Abstract
Mycobacterium bovis BCG prime DNA (Mycobacterium tuberculosis genes)-booster vaccinations have been shown to induce greater protection against tuberculosis (TB) than BCG alone. This heterologous prime-boost strategy is perhaps the most realistic vaccination for the future of TB infection control, especially in countries where TB is endemic. Moreover, a prime-boost regimen using biodegradable microspheres seems to be a promising immunization to stimulate a long-lasting immune response. The alanine proline antigen (Apa) is a highly immunogenic glycoprotein secreted by M. tuberculosis. This study investigated the immune protection of Apa DNA vaccine against intratracheal M. tuberculosis challenge in mice on the basis of a heterologous prime-boost regimen. BALB/c mice were subcutaneously primed with BCG and intramuscularly boosted with a single dose of plasmid carrying apa and 6,6′-trehalose dimycolate (TDM) adjuvant, coencapsulated in microspheres (BCG-APA), and were evaluated 30 and 70 days after challenge. This prime-boost strategy (BCG-APA) resulted in a significant reduction in the bacterial load in the lungs, thus leading to better preservation of the lung parenchyma, 70 days postinfection compared to BCG vaccinated mice. The profound effect of this heterologous prime-boost regimen in the experimental model supports its development as a feasible strategy for prevention of TB.
INTRODUCTION
Tuberculosis (TB) is still one of the leading causes of death from a single infectious agent, causing 1.6 million deaths each year, mostly in developing countries (1, 2). Preventive efforts to control the spread of TB in various parts of the world rely on the use of Mycobacterium bovis bacillus Calmette-Guérin (BCG) vaccine. Over 90% of children are immunized with BCG annually in most developing countries, making it the most widely used human vaccine. This vaccine offers protection against severe manifestations of childhood TB, including meningeal and miliary TB. Moreover, application of the BCG vaccine has considerably reduced the incidence of infant TB in areas where TB is endemic (3, 4, 5). However, its protective effect decreases with advancing age, and it is insufficient to control pulmonary TB, the most frequent occurrence of the disease, in adults. Heterologous prime-boost vaccines are proposed to enhance specific immunity primed by childhood BCG vaccination (6) and are one of the best candidates for novel TB vaccines. Specifically, DNA-based vaccines could be a good choice for improving BCG efficacy because they can stimulate both humoral and cell-mediated immunity in experimental TB models (7).
Antigens present in the mycobacterial culture filtrate (CF) are inducers of protective immunity against Mycobacterium tuberculosis challenge in mice (8, 9, 10), guinea pigs (11, 12), and nonhuman primates (13). Alanine proline antigen (Apa) is a major immunodominant antigen secreted by the M. tuberculosis complex (14, 15). This glycoprotein is a fibronectin attachment protein (FAP) and can mediate mycobacterial binding to host cells as a potential adhesin (16). Apa was selected based on its ability to be recognized by lymphocytes and by antibodies raised in guinea pigs immunized with living mycobacteria (17). This antigen has been previously shown to be highly antigenic in humans, in vitro (18, 19). Additionally, an Apa-encoding DNA vaccine (Apa DNA) induces strong Th1 and Th2 responses and is capable of activating significant protective responses in mice (20) and guinea pigs (18).
To further improve the effectiveness of subunit vaccines, adjuvants can be introduced in the vaccine formulation. 6,6′-Trehalose dimycolate (TDM) is present in M. tuberculosis cell walls and acts as a potent immunomodulator, preferentially inducing Th1 responses, thus making it an interesting adjuvant for TB vaccines (21). Combined with delivery systems such as microspheres, antigens (DNA, proteins, or peptides) can be encapsulated and protected from harsh conditions. The microparticles can be coencapsulated with or without immune stimulants and can be administered by different routes. Moreover, microspheres do not induce the toxicity that is generally associated with adjuvants (22, 23, 24). The choice of an appropriate adjuvant formulation and delivery system is crucial in obtaining optimal vaccine stability and effectiveness (25).
In this study, we investigated the immune protection against intratracheal M. tuberculosis challenge by priming with BCG and boosting with a single dose of Apa DNA vaccine, containing TDM adjuvant, coencapsulated in biodegradable poly(d,l-lactic-co-glycolic acid) (PGLA) microspheres, and compared the findings with those obtained with BCG-vaccinated mice.
MATERIALS AND METHODS
Animals.
Specific-pathogen-free female BALB/c mice 6 to 8 weeks of age were purchased from the breeding facility of Oswaldo Cruz Foundation (Fiocruz) and University of São Paulo (USP) at Ribeirão Preto School of Medicine, Brazil. The mice were maintained under barrier conditions in a biosafety level 3 (BSL3) facility, and they received free access to food and sterile water throughout the study period. The research protocol was reviewed and approved by and conducted according to the institutional ethical guidelines of Fiocruz (CEUA L-013/05) and USP (CETEA 05/2010).
Bacteria.
The H37Rv strain of M. tuberculosis (American Type Culture Collection, Manassas, VA) was grown in 7H9 Middlebrook broth (Difco Laboratories, Detroit, MI) at 37°C for 7 days and used as previously described (26, 27). The BCG Moreau vaccine was donated by Ataulpho de Paiva Foundation (Rio de Janeiro, Brazil) and was prepared according to its instructions. Briefly, lyophilized BCG Moreau vaccine was resuspended in vaccine diluent (0.9% saline) provided by the supplier, and this suspension (5 × 105 CFU) was used for vaccination of mice.
Plasmid construction.
An LB liquid medium (Gibco BRL, Gaithersburg, MD) containing ampicillin (100 μg/ml) was used to culture Escherichia coli DH5α that was transformed with either empty pVAX1 plasmid (Invitrogen, Carlsbad, CA) or plasmid containing apa (pVAXapa). The apa gene without its signal sequence was cloned by PCR from pAG831 (11) using the following primers: F 5′-TAG GAATCC ACC ATG GAT CCG GAG CCA GCG CC-3′ and R 5′-TAG CTCGAG TCA GGC CGG TAA GGT CCG-3′. Italic and underlined sequences indicate EcoRI and XhoI (Gibco BRL, Gaithersburg, MD) restriction sites, respectively. The amplified fragment was subcloned into pGEM-T Easy (Promega Corp., Madison, WI) and further cloned into pVAX1 using EcoRI and XhoI restriction sites. The consensus ACC Kozak sequence for vertebrate mRNAs was added before the ATG start codon. The DNA sequence was analyzed and confirmed with enzyme digestion and an ABI Prism DNA sequencer (PE Applied Biosystems, Foster City, CA). Plasmids were purified using the Endofree Plasmid Giga kit (Qiagen Inc., Chatsworth, CA). Furthermore, endotoxin levels were measured using the Limulus amebocyte lysate QCL-1000 kit (BioWhittaker, Walkersville, MD), and no endotoxin was detected.
Antigen expression in transfected mammalian cells.
In vitro expression of Apa protein was analyzed in BHK-21 (baby hamster kidney) cells by using Lipofectamine (Life technologies). Expression was characterized by Western blotting of total extracts of BHK-21 cells 1 day after transfection using standard procedures (28). Approximately 50 μg of protein extract was separated by SDS-PAGE and electrotransferred onto a nitrocellulose membrane. Rabbit polyclonal anti-Apa serum (donated by Gilles Marchal, Pasteur Institute) and horseradish peroxidase-conjugated goat anti-rabbit IgG (Sigma) were used, and detection was performed by using an ECL kit (Amersham-Pharmacia Biotech).
Preparation of microspheres and plasmid encapsulation.
Microspheres were obtained by the double emulsion/solvent evaporation technique. Briefly, 30 ml of dichloromethane solution containing 400 mg of polymer PLGA (50:50; Resomer from Boehringer Ingelheim, Ingelheim, Germany) and 0.5 mg of TDM (Sigma, St. Louis, MO) was emulsified with 0.3 ml of an inner aqueous phase containing 5 mg of DNA (pVAX or pVAXapa) using a T25 Ultra-Turrax homogenizer (IKA Labortechnik, Germany) to produce a primary water-in-oil emulsion. This emulsion was then mixed with 100 ml of an external aqueous phase containing 1 to 3% polyvinyl alcohol (Mowiol 40-88; Aldrich Chemicals, Wankee, WI) as a surfactant to form a stable water-in-oil emulsion. The mixture was stirred for 6 h with an RW20 IKA homogenizer to evaporate the solvent. Microspheres were collected and washed 3 times with sterile water; then they were freeze-dried and stored at 4°C until use. Plasmid encapsulation efficiency was determined as described by Diwan and Park (29, 30). Briefly, 10 mg of microspheres was dissolved in 1 ml of acetonitrile. After centrifugation, the precipitated plasmid was pelleted down and supernatant containing polymer was discarded. The pellet was redispersed in phosphate-buffered saline (PBS) (50 mM, pH 7.4) and the samples (in triplicate) were centrifuged again. The supernatant was preserved and the residue was further dissolved in 0.1 N NaOH. The plasmid in aqueous and alkaline extractions was estimated using a NanoDrop ND-1000 (Thermo).
Culture filtrate antigens.
The CF was kindly donated by Gilles Marchal of Institut Pasteur, Paris, France. It was prepared using an M. tuberculosis H37Rv 15-day culture, as previously described (15).
Immunization and M. tuberculosis infection.
BALB/c mice were separated into 5 experimental groups: (i) PBS (received saline, nonvaccinated and noninfected), (ii) BCG (received 1 dose of BCG), (iii) BCG-PVAX (received prime BCG-boost pVAX-TDM-Me), (iv) BCG-APA (received prime BCG-boost pVAXapa-TDM-Me), and (v) TB (nonvaccinated and infected with M. tuberculosis H37Rv). Immunizations were performed according to a heterologous schedule (see below). The PBS group received 50 μl of saline in each quadriceps subcutaneously (s.c.) and intramuscularly (i.m.) in 2 doses, and mice were not infected. The BCG group received a single vaccination with 5 × 105 BCG Moreau bacilli and 1 dose of saline, which were administered s.c. For heterologous prime-boost vaccination schedules, mice were primed with 5 × 105 BCG Moreau bacilli s.c. and boosted i.m. after 30 days with 100 μg of pVAX-TDM-Me (BCG-PVAX) or pVAXapa-TDM-Me (BCG-APA) formulation. For the TB control group, mice were injected with 50 μl of saline in each quadriceps s.c. and in 2 doses i.m. Thirty days after the last immunization, mice were anesthetized with 2,2,2-tribromoethanol (Acros) by intraperitoneal administration. The trachea was exposed and inoculated with 1 × 105 viable bacilli of the M. tuberculosis H37Rv strain (ATCC 27294). The incision was sutured with sterile silk. Thirty and 70 days after infection (corresponding to the initial and chronic phases of infection, respectively), mice were euthanized for evaluation of the protective immune response. The microsphere formulations consisted of 30 μg of plasmidial DNA (pVAXapa or pVAX) in TDM-loaded PLGA 50:50 microspheres. All procedures were performed in a BSL3 room facility in the University of São Paulo at the Ribeirão Preto School of Medicine.
CFU determination.
The right lower and middle lobes of lungs were digested using Liberase Blendzyme 2 (Roche, Indianapolis, IN) diluted (0 to 5 μg/ml) in incomplete RPMI 1640 medium (Sigma). Spleens were placed in a petri dish containing incomplete RPMI 1640 medium and then fragmented with the aid of anatomical sterile forceps. Serial dilutions of the digested lungs and macerated spleens were plated on 7H11 Middlebroock agar medium (Difco Laboratories, Detroit, MI) and used for the CFU assay as previously described (25, 26). Colonies were counted after 28 days of incubation at 37°C.
Histopathological analysis.
The right upper lobes of the lungs were fixed in 10% formalin, embedded in paraffin blocks, prepared routinely, and then sectioned for evaluation by light microscopy. For the histopathological analyses, 5-μm-thick sections were stained with hematoxylin and eosin.
The slides from each of the mouse groups were examined in a blind study by a pathologist, to establish the pattern and extent of the histological changes. A grade was established on the basis of the most severely inflamed section on each slide. After the slides were surveyed to identify the maximal response for each category, which was designated 4+ (intense/severe), additional slides representing intermediate/moderate, low, and very low responses were designated 3+, 2+, and 1+, respectively. The absence of response was designated by a minus sign (Table 1). The results shown in Table 1 are for one representative out of 3 independent experiments.
Table 1.
Qualitative histopathological analysisa
| Group and infection time (days) | Degree of parenchymal damage | Presence of granulomas | Infiltration of macrophages (other cells) | Infiltration of lymphocytes | Degree of inflammation |
|---|---|---|---|---|---|
| PBS | |||||
| 30 | − | − | − | − | − |
| 70 | − | − | − | − | − |
| TB | |||||
| 30 | +++ | +++ | +++ (foam cells +++) | ++++ | +++ |
| 70 | ++++ | ++++ | ++++ (foam cells ++++) | ++ | ++++ |
| BCG | |||||
| 30 | ++ | + | + (giant cells +; foam cells +) | ++ | ++ |
| 70 | ++/+++ | ++ | ++ (giant cells ++; foam cells ++) | ++ | ++ |
| BCG-PVAX | |||||
| 30 | ++ | + | ++ (foam cells ++) | ++ | ++ |
| 70 | ++ | ++ | + (giant cells +/foam cells +) | ++ | +/++ |
| BCG-APA | |||||
| 30 | + | + | + (foam cells +) | + | + |
| 70 | + | + | + (foam cells +) | + | + |
Mice (n = 5) were vaccinated with BCG, BCG-pVAX-TDM-Me, or BCG-pVAXapa-TDM-Me, as described in the legend to Fig. 3. Thirty days after the last immunization, we performed an intratracheal challenge with M. tuberculosis, and at 30 and 70 days after infection, the lungs were collected and processed for histopathological analysis. The symbols indicate the absence (−) or the very low (+), low (++), moderate (+++), or intense (++++) presence of each parameter evaluated. There were rare (+), some (++), many (+++), or numerous (++++) giant cells and foam cells. The results of one representative experiment out of three independent experiments are shown.
Statistical analysis.
Assessment of the experiments and statistical analysis were performed by using analysis of variance (ANOVA) and the Tukey test to determine the difference between the experiment group and the control groups. The data are expressed as means ± standard deviations (SDs), and P values of <0.05 were considered statistically significant. Analysis was performed using the SPSS 16.0 statistical software package (IBM Company, Chicago, IL).
RESULTS
Plasmid construction and expression of Apa antigen.
Apa DNA lacking the signal sequence was cloned by PCR from pAG831 (20). The amplified fragment was subcloned into pGEM-T Easy and further cloned into pVAX1 using EcoRI and XhoI sites and transformed into competent E. coli DH5α cells. The recombinant plasmid was identified by enzyme digestion on an agarose gel stained with ethidium bromide. Figure 1A shows the 900-bp fragment that corresponds to apa and the 3,054-bp fragment that corresponds to the pVAX plasmid (Fig. 1A, lane 3) and pVAX digested by XhoI (Fig. 1A, lane 2).
Fig 1.
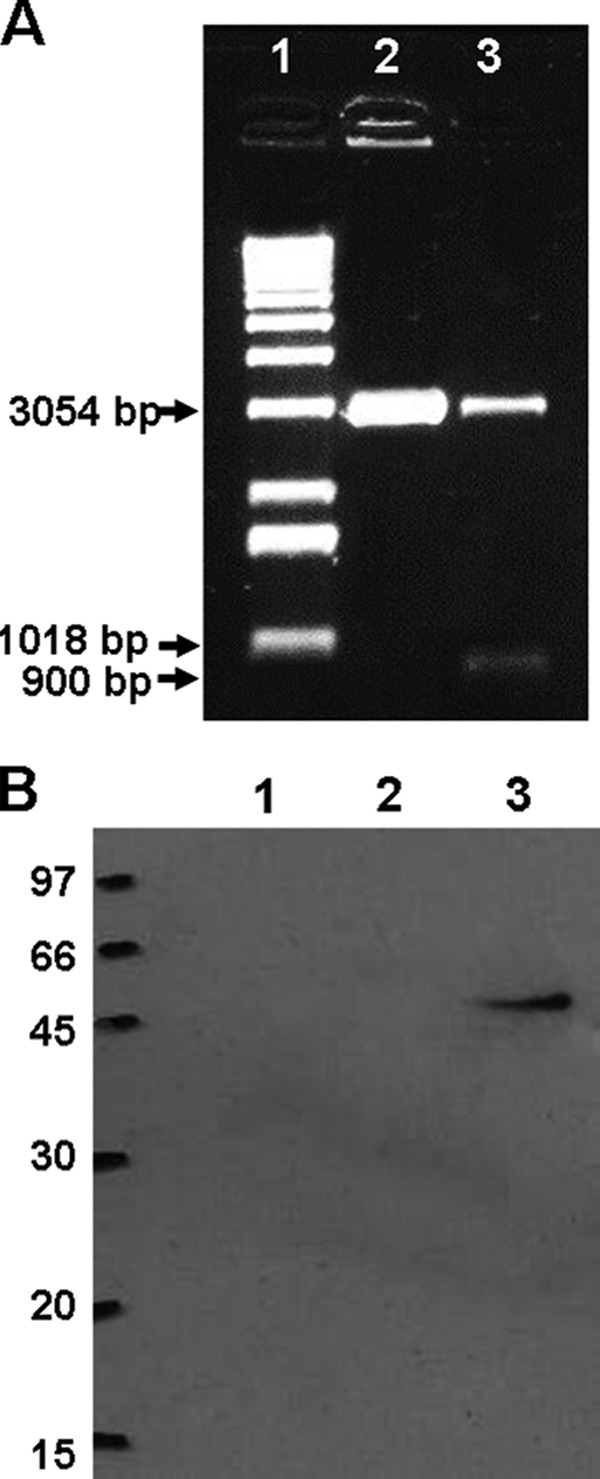
Plasmid construction and expression of Apa antigen. (A) Amplified fragments corresponding to kDNA of pVAXapa plasmid construction. The amplified products were separated by electrophoresis on a 2.5% agarose gel and stained with ethidium bromide. The image of the gel captured under UV light shows the 900-bp fragment corresponding to apa and the 3,054-bp fragment corresponding to the pVAX plasmid. Lane 1, molecular size standard (1 kb of DNA); lane 2: pVAX digested with XhoI; lane 3: pVAX-apa digested with XhoI and EcoRI. (B) Transient expression of Apa by pVAX-apa: Western blot analysis of cell lysates from BHK-21 cells transfected with pVAX-apa (lane 3) or pVAX (lane 2) or not transfected (lane 1). The size of the molecular mass standard is indicated, in kilodaltons. The primary antibody used was rabbit anti-Apa serum.
BHK-21 cells were transfected with pVAX-apa. Total protein extract was obtained from transfected cells, and the presence of Apa in the extract was confirmed by Western blotting using an Apa-specific polyclonal antibody (Fig. 1B). The expression of Apa was confirmed by the presence of a band of 47 kDa, the expected size of the Apa protein (Fig. 1B, lane 3). Total protein extracts from BHK-21 cells transfected with plasmid pVAX (Fig. 1B, lane 2) and from untransfected controls (Fig. 1B, lane 1) did not express Apa protein, as expected.
The heterologous prime (BCG)-boost (pVAXapa-TDM-Me) regimen protects mice against M. tuberculosis infection.
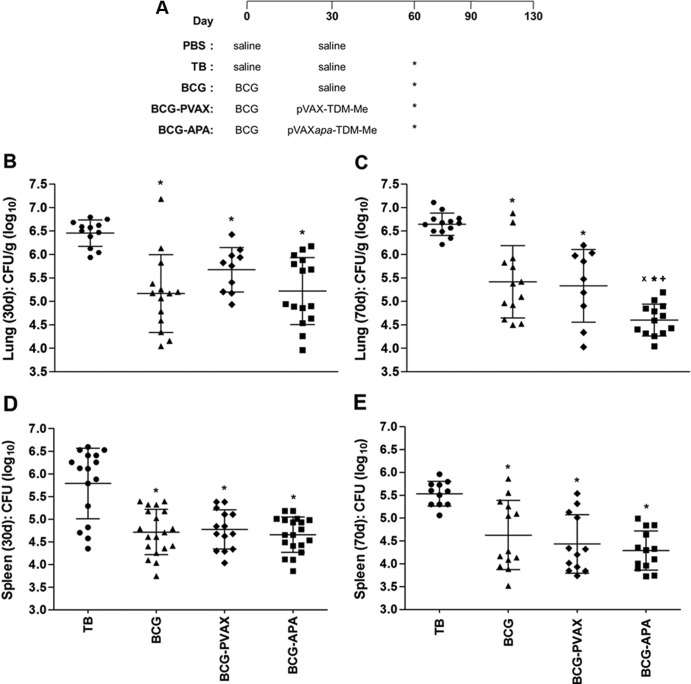
To improve BCG protection and evaluate a heterologous immunization schedule (Fig. 2A) using the pVAXapa-TDM-Me vaccine, mice were challenged with M. tuberculosis. At 30 (Fig. 2B) and 70 (Fig. 2C) days after infection, which correspond to the initial and chronic phases of infection, respectively, the BCG and prime-boost groups showed significant reduction in the bacterial loads in lungs compared with the TB group (30 days, P < 0.05; 70 days, P < 0.001).
Fig 2.
Protection against experimental TB conferred by the different vaccination regimens. (A) The time points for immunizations, infection, and sample collection are shown. Asterisks indicate the days on which mice were infected. BALB/c mice were immunized s.c. with a single dose of BCG (5 × 105 CFU; BCG group) or with one dose of BCG administered s.c. followed by one dose of pVAX-TDM-Me (BCG-PVAX) or pVAXapa-TDM-Me (BCG-APA) administered i.m. after 30 days. Thirty days after vaccination, mice were challenged intratracheally with a virulent strain of M. tuberculosis (1 × 105 CFU). At 30 or 70 days after infection, the lungs (B and C) and spleens (D and E) were processed to determine bacterial loads by counting the CFU. The bacterial load is expressed as log10 CFU/g of lung and spleen, derived from the means ± SDs of serial dilutions individually counted for each group. P values indicating statistically significant differences among the groups are displayed in the representative graphs. *, P < 0.05 versus nonimmunized infected mice (TB group) at 30 days postinfection. The following symbols indicate significance at 70 days postinfection: *, P < 0.001 versus the TB group; X, P < 0.05 versus the BCG-PVAX group; and +, P < 0.005 versus the BGG-vaccinated group. Results of one representative experiment out of three independent experiments are shown. (Three experiments were conducted with various numbers of animals in each group, with a total of 9 to 19 mice in the 3 experiments.)
Most importantly, 70 days postinfection, the strongest protective effect was seen in BCG-APA-vaccinated mice, with a significant inhibition of the growth of M. tuberculosis in the lungs compared with that in mice vaccinated with BCG alone (P < 0.005) or with BCG-pVAX (P < 0.05) (Fig. 2C). The BCG-APA group (4.6 logs) exhibited a reduction in the bacterial load which was nearly 1 order of magnitude lower than that of the BCG (5.4 logs) and BCG-PVAX (5.3 logs) groups, as well as an important reduction of approximately 2.0 logs compared with the TB group (6.6 logs).
Evaluation of the extrapulmonary protective immune response indicated that either heterologous immunization or BGC alone was effective in controlling the bacterial growth in the spleen. In both the early (Fig. 2D) and chronic (Fig. 2E) phases of experimental infection, all groups of immunized animals (BCG, BCG-PVAX, and BCG-APA) exhibited a reduction in bacterial growth of about 1 log compared to the TB infection control group (P < 0.005).
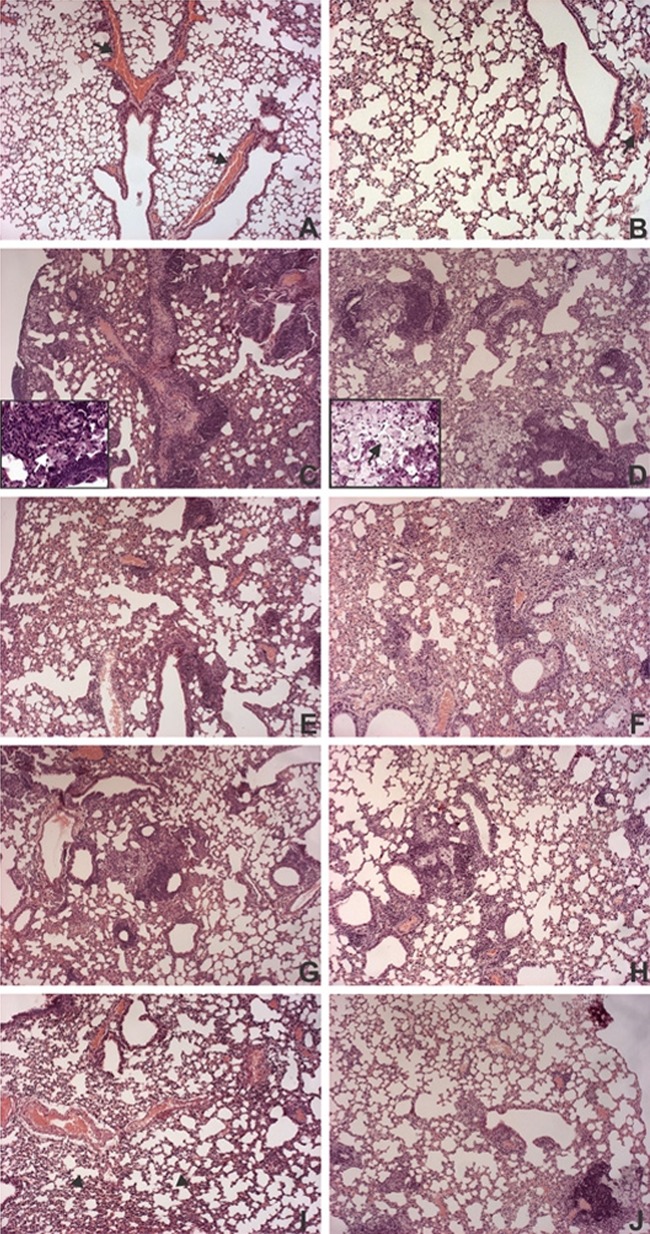
Histological evaluation of the lung specimens from the PBS control mice showed no pathological changes, with well-preserved lung parenchyma, clearly defined alveolar spaces, and no inflammatory cells in the tissue and normal lumen (Fig. 3A and B). In contrast, evaluation of the lung specimens from nonimmunized and infected mice (TB group) showed moderate impairment of the lung parenchyma and moderate inflammation with numerous granulomas 30 days after infection (Fig. 3C and Table 1). These granulomas were formed by many foam cells in their central regions. In addition, intense interstitial chronic inflammatory infiltrates were observed mostly in the peribronchiolar regions. This inflammatory infiltrate was accompanied by diffuse thickening of the alveolar walls and reductions in air space. By the later stage of infection, i.e., at 70 days, severe lung injury was diagnosed on the basis of the presence of intense inflammatory infiltrates, represented by multiple granulomas with many foam cells and some cholesterol clefts (Fig. 3D and Table 1). However, the interstitial inflammatory infiltrate was less intense than that observed 30 days postinfection in the same group.
Fig 3.
Histopathology of lungs from immunized mice at 30 and 70 days after M. tuberculosis infection. BALB/c mice (n = 5) were immunized s.c. with a single dose of BCG (5 × 105 CFU; BCG group) or one dose of BCG administered s.c., followed by one dose of pVAX-TDM-Me (BCG-PVAX) or pVAXapa-TDM-Me (BCG-APA) administered i.m. after 30 days. Thirty days after vaccination, the mice were challenged intratracheally with a virulent strain of M. tuberculosis (1 × 105 CFU). At 30 (A) or 70 (B) days after infection, the lungs were fixed in buffered formalin and stained with hematoxylin and eosin. Representative images of histological sections of lung lobes are shown for the PBS (A and B), TB (C and D), BCG (E and F), BCG-PVAX (G and H), and BCG-APA (I and J) groups. White and black arrows in the insets (TB group) indicate giant cells and xanthomatous macrophages, respectively. Arrows indicate congestion of blood vessels, and arrowheads indicate collapsed pulmonary alveoli. Original magnification, ×100. Magnification of the insets, ×400. The results shown are representative of 3 independent experiments.
The injury of lung tissue in mice immunized with BCG alone (Fig. 3E and F) and the BCG-PVAX group (Fig. 3G and H) was more severe than that seen in the BCG-APA group (Fig. 3I and J), in agreement with the bacterial loads measured at 30 and 70 days postinfection. In both groups (BCG and BCG-PVAX), the presence of peribronchial lymphocytes and few granulomas was observed at 30 days postinfection, with the notable formation of lymphoid aggregates and an increased number of granulomas at 70 days postinfection. However, the lung parenchyma of the BCG-PVAX group had more preserved areas, as well as lower inflammation than BCG-immunized mice. In the group administered BCG alone, giant and foam cells were rarely observed during early infection; however, some foam and giant cells were found in the later stage of infection. Moreover, in the BCG-PVAX group, at 30 days postinfection, few foam cells were found but fewer giant and foam cells were noted at 70 days postinfection compared to the BCG group. When animals were immunized with the Apa DNA formulation in the heterologous immunization regimen (BCG-APA), better preservation of lung parenchyma was observed than that in the other immunized groups following M. tuberculosis infection. In the lung tissue of this group, few granulomas were observed, foam cells were rare (Fig. 3J), and giant cells were absent. Furthermore, the majority of specimens from these immunized mice exhibited few lymphocytic inflammatory infiltrates in the alveolar walls at 30 and at 70 days after infection (Fig. 3I and J and Table 1) mainly adjacent to the rare granulomas observed at 70 days postinfection (Fig. 3J). These findings indicate that mice treated using the BCG-APA strategy showed best preservation of lung parenchyma among all the immunized groups, and especially compared to the TB group, in the chronic phase of infection; the results of the BCG-APA group were similar to those of the PBS group. In addition, these findings corroborate the significant reduction in the number of bacteria detected in the lung tissue of these animals. Images of the lung lobes of the different groups presented in Fig. 4 show a similar pattern and are consistent with the above-mentioned data.
Fig 4.
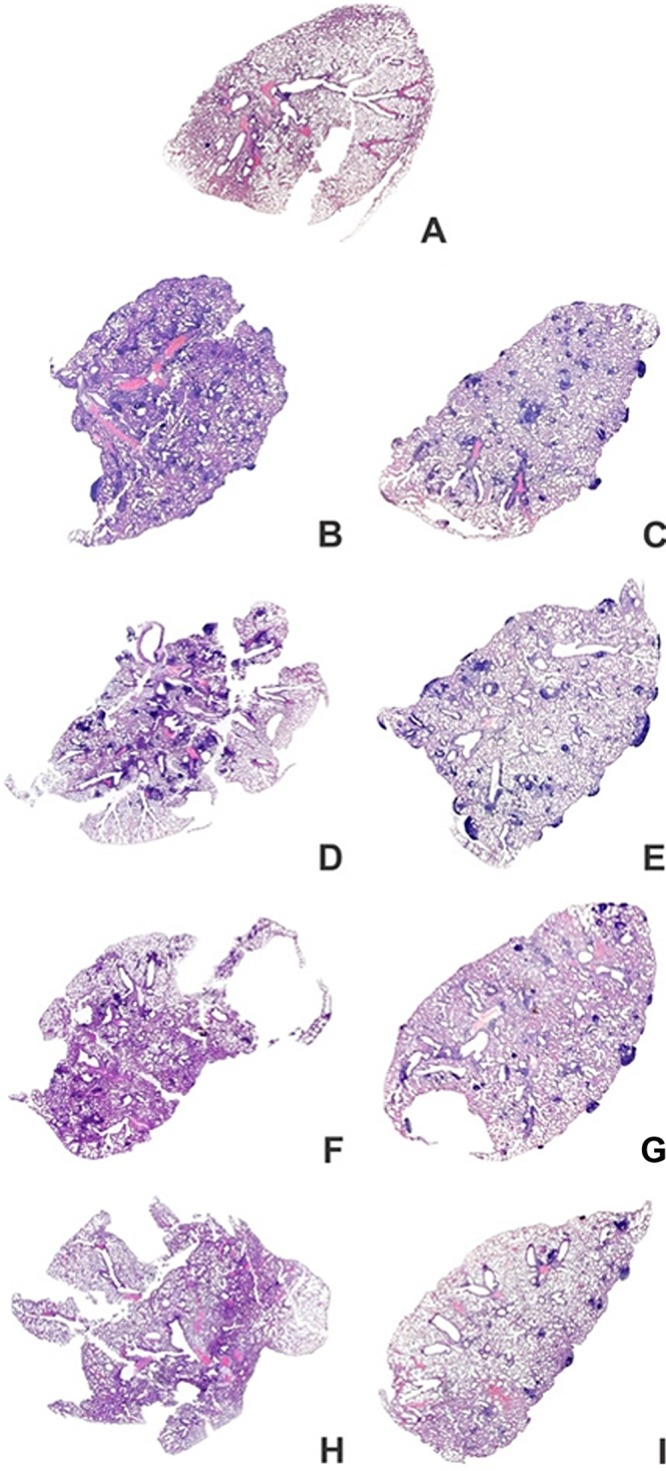
Overview perspective of the whole lungs of mice at 30 and 70 days after M. tuberculosis infection. BALB/c mice were immunized as described in the legend to Fig. 3. The lungs were fixed in buffered formalin and stained with hematoxylin and eosin. Representative images of lung lobes are shown for the PBS (A), TB (B and C), BCG (D and E), BCG-PVAX (F and G), and BCG-APA (H and I) groups. The results shown are representative of 3 independent experiments.
DISCUSSION
In this study, we showed that heterologous prime-boost vaccination with prime BCG administered s.c. followed by pVAXapa-TDM-Me boost immunization (BCG-APA) administered i.m. provided significantly greater protection than that conferred by a single s.c. dose of BCG in controlling bacterial loads, leading to better preservation of the lung parenchyma, after 70 days of infection.
Many studies have shown that using BCG as a prime combined with a novel boost vaccine is more efficacious than BCG alone (31). However, it is also known that revaccination with BCG in humans does not confer additional protection and, in some cases, may be deleterious to protection against TB (32, 33). Therefore, the development of a new vaccine capable of promoting immunity in individuals who have already received the BCG vaccine would be very beneficial (6).
As reported previously, the order of prime-boost vaccination may not be critical for enhancing protection against experimental TB (34). Priming but not boosting with a DNA vaccine could also increase the survival time of BCG-vaccinated mice against challenge with M. tuberculosis (35, 36). This vaccination strategy is often assessed by CFU counting and pathology score, but long-term survival is not always evaluated. Therefore, there is still no consensus in the order of prime-boost vaccination, and the improvement of the BCG vaccine merits further investigation.
Previous studies have shown that plasmids with apa-induced humoral and cell-mediated immunity provided significant protection against TB in mice and guinea pigs (18, 20). Subsequently, immunization with 3 doses of apa was shown to be highly immunogenic in a murine model (36). Recently, among the nine M. tuberculosis recombinant proteins evaluated, the Apa antigen was found to be highly antigenic following BCG vaccination (37). Our data on heterologous prime-boost immunization also confirmed this immunogenicity induced by Apa, after an intratracheal M. tuberculosis infection, and showed that this immunization lasts longer than the one conferred by BCG vaccination alone.
The importance of the preservation of lung morphology along with bacterial clearance has been described previously (38, 39). Although some TB vaccines show highly prospective clearance of the bacillus, they may induce an exacerbated inflammatory response that culminates with lung necrosis (39). On the other hand, it was also reported that some other vaccines do not cause this damage but are ineffective in reducing the bacterial load to levels comparable with that induced by BCG (40). In addition, our group showed that consistent cellular and humoral responses generated by vaccination do not prevent the development of pulmonary injury in immunized mice (27). In contrast to those results, our results in this study showed that the most significant reduction in bacterial load of the lungs was associated with an important reduction of tissue injury in mice vaccinated using the BCG-APA strategy, 30 and 70 days after infection, compared with controls and BCG-vaccinated mice alone.
The advantageous properties of the microsphere-based delivery system likely account for the superior protection obtained in this study compared to that seen in previous studies.
Most importantly, significant protection was accomplished by a boosting immunization with only one dose of pVAXapa-TDM-Me. Indeed, delivery systems such as antigen-encapsulated microspheres enhance the binding, uptake, and half-life of antigens (41), leading to a reduction in the number of doses in the immunization schedule (23). The slow degradation of such a delivery system allows sustained delivery of the antigen. All these data could explain the high level of protection conferred by this formulation compared to the other formulations that used only naked apa DNA (18, 34). Moreover, biodegradable PLGA microspheres have a proven safety record and an established utility in marketed products for controlled delivery of several peptide drugs (42). In this context, the combination of microspheres and the TDM adjuvant, which has been described as a potent immunomodulator (22), is an interesting and potentially beneficial approach to improve vaccine formulations and provide long-lasting protection against TB (23, 43). However, the mechanism of enhanced protection conferred by pVAXapa-TDM-Me, as well as the mechanism of protection conferred by BCG itself, remains to be determined.
In conclusion, the improved protection conferred by the combination of BCG priming followed by a pVAXapa-TDM-Me boosting immunization (BCG-APA) provides evidence for the hypothesis that the efficacy of BCG vaccination can be improved by subsequent boosting with a DNA vaccine. Further investigation on this topic is warranted.
ACKNOWLEDGMENTS
We thank Eliane N. Miyage, Butantan Institute, São Paulo, Brazil, for technical assistance in the gene expression studies. We also thank Elizabeth Albuquerque, Fiocruz, for helpful discussions on statistical analysis. We are grateful to Marcos de Almeida and Francisco Carvalho from the Pathology Service, IPEC-Fiocruz, for their technical assistance in histological sections.
This study was supported by grants from Fiocruz, Rio de Janeiro, Brazil (C.H.), and USP, Ribeirão Preto, São Paulo, Brazil (V.L.D.B.).
Footnotes
Published ahead of print 5 June 2013
REFERENCES
- 1.World Health Organization 2011. Global tuberculosis control: a short update to the 2011 report. World Health Organization, Geneva, Switzerland [Google Scholar]
- 2.World Health Organization 2009. Global tuberculosis control. Epidemiology, strategy, financing. World Health Organziation, Geneva, Switzerland [Google Scholar]
- 3.Soysal A, Millington KA, Bakir M, Dosanjh D, Aslan Y, Deeks JJ, Efe S, Staveley I, Ewer K, Lalvani A. 2005. Effect of BCG vaccination on risk of Mycobacterium tuberculosis infection in children with household tuberculosis contact: a prospective community-based study. Lancet 366:1443–1451 [DOI] [PubMed] [Google Scholar]
- 4.Sadoff JC. 2005. Vaccine risk and benefit in the developing world. Health Aff. (Millwood) 24:1379–1380 [DOI] [PubMed] [Google Scholar]
- 5.Trunz BB, Fine P, Dye C. 2006. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet 367:1173–1180 [DOI] [PubMed] [Google Scholar]
- 6.Kaufmann SH. 2005. Recent findings in immunology give tuberculosis vaccines a new boost. Trends Immunol. 26:660–667 [DOI] [PubMed] [Google Scholar]
- 7.Romano M, Huygen K. 2009. DNA vaccines against mycobacterial diseases. Expert Rev. Vaccines 8:1237–1250 [DOI] [PubMed] [Google Scholar]
- 8.Hubbard RD, Flory CM, Collins FM, Cocito C. 1992. Immunization of mice with the antigen A60 of Mycobacterium bovis BCG. Clin. Exp. Immunol. 88:129–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersen P. 1994. Effective vaccination of mice against Mycobacterium tuberculosis infection with a soluble mixture of secreted mycobacterial proteins. Infect. Immun. 62:2536–2544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts AD, Sonnenberg MG, Ordway DJ, Furney SK, Brennan PJ, Belisle JT, Orme IM. 1995. Characteristics of protective immunity engendered by vaccination of mice with purified culture filtrate protein antigens of Mycobacterium tuberculosis. Immunology 85:502–508 [PMC free article] [PubMed] [Google Scholar]
- 11.Pal PG, Horwitz MA. 1992. Immunization with extracellular proteins of Mycobacterium tuberculosis induces cell-mediated immune responses and substantial protective immunity in a guinea pig model of pulmonary tuberculosis. Infect. Immun. 60:4781–4792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horwitz MA, Lee BW, Dillon BJ, Harth G. 1995. Protective immunity against tuberculosis induced by vaccination with major extracellular proteins of Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 92:1530–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verreck FA, Vervenne RA, Kondova I, van Kralingen KW, Remarque EJ, Braskamp G, van der Werff NM, Kersbergen A, Ottenhoff TH, Heidt PJ, Gilbert SC, Gicquel B, Hill AV, Martin C, McShane H, Thomas AW. 2009. MVA85A boosting of BCG and an attenuated, phoP deficient M. tuberculosis vaccine both show protective efficacy against tuberculosis in rhesus macaques. PLoS One 4:e5264. 10.1371/journal.pone.0005264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laqueyrerie A, Militzer P, Romain F, Eiglmeier K, Cole S, Marchal G. 1995. Cloning, sequencing, and expression of the apa gene coding for the Mycobacterium tuberculosis 45/47-kilodalton secreted antigen complex. Infect. Immun. 63:4003–4010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horn C, Namane A, Pescher P, Riviere M, Romain F, Puzo G, Barzu O, Marchal G. 1999. Decreased capacity of recombinant 45/47-kDa molecules (Apa) of Mycobacterium tuberculosis to stimulate T lymphocyte responses related to changes in their mannosylation pattern. J. Biol. Chem. 274:32023–32030 [DOI] [PubMed] [Google Scholar]
- 16.Ragas A, Roussel L, Puzo G, Riviere M. 2007. The Mycobacterium tuberculosis cell-surface glycoprotein apa as a potential adhesin to colonize target cells via the innate immune system pulmonary C-type lectin surfactant protein A. J. Biol. Chem. 282:5133–5142 [DOI] [PubMed] [Google Scholar]
- 17.Romain F, Laqueyrerie A, Militzer P, Pescher P, Chavarot P, Lagranderie M, Auregan G, Gheorghiu M, Marchal G. 1993. Identification of a Mycobacterium bovis BCG 45/47-kilodalton antigen complex, an immunodominant target for antibody response after immunization with living bacteria. Infect. Immun. 61:742–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar P, Amara RR, Challu VK, Chadda VK, Satchidanandam V. 2003. The Apa protein of Mycobacterium tuberculosis stimulates gamma interferon-secreting CD4+ and CD8+ T cells from purified protein derivative-positive individuals and affords protection in a guinea pig model. Infect. Immun. 71:1929–1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diagbouga S, Fumoux F, Zoubga A, Sanou PT, Marchal G. 1997. Immunoblot analysis for serodiagnosis of tuberculosis using a 45/47-kilodalton antigen complex of Mycobacterium tuberculosis. Clin. Diagn. Lab. Immunol. 4:334–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garapin A, Ma L, Pescher P, Lagranderie M, Marchal G. 2001. Mixed immune response induced in rodents by two naked DNA genes coding for mycobacterial glycosylated proteins. Vaccine 19:2830–2841 [DOI] [PubMed] [Google Scholar]
- 21.Lima VM, Bonato VL, Lima KM, Dos Santos SA, Dos Santos RR, Goncalves ED, Faccioli LH, Brandao IT, Rodrigues-Junior JM, Silva CL. 2001. Role of trehalose dimycolate in recruitment of cells and modulation of production of cytokines and NO in tuberculosis. Infect. Immun. 69:5305–5312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang D, Robinson DR, Kwon GS, Samuel J. 1999. Encapsulation of plasmid DNA in biodegradable poly(D, L-lactic-co-glycolic acid) microspheres as a novel approach for immunogene delivery. J. Control. Release 57:9–18 [DOI] [PubMed] [Google Scholar]
- 23.Lima KM, Santos SA, Lima VM, Coelho-Castelo AA, Rodrigues JM, Jr, Silva CL. 2003. Single dose of a vaccine based on DNA encoding mycobacterial hsp65 protein plus TDM-loaded PLGA microspheres protects mice against a virulent strain of Mycobacterium tuberculosis. Gene Ther. 10:678–685 [DOI] [PubMed] [Google Scholar]
- 24.Griffiths G, Nystrom B, Sable SB, Khuller GK. 2010. Nanobead-based interventions for the treatment and prevention of tuberculosis. Nat. Rev. Microbiol. 8:827–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pérez O, Harandi AM. 2008. 4th International Workshop in Vaccine Adjuvants and Parasitic Vaccines (adjuvant 2008). Expert Rev. Vaccines 7:1151–1153 [DOI] [PubMed] [Google Scholar]
- 26.Bonato VL, Goncalves ED, Soares EG, Santos Junior RR, Sartori A, Coelho-Castelo AA, Silva CL. 2004. Immune regulatory effect of pHSP65 DNA therapy in pulmonary tuberculosis: activation of CD8+ cells, interferon-gamma recovery and reduction of lung injury. Immunology 113:130–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fonseca DM, Silva CL, Paula MO, Soares EG, Marchal G, Horn C, Bonato VL. 2007. Increased levels of interferon-gamma primed by culture filtrate proteins antigen and CpG-ODN immunization do not confer significant protection against Mycobacterium tuberculosis infection. Immunology 121:508–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 29.Diwan M, Park TG. 2001. Pegylation enhances protein stability during encapsulation in PLGA microspheres. J. Control. Release 73:233–244 [DOI] [PubMed] [Google Scholar]
- 30.Diwan M, Park TG. 2003. Stabilization of recombinant interferon-alpha by pegylation for encapsulation in PLGA microspheres. Int. J. Pharm. 252:111–122 [DOI] [PubMed] [Google Scholar]
- 31.Brennan MJ, Clagett B, Fitzgerald H, Chen V, Williams A, Izzo AA, Barker LF. 2012. Preclinical evidence for implementing a prime-boost vaccine strategy for tuberculosis. Vaccine 30:2811–2823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodrigues LC, Pereira SM, Cunha SS, Genser B, Ichihara MY, de Brito SC, Hijjar MA, Dourado I, Cruz AA, Sant'Anna C, Bierrenbach AL, Barreto ML. 2005. Effect of BCG revaccination on incidence of tuberculosis in school-aged children in Brazil: the BCG-REVAC cluster-randomised trial. Lancet 366:1290–1295 [DOI] [PubMed] [Google Scholar]
- 33.Dantas OM, Ximenes RA, de Albuquerque Mde F, da Silva NL, Montarroyos UR, de Souza WV, Pereira TC, Campelo AR, Rodrigues LC. 2006. A case-control study of protection against tuberculosis by BCG revaccination in Recife, Brazil. Int. J. Tuberc. Lung Dis. 10:536–541 [PubMed] [Google Scholar]
- 34.Skinner MA, Wedlock DN, de Lisle GW, Cooke MM, Tascon RE, Ferraz JC, Lowrie DB, Vordermeier HM, Hewinson RG, Buddle BM. 2005. The order of prime boost vaccination of neonatal calves with Mycobacterium bovis BCG and a DNA vaccine encoding mycobacterial proteins hsp65, hsp70, and Apa is not critical for enhancing protection against bovine tuberculosis. Infect. Immun. 73:4441–4444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romano M, D'Souza S, Adnet PY, Laali R, Jurion F, Palfliet K, Huygen K. 2006. Priming but not boosting with plasmid DNA encoding mycolyl-transferase Ag85A from Mycobacterium tuberculosis increases the survival time of Mycobacterium bovis BCG vaccinated mice against low dose intravenous challenge with M. tuberculosis H37Rv. Vaccine 24:e3353–e3364 [DOI] [PubMed] [Google Scholar]
- 36.Ferraz JC, Stavropoulos E, Yang M, Coade S, Espitia C, Lowrie DB, Colston MJ, Tascon RE. 2004. A heterologous DNA priming-Mycobacterium bovis BCG boosting immunization strategy using mycobacterial Hsp70, Hsp65, and Apa antigens improves protection against tuberculosis in mice. Infect. Immun. 72:6945–6950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sable SB, Cheruvu M, Nandakumar S, Sharma S, Bandyopadhyay K, Kellar KL, Posey JE, Plikaytis BB, Amara RR, Shinnick TM. 2011. Cellular immune responses to nine Mycobacterium tuberculosis vaccine candidates following intranasal vaccination. PLoS One 6(7):e22718. 10.1371/journal.pone.0022718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor JL, Turner OC, Basaraba RJ, Belisle JT, Huygen K, Orme IM. 2003. Pulmonary necrosis resulting from DNA vaccination against tuberculosis. Infect. Immun. 71:2192–2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor JL, Ordway DJ, Troudt J, Gonzalez-Juarrero M, Basaraba RJ, Orme IM. 2005. Factors associated with severe granulomatous pneumonia in Mycobacterium tuberculosis-infected mice vaccinated therapeutically with hsp65 DNA. Infect. Immun. 73:5189–5193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu J, Wang C, Zhou Z, Zhang Y, Cao T, Shi C, Chen Z, Chen L, Cai C, Fan X. 2011. Immunogenicity and protective efficacy against murine tuberculosis of a prime-boost regimen with BCG and a DNA vaccine expressing ESAT-6 and Ag85A fusion protein. Clin. Dev. Immunol. 2011:617892. 10.1155/2011/617892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moreno-Mendieta SA, Rocha-Zavaleta L, Rodriguez-Sanoja R. 2010. Adjuvants in tuberculosis vaccine development. FEMS Immunol. Med. Microbiol. 58:75–84 [DOI] [PubMed] [Google Scholar]
- 42.Jiang W, Gupta RK, Deshpande MC, Schwendeman SP. 2005. Biodegradable poly(lactic-co-glycolic acid) microparticles for injectable delivery of vaccine antigens. Adv. Drug Deliv. Rev. 57:391–410 [DOI] [PubMed] [Google Scholar]
- 43.de Paula L, Silva CL, Carlos D, Matias-Peres C, Sorgi CA, Soares EG, Souza PR, Blades CR, Galleti FC, Bonato VL, Goncalves ED, Silva EV, Faccioli LH. 2007. Comparison of different delivery systems of DNA vaccination for the induction of protection against tuberculosis in mice and guinea pigs. Genet. Vaccines Ther. 5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]