Abstract
Oculorespiratory syndrome (ORS) is an infrequent adverse event following influenza vaccination. Its clinical presentation suggests that ORS is an immune-mediated phenomenon, but studies of symptomatic individuals have been few. This study measured cytokine levels in peripheral blood samples following influenza vaccination in those with and without current ORS symptoms. Canadian adults receiving the 2010-2011 seasonal influenza vaccine were recruited and asked to promptly report any adverse effects. ORS symptoms occurring 4 to 48 h after vaccination were identified using previously published criteria. Two blood samples were collected from each subject to measure blood plasma cytokine and hemagglutination inhibition antibody (HAI) titers; visit 1 occurred during the acute disease phase or 4 to 72 h after vaccination for controls, and visit 2 occurred another 21 days postimmunization. Nine ORS cases and 35 controls were enrolled. The median age of ORS cases was 49 years, and 89% were female. Most cases had multiple symptoms, but none required medical care. HAI titers before and after vaccination were similar for the cases and controls. Blood plasma cytokine concentrations did not differ between the ORS cases and controls for most cytokines measured (interleukin 4 [IL-4], IL-5, IL-10, IL-13, IL-1α, IL-8, tumor necrosis factor alpha [TNF-α], gamma interferon [IFN-γ], and IL-17A). However, ORS cases had higher levels of IL-10 and IL-3 than the controls at visits 1 and 2, even after all symptoms had subsided. Persistent higher levels of IL-10 and IL-3 in ORS cases suggest that host factors may have predisposed these individuals to develop ORS following influenza vaccination. Further investigations are warranted, as they might identify subjects who are at risk for ORS prior to vaccination.
INTRODUCTION
Influenza infection is a major cause of morbidity and mortality worldwide, and vaccination is the cornerstone of infection prevention. Administration of the seasonal influenza vaccine is associated with a varied range of adverse events following immunization (AEFI) that include local (injection-site reaction) and systemic manifestations. Oculorespiratory syndrome (ORS) is an influenza vaccine-associated adverse event that was first described in Canada during the 2000-2001 influenza immunization campaign (1). Patients usually presented within 24 h after vaccination with bilateral red eyes, facial edema, and/or respiratory symptoms (Table 1). Manifestations frequently resolved within 48 to 72 h.
Table 1.
Clinical manifestations of ORSa
| Symptom type | Specific symptoms |
|---|---|
| Ocular | Bilateral red eyes (conjunctival erythema) |
| Respiratory | Cough, sore throat, hoarseness, wheezing, chest tightness, difficulty breathing, difficulty swallowing |
| Facial edema | Lip, tongue, or eyelid swelling |
ORS cases can present with symptoms from one or more categories, and ORS symptoms can be associated with other systemic manifestations.
The specific cause of ORS is not understood. Studies in Canada during the 2000-2001 season linked most ORS cases to a domestic manufacturer's vaccine that contained a higher-than-expected content of unsplit and aggregated influenza virions (2). In subsequent seasons, although at a lower frequency, cases of ORS were also associated with influenza vaccines from other manufacturers (3). Although many ORS manifestations resemble allergic reactions, results from affected individuals who were given skin testing suggest that ORS is not a type-I hypersensitivity reaction (4). However, an immune-based pathogenesis of ORS appears likely, given the types and timing of symptoms (5).
The role of cytokine production has been investigated in a few influenza vaccine-associated adverse events. A recent Australian study demonstrated significantly higher levels of gamma interferon (IFN-γ)-induced protein 10 (IP-10) and macrophage inflammatory protein 1 alpha (MIP-1α) in children presenting with febrile convulsions after trivalent influenza vaccine (TIV) immunization than in healthy controls (6). In vitro stimulation of peripheral blood mononuclear cells (PBMCs) using the same influenza vaccine as had been administered to the subjects resulted in significantly higher levels of IFN-α, IL-1β, IL-6, IL-10, IP-10, and MIP-1α than with use of other TIV vaccines, suggesting that this pyrogenic response was related to a component of the implicated vaccine (6). Skowronski et al. (7) conducted a study to assess the association between in vitro cytokine balance (after stimulation of PBMCs) and clinical ORS 6 months after influenza vaccination; significantly more IFN-γ was produced by individuals who received the influenza vaccine than by nonvaccinated individuals, but the data failed to show any significant difference in IFN-γ levels between ORS-affected and -unaffected vaccinees.
To our knowledge, no studies have been done to assess cytokine responses in vivo during the acute symptom phase of ORS or other allergy-like AEFI. A preseason evaluation in Canada of the 2010/2011 TIV in adults identified a small number of cases that met the ORS criteria. We aimed to evaluate a broad panel of in vivo inflammatory mediators in subjects with acute ORS symptoms compared to unaffected individuals following vaccination. We also aimed to evaluate hemagglutination inhibition (HAI) antibody responses in subjects experiencing ORS compared to unaffected individuals following seasonal TIV vaccination, as titers might differ between those with and without ORS.
MATERIALS AND METHODS
Study design.
This was a prospective observational study conducted during employee influenza immunization campaigns between October and December 2010 at two participating Canadian centers. The study was approved by the research ethics board of each center, and each participant provided informed consent.
Study population.
Adults aged 20 to 65 years who experienced ORS shortly after receiving the seasonal influenza vaccine (Fluviral, GlaxoSmithKline, Inc.) and who were still symptomatic were enrolled as cases at one study center (Vancouver). Similarly vaccinated adults without symptoms were enrolled as controls at two study centers (Halifax and Vancouver). To identify cases, participants were given an information card containing a list of ORS symptoms at occupational health-based influenza immunization clinics and were asked to call a study nurse by telephone if they experienced any of the listed symptoms after immunization.
Adults who reported postimmunization symptoms were eligible as cases if they experienced symptoms consistent with ORS starting 4 to 48 h after vaccination that were still present at the time of the first blood draw. Control subjects (those who were asymptomatic) were recruited from employee immunization clinics.
ORS cases were defined according to the 2001-2002 National Advisory Committee on Immunization ORS criteria (8), with the exclusion of the presence of coryza, to minimize the possibility of enrolling cases with symptoms related to infection. Adults were excluded if they had received blood or any blood-derived products within the past 3 months, had an active disease of the immune system (such as transplantation, HIV infection, or congenital immune defects), or received immunosuppressive medications (chemotherapeutic agents for the treatment of cancer or autoimmune diseases, or systemic steroids for >2 weeks). Adults were also excluded if they had recovered from ORS symptoms, had any preexisting ocular or respiratory symptoms at the time of vaccination, or developed symptoms in <4 h after vaccination.
Study procedures.
A blood sample was drawn at visit 1 to measure inflammatory cytokines and HAI titers during the acute disease phase (for cases) or approximately 4 to 72 h postimmunization (for controls). A second blood sample was obtained 21 to 28 days postimmunization (visit 2) to measure inflammatory mediators and HAI titers. Case subjects were given memory aids during the first visit to record the perceived severity as mild (present, but does not interfere with daily activities), moderate (interferes with daily activities, but does not prevent them), or severe (prevents daily activities), date of maximum intensity, and duration of their symptoms for 7 days after vaccination. They were also asked to record any associated general symptoms (malaise, myalgia, arthralgia, fatigue, headache, rash, and itchiness) and to document the occurrence of any new symptoms, including a runny nose, after the first visit. Cases were contacted by telephone 7 days after vaccination for safety debriefing and to assess the resolution of symptoms. Those with ongoing symptoms were asked to continue documenting their symptoms, and their records were collected at the second visit. A third blood sample was drawn to measure select inflammatory cytokines 9 to 12 months after TIV immunization.
Subject characteristics, including demographic data, underlying medical conditions (including asthma or allergies), medication received prior to the first blood draw, previous influenza immunization history (including the pandemic influenza vaccine and/or seasonal influenza vaccine), and history of ORS were documented for all participants.
Blood sample processing.
Blood samples were processed promptly to recover plasma and serum fractions, which were stored at −70°C pending subsequent assays. Samples were transferred between sites on dry ice. Laboratory personnel were blinded to the case and control statuses of the participants. Paired samples were analyzed concurrently.
Plasma cytokines.
Cytokines were measured in pg/ml using a commercial kit (Milliplex MAP human cytokine/chemokine, premixed 39-plex; Millipore, Billerica, MA) on the Luminex platform (Luminex, Austin, TX), as described in previous studies (9). Undetectable cytokines were assigned a value of half the minimal level of detection. Samples were analyzed for 39 different plasma cytokines: epidermal growth factor (EGF), eotaxin, basic fibroblast growth factor (FGF-2), FMS-like tyrosine kinase-3 (Flt-3) ligand, fractalkine, granulocyte colony-stimulating factor (G-CSF), granulocyte macrophage colony-stimulating factor (GM-CSF), growth regulated oncogene (GRO), IFN-α2, IFN-γ, IL-10, IL-12 (p40), IL-12 (p70), IL-13, IL-15, IL-17, IL-1Rα, IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IP-10, monocyte chemoattractant protein-1 (MCP-1), MCP-3, macrophage-derived chemokine (MDC) (CCL22), MIP-1α, MIP-1β, transforming growth factor-alpha (TGF-α), TNF-α, TNF-β, vascular endothelial growth factor (VEGF), soluble CD40 ligand (sCD40L), and soluble IL-2 receptor alpha (sIL-2Rα).
Hemagglutination inhibition test (HAI).
Serum samples were tested for the presence of HAI antibodies against the homologous antigens of the 3 influenza strains in the 2010/2011 vaccine (A/California/07/09 [H1N1], A/Perth/16/09 [H3N2], and B/Brisbane/60/08) with chicken red blood cells, following standard procedures (10). Seroprotection was defined as an HAI titer of ≥40.
Statistical analysis.
Descriptive statistics were used to report the baseline characteristics of the participants. Continuous variables were compared using a two-sample Student's t test, and categorical variables were compared using the chi-square or Fisher's exact test.
The primary outcome of the study was the difference in mean individual blood plasma cytokine concentrations between ORS cases and controls at visit 1. To assess the normalization of cytokine concentrations, mean differences between acute and convalescent values (visit 1 − visit 2) were calculated. Logarithmic transformations were used prior to analysis to ensure the assumptions of normality, and comparison was done using a two-sample t test. Calculations included the 95% confidence interval. For the primary outcome, significance was determined using a Bonferroni correction for the adjustment of multiple comparisons; an alpha of 0.0013 was selected as the corrected level of statistical significance.
The secondary outcome was the mean difference in HAI titers between visit 1 and visit 2 for cases and controls. Logarithmic transformation to geometric mean titers (GMT) was done to meet a normality assumption, and the two-sample t test was used. Seroconversion rates, final HAI titers, and GMTs in cases and controls were compared among H1N1, H3N2, and B strains, including the calculation of the 95% confidence intervals. Data analysis was done using SAS 9.2 software (Cary, NC).
RESULTS
Description of study sample.
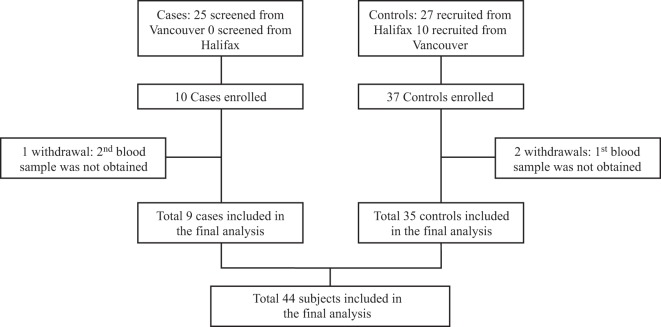
Nine ORS cases and 35 controls were enrolled (Fig. 1), and all attended the follow-up visit between days 21 and 28 postvaccination. All cases came from one center (Vancouver), where the study information was provided to 3,172 immunized individuals. The mean time from vaccination to the first blood draw was similar in cases and controls (49.4 h versus 48.3 h, respectively; P = 0.89). Baseline characteristics were similar between cases and controls in all clinical categories (Table 2). Two of the ORS cases reported a history of ORS following previous influenza immunizations.
Fig 1.
Included cases and controls.
Table 2.
Demographic characteristics of study participants
| Characteristic | Total | ORS cases | Controls | P |
|---|---|---|---|---|
| n | 44 | 9 | 35 | |
| Female (no. [%]) | 26 (59) | 8 (89) | 18 (51) | 0.06 |
| Age (yr) | ||||
| Mean | 42.8 | 45.2 | 42.2 | 0.43 |
| Median | 43.5 | 49.0 | 41.0 | |
| Range | 24–65 | 29–54 | 24–65 | |
| Ethnic background (no. [%]) | ||||
| White | 39 (89) | 8 (89) | 31 (89) | >0.99 |
| Other | 5 (11) | 1 (11) | 4 (11) | |
| Underlying medical conditiona (no. [%]) | 24 (55) | 7 (78) | 17 (49) | 0.15 |
| Hypertension | 3 | 1 | 2 | — |
| Asthma | 6 | 4 | 2 | 0.01 |
| Allergies | 7 | 3 | 4 | 0.14 |
| Other | 17 | 5 | 12 | — |
| Previous ORSb (no. [%]) | 2 (5)c | 2 (22)c | 0 | — |
| Influenza vaccination status (no. [%]) | ||||
| Previous vaccination | 44 (100) | 9 (100) | 35 (100) | — |
| Pandemic 2009/2010 vaccine | 39 (89) | 8 (89) | 31 (89) | >0.99 |
| Seasonal vaccine last year | 40 (91) | 9 (100) | 31 (89) | 0.57 |
| Received medication within the past 24 ha (no. [%]) | 20 (48)e | 6 (86)e | 14(40) | 0.04 |
| NSAIDd | 8 | 4 | 4 | 0.08 |
| Antihistamine | 5 | 2 | 3 | 0.53 |
| Birth control or hormonal therapy | 5 | 2 | 3 | 0.27 |
| Other | 8 | 3 | 5 | — |
Some subjects had more than one condition.
Previous ORS was following a past influenza vaccine. One of the cases experienced ORS twice, and one had it once previously.
One missing response from an ORS case (43 total valid responses, 8 among ORS cases).
NSAID, nonsteroidal anti-inflammatory drugs.
Two missing responses in ORS cases (42 total valid responses, 7 among ORS cases).
Description of AEFI symptoms.
Among the nine ORS cases, the most commonly reported symptom was chest tightness, followed by sore throat, hoarseness, and cough (Table 3). ORS symptoms ranged from mild to moderate in severity and were generally well tolerated. One case who rated her symptoms as moderate reported staying at home for 4 days due to symptoms. This case was the only one with ORS symptoms persisting for >21 days and she had experienced ORS twice previously after TIV vaccination. None of the ORS cases sought medical advice or required hospitalization.
Table 3.
ORS symptoms among the ORS-affected group and their severity
| ORS symptom | No. experiencing symptom (%) (total n = 9) | Median duration (range [days])a | No. (%) experiencing maximum severity of symptomsb |
||
|---|---|---|---|---|---|
| Mild | Moderate | Severe | |||
| Redness in both eyes | 4 (44) | 3 (2 to >21)c | 3 (33) | 1 (11) | 0 |
| Respiratory | |||||
| Coughing | 5 (56) | 4 (3–6) | 3 (33) | 2 (22) | 0 |
| Sore throat | 6 (67) | 3 (2 to >21)c | 3 (33) | 3 (33) | 0 |
| Hoarseness | 5 (56) | 4 (3–6) | 4 (44) | 1 (11) | 0 |
| Wheezing | 2 (22) | 4.5 (3–6) | 1 (11) | 1 (11) | 0 |
| Chest tightness | 7 (78) | 4 (2–7) | 5 (56) | 2 (22) | 0 |
| Difficult breathing | 1 (11) | 4 | 0 | 1 (11) | 0 |
| Difficulty swallowing | 3 (33) | 8 (2–17) | 0 | 3 (33) | 0 |
| ≥2 symptoms | 8 (89) | — | — | — | — |
| Facial swelling | |||||
| Lip swelling | 1 (11) | 2 | 0 | 1 (11) | 0 |
| Other swelling | 0 | — | — | — | — |
All cases had onset of symptoms 4 to 48 h after vaccination, as defined by the study protocol.
Mild, symptom(s) was present but did not interfere with daily activities; moderate, symptom(s) interfered with daily activities but did not prevent an individual from engaging in them; severe, symptom(s) prevented engagement in daily activities.
One subject had ongoing symptoms at 21 days postvaccination; the end date of those symptoms is unknown.
Seven of the ORS cases (78%) had systemic symptoms following vaccination, with fatigue (67%), headache (56%), and myalgia/malaise (44%) reported most commonly. Most of these cases rated their symptoms as mild or moderate, with only four reporting severe symptoms (two with headache, one with malaise, and one with itchiness).
Description of study outcomes. (i) Cytokine levels.
At visit 1, we found that the mean production level of IL-10 in ORS-affected cases was higher than that in controls, 33.6 pg/ml (95% confidence interval [CI], 22.4 to 50.5) versus 4.9 pg/ml (2.5 to 9.5), respectively (P < 0.0001), as was the mean IL-3 concentration: 26.8 pg/ml (95% CI, 16.3 to 44.1) versus 6.0 pg/ml (3.41 to 10.7) (P = 0.0002) (Fig. 2). We found a slightly higher concentration of MCP-1 (P = 0.0049) in cases than in controls during acute disease, although the difference fell short of statistical significance. None of the other 36 cytokine concentration levels evaluated were found to be elevated in cases compared with controls. Of note, the concentrations of IL-10, IL-3, and MCP-1 remained elevated in cases (P < 0.0001, P = 0.0002, and P = 0.001, respectively) 21 to 28 days after vaccination. Seven ORS cases had an additional blood draw 9 to 12 months after vaccination to measure IL-10 and IL-3 concentrations; no change in the mean cytokine levels was evident (data not shown).
Fig 2.
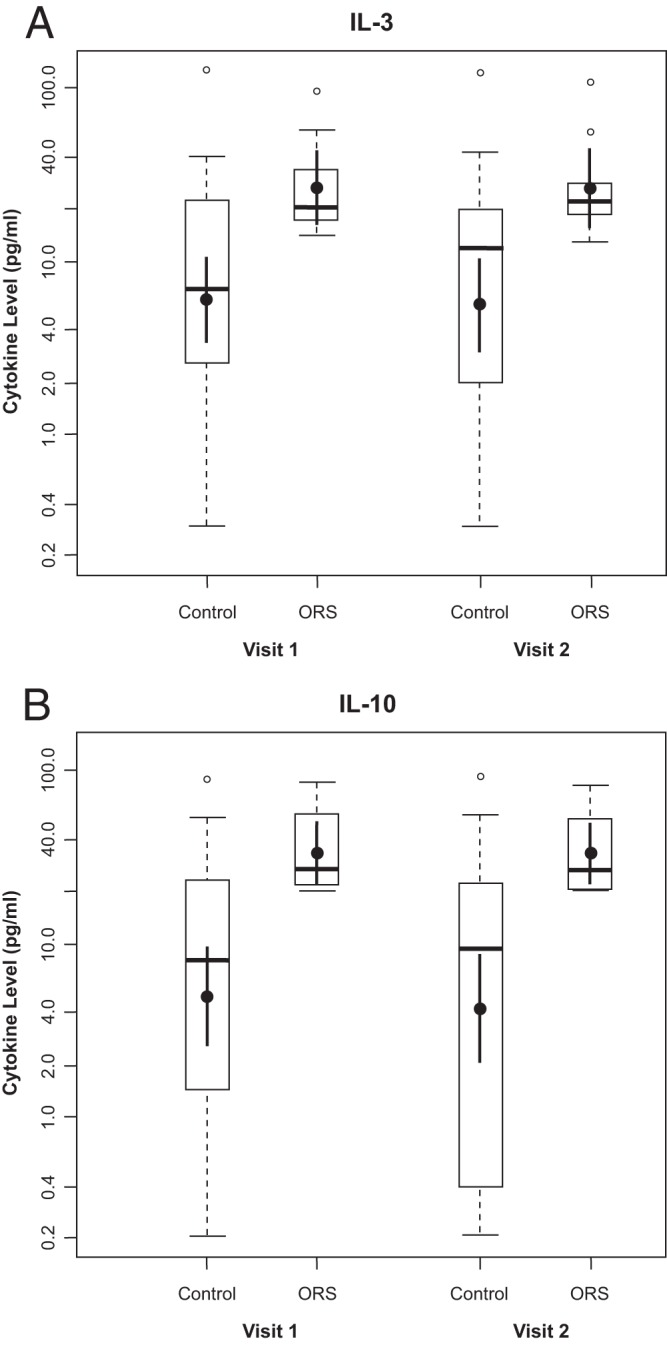
IL-10 and IL-3 levels at visits 1 and 2 for ORS cases and controls. (A and B) Mean and median plasma levels (pg/ml) (means and 95% confidence intervals are shown with closed circles and vertical lines, respectively, and medians and quartiles are shown with horizontal lines and boxes, respectively). Open circles represent outliers, which are values more than 1.5 times higher than 75% of the values.
To determine if certain cytokine concentrations were acutely elevated following vaccination in both cases and controls, the mean difference in measured values between the first and second samples was computed for all 39 measured cytokines. No significant change (including decline) in values was evident with any cytokine (data not shown).
(ii) Hemagglutination inhibition (HAI) antibody response.
Seroprotection rates were similar in cases and controls for all three strains contained in the influenza vaccine. In general, the baseline seroprotection rate was higher for the B strain than the A strain in both cases and controls. The postimmunization seroprotection rates were highest for the B strains (100% and 100%), followed by H1N1 (100% and 88%) and H3N2 (67% and 57%) in cases and controls, respectively, but the differences between the groups were not significant. The fold increase in GMTs was not significantly different between ORS cases and controls for the H1N1, H3N2, or B influenza strains (Fig. 3).
Fig 3.
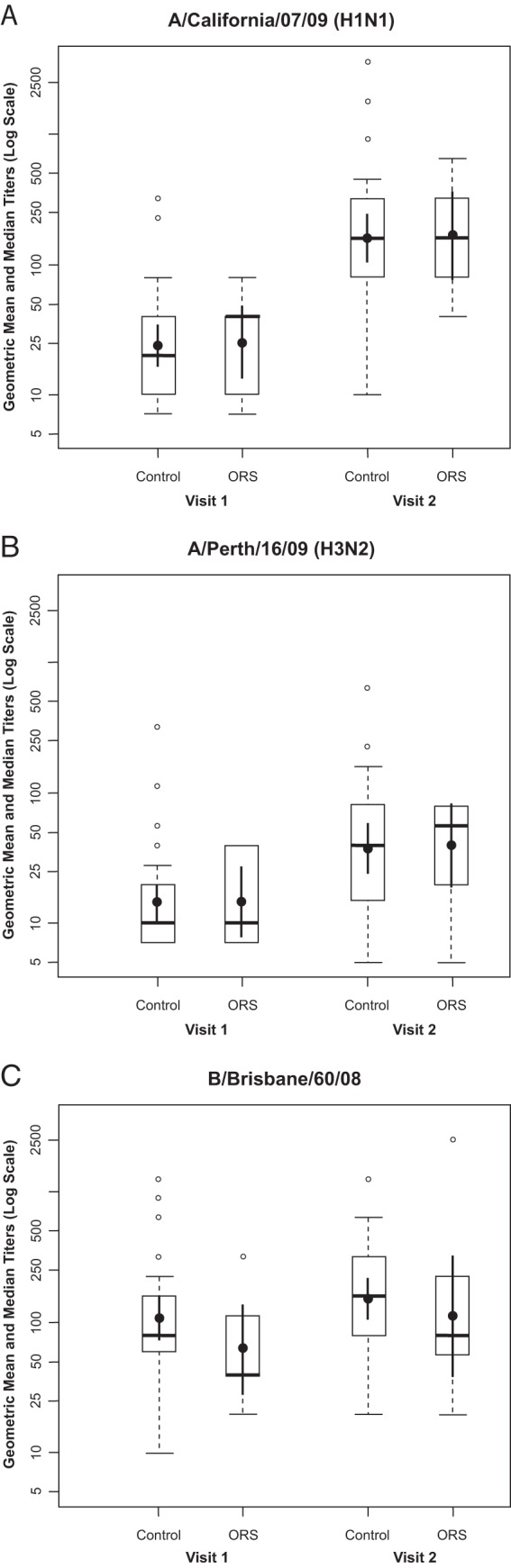
Hemagglutination inhibition (HAI) antibody response in ORS cases and controls. Means and 95% confidence intervals are shown with closed circles and vertical lines, respectively, and medians and quartiles are shown with horizontal lines and boxes, respectively. Open circles represent outliers, which are values more than 1.5 times higher than 75% of the values. (A) Mean and median HAI titers for the A/California H1N1 strain; (B) mean and median HAI titers for the A/Perth H3N2 strain; (C) mean and median HAI titers for the B/Brisbane strain.
DISCUSSION
The mechanism for developing ORS post-influenza immunization is not well understood. Our results indicate that ORS cases had greater concentrations of particular cytokines (IL-10 and IL-3) than did controls. No elevations were evident between cases or controls for the IL-4, IL-5, IL-13, IL-1α, IL-8, TNF-α, IFN-γ, or IL-17A cytokines. However, the cytokines that were elevated in symptomatic ORS cases were still high 21 to 28 days and 9 to 12 months later. This suggests that the symptoms of ORS in affected subjects were not due to a sudden increased production of inflammatory cytokines in response to influenza vaccination. Instead, elevated concentrations of these cytokines may represent a biomarker for susceptibility to ORS without necessarily playing a direct role in its pathogenesis.
We also found that there was no difference in the mean antibody response from baseline to 21 to 28 days after immunization in ORS cases compared to controls for any of the three influenza strains contained in the vaccine; this indicates that ORS does not result in higher antibody responses. The increase in cytokines may be related to genetically determined differences in the baseline production of these two cytokines.
The mechanism by which IL-10 and IL-3 might be associated with the development of ORS is not clear. IL-3 has been described to play an important role in inducing chronic inflammation by supporting cell-mediated immune responses, and it is also involved in eosinophil activation, B-cell differentiation, and control of IgE synthesis (11). In contrast, IL-10 is a potent anti-inflammatory cytokine with several important immunoregulatory functions (12). It has the capability of inhibiting proinflammatory cytokines, such as TNF-α, IL-1, and IL-6. IL-10 also has an anti-inflammatory effect on eosinophils, basophils, and mast cells, and thus, it plays a major role in the control and regulation of allergy and asthma (12, 13). It is thus difficult to implicate these cytokines in specific cause-and-effect relationships with ORS.
An association between host genetic factors and the development of AEFI has been hypothesized (14–16). Stanley et al. (19) demonstrated that certain haplotypes in the IL-1 gene complex and in the IL-18 gene were associated with the development of fever after smallpox vaccination, whereas a haplotype in the IL-4 gene was associated with a significant reduction in susceptibility to such an event. In addition, Vestergaard et al. (15) found that at 15 to 17 months of age, the risk difference of febrile seizures within 2 weeks following measles, mumps, and rubella (MMR) vaccination was 3.97 per 1,000 (95% CI, 2.90 to 5.40) for siblings of children with a history of febrile seizures compared to siblings of children with no history of febrile seizure, strongly suggesting an underlying genetic predisposition. Finally, the Finnish data, which found the occurrence of narcolepsy following immunization with a pandemic influenza vaccine to be increased among individuals with a particular genetic factor (human leukocyte antigen DQB1*0602 allele), support a potential genetic basis for this rare adverse event (17).
Our study was exploratory, and its major limitation is its small sample size. Our numbers in this pilot study were insufficient to detect all but the greatest differences between cases and controls for most cytokines. The reliance on self-reporting of ORS symptoms is another limitation of our study. This limited our ability to identify all ORS cases that occurred during the campaign season. It is unclear to us why no ORS cases were documented in Halifax, as the same vaccine was used in both provinces; this may be due to an actual difference in the incidence of ORS between Nova Scotia and British Columbia and deserves further investigation. Finally, although our proportion of females to males was not significantly unbalanced among cases and controls, female predominance in ORS cases has been documented in all previous reports (1–3, 8, 16, 18), and the effect of gender should be examined in future studies.
This study demonstrates that in adults with ORS, a persistent elevation in blood plasma levels of specific cytokines (IL-10 and IL-3) compared to vaccine recipients without ORS can be detected. The persistent nature of the elevation suggests that underlying host factors may predispose certain individuals to develop ORS following influenza vaccination. Given the exploratory nature of our data, further investigation of these phenomena in a study with a larger sample size may be warranted.
ACKNOWLEDGMENTS
The Public Health Agency of Canada and the Canadian Institutes of Health Research provided the funding for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. T.R.K. is supported in part by a Burroughs Wellcome Career Award in the biomedical sciences. T.R.K and J.A.B. are supported by Michael Smith Foundation for Health Research Career Investigator Awards.
The Canadian Association for Immunization Research and Evaluations (CAIRE) provided networking assistance for this study. We gratefully acknowledge the expert assistance provided by the Vaccine Evaluation Center (Carol LaJeunesse, Arlene Kallos, Kim Marty, and Shu Yu Fan), the Canadian Center for Vaccinology, and the National Microbiology Laboratory. We also acknowledge the laboratory personnel and technicians at BC Children's Hospital, the Wadsworth Center, and the National Microbiology Laboratory, as well as all participants in the study. Mona Al-Dabbagh, Keswadee Lapphra, and Patricia Cho were PCIRN trainees.
M.A.-D., J.A.B., T.R.K., P.C., K.L., E.S.F., and Y.L. disclose no conflicts of interest. D.W.S., S.A.H., and J.M.L. disclose that they have received funding from GlaxoSmithKline, Sanofi Pasteur, and Novartis Vaccines for performance of other influenza-related clinical trials and have served on ad hoc advisory boards for these companies. G.D.S. has received funding from GSK and Sanofi Pasteur for clinical trials not related to influenza and reimbursement for travel expenses to attend a GSK advisory board meeting.
Footnotes
Published ahead of print 22 May 2013
REFERENCES
- 1. Skowronski DM, Strauss B, De Serres G, MacDonald D, Marion SA, Naus M, Patrick DM, Kendall P. 2003. Oculo-respiratory syndrome: a new influenza vaccine-associated adverse event? Clin. Infect. Dis. 36:705–713 [DOI] [PubMed] [Google Scholar]
- 2.Public Health Agency of Canada 2001. An Advisory Committee Statement (ACS). National Advisory Committee on Immunization (NACI). Statement on influenza vaccination for the 2001-2002 season. Can. Commun. Dis. Rep. 27:1–24 [PubMed] [Google Scholar]
- 3. De Serres G, Boulianne N, Duval B, Rochette L, Grenier JL, Roussel R, Donaldson D, Tremblay M, Toth E, Ménard S, Landry M, Robert Y. 2003. Oculo-respiratory syndrome following influenza vaccination: evidence for occurrence with more than one influenza vaccine. Vaccine 21:2346–2353 [DOI] [PubMed] [Google Scholar]
- 4. Skowronski DM, De Serres G, Hebert J, Stark D, Warrington R, Macnabb J, Shadmani R, Rochette L, MacDonald D, Patrick DM, Duval B. 2002. Skin testing to evaluate oculo-respiratory syndrome (ORS) associated with influenza vaccination during the 2000-2001 season. Vaccine 20:2713–2719 [DOI] [PubMed] [Google Scholar]
- 5. Spellberg B, Edwards JE., Jr 2001. Type 1/type 2 immunity in infectious diseases. Clin. Infect. Dis. 32:76–102 [DOI] [PubMed] [Google Scholar]
- 6. Blyth CC, Currie AJ, Wiertsema SP, Conway N, Kirkham LA, Fuery A, Mascaro F, Geelhoed GC, Richmond PC. 2011. Trivalent influenza vaccine and febrile adverse events in Australia, 2010: clinical features and potential mechanisms. Vaccine 29:5107–5113 [DOI] [PubMed] [Google Scholar]
- 7. Skowronski DM, Lu H, Warrington R, Hegele RG, De Serres G, HayGlass K, Stark D, White R, Macnabb J, Li Y, Manson HE, Brunham RC. 2003. Does antigen-specific cytokine response correlate with the experience of oculorespiratory syndrome after influenza vaccine? J. Infect. Dis. 187:495–499 [DOI] [PubMed] [Google Scholar]
- 8.Public Health Agency of Canada 2001. An Advisory Committee Statement (ACS). National Advisory Committee on Immunization (NACI). Supplementary statement for the 2001-2002 season: influenza vaccination of persons who experienced oculo-respiratory syndrome following previous influenza vaccination. Can. Commun. Dis. Rep. 27:1–7 [PubMed] [Google Scholar]
- 9. Kollmann TR, Crabtree J, Rein-Weston A, Blimkie D, Thommai F, Wang XY, Lavoie PM, Furlong J, Fortuno ES, III, Hajjar AM, Hawkins NR, Self SG, Wilson CB. 2009. Neonatal innate TLR-mediated responses are distinct from those of adults. J. Immunol. 183:7150–7160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kendal AP, Pereira MS, Skehel J. 1982. Concepts and procedures for laboratory-based influenza surveillance. World Health Organization, Geneva, Switzerland [Google Scholar]
- 11. Feghali CA, Wright TM. 1997. Cytokines in acute and chronic inflammation. Front. Biosci. 2:d12–d26 [DOI] [PubMed] [Google Scholar]
- 12. Donnelly RP, Dickensheets H, Finbloom DS. 1999. The interleukin-10 signal transduction pathway and regulation of gene expression in mononuclear phagocytes. J. Interferon Cytokine Res. 19:563–573 [DOI] [PubMed] [Google Scholar]
- 13. Chung F. 2001. Anti-inflammatory cytokines in asthma and allergy: interleukin-10, interleukin-12, interferon-gamma. Mediators Inflamm. 10:51–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Poland GA, Ovsyannikova IG, Jacobson RM. 2008. Personalized vaccines: the emerging field of vaccinomics. Expert Opin. Biol. Ther. 8:1659–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vestergaard M, Hviid A, Madsen KM, Wohlfahrt J, Thorsen P, Schendel D, Melbye M, Olsen J. 2004. MMR vaccination and febrile seizures: evaluation of susceptible subgroups and long-term prognosis. JAMA 292:351–357 [DOI] [PubMed] [Google Scholar]
- 16. De Serres G, Toth E, Ménard S, Grenier JL, Roussel R, Tremblay M, Landry M, Robert Y, Rochette L, Skowronski DM. 2005. Oculo-respiratory syndrome after influenza vaccination: trends over four influenza seasons. Vaccine 23:3726–3732 [DOI] [PubMed] [Google Scholar]
- 17.National Institute for Health and Welfare 2011. National Narcolepsy Task Force Interim Report. National Institute for Health and Welfare (THL), Helsinki, Finland: http://www.thl.fi/thl-client/pdfs/dce182fb-651e-48a1-b018-3f774d6d1875 [Google Scholar]
- 18. Ahmadipour N, Tam T, Walop W, King A, Pless R. 2005. Oculo-respiratory syndrome following influenza vaccination: review of post-marketing surveillance through four influenza seasons in Canada. Can. Commun. Dis. Rep. 31:217–225 [PubMed] [Google Scholar]
- 19. Stanley SL, Jr, Frey SE, Taillon-Miller P, Guo J, Miller RD, Koboldt DC, Elashoff M, Christensen R, Saccone NL, Belshe RB. 2007. The immunogenetics of smallpox vaccination. J. Infect. Dis. 196:212–219 [DOI] [PubMed] [Google Scholar]