Abstract
Immunization and nutritional interventions are mainstays of child health programs in sub-Saharan Africa, yet few published data exist on their interactions. HIV-exposed (but uninfected) infants enrolled in a randomized placebo-controlled trial of multivitamin supplements (vitamins B complex, C, and E) conducted in Tanzania were sampled for an assessment of measles IgG quantity and avidity at 15 to 18 months. Infants were vaccinated between 8.5 and 12 months of age, and all mothers received high-dose multivitamins as the standard of care. Of 201 HIV-exposed infants who were enrolled, 138 (68.7%) were seropositive for measles. There were no effects of infant multivitamin supplementation on measles seroconversion proportions, IgG concentrations, or IgG avidity (P > 0.05). The measles seroconversion proportion was greater for HIV-exposed infants vaccinated at 10 to 11 months of age than for those vaccinated at 8.5 to 10 months (P = 0.032) and greater for infants whose mothers had a CD4 T-cell count of <200 cells/μl than for infants whose mothers had a CD4 T-cell count of >350 cells/μl (P = 0.039). Stunted infants had a significantly decreased IgG quantity compared to nonstunted infants (P = 0.012). As for measles avidity, HIV-exposed infants vaccinated at 10 to 11 months had increased antibody avidity compared to those vaccinated at 8.5 to 10 months (P = 0.031). Maternal CD4 T-cell counts of <200 cells/μl were associated with decreased avidity compared to counts of >350 cells/μl (P = 0.047), as were lower infant height-for-age z-scores (P = 0.016). Supplementation with multivitamins containing B complex, C, and E does not appear to improve measles vaccine responses for HIV-exposed infants. Studies are needed to better characterize the impact of maternal HIV disease severity on the immune system development of HIV-exposed infants and the effect of malnutrition interventions on vaccine responses. (This study has been registered at ClinicalTrials.gov under registration no. NCT00197730.)
INTRODUCTION
HIV-infected infants are well documented to have reduced seroconversion rates and more rapid declines in antibody levels following routine childhood vaccinations than infants who are not exposed to HIV (1). Comparatively few studies have also suggested that HIV-exposed (but uninfected) infants may have impaired immune responses following vaccination (2, 3). HIV proteins from an infected mother can cross the placenta and induce a state of persistent immune activation in the fetus, which may impair immune system development (4). Maternal receipt of antiretrovirals can also alter the placental barrier and change cytokine expression in the fetus (5). Further study of vaccine responses in HIV-exposed (uninfected) infants is needed, since the number of these children worldwide is increasing due to the success of programs that prevent mother-to-child transmission (6).
Immunization and nutritional interventions are the foundation for most child health programs worldwide, yet limited data are available on the interaction between vaccine responses and nutrition (7). Micronutrients are known to have a wide range of effects on immune responses (8, 9). The effect of vitamin A on measles vaccine responses has been studied in multiple clinical trials, but the results are unclear (10). Vitamin A may improve measles vaccine responses among boys when administered with the vaccine at 9 months of age but may worsen responses when administered at 6 months of age (10–12). Randomized controlled trials of vitamin E supplementation have found an improved innate immune activity, lymphocyte proliferation, and tetanus vaccine response among adults and elderly populations (9). Only one randomized trial of vitamin E supplementation and vaccine responses has been conducted in infants, and it reported no effect of supplementation on IgG titers following tetanus vaccination (13). To our knowledge, no trials have assessed the effect of vitamin B complex, C, or E supplementation on measles vaccine or other live attenuated vaccine responses in infants.
We hypothesized that multivitamins containing vitamins B complex, C, and E provided to HIV-exposed infants would increase measles IgG quantity and avidity compared to a placebo. We included HIV-infected infants in the trial as a secondary comparison group to determine the effectiveness of measles vaccination in this population. We also examined correlates of the measles vaccine response, including infant HIV infection, age at vaccination, breastfeeding duration, nutritional status, severity of maternal HIV disease, and maternal receipt of highly active antiretroviral therapy (HAART).
MATERIALS AND METHODS
Parent trial design.
This study consists of infants who were enrolled in a randomized double-blind placebo-controlled trial of multivitamin supplementation conducted in Dar es Salaam, Tanzania (ClinicalTrials.gov registration no. NCT00197730) (14). Briefly, the trial enrolled infants between 5 to 7 weeks of age who were born to HIV-infected mothers. Infants were excluded from the trial if they were of multiple gestation or had a serious congenital anomaly or other conditions that would affect study procedures, including an inability to take a daily micronutrient supplement. Infants were randomized to receive one capsule of either placebo or multivitamin from 6 weeks to 6 months of age, the latter of which contained vitamin C (60 mg), vitamin E (8 mg), thiamine (0.5 mg), riboflavin (0.6 mg), niacin (4 mg), vitamin B6 (0.6 mg), folate (130 μg), and vitamin B12 (1 mg). From 7 months of age to the end of follow-up at 24 months postrandomization, two capsules of the multivitamin or placebo were given daily. The multivitamin doses were selected to be between 150% to 600% and 200% to 400% of the U.S. adequate intake for children aged 0 to 6 months and 7 to 12 months, respectively (15, 16). The tolerable upper-intake levels of these nutrients have not been defined for infants <1 year of age, and the tolerable upper-intake levels were not exceeded for infants aged ≥1 year (15, 16). The placebo and multivitamin capsules contained an orange powder that made the preparations identical in taste and appearance. Mothers were instructed on how to open the capsules and decant the powder into a small plastic cup containing sterile water (5 ml) that was supplied with the capsules. The dose was then given to the child orally. Compliance was measured by research nurses who counted the number of unused capsules that were returned at each monthly clinic visit. The median regimen compliance, expressed as the percentage of monthly doses missing at the subsequent visit, was 96% (25th percentile, 91%; 75th percentile, 99%). All mothers in this trial received oral multivitamins during pregnancy and postpartum period as part of the HIV treatment standard of care in Tanzania, which is based on our previous findings in this population (17). Maternal multivitamin doses were mostly several times the adult recommended dietary allowance (RDA) for vitamins B complex, C, and E, but mothers who were initiated on HAART were switched to a single-RDA multivitamin supplement.
Infants were followed at monthly clinic visits starting at 6 weeks of age for a total of 24 months. At each clinic visit, the study physicians performed a clinical examination. Infants were tested for HIV infection at 6 weeks of age by PCR using the Amplicor HIV-1 DNA assay version 1.5 (Roche Molecular Systems, Inc., Branchburg, NJ) and at 18 months of age using HIV enzyme-linked immunosorbent assays (ELISAs) and the rapid Enzygnost anti-HIV-1/2 plus (Dade Behring, Marburg, Germany); discordant results were resolved by Western blotting. During the trial, the availability of HAART significantly increased for infants and adults per Tanzanian national guidelines. Starting in July 2005, children aged <18 months with a CD4 percentage of <20% or pediatric WHO HIV disease stage III and children aged ≥18 months with pediatric WHO HIV disease stage III or CD4 percentage of <15% were eligible for initiation of HAART. Adults were eligible if they had WHO stage IV HIV disease, a CD4 cell count of <200 cells/μl, or WHO stage III and a CD4 cell count of <350 cells/μl. The standard first-line regimen was stavudine (d4T), lamivudine (3TC), and nevirapine (NVP) for adults, and zidovudine (AZT), 3TC, and NVP for children.
At each clinic visit, trained research nurses took anthropometric measurements. Each infant was weighed on a digital infant balance with 10-gram precision (Tanita, Japan), and height was measured with 1-mm precision using a rigid length board with a movable foot piece. Weight-for-height, weight-for-age, and height-for-age z-scores were calculated using WHO child growth standards (18). Wasting was defined as a weight-for-height z-score of 2 or more standard deviations below the WHO reference population mean, stunting was defined as a height-for-age z-score of 2 or more standard deviations below the mean, and underweight was defined as a weight-for-age z-score of 2 or more standard deviations below the mean. At clinic visits, nurses also asked mothers about their breastfeeding and complementary feeding methods during the previous month.
All infants in the study received routine childhood immunizations and medical care. An Edmonston-Zagreb measles vaccine was administered at the clinic visit that was closest to 9 months of age, per Tanzanian national guidelines. A routine second opportunity for measles vaccination is not included in national guidelines, and there were no measles vaccination guidelines specific to HIV-infected infants. No infants included in this study were documented to have received a second dose of the measles vaccine before 18 months. All infants received high-dose vitamin A supplements of 100,000 IU at 9 months of age and 200,000 IU at 15 and 21 months of age. The trial protocol was approved by the institutional review boards at Muhimbili University of Health and Allied Sciences and the Harvard School of Public Health.
Study population.
The primary study population consisted of HIV-exposed and uninfected (referred to as HIV-exposed) infants enrolled in the parent trial. We randomly selected 225 HIV-exposed infants who were not infected with HIV at 6 weeks of age, remained uninfected at 18 months of age, and were documented to have received the measles vaccine between 8.5 and 12 months of age. Infants who became infected with HIV between 6 weeks and 18 months were excluded due to an unclear date of seroconversion. Of these 225 selected infants, 201 (89.3%) were alive, enrolled in the study, and had a blood plasma sample available for measles testing from a clinic visit that occurred between 15 and 18 months of age. Among the 24 selected HIV-exposed infants without outcome data, there were 3 deaths (1.3%), 6 (2.7%) lost to follow-up, and 15 (6.7%) infants who did not have a blood plasma sample available. We selected 225 HIV-exposed infants in order to obtain measles response data on 200 infants based on the ability to detect a relative risk (RR) of seroconversion of 1.25 with multivitamin supplements, assuming a 70% seroconversion rate in the placebo group.
We also included HIV-infected children as a secondary comparison group. We selected all infants who were HIV infected at 6 weeks of age and were documented to have received a measles vaccine at between 8.5 and 12 months of age (n = 48). Of these selected infants, 35 (89.7%) were alive, enrolled in the study, and had a blood plasma sample available for measles testing from a clinic visit that occurred between 15 and 18 months of age. Among the 13 infants without outcome data, there were 8 (16.7%) deaths, 1 (2.1%) infant was lost to follow-up, and 4 (8.3%) infants did not have a blood plasma sample available. We recognize that the study had limited statistical power to detect any models effect of the multivitamin supplements among HIV-infected infants. We had 80% power to detect a relative risk of 2.0 with multivitamins for measles seroconversion. As a result, our treatment estimates should be considered exploratory for HIV-infected infants.
Measles IgG concentration.
Plasma samples collected between 15 and 18 months of age were stored at −70°C. If an infant had more than one sample available for testing, the sample from the oldest age within the 15- to 18-month time window was selected. We utilized a commercially available ELISA for measles IgG quantification (IBL, Hamburg, Germany). The assay includes internal standards for the quantitative evaluation of measles IgG concentrations that have been adjusted to a WHO international standard (3rd International Standard for Anti-Measles, National Institute for Biological Standards and Control [NIBSC] code 97/648). All samples were plated in duplicate. The results of the assay are expressed as mIU/ml, and measles IgG seropositivity was defined as an IgG titer of ≥200 mIU/ml (19).
Measles IgG avidity.
Avidity maturation is a product of somatic hypermutation, a mutation process of B lymphocytes affecting the variable regions of immunoglobulins, and is the programmed selection of antigen-specific B cells in the lymphoid tissues based on the strength of bonding (20). Measles IgG avidity was assessed in tandem with IgG quantity using an ammonium thiocyanate (NH4SCN) elution assay (21). Briefly, 96-well plates were coated with 1 μg of Vero cells infected with the Edmonston strain of the measles virus (Advanced Biotechnologies), diluted in NaHCO3, and incubated overnight at 4°C. The next day, the plates were washed with phosphate-buffered saline (PBS) containing 0.05% Tween 20 and were incubated in blocking buffer (2% skim milk in PBS) for 2 h at 37°C. Serum samples were diluted 1:100 in blocking buffer, plated in duplicate, and incubated for 1 h at 37°C. All serum samples were plated in duplicate for all dilutions. Plates were then washed and incubated with 50 μl of 0- to 4.0-mol/liter NH4SCN in 0.5-mol/liter increments at room temperature for 15 min. Plates were washed and incubated with alkaline phosphatase-conjugated rabbit antibody against human IgG (Accurate Chemical and Scientific). Plates were washed and then incubated in the dark for 30 min with 50 μl of p-nitrophenyl phosphate (Sigma, St. Louis, MO). Absorbance was read at 405 nm, and the avidity index (AI) for each infant sample was defined as the concentration of NH4SCN required to reduce antibody binding by 50%. Samples with absorbance readings of <0.3 in the control well (0 mol/liter NH4SCN) were excluded from avidity analysis.
Statistical analysis.
The χ2 test for categorical variables and Wilcoxon rank sum tests for continuous variables were utilized to assess differences in sex, infant age, breastfeeding duration, nutritional status, maternal CD4 T-cell count at randomization, infant receipt of HAART, and maternal receipt of HAART during pregnancy between the randomized treatment regimens.
The effect of randomization to multivitamin supplements versus placebo on the proportion of measles IgG-seropositive (≥200 mIU/ml) infants was assessed with the χ2 test, stratified by HIV infection status. The Shapiro-Wilk test was used to assess the normality of continuous outcomes, namely, measles IgG concentration (mIU/ml) and measles IgG avidity index (22). Wilcoxon rank sum tests were used to compare nonnormally distributed log measles IgG concentrations (log mIU/ml) and untransformed avidity indices between infants who were randomized to multivitamins and those assigned to receive placebo, stratified by HIV infection status. The effect modification of any randomized-treatment effect by sex, age at vaccination, breastfeeding duration, nutritional status, maternal CD4 T-cell count, and maternal receipt of HAART was assessed by stratification. We also examined the effect modification by the duration of breastfeeding (as reported by the mother), dichotomized by any breastfeeding versus no breastfeeding reported at 6 and 8 months postpartum. In secondary analyses, we investigated the impact of a possible imbalance in age at vaccination between the randomized treatment arms for HIV-infected infants on treatment-effect estimates. We utilized log-binomial regression models in order to obtain age-at-vaccination adjusted-risk-ratio treatment effect estimates for measles seropositivity (23, 24). Linear regression models were used to obtain age-at-vaccination adjusted mean differences in the log measles IgG concentration and avidity index for infants randomized to multivitamins versus placebo. A robust empirical variance structure was used to overcome slight deviations from normality.
We then examined nonrandomized correlates of the measles vaccine response, including HIV status, sex, age at vaccination, breastfeeding duration, nutritional status at vaccination, maternal CD4 T-cell count, and maternal receipt of HAART during pregnancy. Log-binomial regression models were used in order to obtain risk-ratio estimates for measles seropositivity (23, 24). In a few instances, the models did not converge, and log-Poisson models, which provide consistent but not fully efficient estimates of the relative risk and its confidence intervals (CIs), were used (25). Linear regression models with a robust empirical variance structure were used to account for slight deviations from normality to obtain the mean differences in log measles IgG concentration and avidity index.
The associations of HIV status with measles seropositivity, log measles IgG concentration, and IgG avidity index were assessed among all 236 HIV-infected and HIV-exposed infants. Nutritional status indicators at the vaccination visit were not included in the HIV infection analysis, since poor nutritional status may be a mediator between HIV infection status at 6 weeks of age and measles vaccine response at 15 to 18 months of age. Adjustment for mediators may result in bias by unmeasured variables that are associated with both the mediator and outcome (26).
The associations of sex, age at vaccination, breastfeeding duration, nutritional status indicators (stunting, wasting, and being underweight), maternal CD4 T-cell count at randomization, and maternal receipt of HAART during pregnancy with measles seropositivity, log IgG concentration, and IgG avidity index were examined among HIV-exposed infants (n = 201). HIV-infected individuals were excluded from this analysis due to possible effect modification. Secondarily, we also analyzed nutritional z-scores continuously, since a significant proportion of the infants in the nonstunted group had mild and moderate malnutrition (39.8% had mild to moderate stunting or height-for-age z-score [HAZ] of −1 standard deviation [SD] to −2 SD). Each nutritional indicator or z-score was entered into the model separately. Interaction terms were used in the multivariate models to examine any effect modification for nonrandomized correlates by all other covariates. In a sensitivity analysis, we also included an assessment of infant age at measles vaccine response (15 to 18 months) in the models, and there was no association of the timing of outcome assessment with seroconversion, IgG quantity, or IgG avidity and no discernible difference in other covariates of interest. Missing data for covariates were retained in the analysis using the missing indicator method for variables. All P values were 2-sided, and a P value of <0.05 was considered statistically significant. Statistical analyses were performed using SAS v9.2 (SAS Institute Inc., Cary, NC).
RESULTS
A total of 201 HIV-exposed infants and 35 HIV-infected infants enrolled in a randomized trial of multivitamin supplementation were selected for an assessment of measles vaccine responses at 15 to 18 months of age. The baseline characteristics of the infants by randomized treatment group are presented in Table 1, stratified by HIV infection status. We did not detect any statistically significant imbalances in sex, age at vaccination, breastfeeding duration, anthropometric status indicators, or baseline maternal CD4 T-cell count between the randomization groups stratified by HIV infection status. There was some indication that HIV-infected infants randomized to the placebo group were vaccinated at a younger age than infants who were randomized to the multivitamin group (P = 0.071).
Table 1.
Baseline characteristics of infants by randomized treatment group, stratified by HIV infection statusa
| Infant characteristic | HIV-infected infants (n = 35) given: |
HIV-exposed infants (n = 201) given: |
||
|---|---|---|---|---|
| Multivitamin | Placebo | Multivitamin | Placebo | |
| n | 17 | 18 | 101 | 100 |
| Female | 9 (52.9) | 7 (38.9) | 49 (48.5) | 48 (48.0) |
| Age at vaccination | ||||
| 8.5–10 mo | 6 (35.3)b | 13 (72.2)b | 60 (59.4) | 54 (54.0) |
| 10–11 mo | 10 (58.8)b | 4 (22.2)b | 32 (31.7) | 32 (32.0) |
| 11–12 mo | 1 (5.8)b | 1 (5.6)b | 9 (8.9) | 14 (14.0) |
| Any breastfeeding at: | ||||
| 6 mo | 6 (41.2) | 7 (38.9) | 26 (25.7) | 26 (26.0) |
| 8 mo | 2 (11.8) | 0 (0.0) | 9 (8.9) | 5 (5.0) |
| Time of vaccination | 0 (0.0) | 0 (0.0) | 3 (2.9) | 1 (1.0) |
| Nutritional status at vaccination | ||||
| Stunting | 9 (52.9) | 7 (38.9) | 11 (10.9) | 15 (15.0) |
| Wasting | 4 (23.5) | 4 (22.2) | 13(12.9) | 15 (15.0) |
| Underweight | 8 (47.1) | 8 (44.4) | 14 (13.9) | 20 (20.0) |
| Receiving ARTc | 3 (17.6) | 6 (33.3) | ||
| Maternal CD4 T-cell count at randomization (cells/μl) | ||||
| <200 | 2 (11.8) | 1 (5.6) | 12 (11.9) | 6 (6.0) |
| 200–350 | 7 (41.2) | 7 (38.9) | 13 (12.9) | 18 (18.0) |
| >350 | 6 (35.3) | 9 (50.0) | 66 (65.3) | 67 (67.0) |
| Data missing | 2 (11.8) | 1 (5.6) | 10 (9.9) | 9 (9.0) |
| Mother received HAART during pregnancy | 4 (23.5) | 6 (33.3) | 26 (25.7) | 15 (15.0) |
Data are presented as no. (%).
P < 0.10.
ART, antiretroviral therapy.
The measles IgG quantity and avidity index were assessed at 15 to 18 months of age. Untransformed measles IgG concentration was significantly nonnormal (P < 0.001), as was the log-transformed IgG concentration (P = 0.028). As for avidity index, the untransformed data were borderline nonnormal (P = 0.045), whereas the log-transformed avidity index was significantly nonnormal (P < 0.001). As a result, we used the log-transformed IgG concentration and untransformed avidity index in all analyses, since the selection of the outcome with a more normal distribution may slightly increase power using the robust empirical variance structure.
Effect of multivitamin supplementation on measles vaccine response.
At 15 to 18 months of age, 68.7% of the vaccinated HIV-exposed infants (n = 201) were measles IgG seropositive as defined by an anti-measles IgG concentration of ≥200 mIU/ml (19). There was no significant difference in the proportion of infants who were measles seropositive between those randomized to multivitamins (70 of 101 infants [69%]) and those randomized to placebo (68 of 100 infants [68%]) (P = 0.842). There was also no significant difference in the log measles IgG concentration between infants by randomized treatment regimen (multivitamin median, 5.7; interquartile range [IQR], 5.1 to 6.1 and placebo median, 5.6; IQR, 5.1 to 6.1) (P = 0.291) (Fig. 1). As for the measles IgG avidity index, no significant difference was found for infants randomized to multivitamins (median, 1.37; IQR, 1.06 to 1.68) compared to placebo (median, 1.40; IQR, 1.16 to 1.75) (P = 0.526) (Fig. 2). There was no evidence of effect modification of the randomized treatment for any measles vaccine response outcome by sex, age at vaccination, nutritional status, breastfeeding at 6 or 8 months postpartum, maternal CD4 T-cell count, or maternal receipt of HAART for both the HIV-infected and HIV-exposed infants (all P > 0.20).
Fig 1.
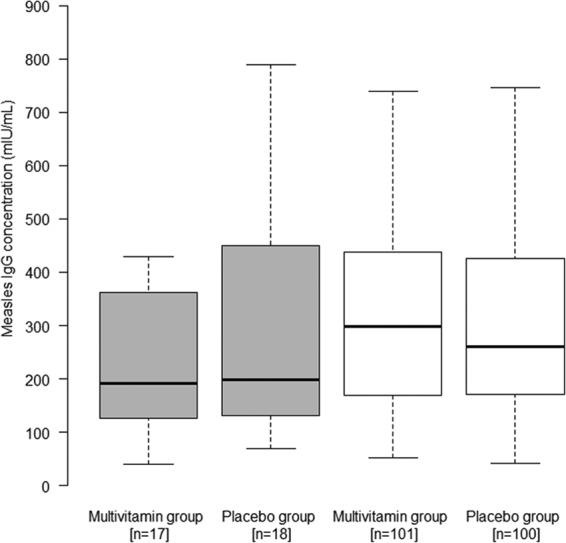
Effect of multivitamins versus placebo on log measles IgG concentration (log mIU/ml), stratified by HIV infection status. The box plots for HIV-infected infants are gray, while the box plots for HIV-exposed infants are white. The boxes show the median, quartile 1 (Q1) and Q3, with the whiskers indicating the minimum and maximum of the range of concentrations. Results were obtained using the Wilcoxon rank sum test.
Fig 2.
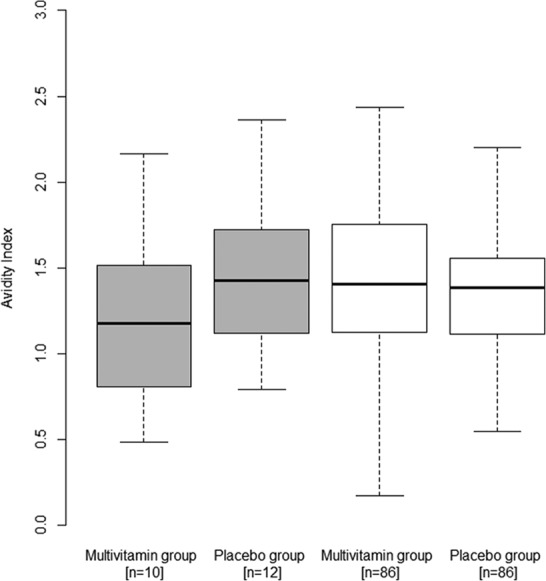
Effect of multivitamins versus placebo on measles IgG avidity index, stratified by HIV infection status. The box plots for HIV-infected infants are gray, while the box plots for HIV-exposed infants are white. The boxes show the median, Q1, and Q3, with the whiskers indicating the minimum and maximum of the avidity index range. Results were obtained using the Wilcoxon rank sum test.
In an exploratory analysis of HIV-infected infants (n = 35), 45.7% were measles IgG seropositive (18). There was no significant difference in the proportion of infants who were measles seropositive between infants who were randomized to multivitamins (7 of 17 infants [41%]) and those randomized to placebo (9 of 18 infants [50%]) (P = 0.601). There was also no significant difference in the log measles IgG concentration (log mIU/ml) between the randomized treatment groups (multivitamin median, 5.3, and IQR, 4.8 to 5.9; placebo median, 5.3, and IQR, 4.9 to 6.0) (P = 0.492) (Fig. 1). In addition, there was no significant difference in the measles IgG avidity index between HIV-infected infants who were randomized to multivitamins (median, 1.18; IQR, 0.81 to 1.51) and those randomized to placebo (median, 1.43; IQR, 1.12 to 1.72) (P = 0.099) (Fig. 2). In secondary analyses, there were no significant differences in measles seropositivity (P = 0.262), log measles IgG concentration (P = 0.393), or avidity index (P = 0.737) between the randomized treatment regimens among HIV-infected infants, after adjustment for age at vaccination.
Association of HIV infection with measles vaccine response.
We examined the association of HIV infection status with measles vaccine responses among all infants (n = 236). HIV-infected infants had a 43% (95% CI, 22 to 58%) (P = 0.008) lower risk of measles seroconversion at 15 to 18 months compared to HIV-exposed infants, after multivariate adjustment (Table 2). Correspondingly, HIV-infected infants also appeared to have a lower measles IgG concentration (difference log IgG concentration, −0.28; 95% CI, −0.59 to 0.04) (P = 0.083), but the results were not statistically significant (Table 3). As for measles IgG avidity, there was no significant difference between HIV-infected infants and HIV-exposed infants, after multivariate adjustment (difference avidity index, −0.02; 95% CI, −0.21 to 0.17) (P = 0.857) (Table 4).
Table 2.
Association of HIV status, sex, age at vaccination, nutritional status, and maternal CD4 T-cell count with measles seropositivitya
| Infant characteristic | Univariate RR (95% CI) for seropositivityb | P | Multivariate RR (95% CI) for seropositivityb,c | P |
|---|---|---|---|---|
| All infants (n = 236) | ||||
| HIV status | ||||
| HIV exposed | Ref. | Ref. | ||
| HIV infected | 0.67 (0.46–0.97) | 0.033 | 0.57 (0.42–0.78) | 0.008 |
| HIV-exposed infants (n = 201) | ||||
| Sex | ||||
| Female | Ref. | Ref. | ||
| Male | 0.99 (0.82–1.19) | 0.902 | 1.00 (0.83–1.20) | 0.991 |
| Age at vaccination (mo) | ||||
| 8.5–10 | Ref. | Ref. | ||
| 10–11 | 1.21 (1.01–1.45) | 0.038 | 1.23 (1.02–1.48) | 0.032 |
| 11–12 | 0.79 (0.52–1.20) | 0.271 | 0.83 (0.55–1.24) | 0.353 |
| Any breastfeeding at 8 mo | ||||
| No | Ref. | Ref. | ||
| Yes | 0.82 (0.52–1.31) | 0.407 | 0.81 (0.52–1.27) | 0.357 |
| Stunting at vaccination | ||||
| No | Ref. | Ref. | ||
| Yes | 0.95 (0.70–1.27) | 0.712 | 0.90 (0.68–1.19) | 0.457 |
| Wasting at vaccination | ||||
| No | Ref. | Ref. | ||
| Yes | 1.11 (0.87–1.41) | 0.393 | 1.09 (0.87–1.37) | 0.430 |
| Underweight at vaccination | ||||
| No | Ref. | Ref. | ||
| Yes | 0.98 (0.76–1.27) | 0.891 | 0.99 (0.79–1.25) | 0.948 |
| Maternal CD4 T-cell count at randomization (cells/μl) | ||||
| <200 | 1.29 (1.05–1.57) | 0.014 | 1.24 (1.01–1.53) | 0.039 |
| 200–350 | 0.98 (0.75–1.28) | 0.879 | 1.00 (0.75–1.32) | 0.981 |
| >350 | Ref. | Ref. | ||
| Mother received HAART during pregnancy | ||||
| Yes | 1.04 (0.83–1.30) | 0.742 | 1.02 (0.81–1.29) | 0.881 |
| No | Ref. | Ref. |
Seropositivity defined as an IgG titer of ≥200 mIU/ml.
RR, relative risk; Ref., reference group.
Multivariate models for HIV status among all infants included sex, age at vaccination, any breastfeeding at 8 months, maternal CD4 T-cell count, and maternal receipt of HAART as covariates. Multivariate models among HIV-exposed infants included sex, age at vaccination, any breastfeeding at 8 months, maternal CD4 T-cell count, maternal receipt of HAART, and a single indicator of nutritional status (stunting, wasting, or underweight). Each nutritional status indicator was entered into a separate multivariate model.
Table 3.
Association of HIV status, sex, age at vaccination, nutritional status, and maternal CD4 T-cell count with log measles IgG concentration
| Infant characteristic | Log IgG concn (log mIU/ml) (mean ± SE) | Univariate difference in log IgG concn (95% CI)a | P | Multivariatea,b difference in log IgG concn (95% CI) | P |
|---|---|---|---|---|---|
| All infants (n = 236) | |||||
| HIV status | |||||
| HIV exposed | 5.67 ± 0.84 | Ref. | Ref. | ||
| HIV infected | 5.46 ± 1.02 | −0.21 (−0.56 to 0.14) | 0.248 | −0.28 (−0.59 to 0.04) | 0.083 |
| HIV-exposed infants (n = 201) | |||||
| Sex | |||||
| Female | 5.67 ± 0.85 | Ref. | Ref. | ||
| Male | 5.67 ± 0.83 | 0.00 (−0.23 to 0.23) | 0.985 | −0.04 (−0.27 to 0.20) | 0.763 |
| Age at vaccination (mo) | |||||
| 8.5–10 | 5.61 ± 0.79 | Ref. | Ref. | ||
| 10–11 | 5.80 ± 0.81 | 0.19 (−0.05 to 0.44) | 0.962 | 0.23 (−0.02 to 0.48) | 0.076 |
| 11–12 | 5.62 ± 1.13 | 0.01 (−0.46 to 0.49) | 0.120 | 0.08 (−0.38 to 0.53) | 0.742 |
| Any breastfeeding at 8 mo | |||||
| No | 5.67 ± 0.81 | Ref. | Ref. | ||
| Yes | 5.70 ± 1.22 | 0.04 (−0.59 to 0.67) | 0.908 | -0.02 (−0.64 to 0.60) | 0.954 |
| Stunting at vaccination | |||||
| No | 5.70 ± 0.87 | Ref. | Ref. | ||
| Yes | 5.44 ± 0.60 | −0.26 (−0.52 to 0.00) | 0.047 | −0.34 (−0.60 to −0.07) | 0.012 |
| Wasting at vaccination | |||||
| No | 5.63 ± 0.85 | Ref. | Ref. | ||
| Yes | 5.94 ± 0.76 | 0.31 (0.01 to 0.61) | 0.046 | 0.29 (−0.01 to 0.60) | 0.062 |
| Underweight at vaccination | |||||
| No | 5.63 ± 0.81 | Ref. | Ref. | ||
| Yes | 5.86 ± 0.95 | 0.23 (−0.10 to 0.57) | 0.175 | 0.23 (−0.11 to 0.56) | 0.184 |
| Maternal CD4 T-cell count at randomization (cells/μl) | |||||
| <200 | 5.78 ± 0.37 | 0.08 (−0.14 to 0.31) | 0.470 | 0.04 (−0.21 to 0.27) | 0.756 |
| 200–350 | 5.67 ± 0.91 | −0.03 (−0.38 to 0.32) | 0.876 | −0.01 (−0.33 to 0.32) | 0.975 |
| >350 | 5.70 ± 0.89 | Ref. | Ref. | ||
| Mother received HAART during pregnancy | |||||
| Yes | 5.67 ± 0.85 | 0.00 (−0.28 to 0.28) | 0.995 | 0.00 (−0.27 to 0.27) | 0.986 |
| No | 5.67 ± 0.82 | Ref. | Ref. |
Differences in log measles IgG concentration were estimated from linear regression models with robust empirical variance. Ref. indicates the reference group.
Multivariate models for HIV status among all infants included sex, age at vaccination, any breastfeeding at 8 months, maternal CD4 T-cell count, and maternal receipt of HAART as covariates. Multivariate models among HIV-exposed infants included sex, age at vaccination, any breastfeeding at 8 months, maternal CD4 T-cell count, maternal receipt of HAART, and a single indicator of nutritional status (stunting, wasting, or underweight). Each nutritional status indicator was entered into a separate multivariate model.
Table 4.
Association of HIV status, sex, age at vaccination, nutritional status, maternal CD4 T-cell count, and maternal receipt of HAART during pregnancy with measles IgG avidity index
| Infant characteristic | Avidity index (mean ± SE) | Univariate difference (95% CI)a | P | Multivariatea,b difference (95% CI) | P |
|---|---|---|---|---|---|
| All infants (n = 204) | |||||
| HIV status | |||||
| HIV exposed | 1.40 ± 0.46 | Ref. | Ref. | ||
| HIV infected | 1.38 ± 0.54 | −0.02 (−0.21 to 0.18) | 0.854 | −0.02 (−0.21 to 0.17) | 0.857 |
| HIV-exposed infants (n = 172) | |||||
| Sex | |||||
| Female | 1.42 ± 0.48 | Ref. | Ref. | ||
| Male | 1.38 ± 0.43 | −0.03 (−0.17–0.10) | 0.577 | −0.07 (−0.20 to 0.07) | 0.354 |
| Age at vaccination (mo) | |||||
| 8.5–10 | 1.35 ± 0.48 | Ref. | Ref. | ||
| 10–11 | 1.48 ± 0.41 | 0.13 (−0.01 to 0.27) | 0.079 | 0.17 (0.02–0.32) | 0.031 |
| 11–12 | 1.42 ± 0.43 | 0.07 (−0.14 to 0.28) | 0.510 | 0.08 (−0.13 to 0.28) | 0.477 |
| Any breastfeeding at 8 mo | |||||
| No | 1.39 ± 0.47 | Ref. | Ref. | ||
| Yes | 1.51 ± 0.49 | 0.12 (−0.13 to 0.38) | 0.351 | −0.03 (−0.21 to 0.14) | 0.705 |
| Stunting at vaccination | |||||
| No | 1.41 ± 0.46 | Ref. | Ref. | ||
| Yes | 1.31 ± 0.40 | −0.10 (−0.29 to 0.09) | 0.292 | −0.14 (−0.34 to 0.06) | 0.157 |
| Wasting at vaccination | |||||
| No | 1.41 ± 0.47 | Ref. | Ref. | ||
| Yes | 1.36 ± 0.41 | −0.05 (−0.22 to 0.12) | 0.584 | −0.08 (−0.25 to 0.10) | 0.392 |
| Underweight at vaccination | |||||
| No | 1.41 ± 0.47 | Ref. | Ref. | ||
| Yes | 1.33 ± 0.41 | −0.09 (−0.25 to 0.08) | 0.316 | −0.11 (−0.29 to 0.06) | 0.192 |
| Maternal CD4 T-cell count at randomization (cells/μl) | |||||
| <200 | 1.24 ± 0.44 | −0.18 (−0.39 to 0.03) | 0.096 | −0.21 (−0.42 to 0.00) | 0.047 |
| 200–350 | 1.41 ± 0.37 | −0.02 (−0.18 to 0.15) | 0.847 | −0.01 (−0.18 to 0.15) | 0.874 |
| >350 | 1.42 ± 0.47 | Ref. | Ref. | ||
| Mother received HAART during pregnancy | |||||
| Yes | 1.41 ± 0.48 | −0.06 (−0.20 to 0.08) | 0.405 | −0.03 (−0.18 to 0.12) | 0.683 |
| No | 1.35 ± 0.68 | Ref. | Ref. |
Differences in log measles IgG concentration were estimated from linear regression models with robust empirical variance. Ref. indicates the reference group.
Multivariate models for HIV status among all infants included sex, age at vaccination, any breastfeeding at 8 months, maternal CD4 T-cell count, and maternal receipt of HAART as covariates. Multivariate models among HIV-exposed infants included sex, age at vaccination, any breastfeeding at 8 months, maternal CD4 T-cell count, maternal receipt of HAART, and a single indicator of nutritional status (stunting, wasting, underweight). Each nutritional status indicator was entered into a separate multivariate model.
Correlates of measles vaccine response among HIV-exposed infants.
We also investigated the associations of sex, age at vaccination, breastfeeding duration, nutritional status, maternal CD4 T-cell count, and maternal receipt of HAART with measles vaccine responses among HIV-exposed infants (n = 201). After multivariate adjustment, the risk of measles seroconversion was significantly increased for HIV-exposed infants who were vaccinated at 10 to 11 months rather than at 8.5 to 10 months (RR, 1.23; 95% CI, 1.02 to 1.48) (P = 0.032) and for infants whose mothers had a CD4 T-cell count of <200 cells/μl at randomization compared to a CD4 T-cell count of >350 cells/μl (RR, 1.24; 95% CI, 1.01 to 1.53) (P = 0.039) (Table 2). The only variable that was significantly associated with measles IgG concentration after multivariate adjustment was stunting (Table 3). Infants who were considered stunted at the vaccination visit had a mean difference in log measles IgG concentration of −0.34 (95% CI, −0.60 to −0.07) (P = 0.012) compared to the nonstunted infants.
The correlates of measles IgG avidity among the HIV-exposed infants are presented in Table 4. After multivariate adjustment, HIV-exposed infants who were vaccinated at 10 to 11 months had increased avidity compared to those who were vaccinated at 8.5 to 10 months of age (difference, 0.17; 95% CI, 0.02 to 0.32) (P = 0.031). HIV-exposed infants whose mothers had a CD4 T-cell count of <200 cells/μl at baseline also had a significantly lower avidity index than infants whose mothers had a CD4 T-cell count of >350 cells/μl (difference, −0.21; 95% CI, −0.42 to −0.00) (P = 0.047). There was some indication that stunted infants had a decreased avidity index; however, the results were not statistically significant (difference, −0.14; 95% CI, −0.34 to 0.06) (P = 0.157). In a secondary analysis, each 1-unit decrease in height-for-age z-score was significantly associated with a 0.08 decrease (95% CI of difference, −0.01 to −0.14) (P = 0.016) in measles avidity, after multivariate adjustment. Among the HIV-exposed infants, there was no evidence of effect modification of any seroconversion proportion, IgG concentration, or avidity index association by other covariates, including sex, age at vaccination, nutritional status, maternal CD4 T-cell count, and maternal receipt of HAART.
DISCUSSION
We found no effect of supplementation with vitamins B complex, C, and E on measles vaccine seroconversion, measles IgG quantity, or IgG avidity among HIV-exposed infants at 15 to 18 months of age. An important factor to highlight in our study is that all mothers received high-dose micronutrient supplements containing vitamins B complex, C, and E. Maternal multivitamin supplements may improve breast milk micronutrient levels, and as a result, infants who breastfeed may have had an adequate intake of vitamins B, C, and E (27). Nevertheless, we found no difference in the effect of multivitamin supplementation on measles vaccine response for infants who terminated breastfeeding prior to measles vaccination. The low measles seroconversion proportion (68.7%) among HIV-exposed infants in this study is also important to note. Even though we did not have an HIV-unexposed comparison group in this study, 85% of HIV-unexposed infants are expected to seroconvert following a measles vaccination prior to 12 months of age (28).
Previous randomized trials examining the effect of micronutrient supplementation with vitamin B complex, C, or E on vaccine responses have found mixed results. A 2-by-2 factorial trial of vitamins A and E on tetanus vaccine responses among Turkish infants found no effect of vitamin E supplementation alone or concurrently with vitamin A on anti-tetanus IgG titers at 2, 5, 16, or 18 months of age (13). A trial of supplementation with vitamins C, E, and β-carotene conducted among elderly nursing home residents and a trial of vitamin E alone among adult chronic care patients found no effect of supplementation on influenza vaccination titers compared to those given a placebo (29, 30). In contrast, some randomized trials have found a positive effect of vitamin E supplementation on vaccine responses. A randomized trial conducted among healthy Malaysian women aged 18 to 25 determined that vitamin E significantly increased anti-tetanus antibody titers following revaccination compared to placebo (31). A trial conducted in healthy U.S. elderly subjects also found that high-dose vitamin E supplementation increased anti-tetanus titers and antibody responses to hepatitis B following vaccination, but there was no effect on diphtheria or pneumococcal polysaccharide vaccine responses (32). In this trial, a significant effect was found in the group that received 200 mg/day (∼600% RDA) but not in the group that received 60 mg/day (∼200% RDA). Accordingly, the vitamin E dose in our study may have been too low to impact the vaccine response; other possibilities for this effect are that vitamin E may only improve immune response deficits that are due to aging or that only a few infants in our study were vitamin E deficient.
We also examined nonrandomized correlates of measles immune responses among the study cohort. HIV-infected infants had a significantly lower rate of measles seroconversion than HIV-exposed infants, which is well documented, but the mechanism by which HIV impairs IgG responses is not completely clear (1). HIV can directly cause B-lymphocyte dysregulation, increased B-lymphocyte activation and turnover, and loss of memory B lymphocytes, but the relative contribution of these impairments, as well as others, to reduced measles vaccine responses is not known (33, 34). We did not find any association of HIV infection with measles IgG avidity. This finding is not in concordance with a previous study that found significantly decreased measles avidity among HIV-infected infants compared to HIV-unexposed infants (35). These contrasting findings may be explained by differences in the comparison group, since HIV-exposed infants may have decreased measles avidity compared to HIV-unexposed infants. Avidity maturation is T-cell dependent, and intrauterine HIV exposure has been shown to impair infant thymic and progenitor cell function, decrease T-cell counts, and alter dendritic cell antigen presentation (36, 37). The sum of these effects may decrease antibody avidity in HIV-exposed infants compared to HIV-unexposed infants and therefore may have reduced the contrast between the groups in our study; or, we may have also had a limited sample size to detect a difference in our study. Future research is needed to compare measles vaccine avidities in HIV-exposed and HIV-unexposed infants. It is also unclear if the use of HAART in mothers or infants can improve measles vaccine responses. HAART became available during this trial, and we were unable to assess the differences in associations by receipt of HAART due to the small sample size.
Among HIV-exposed infants, vaccination at 10 to 11 months of age was associated with increased measles seroconversion and IgG avidity compared to infants vaccinated at 8.5 to 10 months. Healthy infants vaccinated at younger ages are well documented to have decreased seroconversion due to the presence of maternal antibodies (38). As for avidity, a study of HIV-unexposed infants found that those who received a measles vaccine at 6 months had significantly lower IgG avidity than infants who were vaccinated at 9 months (39). The mechanism leading to this difference has not been completely determined, but defects in somatic hypermutation at younger ages, which appear to be independent of the presence of maternal antibodies, may lead to reduced antibody avidity (39, 40). This trend of older age leading to increased IgG quantity and avidity was not found for infants in our study who were vaccinated at 11 to 12 months. Infants vaccinated at 11 to 12 months had to miss at least one clinic visit around 9 months in order to be vaccinated late. As a result, these infants may be more likely to be of poorer health or have other features that are predictive of nonresponse to the measles vaccine. Further, only 23 HIV-exposed infants (11%) were vaccinated at 11 to 12 months, and statistical variability may also partially explain the null findings.
We also found that a lower maternal CD4 T-cell count at 5 to 7 weeks postpartum was significantly associated with increased infant measles seroconversion but lower IgG avidity among HIV-exposed infants. HIV-infected pregnant women are characterized by decreased placental transfer of maternal measles antibodies compared to HIV-uninfected pregnant women (41). Further, a study of HIV-infected Kenyan mothers found that women with high HIV load during the last trimester of pregnancy had decreased transfer levels of placental measles IgG (42). As a result, decreased placental transfer of antibodies for mothers with more-severe HIV disease may explain our findings. To our knowledge, there are no biological studies of the impact of maternal HIV disease severity on avidity maturation in HIV-exposed (uninfected) infants. Severe maternal HIV disease may significantly impair the development of the immune system in utero, and these deficits may lead to decreased avidity maturation following vaccination. Future studies are needed to examine the impact of maternal HIV disease severity and the receipt of HAART on the development of the HIV-exposed infant immune system.
As for markers of nutritional status, low height-for-age z-score was significantly associated with reduced measles vaccine responses among HIV-exposed infants. Stunted infants had significantly lower measles IgG concentrations than nonstunted infants, which has also been reported in previous studies; however, these studies did not completely adjust for potential confounders (43, 44). Immune system dysfunction is a well-documented sequela of protein energy malnutrition characterized by thymic atrophy and reduced T-cell proliferation and function (45). Stunting may also reduce the production of Th2 cytokines and B-lymphocyte counts, but published studies offer inconclusive findings (7). In our study, stunting also appeared to be correlated with reduced measles avidity, but the results were not statistically significant. Nevertheless, decreasing height-for-age analyzed continuously was significantly associated with decreased antibody avidity. To our knowledge, only one previous study has examined the association of malnutrition with antibody avidity following vaccination. This study determined that infants with marasmus or kwashiorkor had significantly lower antibody avidity following tetanus vaccination than did well-nourished infants (46). Future studies examining the impact of interventions for stunting on antibody quantity and avidity maturation appear to be warranted.
There are several limitations to this study. First, we did not have an HIV-unexposed control group, and as a result, we are unable to directly assess the impact of HIV exposure in utero on measles vaccine responses. Despite this limitation, we found that HIV-exposed infants whose mothers had lower CD4 T-cell counts had impaired avidity responses, which suggests that HIV-exposed infants born to mothers with severe HIV disease will likely differ in avidity maturation compared to HIV-unexposed infants. We also did not utilize a plaque reduction neutralization (PRN) assay, which is the best correlate of protection against measles infection (19). Nevertheless, the use of an enzyme immunoassay still indicates differences in the quality of the immune responses following measles vaccination. Third, we only had data on the effect of micronutrients and correlates of vaccine responses at 15 to 18 months of age following a single dose of the measles vaccine. The effect of micronutrient supplementation and the correlates of protection may differ at older ages or with a two-dose measles vaccination strategy. All infants also received vitamin A supplements, and consequently, we were unable to assess the potential interaction or the effect of vitamins B complex, C, and E in the absence of vitamin A supplements. Furthermore, we did not have data on the mothers' measles antibody levels, which may have helped explain the CD4 T-cell count findings.
Overall, we found no effect of infant supplementation with multivitamins containing vitamins B complex, C, and E on measles vaccine-induced IgG quantity or avidity for HIV-exposed infants. Nevertheless, the nonrandomized exposures in this study indicated important areas for future research. Stunted HIV-exposed infants had reduced measles IgG quantity and avidity following vaccination; however, whether these associations directly translate into an increased risk of acquiring measles cannot be directly inferred. Studies and trials are needed to determine if enhanced integration of interventions to prevent or treat malnutrition support the achievement of measles control goals in resource-limited settings. HIV-exposed infants whose mothers had lower CD4 T-cell counts had low measles antibody avidity but a higher seroconversion proportion. Future studies examining the impact of maternal HIV disease severity and the receipt of HAART on development of the HIV-exposed uninfected infant immune system are also warranted.
ACKNOWLEDGMENTS
We thank the mothers, children, and field teams, including physicians, nurses, midwives, supervisors, laboratory staff, and the administrative staff, who made this study possible; we also thank the Muhimbili National Hospital, Muhimbili University of Health and Allied Sciences, and the National AIDS Control Program in Dar es Salaam for their institutional support.
This work was made possible with support from the Harvard Global Health Institute (HGHI), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD R01 HD043688-01 and K24HD058795), and the National Institute of Allergy and Infectious Diseases (T32AI007358).
Footnotes
Published ahead of print 29 May 2013
REFERENCES
- 1. Mphahlele MJ, Mda S. 2012. Immunising the HIV-infected child: a view from sub-Saharan Africa. Vaccine 30:C61–C65. 10.1016/j.vaccine.2012.02.040 [DOI] [PubMed] [Google Scholar]
- 2. Abramczuk BM, Mazzola TN, Moreno YM, Zorzeto TQ, Quintilio W, Wolf PS, Blotta MH, Morcillo AM, da Silva MT, Dos Santos Vilela MM. 2011. Impaired humoral response to vaccines among HIV-exposed uninfected infants. Clin. Vaccine Immunol. 18:1406–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jones CE, Naidoo S, De Beer C, Esser M, Kampmann B, Hesseling AC. 2011. Maternal HIV infection and antibody responses against vaccine-preventable diseases in uninfected infants. JAMA 305:576–584 [DOI] [PubMed] [Google Scholar]
- 4. Hygino J, Lima PG, Filho RG, Silva AA, Saramago CS, Andrade RM, Andrade DM, Andrade AF, Brindeiro R, Tanuri A, Bento CA. 2008. Altered immunological reactivity in HIV-1-exposed uninfected neonates. Clin. Immunol. 127:340–347 [DOI] [PubMed] [Google Scholar]
- 5. Faye A, Pornprasert S, Mary JY, Dolcini G, Derrien M, Barré-Sinoussi F, Chaouat G, Menu EANRS 1267 Study Team, HIV-1 PMTCT-PlaNet 2007. Characterization of the main placental cytokine profiles from HIV-1-infected pregnant women treated with anti-retroviral drugs in France. Clin. Exp. Immunol. 149:430–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Filteau S. 2009. The HIV-exposed, uninfected African child. Trop. Med. Int. Health 14:276–287 [DOI] [PubMed] [Google Scholar]
- 7. Savy M, Edmond K, Fine PE, Hall A, Hennig BJ, Moore SE, Mulholland K, Schaible U, Prentice AM. 2009. Landscape analysis of interactions between nutrition and vaccine responses in children. J. Nutr. 139:2154S–218S. 10.3945/jn.109.105312 [DOI] [PubMed] [Google Scholar]
- 8. Webb AL, Villamor E. 2007. Update: effects of antioxidant and non-antioxidant vitamin supplementation on immune function. Nutr. Rev. 65:181–217 [DOI] [PubMed] [Google Scholar]
- 9. Meydani SN, Han SN, Wu D. 2005. Vitamin E and immune response in the aged: molecular mechanisms and clinical implications. Immunol. Rev. 205:269–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Villamor E, Fawzi WW. 2005. Effects of vitamin A supplementation on immune responses and correlation with clinical outcomes. Clin. Microbiol. Rev. 18:446–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Benn CS, Aaby P, Balé C, Olsen J, Michaelsen KF, George E, Whittle H. 1997. Randomised trial of effect of vitamin A supplementation on antibody response to measles vaccine in Guinea-Bissau, west Africa. Lancet 350:101–105 [DOI] [PubMed] [Google Scholar]
- 12. Semba RD, Munasir Z, Beeler J, Akib A, Muhilal , Audet S, Sommer A. 1995. Reduced seroconversion to measles in infants given vitamin A with measles vaccination. Lancet 345:1330–1332 [DOI] [PubMed] [Google Scholar]
- 13. Kutukculer N, Akil T, Egemen A, Kurugöl Z, Akşit S, Ozmen D, Turgan N, Bayindir O, Cağlayan S. 2000. Adequate immune response to tetanus toxoid and failure of vitamin A and E supplementation to enhance antibody response in healthy children. Vaccine 18:2979–2984 [DOI] [PubMed] [Google Scholar]
- 14. Duggan C, Manji KP, Kupka R, Bosch RJ, Aboud S, Kisenge R, Okuma J, Fawzi WW. 2012. Multiple micronutrient supplementation in Tanzanian infants born to HIV-infected mothers: a randomized, double-blind, placebo-controlled clinical trial. Am. J. Clin. Nutr. 96:1437–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Food and Nutrition Board, Institute of Medicine 2000. Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids: a report of the Panel on Dietary Antioxidants and Related Compounds, Subcommittees on Upper Reference Levels of Nutrients and Interpretation and Uses of Dietary Reference Intakes, and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. National Academy Press, Washington, DC [Google Scholar]
- 16.Food and Nutrition Board, Institute of Medicine 2000. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline: a report of the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline and Subcommittee on Upper Reference Levels of Nutrients, Food and Nutrition Board, Institute of Medicine. National Academy Press, Washington, DC [Google Scholar]
- 17. Fawzi WW, Msamanga GI, Spiegelman D, Urassa EJ, McGrath N, Mwakagile D, Antelman G, Mbise R, Herrera G, Kapiga S, Willett W, Hunter DJ. 1998. Randomised trial of effects of vitamin supplements on pregnancy outcomes and T cell counts in HIV-1-infected women in Tanzania. Lancet 351:1477–1482 [DOI] [PubMed] [Google Scholar]
- 18.WHO Multicentre Growth Reference Study Group 2006. WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. World Health Organization, Geneva, Switzerland [Google Scholar]
- 19. Chen RT, Markowitz LE, Albrecht P, Stewart JA, Mofenson LM, Preblud SR, Orenstein WA. 1990. Measles antibody: reevaluation of protective titers. J. Infect. Dis. 162:1036–1042 [DOI] [PubMed] [Google Scholar]
- 20. Phan TG, Paus D, Chan TD, Turner ML, Nutt SL, Basten A, Brink R. 2006. High affinity germinal center B cells are actively selected into the plasma cell compartment. J. Exp. Med. 203:2419–2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Macdonald RA, Hosking CS, Jones CL. 1988. The measurement of relative antibody affinity by ELISA using thiocyanate elution. J. Immunol. Methods 106:191–194 [DOI] [PubMed] [Google Scholar]
- 22. Shapiro SS, Wilk MB. 1965. An analysis of variance test for normality (complete samples). Biometrika 52:591–611 [Google Scholar]
- 23. Wacholder S. 1986. Binomial regression in GLIM: estimating risk ratios and risk differences. Am. J. Epidemiol. 123:174–184 [DOI] [PubMed] [Google Scholar]
- 24. Spiegelman D, Hertzmark E. 2005. Easy SAS calculations for risk or prevalence ratios and differences. Am. J. Epidemiol. 162:199–200 [DOI] [PubMed] [Google Scholar]
- 25. Zou G. 2004. A modified Poisson regression approach to prospective studies with binary data. Am. J. Epidemiol. 159:702–706 [DOI] [PubMed] [Google Scholar]
- 26. Cole SR, Hernán MA. 2002. Fallibility in estimating direct effects. Int. J. Epidemiol. 31:163–165 [DOI] [PubMed] [Google Scholar]
- 27. Baylin A, Villamor E, Rifai N, Msamanga G, Fawzi WW. 2005. Effect of vitamin supplementation to HIV-infected pregnant women on the micronutrient status of their infants. Eur. J. Clin. Nutr. 59:960–968 [DOI] [PubMed] [Google Scholar]
- 28. Sudfeld CR, Navar AM, Halsey NA. 2010. Effectiveness of measles vaccination and vitamin A treatment. Int. J. Epidemiol. 39(Suppl 1):i48–i55. 10.1093/ije/dyq021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Girodon F, Galan P, Monget AL, Boutron-Ruault MC, Brunet-Lecomte P, Preziosi P, Arnaud J, Manuguerra JC, Herchberg S. 1999. Impact of trace elements and vitamin supplementation on immunity and infections in institutionalized elderly patients: a randomized controlled trial. MIN. VIT. AOX. geriatric network. Arch. Intern. Med. 159:748–754 [DOI] [PubMed] [Google Scholar]
- 30. Harman D, Miller RW. 1986. Effect of vitamin E on the immune response to influenza virus vaccine and the incidence of infectious disease in man. Age 9:21–23 [Google Scholar]
- 31. Mahalingam D, Radhakrishnan AK, Amom Z, Ibrahim N, Nesaretnam K. 2011. Effects of supplementation with tocotrienol-rich fraction on immune response to tetanus toxoid immunization in normal healthy volunteers. Eur. J. Clin. Nutr. 65:63–69 [DOI] [PubMed] [Google Scholar]
- 32. Meydani SN, Meydani M, Blumberg JB, Leka LS, Siber G, Loszewski R, Thompson C, Pedrosa MC, Diamond RD, Stollar BD. 1997. Vitamin E supplementation and in vivo immune response in healthy elderly subjects. A randomized controlled trial. JAMA 277:1380–1386 [DOI] [PubMed] [Google Scholar]
- 33. Moir S, Fauci AS. 2009. B cells in HIV infection and disease. Nat. Rev. Immunol. 9:235–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cagigi A, Nilsson A, De Millito A, Chiodi F. 2008. B cell immunopathology during HIV-1 infection: lessons to learn for HIV-1 vaccine design. Vaccine 26:3016–3025 [DOI] [PubMed] [Google Scholar]
- 35. Nair N, Moss WJ, Scott S, Mugala N, Ndhlovu ZM, Lilo K, Ryon JJ, Monze M, Quinn TC, Cousens S, Cutts F, Griffin DE. 2009. HIV-1 infection in Zambian children impairs the development and avidity maturation of measles virus-specific immunoglobulin G after vaccination and infection. J. Infect. Dis. 200:1031–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nielsen SD, Jeppesen DL, Kolte L, Clark DR, Sørensen TU, Dreves AM, Ersbøll AK, Ryder LP, Valerius NH, Nielsen JO. 2001. Impaired progenitor cell function in HIV-negative infants of HIV-positive mothers results in decreased thymic output and low CD4 counts. Blood 98:398–404 [DOI] [PubMed] [Google Scholar]
- 37. Miyamoto M, Pessoa SD, Ono E, Machado DM, Salomão R, Succi RC, Pahwa S, de Moraes-Pinto MI. 2010. Low CD4T+-cell levels and B-cell apoptosis in vertically HIV-exposed noninfected children and adolescents. J. Trop. Pediatr. 56:427–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cáceres VM, Strebel PM, Sutter RW. 2000. Factors determining prevalence of maternal antibody to measles virus throughout infancy: a review. Clin. Infect. Dis. 31:110–119 [DOI] [PubMed] [Google Scholar]
- 39. Nair N, Gans H, Lew-Yasukawa L, Long-Wagar AC, Arvin A, Griffin DE. 2007. Age-dependent differences in IgG isotype and avidity induced by measles vaccine received during the first year of life. J. Infect. Dis. 196:1339–1345 [DOI] [PubMed] [Google Scholar]
- 40. Ridings J, Dinan L, Williams R, Roberton D, Zola H. 1998. Somatic mutation of immunoglobulin V(H)6 genes in human infants. Clin. Exp. Immunol. 114:33–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Scott S, Moss WJ, Cousens S, Beeler JA, Audet SA, Mugala N, Quinn TC, Griffin DE, Cutts FT. 2007. The influence of HIV-1 exposure and infection on levels of passively acquired antibodies to measles virus in Zambian infants. Clin. Infect. Dis. 45:1417–1424 [DOI] [PubMed] [Google Scholar]
- 42. Farquhar C, Nduati R, Haigwood N, Sutton W, Mbori-Ngacha D, Richardson B, John-Stewart G. 2005. High maternal HIV-1 viral load during pregnancy is associated with reduced placental transfer of measles IgG antibody. J. Acquir. Immune Defic. Syndr. 40:494–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Waibale P, Bowlin SJ, Mortimer EA, Jr, Whalen C. 1999. The effect of human immunodeficiency virus-1 infection and stunting on measles immunoglobulin-G levels in children vaccinated against measles in Uganda. Int. J. Epidemiol. 28:341–346 [DOI] [PubMed] [Google Scholar]
- 44. Moss WJ, Scott S, Mugala N, Ndhlovu Z, Beeler JA, Audet SA, Ngala M, Mwangala S, Nkonga-Mwangilwa C, Ryon JJ, Monze M, Kasolo F, Quinn TC, Cousens S, Griffin DE, Cutts FT. 2007. Immunogenicity of standard-titer measles vaccine in HIV-1-infected and uninfected Zambian children: an observational study. J. Infect. Dis. 196:347–355 [DOI] [PubMed] [Google Scholar]
- 45. Scrimshaw NS, SanGiovanni JP. 1997. Synergism of nutrition, infection, and immunity: an overview. Am. J. Clin. Nutr. 66:464S–477S [DOI] [PubMed] [Google Scholar]
- 46. Chandra RK, Chandra S, Gupta S. 1984. Antibody affinity and immune complexes after immunization with tetanus toxoid in protein-energy malnutrition. Am. J. Clin. Nutr. 40:131–134 [DOI] [PubMed] [Google Scholar]


