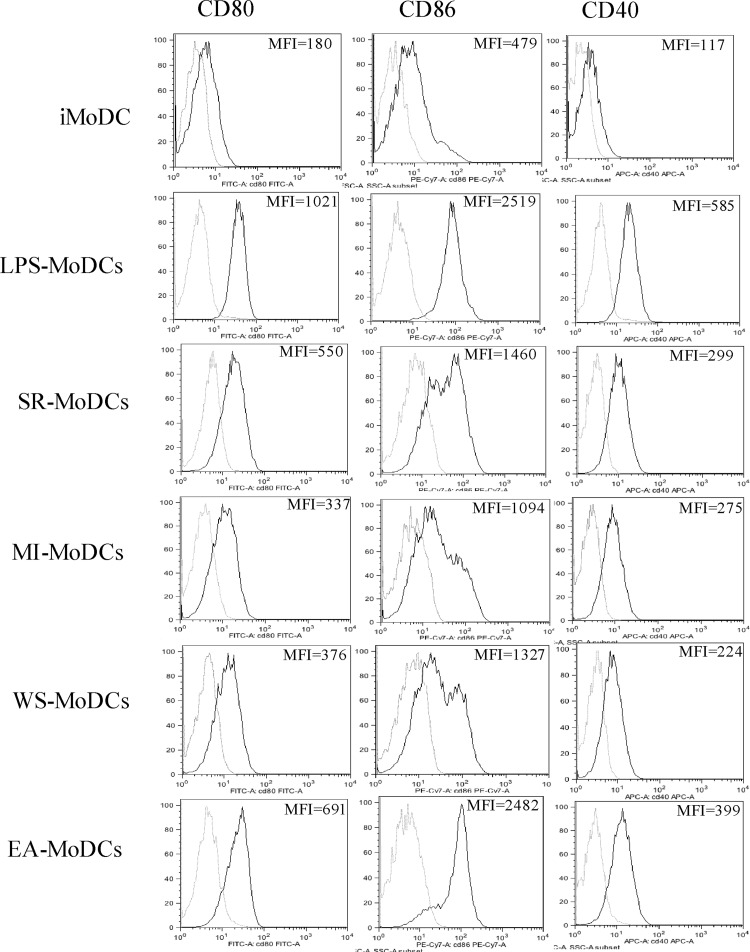
We observed slight T lymphocyte contamination in each preparation (3.26% ± 1.4% T lymphocytes). MoDCs were selected on the basis of their CD11c expression. Exposure to microbial extracts may lead to changes in the expression of costimulatory molecules present on the surface of MoDCs. The percentages of CD80, CD86, and CD40 obtained for immature MoDCs (iMoDCs) and MoDCs exposed to either LPS, E. amstelodami (EA-MoDCs), W. sebi (WS-MoDCs), S. rectivirgula (SR-MoDCs), or M. immunogenum (MI-MoDCs) were measured by flow cytometry, and an example of the results observed is presented in Fig. 1. The percentages of activation of the costimulatory molecules are detailed in Table 1 and the corresponding statistical analysis in Table 2. The analysis revealed that EA-MoDCs expressed higher percentages of costimulatory molecules (CD80, CD86, and CD40) than did WS-MoDCs, SR-MoDCs, and MI-MoDCs (P < 0.01, Wilcoxon signed-rank test).
Fig 1.
Expression of the costimulatory molecules CD80, CD86, and CD40 by immature MoDCs (iMoDCs) and MoDCs exposed to LPS (positive control), S. rectivirgula (SR), M. immunogenum (MI), W. sebi (WS), or E. amstelodami (EA). White peaks, isotypes; gray peaks, corresponding antibodies. The mean fluorescence intensity (MFI) is indicated for each condition.
Table 1.
Percentages of costimulatory molecules CD80, CD86, and CD40 measured on human MoDCs before (iMoDCs) and after (positive control) exposure to LPS, E. amstelodami, W. sebi, S. rectivirgula, or M. immunogenum
| Cells tested | % of expression (mean ± SD) |
||
|---|---|---|---|
| CD80 | CD86 | CD40 | |
| iMoDCs | 12 ± 10 | 10 ± 5 | 9 ± 5 |
| LPS-exposed MoDCs | 94 ± 10 | 97 ± 13 | 98 ± 12 |
| EA-MoDCs | 60 ± 18 | 66 ± 16 | 78 ± 16 |
| WS-MoDCs | 36 ± 13 | 32 ± 15 | 41 ± 12 |
| SR-MoDCs | 51 ± 19 | 45 ± 18 | 41 ± 15 |
| MI-MoDCs | 26 ± 16 | 25 ± 13 | 31 ± 12 |
Table 2.
Statistical analysis of differences in the expression of costimulatory molecules among MoDCs pulsed with E. amstelodami, W. sebi, S. rectivirgula, or M. immunogenum
| Comparison |
P for expression of: |
||
|---|---|---|---|
| CD80 | CD86 | CD40 | |
| EA-MoDCs vs WS-MoDCs | 0.002 | 0.002 | 0.008 |
| EA-MoDCs vs SR-MoDCs | 0.002 | 0.002 | 0.008 |
| EA-MoDCs vs MI-MoDCs | 0.001 | 0.001 | 0.001 |
| WS-MoDCs vs SR-MoDCs | NSa | NS | NS |
| WS-MoDCs vs MI-MoDCs | NS | NS | NS |
| SR- MoDCs vs MI-MoDCs | 0.03 | 0.04 | NS |
NS, not significant.
(ii) Cytokine/chemokine production.
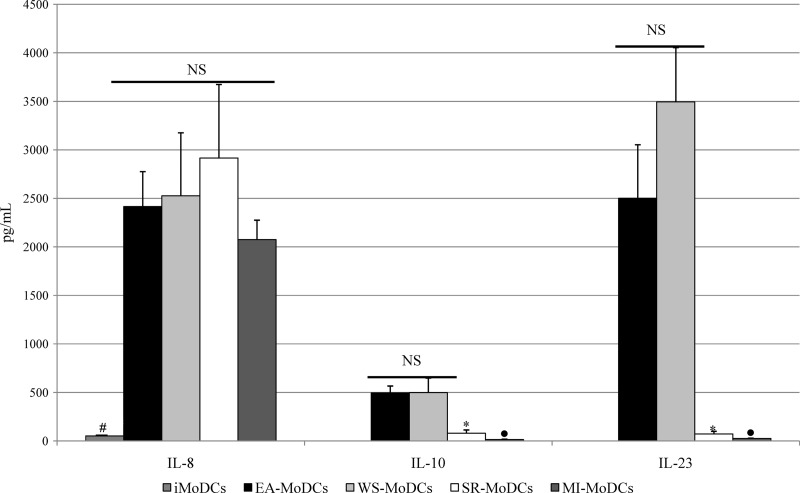
Exposure to microbial extracts involved in HP may induce the secretion of chemokines and cytokines such as IL-8, IL-23, IL-12, and IL-10. A basal level of IL-8 (122 ± 41 pg/ml) was detected in the supernatants of iMoDCs, but IL-23, IL-12, and IL-10 were undetectable. LPS-exposed MoDCs produced 10,060 ± 651 pg/ml IL-8, 1,170 ± 350 pg/ml IL-23, 246 ± 80 pg/ml IL-12, and 491 ± 80 pg/ml IL-10. EA-MoDCs, WS-MoDCs, SR-MoDCs, and MI-MoDCs all produced high levels of IL-8 (means, 2,076 pg/ml to 2,931 pg/ml). MoDCs exposed to filamentous fungi (EA-MoDCs and WS-MoDCs) produced higher levels of IL-10 and IL-23 than did MoDCs exposed to bacteria (SR-MoDCs and MI-MoDCs) (P < 0.05, Wilcoxon signed-rank test). These results are presented in Fig. 2. The levels of IL-12 detected were low for EA-MoDCs, WS-MoDCs, and SR-MoDCs (means ranging from 6.8 pg/ml to 21.5 pg/ml) and undetectable for MI-MoDCs (Table 3).
Fig 2.
Levels of IL-8, IL-10, and IL-23 (means ± SDs) in supernatants of EA-MoDCs, WS-MoDCs, SR-MoDCs, and MI-MoDCs, in six separate experiments. EA-MoDCs and WS-MoDCs both produced significantly higher levels of IL-10 and IL-23 than did SR-MoDCs (*, P < 0.05, Wilcoxon signed-rank test) and MI-MoDCs (●, P < 0.05, Wilcoxon signed-rank test). iMoDCs did produce significantly lower levels of IL-8 than EA-MoDCs, WS-MoDCs, MI-MoDCs, and SR-MoDCs (#, P < 0.05, Wilcoxon signed-rank test). NS, not significant.
Table 3.
Levels of IL-12 (p70) in supernatants in assays investigating phenotypic and functional activation of MoDCs
| Condition tested | IL-12 level (pg/ml [mean ± SD]) |
|---|---|
| Phenotypic assays | |
| iMoDCs | 0 ± 0 |
| LPS-exposed MoDCs | 246 ± 80 |
| EA-MoDCs | 21 ± 4.5 |
| WS-MoDCs | 7.2 ± 2.9 |
| SR-MoDCs | 6.7 ± 2.2 |
| MI-MoDCs | 0 ± 0 |
| Functional assays | |
| Allogeneic T lymphocytes + iMoDCs | 1.4 ± 0.1 |
| Naive CD4+ T lymphocytes + iMoDCs | 1.5 ± 0.2 |
| Naive CD4+ T lymphocytes + LPS-exposed MoDCs | 10.8 ± 4.1 |
| Naive CD4+ T lymphocytes + EA-MoDCs | 11.5 ± 4.7 |
| Naive CD4+ T lymphocytes + WS-MoDCs | 11.9 ± 3.7 |
| Naive CD4+ T lymphocytes + SR-MoDCs | 9.5 ± 3.6 |
| Naive CD4+ T lymphocytes + MI-MoDCs | 9.8 ± 2.5 |
Functional activation of MoDCs. (i) MLR with total allogeneic PBLs.
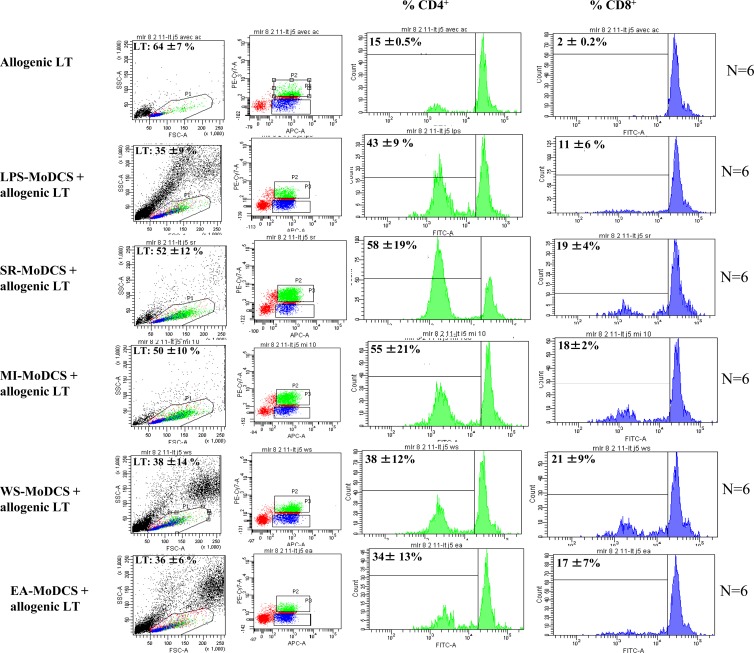
The capacity of exposed MoDCs to stimulate T cell proliferation was initially investigated using total allogeneic PBLs, in order to determine whether the lymphocytic response involved mostly CD4+ or CD8+ lymphocytes. The results are presented in Fig. 3. Cocultivation of total allogeneic PBLs with LPS-exposed MoDCs, EA-MoDCs, WS-MoDCs, SR-MoDCs, or MI-MoDCs for 5 days, in three separate experiments, induced greater proliferation of CD4+ cells (means, 34% ± 13% to 58% ± 19%) than CD8+ cells (means, 11% ± 6% to 21% ± 9%). Moreover, greater proliferation of PBLs was observed in the case of stimulation with bacteria (S. rectivirgula and M. immunogenum) (P = 0.028, Wilcoxon signed-rank test).
Fig 3.
Percentages of proliferation of CD4+ (green) and CD8+ (blue) lymphocytes after cultivation of total allogeneic T lymphocytes (LT) (MLR negative control) and after cocultivation of T lymphocytes plus LPS-exposed MoDCs, T lymphocytes plus SR-MoDCs, T lymphocytes plus MI-MoDCs, T lymphocytes plus WS-MoDCs, and T lymphocytes plus EA-MoDCs. Red, lymphocytes neither CD4+ nor CD8+. Greater proliferation of PBLs was observed in the case of stimulation with bacteria (S. rectivirgula and M. immunogenum) (P = 0.028, Wilcoxon signed-rank test). FSC, forward scatter; SSC, side scatter.
(ii) MLR with purified naive CD4+ T lymphocytes. (a) Transcription factors.
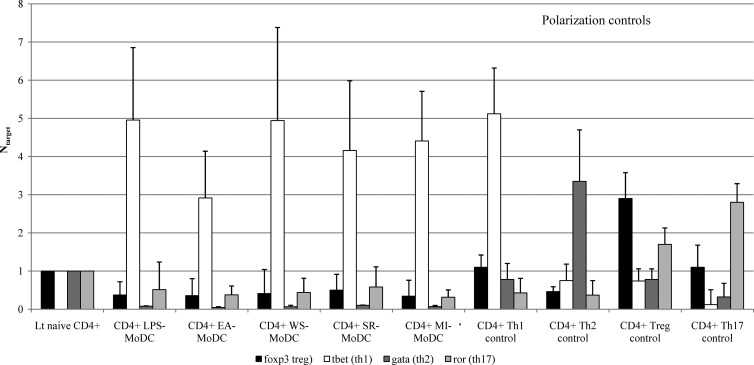
In order to determine the polarization of the proliferating CD4+ lymphocytes, the MLR was repeated with only CD4+ T cells and the expression of four transcription factors (target genes), each representative of a polarization pathway, i.e., T-bet (Th1), Gata-3 (Th2), FoxP3 (Treg), and RORc (Th17), was measured. Cocultivation of naive CD4+ T lymphocytes with LPS-exposed MoDCs, EA-MoDCs, WS-MoDCs, SR-MoDCs, or MI-MoDCs for 3 days, in three separate experiments, induced marked upregulation of the T-bet gene (NT-bet means, 3.1 to 4.96) (Fig. 4). The expression levels of the three other target genes were downregulated (means, 0.04 to 0.1 [NGata-3], 0.34 to 0.5 [NFoxP3], and 0.31 to 0.58 [NRORc]). Levels of mRNA expression for polarization controls are presented in the right part of Fig. 4. The results showed that all microbial extracts promoted Th1 polarization, as measured by T-bet.
Fig 4.
Standardized mRNA expression of the FoxP3, T-bet, Gata-3, and RORc genes after exposure of MoDCs to LPS, E. amstelodami, W. sebi, S. rectivirgula, or M. immunogenum, calculated with the ΔΔCq method, with GAPDH as the reference gene. The data are presented as means ± SEMs from three separate experiments. In three separate experiments, levels of T-bet produced by T lymphocytes (Lt) after cocultivation with LPS-exposed MoDCs, EA-MoDCs, WS-MoDCs, SR-MoDCs, or MI-MoDCs were significantly higher (P < 0.05, Wilcoxon signed-rank test) than those produced by T lymphocytes alone and also were higher than levels of the three other genes investigated (FoxP3, RORc, and Gata-3). Transcription factor mRNA levels are presented for the four polarization controls (Th1, Th2, Th17, and Treg) at the right.
(b) Intracellular markers.
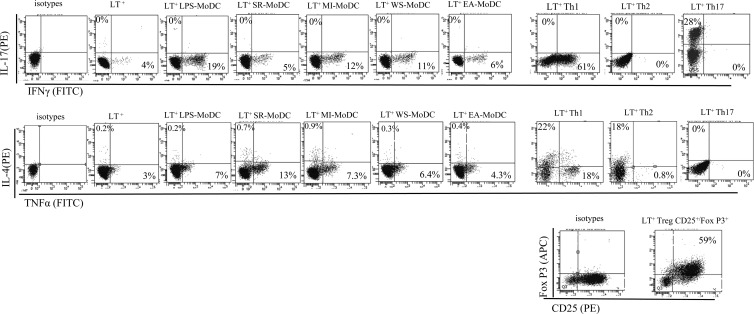
In order to determine the polarization of the proliferating CD4+ lymphocytes, the MLR was repeated with only CD4+ T cells and the presence of intracellular markers such as IL-17a, IL-4, TNF-α, and IFN-γ in CD4+ T cells was investigated by flow cytometry. After 5 days of cocultivation of naive CD4+ T lymphocytes with LPS-exposed MoDCs, EA-MoDCs, WS-MoDCs, SR-MoDCs, or MI-MoDCs, the percentages of IFN-γ (means ranging from 6.5% to 13.5%) and TNF-α (means ranging from 4.3% to 7.9%) were high (Table 4). The percentages of IL-17a (means ranging from 0.13% to 0.17%) and IL-4 (means ranging from 0.7% to 1.5%) remained low (Table 4). In the case of the negative control (CD4+ T cells alone), the percentages of intracellular markers detected were 0.1% ± 0.08% for IL-17a, 1.2% ± 0.7% for IFN-γ, 3% ± 0.5% for TNF-α, and 0.7% ± 1.2% for IL-4. Concerning the polarization controls, the Th1 control showed high percentages of IFN-γ, TNF-α, and IL-4, the Th2 control showed a high percentage only of IL-4, the Th17 control showed a high percentage only of IL-17a, and the Treg polarization control showed a high percentage only of CD4+ CD25+ FoxP3+ cells. All of these results are detailed in fluorescence-activated cell sorting plots (Fig. 5) and in Table 4.
Table 4.
Intracellular staining for IL-17a, IL-4, IFN-γ, and TNF-α for allogeneic T lymphocytes and naive CD4+ T lymphocytes cocultured with MoDCs previously exposed to LPS, E. amstelodami, W. sebi, S. rectivirgula, or M. immunogenum
| Cells tested | % (mean ± SD) |
CD4+ CD25+ FoxP3+ cellsa (% [mean ± SD] of parents) | |||
|---|---|---|---|---|---|
| IL-17a | IFN-γ | IL-4 | TNF-α | ||
| T lymphocytes cocultured with MoDCs exposed to microbial extracts | |||||
| Naive CD4+ T lymphocytes | 0.1 ± 0.08 | 1.2 ± 0.7 | 0.7 ± 1.2 | 3 ± 0.5 | NR |
| Naive CD4+ T lymphocytes + LPS-exposed MoDCs | 0.17 ± 0.1 | 13.5 ± 5 | 1.1 ± 1.5 | 7.7 ± 0.7 | NR |
| Naive CD4+ T lymphocytes + EA-MoDCs | 0.1 ± 0 | 6.8 ± 1.9 | 1.5 ± 2 | 6.6 ± 0.9 | NR |
| Naive CD4+ T lymphocytes + WS-MoDCs | 0.13 ± 0.06 | 7.9 ± 4.9 | 0.7 ± 0.6 | 5 ± 0.4 | NR |
| Naive CD4+ T lymphocytes + SR-MoDCs | 0.1 ± 0 | 6.5 ± 1.1 | 0.9 ± 5 | 7.9 ± 1.3 | NR |
| Naive CD4+ T lymphocytes + MI-MoDCs | 0.1 ± 0 | 8.9 ± 0.4 | 1.5 ± 3 | 4.3 ± 0.4 | NR |
| Polarization controls | |||||
| Naive CD4+ T lymphocytes, Th1 | 0.1 ± 0 | 60.8 ± 1.5 | 21.2 ± 2.3 | 18.4 ± 1.6 | NR |
| Naive CD4+ T lymphocytes, Th2 | 0.1 ± 0 | 0.1 ± 0 | 19.2 ± 0.8 | 0.9 ± 0.3 | NR |
| Naive CD4+ T lymphocytes, Th17 | 25 ± 7.5 | 0.3 ± 0.1 | 0 ± 0 | 0 ± 0 | NR |
| Naive CD4+ T lymphocytes, Treg | 0 ± 0 | 0.4 ± 0.08 | 0 ± 0 | 0 ± 0 | 55 ± 9.8 |
Specific CD25/FoxP3 staining was added for the Treg polarization control. NR, no response.
Fig 5.
Intracellular staining for IL-17a, IL-4, IFN-γ, and TNF-α in allogeneic T lymphocytes (LT) and naive CD4+ T cells cocultured with MoDCs that had been previously exposed to LPS, S. rectivirgula, M. immunogenum, W. sebi, or E. amstelodami. Polarization controls for Th1, Th2, Th17, and Treg are presented at the right. Specific CD25/FoxP3 staining was added for the Treg polarization control.
(c) Cytokine/chemokine levels in supernatants.
In order to determine the polarization of the proliferating CD4+ lymphocytes, the MLR was repeated with only CD4+ T cells and the presence of 8 different cytokines and chemokines in culture supernatants was investigated using Luminex technology. After 5 days of cocultivation of naive CD4+ T lymphocytes with LPS-exposed MoDCs, EA-MoDCs, WS-MoDCs, SR-MoDCs, or MI-MoDCs, high levels of IFN-γ (means, 51,254 pg/ml to 98,266 pg/ml), TNF-α (means, 2,703 pg/ml to 3,529 pg/ml), IL-8 (means, 3,861 pg/ml to 4,172 pg/ml), and IL-6 (means, 1,673 pg/ml to 2,279 pg/ml) (Table 5) were found, in contrast to levels of IL-17a (means, 9 pg/ml to 31 pg/ml), IL-10 (means, 27 pg/ml to 156 pg/ml), IL-12 (means, 9.5 pg/ml to 11.9 pg/ml) (Table 3), and IL-4 (undetectable). Statistical analyses comparing the production of cytokines according to the microbial extracts tested (E. amstelodami, W. sebi, S. rectivirgula, or M. immunogenum) showed no significant differences in IL-8, IL-6, and IFN-γ production, while lower production of TNF-α was observed in the case of coculture of CD4+ T lymphocytes with SR-MoDCs (P < 0.05, Wilcoxon signed-rank test) and higher production of IL-10 and IL-17a was observed in the case of coculture of CD4+ T lymphocytes with EA-MoDCs (P < 0.05, Wilcoxon signed-rank test).
Table 5.
Cytokine/chemokine levels in supernatants of allogeneic T lymphocytes, naive CD4+ T cells cocultured with MoDCs previously exposed to LPS, S. rectivirgula, M. immunogenum, W. sebi, or E. amstelodami, and T cell polarization controlsa
| Cells tested | Cytokine/chemokine levels (pg/ml [mean ± SD]) in: |
||||||
|---|---|---|---|---|---|---|---|
| IL-8 | IL-6 | IFN-γ | TNF-α | IL-10 | IL-17a | IL-4 | |
| T lymphocytes cocultured with MoDCs exposed to microbial extracts | |||||||
| Naive CD4+ T lymphocytes | 34 ± 7 | 0 ± 0 | 19 ± 4.4 | 7 ± 4.5 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Naive CD4+ T lymphocytes + LPS-exposed MoDCs | 3,888 ± 277 | 2,005 ± 577 | 98,266 ± 4,520 | 2,343 ± 423 | 27 ± 5 | 9 ± 4 | 0 ± 0 |
| Naive CD4+ T lymphocytes + EA-MoDCs | 4,172 ± 550 | 1,983 ± 346 | 67,676 ± 2,732 | 3,529 ± 637 | 156 ± 39 | 31 ± 12 | 0 ± 0 |
| Naive CD4+ T lymphocytes + WS-MoDCs | 4,169 ± 139 | 2,223 ± 737 | 56,123 ± 2,767 | 3,451 ± 470 | 46 ± 18 | 16 ± 3 | 0 ± 0 |
| Naive CD4+ T lymphocytes + SR-MoDCs | 3,861 ± 315 | 1,673 ± 305 | 51,254 ± 2,223 | 1,769 ± 310 | 29 ± 10 | 12 ± 4 | 0 ± 0 |
| Naive CD4+ T lymphocytes + MI-MoDCs | 4,041 ± 190 | 2,279 ± 508 | 56,704 ± 2,895 | 2,703 ± 563 | 33 ± 12 | 15 ± 6 | 0 ± 0 |
| Polarization controls | |||||||
| Naive CD4+ T lymphocytes, Th1 | 6,058 ± 358 | 2,247 ± 252 | 4,714 ± 547 | 2,813 ± 926 | 393 ± 170 | 47 ± 11 | 0 ± 0 |
| Naive CD4+ T lymphocytes, Th2 | 44 ± 16 | 276 ± 56 | 116 ± 46 | 946 ± 329 | 1,320 ± 344 | 42 ± 16 | 36,957 ± 6,276 |
| Naive CD4+ T lymphocytes, Th17 | 443 ± 130 | 1,743 ± 712 | 177 ± 86 | 1,082 ± 435 | 1,500 ± 522 | 511 ± 218 | 0 ± 0 |
| Naive CD4+ T lymphocytes, Treg | 1,620 ± 780 | 852 ± 290 | 62 ± 17 | 2,081 ± 637 | 223 ± 78 | 42 ± 18 | 0 ± 0 |
Cytokine/chemokine levels were measured in supernatants using the Luminex technique after 6 days of incubation.
All of the results are detailed in Tables 3 and 5, including those obtained for the negative control (CD4+ T cells alone) and polarization controls. These results support the fact that all microbial extracts promoted Th1 polarization, as measured by IFN-γ, TNF-α, IL-8, and IL-6 levels.
Endotoxin levels.
The presence of endotoxins was measured in the total extracts using the Chromo-LAL method (Biogenic, Perols, France). Microbial extracts were used at 10 μg/ml for MoDC exposure. Endotoxin levels for the W. sebi and M. immunogenum extracts were 0.007 endotoxin units (EU)/ml and 0.0006 EU/ml, respectively. A first set of experiments was performed using extracts of S. rectivirgula and E. amstelodami with endotoxin levels higher than 0.01 EU/ml (18). A second set of experiments was performed using S. rectivirgula and E. amstelodami extracts with levels under the 0.01 EU/ml limit (0.008 EU/ml and 0.0005 EU/ml, respectively). Significant differences were observed between the two sets of experiments in two cases, i.e., (i) higher production of IL-8 in cocultures with the S. rectivirgula extract with <0.01 EU/ml and (ii) higher production of IFN-γ in cocultures with the E. amstelodami extract with >0.01 EU/ml (Wilcoxon signed-rank test) (Table 6). The results were considered similar for phenotypic and functional assays using either set of S. rectivirgula and E. amstelodami total extracts, and we concluded that the presence of endotoxin did not affect the Th1 polarization (Table 6).
Table 6.
Comparison of results obtained in phenotypic and functional assays using different batches of S. rectivirgula and E. amstelodami total extracts, either below or above the 0.01 EU/ml limit
| Polarization result | Extracts with >0.01 EU/mla |
Extracts with <0.01 EU/mla |
Differenceb |
|||
|---|---|---|---|---|---|---|
| SR- MoDCs | EA-MoDCs | SR- MoDCs | EA-MoDCs | SR- MoDCs | EA-MoDCs | |
| Phenotypic assays | ||||||
| % with CD80 | 51 ± 19 | 60 ± 18 | 61.2 ± 15.2 | 58.3 ± 15.2 | NS | NS |
| % with CD86 | 45 ± 18 | 66 ± 16 | 38.8 ± 19 | 58.4 ± 13.1 | NS | NS |
| IL-23 level (pg/ml) | 72 ± 38 | 2,416 ± 875 | 122 ± 46 | 2,590 ± 422 | NS | NS |
| IL-8 level (pg/ml) | 1,987 ± 358 | 2,708 ± 296 | 3,876 ± 823 | 2,189 ± 752 | 0.03 | NS |
| IL-10 level (pg/ml) | 79 ± 52 | 475 ± 89 | 86 ± 48 | 546 ± 66 | NS | NS |
| Functional assays | ||||||
| % with CD4 (CFSE) | 48 ± 19.6 | 36.4 ± 11.2 | 73 ± 27 | 30.2 ± 7.9 | NS | NS |
| T-bet mRNA expression (fold) | 4.16 ± 1.8 | 2.9 ± 1.2 | 4.78 ± 1.3 | 3.3 ± 1.6 | NS | NS |
| % with intracellular TNF-α | 7.6 ± 0.2 | 7 ± 0.5 | 8.2 ± 2.4 | 6.1 ± 0.9 | NS | NS |
| % with intracellular IFN-γ | 6.1 ± 0.2 | 8 ± 2.3 | 6.8 ± 1.8 | 5.6 ± 1.6 | NS | NS |
| Il-8 level (pg/ml) | 3,522 ± 243 | 4,356 ± 487 | 4,200 ± 386 | 3,987 ± 612 | NS | NS |
| Il-6 level (pg/ml) | 1,423 ± 253 | 2,289 ± 423 | 1,923 ± 358 | 1,678 ± 269 | NS | NS |
| IFN-γ level (pg/ml) | 53,620 ± 1,245 | 75,283 ± 2,641 | 48,888 ± 3,200 | 60,069 ± 2,823 | NS | 0.044 |
Values are means ± SD.
Differences were tested using the Wilcoxon signed-rank test. NS, not significant.
DISCUSSION
The present study, which aimed to determine the cross talk between MoDCs and microbial extracts from microorganisms involved in HP, provides evidence that, although differences initially were observed between MoDCs exposed to filamentous fungi and MoDCs exposed to bacteria (high levels of IL-23 and IL-10 for MoDCs stimulated by E. amstelodami or W. sebi), a Th1 response ultimately was promoted by MoDCs regardless of the microbial extract tested. The results of our study are consistent with the hypothesis that upregulation of Th1 signaling plays a critical role in the pathogenesis of HP.
Th17 cells are a recently described subset of Th cells, and they play a pivotal role in immunity against fungi (19, 20). IL-23, a member of the IL-12 cytokine family produced by MoDCs, is involved in the Th17 lineage and plays a critical role in the development of inflammation. A previous study demonstrated that the interaction between MoDCs and the polysaccharide β-glucan, which is present in the cell walls of fungi, triggered the syk-CARD9 signaling pathway and thus the production of IL-23 (21). E. amstelodami and W. sebi are both filamentous fungi with β-glucan in their cell walls, and high levels of IL-23 were detected in supernatants of EA-MoDCs and WS-MoDCs. This result suggested that differentiation of naive CD4+ T cells into Th17 cells could occur. In MLR assays, however, a Th1-polarized immune response was observed for the four microbial extracts tested, including E. amstelodami and W. sebi. This observation is consistent with the recent demonstration that IL-23, while able to maintain a Th17 phenotype, does not mediate commitment to this phenotype (22).
Previous studies, focusing mainly on Aspergillus fumigatus, a filamentous fungi involved in invasive aspergillosis, demonstrated that surface components of fungi stimulate the Toll-like receptors (TLRs) TLR2 and TLR4 (23, 24). Ligands that are present on nearly all fungi and might be responsible for stimulating the TLRs include β-glucans, mannans, and chitin (25). Several studies involving either the genus Mycobacterium (mostly Mycobacterium tuberculosis) or Gram-positive bacteria (but not specifically actinomycetes) also demonstrated interactions between bacterial components and TLRs (also mostly TLR2 and TLR4) (26, 27). Thus, it seems most probable that the mechanism involves interactions with TLR2 and TLR4.
Previous studies have established that HP is caused by a Th1-type immune response; bronchoalveolar lavage fluid (BALF) specimens obtained from individuals with HP displayed abundant IFN-γ, TNF-α, IL-8, and IL-12 (28–30). The low levels of IL-12 detected in our assays, in both phenotypic and functional assays, are probably due to the use of MoDCs instead of macrophages. In patients' BALF, macrophages are well represented and may be responsible for IL-12 production. Data showed that MoDCs were able to induce marked Th1 responses, with strong IFN-γ production, but failed to produce high levels of IL-12 (31).
The results of our study are consistent with the hypothesis that upregulation of Th1 signaling plays a critical role in the pathogenesis of HP. The fact that the nature of the microorganisms (filamentous fungi, actinomycetes, or mycobacteria) tested did not influence the outcome of the final immune response gives weight to the hypothesis that the pathophysiology of HP and the consequent clinical form are due more to the type of exposure (32) and individual genetic susceptibility (33–35) than to the nature of the microbial environment of the patient.
In conclusion, this study provides evidence that, although initial differences are observed between MoDCs exposed to filamentous fungi and MoDCs exposed to bacteria, all of the microbial extracts ultimately lead to a Th1-type immune response. This study also clarifies the interactions between MoDCs and microbial extracts from microorganisms involved in HP, highlighting the fact that MoDCs are useful for acquiring a better understanding of antimicrobial immunity.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the University Hospital of Besançon (MP-PHIA study).
We thank Audrey Dazy and Ahn Poirot for their excellent technical help. We also thank Frances Sheppard (Clinical Investigation Center [INSERM], Besançon) for her editorial assistance.
Footnotes
Published ahead of print 29 May 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00043-13.
REFERENCES
- 1. Girard M, Lacasse Y, Cormier Y. 2009. Hypersensitivity pneumonitis. Allergy 64:322–334 [DOI] [PubMed] [Google Scholar]
- 2. Beckett W, Kallay M, Sood A, Zuo Z, Milton D. 2005. Hypersensitivity pneumonitis associated with environmental mycobacteria. Environ. Health Perspect. 113:767–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tillie-Leblond I, Grenouillet F, Reboux G, Roussel S, Chouraki B, Lorthois C, Dalphin JC, Wallaert B, Millon L. 2011. Hypersensitivity pneumonitis and metalworking fluids contaminated by mycobacteria. Eur. Respir. J. 37:640–647 [DOI] [PubMed] [Google Scholar]
- 4. Murat JB, Grenouillet F, Reboux G, Penven E, Batchili A, Dalphin JC, Thaon I, Millon L. 2012. Factors influencing the microbial composition of metalworking fluids and potential implications for machine operator's lung. Appl. Environ. Microbiol. 78:34–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Denis M, Cormier Y, Fournier M, Tardif J, Laviolette M. 1991. Tumor necrosis factor plays an essential role in determining hypersensitivity pneumonitis in a mouse model. Am. J. Respir. Cell Mol. Biol. 5:477–483 [DOI] [PubMed] [Google Scholar]
- 6. Thorne PS, Adamcakova-Dodd A, Kelly KM, O'Neill ME, Duchaine C. 2006. Metalworking fluid with mycobacteria and endotoxin induces hypersensitivity pneumonitis in mice. Am. J. Respir. Crit. Care Med. 173:759–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Woda BA. 2008. Hypersensitivity pneumonitis: an immunopathology review. Arch. Pathol. Lab. Med. 132:204–205 [DOI] [PubMed] [Google Scholar]
- 8. Gudmundsson G, Bosch A, Davidson BL, Berg DJ, Hunninghake GW. 1998. Interleukin-10 modulates the severity of hypersensitivity pneumonitis in mice. Am. J. Respir. Cell Mol. Biol. 19:812–818 [DOI] [PubMed] [Google Scholar]
- 9. Gudmundsson G, Monick MM, Hunninghake GW. 1998. IL-12 modulates expression of hypersensitivity pneumonitis. J. Immunol. 161:991–999 [PubMed] [Google Scholar]
- 10. Reboux G, Piarroux R, Mauny F, Madroszyk A, Millon L, Bardonnet K, Dalphin JC. 2001. Role of molds in farmer's lung disease in eastern France. Am. J. Respir. Crit. Care Med. 163:1534–1539 [DOI] [PubMed] [Google Scholar]
- 11. Reboux G, Reiman M, Roussel S, Taattola K, Millon L, Dalphin JC, Piarroux R. 2006. Impact of agricultural practices on microbiology of hay, silage and flour on Finnish and French farms. Ann. Agric. Environ. Med. 13:267–273 [PubMed] [Google Scholar]
- 12. Takemura T, Akashi T, Ohtani Y, Inase N, Yoshizawa Y. 2008. Pathology of hypersensitivity pneumonitis. Curr. Opin. Pulm. Med. 14:440–454 [DOI] [PubMed] [Google Scholar]
- 13. Barrios RJ. 2008. Hypersensitivity pneumonitis: histopathology. Arch. Pathol. Lab. Med. 132:199–203 [DOI] [PubMed] [Google Scholar]
- 14. Buentke E, Scheynius A. 2003. Dendritic cells and fungi. APMIS 111:789–796 [DOI] [PubMed] [Google Scholar]
- 15. Borg C, Jalil A, Laderach D, Maruyama K, Wakasugi H, Charrier S, Ryffel B, Cambi A, Figdor C, Vainchenker W, Galy A, Caignard A, Zitvogel L. 2004. NK cell activation by dendritic cells (DCs) requires the formation of a synapse leading to IL-12 polarization in DCs. Blood 104:3267–3275 [DOI] [PubMed] [Google Scholar]
- 16. Pallandre JR, Krzewski K, Bedel R, Ryffel B, Caignard A, Rohrlich PS, Pivot X, Tiberghien P, Zitvogel L, Strominger JL, Borg C. 2008. Dendritic cell and natural killer cell cross-talk: a pivotal role of CX3CL1 in NK cytoskeleton organization and activation. Blood 112:4420–4424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roussel S, Rognon B, Barrera C, Reboux G, Salamin K, Grenouillet F, Thaon I, Dalphin JC, Tillie-Leblond I, Quadroni M, Monod M, Millon L. 2011. Immuno-reactive proteins from Mycobacterium immunogenum useful for serodiagnosis of metalworking fluid hypersensitivity pneumonitis. Int. J. Med. Microbiol. 301:150–156 [DOI] [PubMed] [Google Scholar]
- 18. Wolk K, Docke WD, von Baehr V, Volk HD, Sabat R. 2000. Impaired antigen presentation by human monocytes during endotoxin tolerance. Blood 96:218–223 [PubMed] [Google Scholar]
- 19. Chamilos G, Ganguly D, Lande R, Gregorio J, Meller S, Goldman WE, Gilliet M, Kontoyiannis DP. 2010. Generation of IL-23 producing dendritic cells (DCs) by airborne fungi regulates fungal pathogenicity via the induction of TH-17 responses. PLoS One 5:e12955. 10.1371/journal.pone.0012955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eyerich K, Foerster S, Rombold S, Seidl HP, Behrendt H, Hofmann H, Ring J, Traidi-Hoffmann C. 2008. Patients with chronic mucocutaneous candidiasis exhibit reduced production of Th17-associated cytokines IL-17 and IL-22. J. Invest. Dermatol. 128:2640–2645 [DOI] [PubMed] [Google Scholar]
- 21. LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, Schweighoffer E, Tybulewicz V, Brown GD, Ruland J, Reis e Sousa C. 2007. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat. Immunol. 8:630–638 [DOI] [PubMed] [Google Scholar]
- 22. Stritesky GL, Yeh N, Kaplan MH. 2008. IL-23 promotes maintenance but not commitment to the Th17 lineage. J. Immunol. 181:5948–5955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chai LY, Vonk AG, Kullberg BJ, Verweij PE, Verschueren I, van der Meer JW, Joosten LA, Latge JF, Netea MG. 2011. Aspergillus fumigatus cell wall components differentially modulate host TLR2 and TLR4 responses. Microbes Infect. 13:151–159 [DOI] [PubMed] [Google Scholar]
- 24. Bellocchio S, Montagnoli C, Bozza S, Gaziano R, Rossi G, Mambula SS, Vecchi A, Mantovani A, Levitz SM, Romani L. 2004. The contribution of the Toll-like/IL-1 receptor superfamily to innate and adaptive immunity to fungal pathogens in vivo. J. Immunol. 172:3059–3069 [DOI] [PubMed] [Google Scholar]
- 25. Levitz SM. 2010. Innate recognition of fungal cell walls. PLoS Pathog. 6:e1000758. 10.1371/journal.ppat.1000758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Esin S, Counoupas C, Aulicino A, Brancatisano FL, Maisetta G, Bottai D, Di Luca M, Florio W, Campa M, Batoni G. 2013. Interaction of Mycobacterium tuberculosis cell wall components with the human natural killer cell receptors NKp44 and Toll-like receptor 2. Scand. J. Immunol. 77:460–469 [DOI] [PubMed] [Google Scholar]
- 27. Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. 1999. Differential roles of TLR2 and TLR4 in recognition of Gram-negative and Gram-positive bacterial cell wall components. Immunity 11:443–451 [DOI] [PubMed] [Google Scholar]
- 28. Yamasaki H, Ando M, Brazer W, Center DM, Cruikshank WW. 1999. Polarized type 1 cytokine profile in bronchoalveolar lavage T cells of patients with hypersensitivity pneumonitis. J. Immunol. 163:3516–3523 [PubMed] [Google Scholar]
- 29. Mroz RM, Korniluk M, Stasiak-Barmuta A, Chyczewska E. 2008. Upregulation of Th1 cytokine profile in bronchoalveolar lavage fluid of patients with hypersensitivity pneumonitis. J. Physiol. Pharmacol. 59:499–505 [PubMed] [Google Scholar]
- 30. Ye Q, Nakamura S, Sarria R, Costabel U, Guzman J. 2009. Interleukin 12, interleukin 18, and tumor necrosis factor alpha release by alveolar macrophages: acute and chronic hypersensitivity pneumonitis. Ann. Allergy Asthma Immunol. 102:149–154 [DOI] [PubMed] [Google Scholar]
- 31. Sondergaard JN, Brix S. 2012. Isolation of IL-12p70-competent human monocyte-derived dendritic cells. J. Immunol. Methods 386:112–116 [DOI] [PubMed] [Google Scholar]
- 32. Lalancette M, Carrier G, Laviolette M, Ferland S, Rodrique J, Begin R, Cantin A, Cormier Y. 1993. Farmer's lung: long-term outcome and lack of predictive value of bronchoalveolar lavage fibrosing factors. Am. Rev. Respir. Dis. 148:216–221 [DOI] [PubMed] [Google Scholar]
- 33. Ando M, Hirayama K, Soda K, Okubo R, Araki S, Sasazuki T. 1989. HLA-DQw3 in Japanese summer-type hypersensitivity pneumonitis induced by Trichosporon cutaneum. Am. Rev. Respir. Dis. 140:948–950 [DOI] [PubMed] [Google Scholar]
- 34. Flaherty DK, Braun SR, Marx JJ, Blank JL, Emanuel DA, Rankin J. 1980. Serologically detectable HLA-A, B, and C loci antigens in farmer's lung disease. Am. Rev. Respir. Dis. 122:437–443 [DOI] [PubMed] [Google Scholar]
- 35. Selman M, Teran L, Mendoza A, Camarena A, Martinez-Cordero E, Lezama M, Rubio HM. 1987. Increase of HLA-DR7 in pigeon breeder's lung in a Mexican population. Clin. Immunol. Immunopathol. 44:63–70 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.